Abstract
Dysregulation of insulin action is most often considered in the context of impaired glucose homeostasis, with the defining feature of diabetes mellitus being elevated blood glucose concentration. Complications arising from the hyperglycemia accompanying frank diabetes are well known and epidemiological studies point to higher risk toward development of metabolic disease in persons with impaired glucose tolerance. Although the central role of proper blood sugar control in maintaining metabolic health is well established, recent developments have begun to shed light on associations between compromised insulin action [obesity, prediabetes, and type 2 diabetes mellitus (T2DM)] and altered intermediary metabolism of fats and amino acids. For amino acids, changes in blood concentrations of select essential amino acids and their derivatives, in particular BCAA, sulfur amino acids, tyrosine, and phenylalanine, are apparent with obesity and insulin resistance, often before the onset of clinically diagnosed T2DM. This review provides an overview of these changes and places recent observations from metabolomics research into the context of historical reports in the areas of biochemistry and nutritional biology. Based on this synthesis, a model is proposed that links the FFA-rich environment of obesity/insulin resistance and T2DM with diminution of BCAA catabolic enzyme activity, changes in methionine oxidation and cysteine/cystine generation, and tissue redox balance (NADH/NAD+).
The underlying mechanisms that trigger and exacerbate obesity-associated insulin resistance and transition to frank T2DM3 remain elusive and continue to spark great controversy. Historically, most considerations of the problem have focused on blood sugar control. Indeed, the defining clinical characteristic of insulin resistance and T2DM is disruption of normal glucose homeostasis viz. abnormally low tissue glucose utilization and increased hepatic glucose output. In the clinic, prediabetes and T2DM are widely diagnosed by the FPG concentration (e.g. 126 mg/dL < FPG ≥ 100 mg/dL for prediabetics; FPG ≥ 126 mg/dL for diabetics; ADA guidelines). Chronic hyperglycemia is associated with diabetes complications, including diabetic neuropathy, retinopathy, renal disease, and cardiovascular disease. It follows that the majority of studies aimed at understanding the causes and consequences of insulin resistance and frank T2DM have focused on glucose intermediary metabolism, the mechanisms of insulin-stimulated tissue glucose uptake, and associations between hyperglycemia and pathophysiology. This body of work has been crucial in identifying the components of the canonical insulin-signaling pathway and mechanisms of glucose uptake in cells, and to discover mechanisms driving glucose-stimulated insulin secretion and characterize the natural history and epidemiology of T2DM. Risk toward development of T2DM is significantly higher in overweight and obese persons [e.g. (1)], suggesting that chronic positive energy balance and increased storage of energy as fat are mechanistically linked to impaired glucose homeostasis and development of diabetes. Yet millions in the overweight to moderately obese population have perfectly normal blood glucose, remaining undiagnosed as prediabetics despite underlying dysfunctional metabolism. This highlights the need to look beyond glucose metabolism alone when considering the etiology and consequences of insulin resistance and T2DM.
There is a growing appreciation that insulin resistance and T2DM are conditions of a broad perturbation of metabolic physiology involving considerable changes in fat and amino acid metabolism in addition to glucose. This perspective forms the basis of the current review. Until recently, very little attention has been paid to studying the impact of insulin resistance and T2DM on protein and amino acid metabolism. The goal herein is to provide a synthesis of recent large-scale metabolite profiling studies that have placed a spotlight on the important alterations in essential amino acid metabolism that mark the obese, insulin-resistant phenotype and that track worsening blood sugar control. Integrated with the historical biochemistry literature, a picture will emerge that raises the possibility that, at least for a subset of the essential amino acids, insulin resistance and obesity markedly alters their oxidative catabolism and metabolic fates and leads to a convergence between otherwise disparate metabolic pathways. Although the factors that regulate these aberrant amino acid patterns remain to be confirmed, evidence will be presented that at least some of the observations might be explained by changes in α-ketoacid dehydrogenase catabolic enzyme activities and redox status in metabolically influential tissues such as adipose and liver. The aim is that this model will form a framework for discussion and debate in this area. Finally, several questions will be posed: What are the (patho)physiologic ramifications of altered amino acid catabolism in the obese, insulin-resistant or type 2 diabetic state? Can amino acid-related patterns be leveraged to predict metabolic health and disease or to track the efficacy of interventions designed to thwart or reverse T2DM? Would diets high in protein or rich in BCAA harm or benefit metabolic health?
Current Status of Knowledge
Blood patterns of essential amino acids and their derivatives in obese, insulin-resistant, and diabetic states
Recent metabolomics-based studies using unbiased comprehensive metabolite profiling approaches and more targeted analyses focusing specifically on amino acids and their metabolites have consistently revealed perturbation of normal amino acid metabolism in obese, insulin-resistant states and T2DM. These changes manifest as increases in blood and urine concentrations of specific amino acid classes, meaning that the changes observed do not seem to reflect a global disruption of all amino acid metabolism in these conditions. This section of the review will highlight the most pronounced and consistent alterations of essential amino acid patterns observed in the literature in obesity and insulin resistance. Because the vast majority of published amino acid metabolite profiles in obesity and insulin resistance have focused on samples derived from the overnight-to-extended fasting situation, most of the discussion and interpretation in this section does not consider the immediate postprandial effect of dietary protein on metabolite patterns.
BCAA.
Obesity, insulin resistance, and T2DM are conditions associated with elevated blood concentrations of one or more of the BCAA, BCKA, and/or carnitine esters derived from partial BCAA catabolism in human adults. This phenomenon has been described by many researchers (2–9) since the seminal observations in the late 1960s by Adibi (10) and Felig, Marliss, and Cahill (11). Higher BCAA blood levels have also been described in obese children (12). Increased blood BCAA levels are common in rodent models of obesity (4, 13–17). Because insulin resistance is more prevalent in overweight to obese individuals, however, it is important to consider if higher circulating BCAA and derivatives in the aforementioned studies were also tracking insulin resistance and not just adiposity per se.
Alteration of normal BCAA metabolism leading to elevated blood concentrations of BCAA and their derivatives appears to be an early manifestation of insulin resistance. Cahill et al. (11) found highly significant correlations between fasting plasma insulin concentration and levels of valine, isoleucine, and leucine in a cohort of 20 nonobese and obese individuals. Plasma analyzed from a large nondiabetic cohort (Relationship of Insulin Sensitivity to Cardiovascular Risk) revealed that isoleucine and isovalerylcarnitine concentrations were negatively correlated with insulin sensitivity as measured by the hyperinsulinemic-euglycemic clamp method in nondiabetics (7). Studying a group of participants spanning normal glucose tolerance, impaired glucose tolerance, and T2DM, Menge et al. (8) demonstrated a significant correlation between plasma valine concentration and the Homeostatic Model Assessment insulin resistance score. Studies that utilized age- and BMI-matched, obese, nondiabetic and T2DM participants (6) or that statistically adjusted for age and BMI (9, 18) revealed strong associations between plasma leucine and HbA1c% (a marker of poor blood sugar control) between predisease blood BCAA levels and risk for developing future T2DM, or between plasma BCAA concentrations and the HOMA insulin resistance score. Variances in a subset of plasma metabolites (principal components factor) including the BCAA had a significant correlative association (corrected for age, gender, and waist circumference) with insulin sensitivity variables in sedentary, overweight, nondiabetic men and women: e.g. more insulin resistance was reflected by increasing blood BCAA concentrations (5). Potential mechanisms that drive the relationship between elevated blood BCAA and their derivatives with the insulin-resistant state are discussed later in this review.
Methionine and cysteine-cystine.
In the obese, insulin-resistant state and T2DM, increased circulating concentrations of one or more of the sulfur-containing amino acids, methionine and its catabolic derivative cysteine (and/or its dimeric derivative cystine, formed from oxidation of cysteine residues), have been reported by a number of groups. Probably the best example of a link between obesity and blood concentrations of tCys was the cross-sectional examination of >16,000 individuals participating in the Hordaland Homocysteine Study; the plasma concentration of tCys was highly correlated with BMI (19) and changes in BMI over time were correlated with changes in plasma tCys (20). Subsequent analysis by the same group using body composition data from a subset of participants established that individual differences in plasma tCys levels were associated with body fat and not lean body mass [(21); also see (22)]. Comprehensive metabolite profiling studies have indicated that differences in plasma concentrations of cysteine (7) or methionine (as a component of a metabolite “cluster,” or principal components factor) (5, 9) track insulin resistance and/or predict risk toward developing T2DM. Plasma methionine levels were highly correlated with predisease BCAA concentrations in >2000 persons for which the latter metabolites were predictive of T2DM development (18). Plasma cystine concentration significantly increased in T2DM participants compared to nondiabetics in a cohort of BMI- and age-matched obese African-American women, and although not significant, the plasma cysteine and methionine levels were 21 and 16% higher, respectively (6). Other studies using small numbers of obese nondiabetic individuals also found modest increases of methionine of 9% (10) to 18–22% (8, 11) compared to nonobese controls; increases in plasma cysteine/cystine were 10–14% (10, 11). Taken together, the aggregate of results from the literature supports the idea that in situations characterized by suboptimal insulin action, sulfur amino acid tissue metabolism is altered, reflected by sometimes subtle increases in concentrations of methionine and cysteine/cystine in the blood. As we will see, changes in sulfur amino acid metabolism may intersect with pathways involved with BCAA and phenylalanine/tyrosine catabolism.
Phenylalanine and tyrosine.
Increased circulating concentrations of phenylalanine and the “conditionally essential” amino acid, tyrosine, have often been reported to be increased in the obese, insulin-resistant, or T2DM state in humans and changes in these metabolites often go hand in hand. Similar to the sulfur amino acids, relative increases in plasma levels with insulin resistance can be modest, e.g. ∼15–25% for tyrosine in insulin-resistant obese participants (4, 8, 10, 11). In metabolomics studies, differences in phenylalanine and/or tyrosine concentrations were factors that differentiated groups in comparisons of obese vs. nonobese human participants (4) or insulin-sensitive compared to insulin-resistant individuals (9). Variance in plasma phenylalanine concentration (which was modestly increased in T2DM) contributed to separation of nondiabetics compared to T2DM participants in a principal components analysis (6). In studies determining correlations between blood metabolite levels and insulin sensitivity in a cross-sectional study (5) or using predisease metabolites to predict development of T2DM (18), phenylalanine and tyrosine provided some of the strongest associations. In rodents, the plasma phenylalanine concentration was 55% higher in young, insulin-resistant, ZDF fa/fa rats compared to nonobese Fa/? rats, but this variable did not differ in older diabetic animals and tyrosine levels were not altered under either condition (13). Overall, results to date point to perturbation of phenylalanine and tyrosine metabolism in the insulin-resistant condition, and because tyrosine is the first product of phenylalanine catabolism, it is not surprising that in many reports the directionality of their blood concentration shifts are the same.
Other essential amino acids.
There is little consistent evidence from blood amino acid patterns that obesity, insulin resistance, or T2DM result in marked alterations in histidine, lysine, threonine, or tryptophan metabolism. There were correlations between plasma concentrations of these metabolites and those of the T2DM-predictive BCAA and phenylalanine/tyrosine analytes in predisease baseline samples derived from the Framingham Offspring Study; however, histidine, lysine, threonine, and tryptophan patterns were not associated with subsequent risk for developing diabetes (18). Differences in plasma histidine concentration contributed to a multi-metabolite factor that correlated with insulin resistance, but the specific impact of histidine on this factor was modest (5). A significantly increased plasma concentration of histidine, but not lysine, threonine, or tryptophan, was observed in an obese cohort of T2DM African American women compared to nondiabetic obese controls (6). Thus, on balance, the literature indicates that with the exception of histidine, which might have a modestly altered metabolism in some cases, obesity, insulin resistance, or T2DM have little effect on the metabolism of these essential amino acids.
Origins of essential amino acid patterns in insulin resistance and T2DM: on the potential role of cellular redox balance, oxidative stress, and the #x03B1-ketoacid dehydrogenase complexes
The blood concentration patterns of amino acids in the fasted state are affected by the rates of tissue protein catabolism/release of amino acids to the extracellular space, tissue-specific uptake, oxidative metabolism of amino acids or their derivatives, protein synthesis rates, and excretion of amino acids and their catabolic end-products. This complexity introduces interpretive limitations when one uses amino acid concentration data derived from a static sampling of the blood pool to understand how obesity, insulin resistance, and diabetes influence the dynamics of amino acid metabolism in vivo. Nevertheless, such studies provide a foundation from which to form hypotheses regarding tissue amino acid and protein metabolism and flux under these conditions, and some assumptions may be made from physiological considerations that further aid in interpretation of the origins of blood amino acid patterns. Because most studies that have examined blood amino acid patterns in obesity and T2DM have been conducted in the overnight- to extended-fasting state and there is no evidence that obesity or insulin resistance alters renal processing of blood amino acids (23), observed blood amino acid patterns are not likely a direct reflection of dietary-derived amino acids or differences in urinary excretion of these metabolites. It is not likely that differences in protein synthesis rates play a major role in driving blood amino acid differences, because under fasted conditions net protein anabolism would be minimized compared to the postprandial fed state.
Protein and lean body mass catabolism in the obese, insulin-resistant, or T2DM state could also influence amino acid patterns in the blood. The literature in this area is conflicting, with several studies from Gougeon et al. (24–27) indicating a lower nitrogen balance and/or greater leucine turnover in untreated T2DM that is normalized with insulin treatment and others suggesting no effect in obesity and T2DM when comparing weight-matched individuals (3, 28–33). Thus, on balance, it appears that in T2DM with reasonably controlled blood sugar, lean body mass catabolism differences may not fully explain increased blood concentrations of BCAA and a select few amino acids in the obese, insulin-resistant or diabetic state.
Thus, for purposes of discussion, the focus shall turn to how impaired insulin action and T2DM could affect intracellular-level alterations in the oxidative metabolism rates of select essential amino acids, which would in theory influence circulating concentrations of metabolites reflective of these processes. These perspectives are largely influenced by classic metabolic “crossover” analysis concepts in which a reduction in the activity of a specific enzyme within a biochemical pathway increases and decreases tissue precursor and product concentrations, respectively. The idea that for at least some tissues and select essential amino acids the obese and insulin-resistant or T2DM state (and attendant high FFA availability) is characterized by more limited tissue utilization and oxidative metabolism of amino acids stems from several observations. A recent metabolite profiling paper revealed that the magnitudes of reductions in plasma methionine and BCAA concentrations following an oral glucose tolerance test were inversely correlated with insulin sensitivity indices (34). There are some reports indicating lower plasma (35) or tissue (4, 36) carnitine derivatives of tissue propionyl-CoA in T2DM humans and high-fat–fed rodents, respectively, which would be consistent with limited generation of this metabolite from sources such as valine, isoleucine, or methionine. Furthermore, protein and BCAA oxidation are inversely proportional to FFA oxidation in vivo [e.g. (37, 38)], and short- to long-term–fasted obese rodents (38, 39) and humans (40, 41) display a reduced use of protein for energy compared to their nonobese counterparts. That said, under fed or postabsorptive conditions, the results are not so clear. For instance, whole-body BCAA clearance was reportedly ∼20% reduced in T2DM participants (23), but other reports indicated that leucine oxidation or amino acid turnover is not impaired in obesity or T2DM (27, 29–31, 42–44). Clearly, more research is needed to understand tissue-specific BCAA oxidation dynamics in obesity and with changes in insulin action. Teleologically, a diminution of whole-body essential amino acid utilization during times of compromised or low insulin action and robust FFA oxidation, such as fasting, would be a physiological response to spare these crucial metabolites for reuse in anabolic or other necessary processes.
Potential impact of reduced BCKD in obesity and insulin resistance.
One puzzling feature of blood amino acid patterns altered in the insulin-resistant or diabetic state is that only a select number of amino acids are consistently reported to be affected by these conditions, viz. BCAA, sulfur-containing amino acids, and phenylalanine and tyrosine. At first blush, these amino acids appear to follow quite disparate biochemical catabolic paths, so how can one explain shared directionality in their concentrations in the blood in the insulin-resistant state? The biochemistry literature would suggest that some of the amino acid patterns emanate from attenuation of BCKD activity.
The BCKD enzyme is part of a mitochondrial matrix metabolon that includes an association with BCATm (45). BCKD expression and activity is fairly ubiquitous in humans and monkeys, but in contrast, BCATm activity is negligible in hepatocytes, making liver a poor consumer of BCAA but an active site of BCKA utilization (46, 47). BCKD is most appreciated for its role in BCKA decarboxylation (48–51). As the rate-limiting enzyme in BCAA oxidative catabolism, BCKD is a major player in terms of regulation of complete combustion of BCAA to ultimately form intra-mitochondrial acetyl-CoA (leucine) or the anaplerotic metabolite succinyl-CoA via propionyl-CoA (valine and isoleucine). As with most keto-acid dehydrogenases, BCKD is inhibited by phosphorylation effected through binding to BCKD kinase (51, 52). BCKD kinase expression or phosphorylating activity is decreased by acute exercise (53), clofibrate treatment (54, 55), or BCKA (56) and increased by insulin (57). Thus, alterations in metabolic status or hormonal milieu can influence BCKD activity partly through changes in activity of its associated kinase [reviewed in (58, 59)]. Recently, a BCKD phosphatase was also identified (60, 61). Significant reductions in the activity of BCKD in metabolically important tissues would be predicted to result in increased tissue and circulating BCAA and BCAA-derivative concentrations, but is there evidence for decreased tissue BCKD in obesity or insulin resistance?
A series of reports have demonstrated that in obese, insulin-resistant rodent models, liver and WAT BCKD abundance and/or activity are significantly reduced, with indications that the enzyme phosphorylation may be increased relative to lean controls (14–17). The situation in muscle is less clear, because in rodent obesity, expression of the E1α subunit of the BCKD complex is unchanged (14, 16), but the E1β and E2 subunits may be lower (16) and the BCKD kinase higher (14), suggestive of reduced BCKD activity in obese rodent muscle as well. Recently, elegant experiments using WAT depot transplant strategies in mice firmly established that WAT BCKD contributes importantly to overall BCAA metabolism and influences blood BCAA concentrations in that model (62). Adipocyte BCKD is subject to phosphorylation/dephosphorylation events and has a high percent active state analogous to liver (63, 64). Therefore, it is reasonable to consider that raised blood BCAA and BCKA concentrations in the obese, insulin-resistant state or in T2DM reflect reduced BCKD activity in a variety of metabolically relevant tissues such as liver, WAT, and possibly muscle. The idea that WAT BCKD contributes to shifts in blood BCAA profiles in humans remains to be formally tested, but this idea is supported by the observation that in obese humans blood BCAA levels significantly drop following bariatric surgery (14, 65) and WAT BCKD expression increases following this procedure (14). Notably, from comparative tissue enzyme activity measurements, WAT harbors a substantial amount of the whole-body BCKD activity in humans [∼20%; see (46, 66)].
Metabolite patterns emerging from targeted and untargeted metabolomics strategies characterizing the obese, insulin-resistant state are consistent with a diminution of BCKD activity in humans. A case in point are the increases in blood or urinary concentrations of α-KB and/or its derivative α-HB that are commonly observed in insulin-resistant or T2DM humans (6, 7, 67, 68); increased α-HB was also recently reported in diet-induced obese mice (69). An underappreciated function of BCKD is to oxidize α-KB, the ketoacid by-product along with cysteine of the catabolism of methionine/homocysteine-derived cystathionine via cystathionase (α-KB can also be produced from threonine via a threonine hydratase activity). Partially purified liver BCKD readily oxidized α-KB (to form propionyl-CoA) almost as well as the BCAA-derived BCKA (51, 70, 71), and α-KB could competitively inhibit rat liver mitochondrial oxidation of BCKA and vice versa (48, 72). PDH also acts on α-KB (51, 71, 73, 74), but BCKD appears to be more important, because, unlike pyruvate, oxidation of an α-KB precursor α-aminobutyrate remained intact in fibroblasts derived from PDH-deficient patients (75), and the Km of α-KB toward partially purified PDH is 4–5 times higher than that of BCKD (51, 71). Therefore, BCKD is in essence the primary α-KB dehydrogenase. This concept is bolstered by the finding that α-KB is one of the organic acids marking maple syrup urine disease (76, 77), a disease emanating from dysfunctional BCKD complex and diagnosed by detection of elevated BCAA in the blood or urine. Thus, should BCKD activity be attenuated in the obese and insulin-resistant states, it could contribute to increased blood levels of α-KB and/or its derivative, α-HB (product of LDH action on α-KB), that occur alongside elevated BCAA concentrations. In theory, such a biochemical blockade at BCKD would promote upstream methionine and cysteine-cystine accumulation and hence contribute to their raised blood or tissue levels as well.
Tissue redox balance and oxidative stress in insulin-resistance and T2DM: potential effects on BCAA and sulfur amino acids.
Blood BCAA, sulfur amino acids, or their derivatives are increased in states of impaired insulin action that are often accompanied by the hallmark of elevated tissue and blood FFA concentrations, suggestive of an association between lipid and amino acid metabolism. This FFA-rich environment coupled with reduced insulin action in tissues such as liver and muscle (and perhaps WAT) results in more robust β-oxidation that increases intra-mitochondrial generation of reducing equivalents and acetyl-CoA. These conditions in many ways mimic the fasting condition in which the insulin:glucagon ratio is decreased. For instance, persons with poorly controlled and/or insulin-requiring T2DM display increased blood ketone body concentrations and a higher β-hydroxybutyrate:acetoacetate ratio compared to nondiabetics or very well-controlled T2DM individuals (78–81), reflecting higher liver intra-mitochondrial NADH/NAD+ and accelerated β-oxidation. Under these conditions, mitochondrial pyruvate oxidation is attenuated due to inhibition of the PDH complex via increased activity of the PDH kinase that is activated under conditions of increased intra-mitochondrial NADH/NAD+ and acetyl-CoA (82). With hyperglycemia, cytosolic NADH levels also rise due to a relative increase in glycolysis-derived–reducing equivalents NADPH and NADH, and cytosolic NADPH and NADH are increased by hyperglycemia-induced flux through the sorbitol pathway to form fructose (83). The latter is reflected by increased blood and urine levels of fructose in T2DM (6,84), but the tissues contributing to this remain to be firmly established. Thus, conditions of limited insulin action and T2DM can result in a higher intra-mitochondrial and cytosolic NADH:NAD+ ratio that has a strong inhibitory effect on dehydrogenase enzyme systems and an activating effect on NADH-consuming pathways viz. inhibition of PDH and activation of lactate production through LDH (82, 83).
Several reports point to lower BCKD/α-KB dehydrogenase activity in liver and WAT of fasted or insulin-resistant obese rodents (14–17, 72, 85, 86). In addition to changes in BCKD expression, because the PDH and BCKD complexes share many biochemical properties it is reasonable to consider that under FFA-rich conditions of fasting or compromised insulin action BCKD becomes inactivated through a more reduced intra-mitochondrial redox state coupled to increased FFA combustion similar to what occurs in the PDH-PDH kinase system. Several biochemical studies provide evidence that partially purified BCKD is inhibited by NADH (50, 70, 87, 88) and acetyl-CoA (87). NADH elicits a dissociation of BCKD from BCATm within the BCKD metabolon complex, resulting in more limited enzyme activity of BCKD (45). Regardless of the exact mechanism, a NADH-related depression of BCKD activity in metabolically important tissues would attenuate BCAA and BCKA oxidation, potentially contributing to accumulation of these metabolites. Consistent with this viewpoint, Adibi (10) showed that fasting elicits a marked increase in blood BCAA in humans.
FFA combustion also attenuates mitochondrial BCKD. Rat hepatocyte mitochondrial BCKA catabolism was inhibited by FFA β-oxidation (89, 90); carnitine palmitoyltransferase-1 (the rate-limiting enzyme controlling liver β-oxidation) inhibition restored BCKA oxidation under these conditions (89). In rat L6 myotubes, 500 μmol/L oleate led to markedly lower propionylcarnitine and heptanoylcarnitine accumulation (36), and because these metabolites are derivatives of CoA esters produced from α-KB (propionyl-CoA) and BCKA catabolism, it suggests that FFA inhibited BCKD activity. Again, this effect was abolished by adding an inhibitor of carnitine palmitoyltransferase-1 (36). When the leucine-derived BCKA α-ketoisocaproate was co-infused with sorbitol, ethanol, or octanoate (NADH generators) in rats, there were large increases in blood leucine concentrations reflecting inhibition of BCKA oxidation with subsequent BCKA carbon flow back toward BCAA (91). Altogether, the literature supports the idea that mitochondrial BCKD activity is inhibited in a FFA-rich, reduced environment.
Because BCKD plays an important role in complete catabolism of methionine through its action to oxidize α-KB, increased NADH/NAD+ and FFA would also be expected to reduce disposal of α-KB and promote LDH-associated generation of its reduced derivative, α-HB. Consistent with this, in rat liver mitochondrial preparations, α-KB oxidation was inhibited by FFA and β-hydroxybutyrate (92). Adding to the dynamics of this scenario, experimental diabetes and/or hyperglucagonemia triggers expression and activity of the methionine-metabolizing enzymes betaine:homocysteine methyltransferase, cystathionine synthase, and cystathionase (cystathionine γ-lyase), at least in liver where it has been tested; this would promote catabolism of methionine to cysteine and α-KB as well as activate the salvage pathway of methionine regeneration from homocysteine [(93,94); also see review (95)]. Coupled to a lower BCKD activity, such events would predictably raise tissue pool sizes of α-KB and its derivative, α-HB, and could promote accumulation of upstream metabolites such as cysteine-cystine.
Tissue redox balance and oxidative stress in insulin resistance and T2DM: a consideration of phenylalanine and tyrosine catabolism.
The oxidative stress of diabetes has been implicated as a factor to trigger increased conversion of methionine to cysteine to provide precursor for antioxidant glutathione production (95). But can a plausible link be made between redox status, oxidative stress, and phenylalanine and tyrosine metabolism? The answer may rest in considering a relatively underappreciated aspect of tyrosine catabolism that occurs via TAT. Since the early 1970s a cystine-dependent cytosolic system that strongly inhibits TAT activity has been characterized in rodent liver models and in some human hepatomas [(96) and refs. therein]. This system involves formation of cystine from cysteine, desulfuration of cystine by cystathionase to the persulfide thiocysteine, and reaction of the latter or its derivatives with the TAT enzyme through sulfane binding (97, 98). This phenomenon is largely vitamin B-6 (pyridoxal-5′-phosphate) dependent (99, 100), consistent with involvement of the cysteine generator cystathionase (vitamin B-6–requiring). Diminution of TAT activity in metabolically relevant tissues in the insulin-resistant or diabetic states would conceivably contribute to increases in the tissue and blood concentration of tyrosine and/or its immediate upstream precursor, phenylalanine. Although most cystathionase biochemistry has been characterized in liver preparations, Feng et al. (101) very recently described cystathionase activity in WAT and suggested a role in development of the insulin resistance of obesity. In rat liver, experimental streptozotocin diabetes and hyperglucagonemia increased flux through the transsulfuration pathway and the inactivation of TAT by cytosolic preparations (94, 102). However, it remains to be firmly established whether insulin resistance or T2DM affects cystathionase and associated hydrogen sulfide production from cysteine/cystine, whether cytosolic cystine and cystine-derivative pools rise enough under these conditions to affect TAT in situ and in turn, whether or not this influences body-wide tyrosine/phenylalanine metabolism and blood levels. Such events would go hand in hand with a lower effective insulin:glucagon ratio, a shift in redox balance to a more reduced state with concomitant attenuation of α-ketoacid dehydrogenases (e.g. PDH and BCKD), and higher reactive oxygen species generation, oxidative stress, and glutathione production.
A unified model
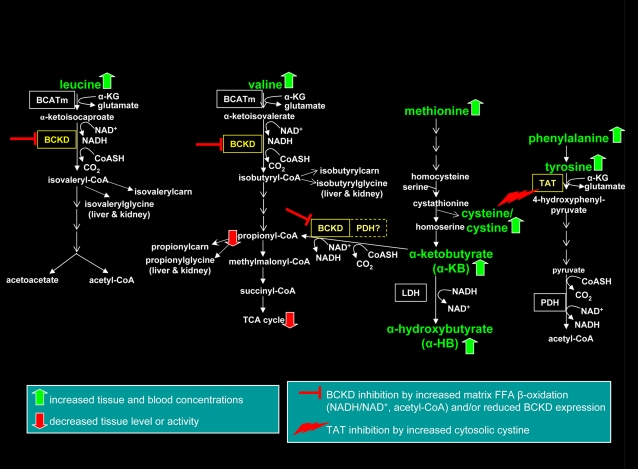
Evidence and a conceptual framework has been presented supporting the concept that at least for some essential amino acids, conditions of impaired insulin action, increased FFA oxidation, and concomitant changes in mitochondrial redox status attenuate specific catabolic pathways that in turn affect tissue and blood concentrations of BCAA, sulfur amino acids, tyrosine/phenylalanine, and related derivatives. A working model was constructed that illustrates this hypothesis (Fig. 1). In contrast, Newgard et al. (4, 65) suggested that the obese, insulin-resistant condition is one of robust BCAA oxidation due to BCAA “overload,” an idea supported by their findings of increased blood carnitine derivatives of BCKA oxidation (e.g. isovaleryl-/2-methylbutaryl-carnitines) in obese human cohorts.
Figure 1.
A model of perturbations in essential amino acid catabolism in tissues under conditions of impaired insulin action and T2DM. Under the FFA-rich environment of diabetes, intra-mitochondrial matrix redox status is shifted toward increased NADH/NAD+ coupled to FFA-derived acetyl-CoA generation, inhibiting the mitochondrial matrix-associated enzymes BCKD and PDH. Oxidative stress and ROS generation promote catabolism of methionine to form glutathione with concomitant production of cysteine-cystine; cystine acts to inhibit the tyrosine catabolic enzyme TAT. Because BCKD and to some extent PDH catabolize the methionine derivative α-KB, inhibition of these enzymes promotes accumulation of α-KB and its derivative, α-HB. These events would selectively increase tissue and blood concentrations of some essential amino acids and their derivatives (BCAA, methionine/cysteine-cystine, α-KB/α-HB, tyrosine, phenylalanine). Secondary outcomes such as anaplerotic stress, reduced TCA cycle intermediate levels, and ultimately suboptimal TCA cycle function leading to a mismatch between FFA delivery and combustion could result. α-HB, α-hydroxybutyrate; α-KB, α-ketobutyrate; BCKD, branched-chain α-ketoacid dehydrogenase; BCATm, mitochondrial branched chain aminotranserase; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase; ROS, reactive oxygen species; TAT, tyrosine aminotransferase; TCA, tricarboxylic acid; T2DM, type 2 diabetes mellitus.
How can these disparate viewpoints regarding amino acid catabolism be reconciled? One possibility is that our interpretations of blood metabolite levels have been too simplistic and need to take into account that the blood metabolite pool is influenced by multiple tissues that could have differing responses to hormonal or dietary cues. For instance, BCKD in rodent liver (and likely WAT) has a large dynamic range in its phosphorylation-regulated activity in response to metabolic challenges, because a large fraction is typically unphosphorylated and active, in stark contrast to muscle in which ∼90% or more of the enzyme is in the inactive form under most conditions (16, 46, 64, 86, 103–106). Total tissue liver and WAT BCKD activities are downregulated in the fasted or obese/insulin-resistant or diabetic states (14–17, 72, 85, 86), but this seems less apparent in muscle. An observation that provides insight into tissue specificity may be gleaned from one study that found that plasma BCKA-derived acylcarnitines were higher in obese, insulin-resistant participants, but BCKA-derived glycine metabolites (isobutyryl- and isovaleryl-glycine) were significantly reduced (4). Because glycine conjugation is thought to be trivial in muscle or WAT (e.g. glycine-N-acyltransferase expression is nil in human or mouse skeletal muscle or WAT), glycine metabolite profiles are largely reflective of liver and kidney metabolism where glycine-N-acyltransferase is expressed robustly. The origins of sometimes elevated BCKA-derived acylcarnitines in obesity (4) are unknown but might reflect increased muscle BCAA catabolism in response to elevated blood BCAA exposure emanating from reduced BCAA catabolism in other tissues. These considerations highlight the need for more research to dissect out how specific tissues handle essential amino acids and related metabolites in the blood of obese, insulin-resistant, or T2DM individuals.
BCAA metabolism, dietary protein, and metabolic health
Insulin resistance, obesity, and elevated BCAA: cause or consequence?
It has been proposed that higher tissue and blood concentrations of BCAA in human obesity cause or exacerbate insulin resistance through mechanisms involving leucine promoting the activation of the mTOR in muscle (4). In that study, rats were fed a high-fat diet (45% of energy) enriched in BCAA that led to a significant increase in plasma valine, isoleucine, and leucine concentrations and increased mTOR phosphorylation and Jun N-terminal kinase activation in muscle. These rats were highly insulin resistant compared to low-fat diet-fed rats despite equal body weight and lower food intake and they displayed insulin receptor substrate-1 serine phosphorylation. The insulin resistance and impaired glucose tolerance of nonobese BCAA-HF rats were equal to those of obese high-fat diet-fed rats and this effect was reversed by provision of the mTOR inhibitor rapamycin. No effect of high dietary BCAA on glucose homeostasis or muscle mTOR or Jun N-terminal kinase activation was apparent when rats were fed low-fat diets, indicating an interaction between BCAA effects and dietary lipid. These results support the idea that high BCAA tissue exposure contributes to the insulin resistance phenotype in diet-induced obese rats through activation of mTOR but that this effect is manifested only in the context of high-fat diet feeding.
Despite such observations, the evidence that BCAA promote obesity-associated insulin resistance and the T2DM metabolic phenotype in humans is not clear cut. For instance, the postsurgery drop in blood BCAA concentrations in bariatric surgery patients (14, 65) is concurrent with improved insulin sensitivity (65), but this association could also result if inhibition of BCAA catabolism is a consequence and not a cause of insulin resistance. Furthermore, it has not been established that the sometimes modest increases in blood BCAA observed in human obesity are of an adequate magnitude to promote activation of mTOR. Many examples from cell culture and rodent model studies also raise questions about a specific association between increased BCAA and insulin resistance. In rat muscle L6 cells in culture, high concentrations of mixed amino acids activated the mTOR system and reduced insulin-stimulated glucose uptake, but these effects were not BCAA or leucine specific; high concentrations of methionine, histidine, threonine, tyrosine, and leucine, but not other amino acids, when provided in isolation could elicit this response (107). Leucine or isoleucine treatment of murine C2C12 myotubes actually stimulated glucose uptake (108). In murine 3T3L1 adipocytes, 5 mmol/L leucine by itself did not activate the mTOR pathway, unlike insulin that triggered mTOR, and leucine only modestly increased mTOR activation above that observed with insulin alone, with limited impact on phospho-Akt (109). In isolated rat adipocytes, leucine activation of the mTOR pathway was accompanied by improvement of glucose uptake when insulin-stimulated PI3-kinase was attenuated by wortmannin (110,111). High-fat feeding elicited whole-body insulin resistance with no change in circulating leucine concentration but higher liver mTOR activation in obese rats (112). Provision of a leucine-enriched, adequate-protein diet or a whey-based 50% protein diet on a low-fat background did not impair glucose homeostasis in C57BL/6J mice (113). Mice fed BCAA for weeks in the drinking water displayed significantly increased muscle mitochondrial biogenesis markers thought to involve mTOR activation, but there was no change in blood insulin concentration (114). Very recently, it was shown that approximately doubling intake of leucine via the drinking water in high-fat–fed mice led to increased blood leucine concentrations and tissue mTOR activation, yet metabolic variables and inflammation phenotypes were improved or normalized (69). In summary, evidence that elevated blood BCAA or leucine concentrations contribute to the insulin resistance often associated with obesity remains equivocal and, as described below, the weight of evidence points to protein- and BCAA-rich diets as actually beneficial to whole-body metabolic health.
Is dietary protein and BCAA-enriched food good or bad for metabolic health?
Based on the discussion above, one must consider whether a high-protein or BCAA-rich diet would be helpful or detrimental in terms of improving insulin sensitivity and other metabolic variables in the obese, insulin-resistant state. In rodent models, most studies in which leucine- or BCAA-rich protein sources (e.g. whey protein isolate, nonfat dry milk) have been administered in the drinking water or food under high-fat, obesogenic conditions have consistently shown a positive effect on metabolic outcomes, including lower adiposity, improved glucose tolerance, and reduced feed efficiency (115–121). Adipocytes isolated from rats fed a high-protein, low-fat diet were more insulin sensitive with respect to lipolysis compared to animals fed a control diet (122). Glycemic control indices (HbA1c%, fasting blood glucose, and insulin levels) were improved and energy expenditure increased in mice fed a modest-fat diet with leucine-enriched drinking water (123). In type 2 diabetic humans, protein-rich diets (i.e. ∼30% of dietary energy) had positive metabolic effects, including reduced fasting glucose and HbA1c% even during weight maintenance over 5 wk to 1 y (124–126). Some studies have shown no effect of high-protein diets on glucose control variables or no difference in the improved metabolic profiles compared to high-carbohydrate controlled diets (127–129). Whether specific types of dietary protein affect metabolism more than others remains to be determined: for instance, a 1-mo, weight-maintenance, dairy protein-based diet (16% of energy) reduced blood markers of oxidative stress and inflammation to a significantly greater degree than a soy protein-based diet in overweight and obese participants (130). Thus, most evidence supports that protein-rich diets do not negatively affect whole-body insulin sensitivity or glucose homeostasis.
Conclusions and Future Directions
Our understanding of changes in essential amino acid metabolism in the insulin-resistant or diabetic states remains in its infancy. A great deal is yet to be learned about the tissue specificity of metabolic shifts and the tissue origins of blood and urinary metabolite patterns. Also unclear are the roles of insulin resistance or the hormonal milieu (e.g. hyperinsulinemia, glucagonemia, insulinopenia following pancreatic β-cell failure in frank T2DM, etc.) in regulating the enzymes controlling utilization of BCAA and other essential amino acids.
This review has highlighted the possibility that diminution of mitochondrial BCKD activity through lowered expression, increased phosphorylation, and/or increased intra-mitochondrial NADH:NAD+ ratio and acetyl-CoA level could underlie many of the essential amino acid metabolite patterns observed in the FFA- and reducing equivalent-rich tissue environment of insulin resistance/obesity and T2DM. If this scenario is true, a plausible outcome is that decreased flux of BCKA and α-KB through BCKD would result in diminished availability of propionyl-CoA–derived metabolites that normally feed into the TCA cycle, leading to “anaplerotic stress” (6) and reduced TCA cycle activity relative to fuel delivery to mitochondria (Fig. 1). That the latter mismatch takes place in obese, insulin-resistant muscle has been recently proposed (36) and is supported by plasma metabolomics analyses of T2DM humans (6, 35). Attenuated TCA capacity of insulin-resistant and T2DM tissues has been reported by many groups (131–135). Such a BCKD/TCA cycle model requires more rigorous evaluation (and may only apply to select tissues), but it provides a foundation for hypothesis testing to explore the origins of changes in essential amino acid metabolism in metabolic disease. In addition, further studies are required to understand if higher tissue BCKA levels activate BCKD, which would in theory temper redox-, phosphorylation-, or expression-related reductions in BCKD activity.
In animal models, liver BCKD activity is markedly reduced by low-dietary protein intakes or fasting and increased or restored by high-protein, high-leucine, or high-BCKA diets (15, 48, 72, 85, 103, 104, 106, 136, 137). Should this manifest in humans, it raises the possibility that improvements in dietary protein intake or quality in prediabetics or in T2DM persons would increase BCKD activity in metabolically relevant tissues. Although speculative, such outcomes would be predicted to lower circulating BCAA metabolite concentrations, affect tissue metabolism of essential amino acids, and increase generation of anaplerotic precursors that would improve TCA cycle function. Recently, improvements in glucose homeostasis were found to accompany increased WAT BCKD gene expression following activation of PPARγ by antidiabetic thiazolidinedione drugs in humans (138), rats (139), and db/db or lean mice (R. Amin and S. Adams, unpublished observations). These results highlight the need for further research to determine how dietary or other interventions alter tissue-specific BCKD and essential amino acid dynamics in humans and to assess whether increased oxidative catabolism of BCAA could ultimately improve mitochondrial function and metabolic health in prediabetes and T2DM.
Acknowledgments
I thank Drs. Norlin J. Benevenga, Tracy G. Anthony, Matthew J. Picklo, Susan M. Hutson, and Dorothy W. Gietzen for valuable comments for drafts of this manuscript, and Dr. Raj Amin for collaboration on db/db mouse studies. Appreciation is also expressed toward partners involved in metabolomics analyses in T2DM participants and animal models that have led to the perspectives outlined in this review: Drs. Oliver Fiehn, John W. Newman, W. Timothy Garvey, Mary-Ellen Harper, and Charles L. Hoppel. The sole author had responsibility for all parts of the manuscript.
Footnotes
Supported by the USDA-ARS Intramural Project 5306-51530-019-00, NIH-NIDDK R01DK078328-01 and R01DK078328-02S1, ILSI Future Leader Award, and the National Dairy Council (grant administered by the Dairy Research Institute). The USDA is an equal opportunity provider and employer.
Author disclosures: S. H. Adams, no conflicts of interest.
Abbreviations used: BCATm, mitochondrial branched chain aminotransferase; BCKA, branched chain α-ketoacid; BCKD, branched chain α-ketoacid dehydrogenase; FPG, fasting plasma glucose; α-HB, α-hydroxybutyrate; HbA1c%, hemoglobin A1c; α-KB, α-ketobutyrate; LDH, lactate dehydrogenase; mTOR, mammalian target of rapamycin; PDH, pyruvate dehydrogenase; TAT, tyrosine aminotransferase; TCA, tricarboxylic acid; tCys, total cysteine+cystine; T2DM, type 2 diabetes mellitus; WAT, white adipose tissue.
Literature Cited
- 1.Diaz VA, Mainous AG III, Baker R, Carnemolla M, Majeed A. How does ethnicity affect the association between obesity and diabetes? Diabet Med. 2007;24:1199–204 [DOI] [PubMed] [Google Scholar]
- 2.Schauder P, Zavelberg D, Langer K, Herbertz L. Sex-specific differences in plasma branched-chain keto acid levels in obesity. Am J Clin Nutr. 1987;46:58–60 [DOI] [PubMed] [Google Scholar]
- 3.Caballero B, Wurtman RJ. Differential effects of insulin resistance on leucine and glucose kinetics in obesity. Metabolism. 1991;40:51–8 [DOI] [PubMed] [Google Scholar]
- 4.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS ONE. 2010;5:e15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE. 2010;5:e10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menge BA, Schrader H, Ritter PR, Ellrichmann M, Uhl W, Schmidt WE, Meier JJ. Selective amino acid deficiency in patients with impaired glucose tolerance and type 2 diabetes. Regul Pept. 2010;160:75–80 [DOI] [PubMed] [Google Scholar]
- 9.Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, Ilkayeva OR, Wenner BR, Bain JR, Lee JJ, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adibi SA. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. 1968;25:52–7 [DOI] [PubMed] [Google Scholar]
- 11.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–6 [DOI] [PubMed] [Google Scholar]
- 12.Zeng M, Liang Y, Li H, Wang M, Wang B, Chen X, Zhou N, Cao D, Wu J. Plasma metabolic fingerprinting of childhood obesity by GC/MS in conjunction with multivariate statistical analysis. J Pharm Biomed Anal. 2010;52:265–72 [DOI] [PubMed] [Google Scholar]
- 13.Wijekoon EP, Skinner C, Brosnan ME, Brosnan JT. Amino acid metabolism in the Zucker diabetic fatty rat: effects of insulin resistance and of type 2 diabetes. Can J Physiol Pharmacol. 2004;82:506–14 [DOI] [PubMed] [Google Scholar]
- 14.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuzuya T, Katano Y, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T, Goto H, Fujita Y, et al. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Biochem Biophys Res Commun. 2008;373:94–8 [DOI] [PubMed] [Google Scholar]
- 16.Bajotto G, Murakami T, Nagasaki M, Sato Y, Shimomura Y. Decreased enzyme activity and contents of hepatic branched-chain alpha-keto acid dehydrogenase complex subunits in a rat model for type 2 diabetes mellitus. Metabolism. 2009;58:1489–95 [DOI] [PubMed] [Google Scholar]
- 17.Doisaki M, Katano Y, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Goto H, Fujita Y, Kadota Y, et al. Regulation of hepatic branched-chain alpha-keto acid dehydrogenase kinase in a rat model for type 2 diabetes mellitus at different stages of the disease. Biochem Biophys Res Commun. 2010;393:303–7 [DOI] [PubMed] [Google Scholar]
- 18.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Khairy L, Ueland PM, Nygard O, Refsum H, Vollset SE. Lifestyle and cardiovascular disease risk factors as determinants of total cysteine in plasma: the Hordaland Homocysteine Study. Am J Clin Nutr. 1999;70:1016–24 [DOI] [PubMed] [Google Scholar]
- 20.El-Khairy L, Vollset SE, Refsum H, Ueland PM. Predictors of change in plasma total cysteine: longitudinal findings from the Hordaland homocysteine study. Clin Chem. 2003;49:113–20 [DOI] [PubMed] [Google Scholar]
- 21.Elshorbagy AK, Nurk E, Gjesdal CG, Tell GS, Ueland PM, Nygard O, Tverdal A, Vollset SE, Refsum H. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: does cysteine link amino acid and lipid metabolism? Am J Clin Nutr. 2008;88:738–46 [DOI] [PubMed] [Google Scholar]
- 22.Elshorbagy AK, Refsum H, Smith AD, Graham IM. The association of plasma cysteine and gamma-glutamyltransferase with BMI and obesity. Obesity (Silver Spring). 2009;17:1435–40 [DOI] [PubMed] [Google Scholar]
- 23.Marchesini G, Bianchi GP, Vilstrup H, Capelli M, Zoli M, Pisi E. Elimination of infused branched-chain amino-acids from plasma of patients with non-obese type 2 diabetes mellitus. Clin Nutr. 1991;10:105–13 [DOI] [PubMed] [Google Scholar]
- 24.Gougeon R, Pencharz PB, Sigal RJ. Effect of glycemic control on the kinetics of whole-body protein metabolism in obese subjects with non-insulin-dependent diabetes mellitus during iso- and hypoenergetic feeding. Am J Clin Nutr. 1997;65:861–70 [DOI] [PubMed] [Google Scholar]
- 25.Gougeon R, Marliss EB, Jones PJ, Pencharz PB, Morais JA. Effect of exogenous insulin on protein metabolism with differing nonprotein energy intakes in Type 2 diabetes mellitus. Int J Obes Relat Metab Disord. 1998;22:250–61 [DOI] [PubMed] [Google Scholar]
- 26.Gougeon R, Styhler K, Morais JA, Jones PJ, Marliss EB. Effects of oral hypoglycemic agents and diet on protein metabolism in type 2 diabetes. Diabetes Care. 2000;23:1–8 [DOI] [PubMed] [Google Scholar]
- 27.Gougeon R, Morais JA, Chevalier S, Pereira S, Lamarche M, Marliss EB. Determinants of whole-body protein metabolism in subjects with and without type 2 diabetes. Diabetes Care. 2008;31:128–33 [DOI] [PubMed] [Google Scholar]
- 28.Glass AR, Bongiovanni R, Smith CE, Boehm TM. Normal valine disposal in obese subjects with impaired glucose disposal: evidence for selective insulin resistance. Metabolism. 1981;30:578–82 [DOI] [PubMed] [Google Scholar]
- 29.Umpleby AM, Scobie IN, Boroujerdi MA, Carson ER, Sonksen PH. Diurnal variation in glucose and leucine metabolism in non-insulin-dependent diabetes. Diabetes Res Clin Pract. 1990;9:89–96 [DOI] [PubMed] [Google Scholar]
- 30.Halvatsiotis PG, Turk D, Alzaid A, Dinneen S, Rizza RA, Nair KS. Insulin effect on leucine kinetics in type 2 diabetes mellitus. Diabetes Nutr Metab. 2002;15:136–42 [PubMed] [Google Scholar]
- 31.Halvatsiotis P, Short KR, Bigelow M, Nair KS. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002;51:2395–404 [DOI] [PubMed] [Google Scholar]
- 32.Pereira S, Marliss EB, Morais JA, Chevalier S, Gougeon R. Insulin resistance of protein metabolism in type 2 diabetes. Diabetes. 2008;57:56–63 [DOI] [PubMed] [Google Scholar]
- 33.Guillet C, Delcourt I, Rance M, Giraudet C, Walrand S, Bedu M, Duche P, Boirie Y. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab. 2009;94:3044–50 [DOI] [PubMed] [Google Scholar]
- 34.Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, Carr SA, Thadhani R, Gerszten RE, Mootha VK. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139:1073–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 37.Tessari P, Nissen SL, Miles JM, Haymond MW. Inverse relationship of leucine flux and oxidation to free fatty acid availability in vivo. J Clin Invest. 1986;77:575–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowell BB, Goodman MN. Protein sparing in skeletal muscle during prolonged starvation. Dependence on lipid fuel availability. Diabetes. 1987;36:14–9 [DOI] [PubMed] [Google Scholar]
- 39.Goodman MN, Larsen PR, Kaplan MM, Aoki TT, Young VR, Ruderman NB. Starvation in the rat. II. Effect of age and obesity on protein sparing and fuel metabolism. Am J Physiol. 1980;239:E277–86 [DOI] [PubMed] [Google Scholar]
- 40.Henry CJ, Rivers JP, Payne PR. Protein and energy metabolism in starvation reconsidered. Eur J Clin Nutr. 1988;42:543–9 [PubMed] [Google Scholar]
- 41.Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes (Lond). 2010;34 Suppl 2:S4–17 [DOI] [PubMed] [Google Scholar]
- 42.Gougeon R, Pencharz PB, Marliss EB. Effect of NIDDM on the kinetics of whole-body protein metabolism. Diabetes. 1994;43:318–28 [DOI] [PubMed] [Google Scholar]
- 43.Staten MA, Matthews DE, Bier DM. Leucine metabolism in type II diabetes mellitus. Diabetes. 1986;35:1249–53 [DOI] [PubMed] [Google Scholar]
- 44.Hoffer LJ, Taveroff A, Hamadeh MJ. Dietary protein restriction alters glucose but not protein metabolism in non-insulin-dependent diabetes mellitus. Metabolism. 1998;47:1145–51 [DOI] [PubMed] [Google Scholar]
- 45.Islam MM, Wallin R, Wynn RM, Conway M, Fujii H, Mobley JA, Chuang DT, Hutson SM. A novel branched-chain amino acid metabolon. Protein-protein interactions in a supramolecular complex. J Biol Chem. 2007;282:11893–903 [DOI] [PubMed] [Google Scholar]
- 46.Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68:72–81 [DOI] [PubMed] [Google Scholar]
- 47.Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab. 2004;286:E64–76 [DOI] [PubMed] [Google Scholar]
- 48.Wohlhueter RM, Harper AE. Coinduction of rat liver branched chain alpha-keto acid dehydrogenase activities. J Biol Chem. 1970;245:2391–401 [PubMed] [Google Scholar]
- 49.Johnson WA, Connelly JL. Cellular localization and characterization of bovine liver branched-chain -keto acid dehydrogenases. Biochemistry. 1972;11:1967–73 [DOI] [PubMed] [Google Scholar]
- 50.Parker PJ, Randle PJ. Partial purification and properties of branched-chain 2-oxo acid dehydrogenase of ox liver. Biochem J. 1978;171:751–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paxton R, Scislowski PW, Davis EJ, Harris RA. Role of branched-chain 2-oxo acid dehydrogenase and pyruvate dehydrogenase in 2-oxobutyrate metabolism. Biochem J. 1986;234:295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obayashi M, Sato Y, Harris RA, Shimomura Y. Regulation of the activity of branched-chain 2-oxo acid dehydrogenase (BCODH) complex by binding BCODH kinase. FEBS Lett. 2001;491:50–4 [DOI] [PubMed] [Google Scholar]
- 53.Xu M, Nagasaki M, Obayashi M, Sato Y, Tamura T, Shimomura Y. Mechanism of activation of branched-chain alpha-keto acid dehydrogenase complex by exercise. Biochem Biophys Res Commun. 2001;287:752–6 [DOI] [PubMed] [Google Scholar]
- 54.Paul HS, Liu WQ, Adibi SA. Alteration in gene expression of branched-chain keto acid dehydrogenase kinase but not in gene expression of its substrate in the liver of clofibrate-treated rats. Biochem J. 1996;317:411–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi R, Murakami T, Obayashi M, Nakai N, Jaskiewicz J, Fujiwara Y, Shimomura Y, Harris RA. Clofibric acid stimulates branched-chain amino acid catabolism by three mechanisms. Arch Biochem Biophys. 2002;407:231–40 [DOI] [PubMed] [Google Scholar]
- 56.Paxton R, Harris RA. Regulation of branched-chain alpha-ketoacid dehydrogenase kinase. Arch Biochem Biophys. 1984;231:48–57 [DOI] [PubMed] [Google Scholar]
- 57.Nellis MM, Doering CB, Kasinski A, Danner DJ. Insulin increases branched-chain alpha-ketoacid dehydrogenase kinase expression in Clone 9 rat cells. Am J Physiol Endocrinol Metab. 2002;283:E853–60 [DOI] [PubMed] [Google Scholar]
- 58.Harris RA, Kobayashi R, Murakami T, Shimomura Y. Regulation of branched-chain alpha-keto acid dehydrogenase kinase expression in rat liver. J Nutr. 2001;131:S841–5 [DOI] [PubMed] [Google Scholar]
- 59.Shimomura Y, Obayashi M, Murakami T, Harris RA. Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr Opin Clin Nutr Metab Care. 2001;4:419–23 [DOI] [PubMed] [Google Scholar]
- 60.Joshi M, Jeoung NH, Popov KM, Harris RA. Identification of a novel PP2C-type mitochondrial phosphatase. Biochem Biophys Res Commun. 2007;356:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, Vondriska TM, Cai H, Lynch CJ, Wang Y. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. 2009;119:1678–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem. 2010;285:11348–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones SM, Yeaman SJ. Phosphorylation of branched-chain 2-oxo acid dehydrogenase complex in isolated adipocytes. Effects of 2-oxo acids. Biochem J. 1986;236:209–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papet I, Lezebot N, Barre F, Arnal M, Harper AE. Influence of dietary leucine content on the activities of branched-chain amino acid aminotransferase (EC 2.6.1.42) and branched-chain alpha-keto acid dehydrogenase (EC 1.2.4.4) complex in tissues of preruminant lambs. Br J Nutr. 1988;59:475–83 [DOI] [PubMed] [Google Scholar]
- 65.Laferrere B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, Bose M, Teixeira J, Stevens RD, Wenner BR, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3:80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr. 2006;136:S207–11 [DOI] [PubMed] [Google Scholar]
- 67.Salek RM, Maguire ML, Bentley E, Rubtsov DV, Hough T, Cheeseman M, Nunez D, Sweatman BC, Haselden JN, Cox RD, et al. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol Genomics. 2007;29:99–108 [DOI] [PubMed] [Google Scholar]
- 68.Li X, Xu Z, Lu X, Yang X, Yin P, Kong H, Yu Y, Xu G. Comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry for metabonomics: biomarker discovery for diabetes mellitus. Anal Chim Acta. 2009;633:257–62 [DOI] [PubMed] [Google Scholar]
- 69.Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Gall W, Kahn CR. Dietary leucine-an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE. 2011;6:e21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pettit FH, Yeaman SJ, Reed LJ. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci USA. 1978;75:4881–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones SM, Yeaman SJ. Oxidative decarboxylation of 4-methylthio-2-oxobutyrate by branched-chain 2-oxo acid dehydrogenase complex. Biochem J. 1986;237:621–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steele RD, Weber H, Patterson JI. Characterization of alpha-ketobutyrate metabolism in rat tissues: effects of dietary protein and fasting. J Nutr. 1984;114:701–10 [DOI] [PubMed] [Google Scholar]
- 73.Bremer J. Pyruvate dehydrogenase, substrate specificity and product inhibition. Eur J Biochem. 1969;8:535–40 [DOI] [PubMed] [Google Scholar]
- 74.Kanzaki T, Hayakawa T, Hamada M, Fukuyoshi Y, Koike M. Mammalian alpha-keto acid dehydrogenase complexes. IV. Substrate specificities and kinetic properties of the pig heart pyruvate and 2-oxyoglutarate dehydrogenase complexes. J Biol Chem. 1969;244:1183–7 [PubMed] [Google Scholar]
- 75.Borud O, Pettersen JE. Normal 2-aminobutyrate oxidation and increased valine oxidation in fibroblasts deficient in pyruvate dehydrogenase. J Inherit Metab Dis. 1982;5:55–7 [DOI] [PubMed] [Google Scholar]
- 76.Smith AJ, Strang LB. An inborn error of metabolism with the urinary excretion of alpha-hydroxy-butyric acid and phenylpyruvic acid. Arch Dis Child. 1958;33:109–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jakobs C, Solem E, Ek J, Halvorsen K, Jellum E. Investigation of the metabolic pattern in maple syrup urine disease by means of glass capillary gas chromatography and mass spectrometry. J Chromatogr A. 1977;143:31–8 [Google Scholar]
- 78.Okuda Y, Kawai K, Murayama Y, Yamashita K. Postprandial changes in plasma ketone body and carnitine levels in normal and non-insulin-dependent diabetic subjects. Endocrinol Jpn. 1987;34:415–22 [DOI] [PubMed] [Google Scholar]
- 79.Ubukata E. Diurnal variation of blood ketone bodies in insulin-dependent diabetes mellitus and noninsulin-dependent diabetes mellitus patients: the relationship to serum C-peptide immunoreactivity and free insulin. Ann Nutr Metab. 1990;34:333–42 [DOI] [PubMed] [Google Scholar]
- 80.Robertson DA, Singh BM, Nattrass M. Effect of obesity on circulating intermediary metabolite concentrations in the absence of impaired glucose tolerance. Int J Obes. 1991;15:635–45 [PubMed] [Google Scholar]
- 81.Avogaro A, Crepaldi C, Miola M, Maran A, Pengo V, Tiengo A, Del Prato S. High blood ketone body concentration in type 2 non-insulin dependent diabetic patients. J Endocrinol Invest. 1996;19:99–105 [DOI] [PubMed] [Google Scholar]
- 82.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003;31:1143–51 [DOI] [PubMed] [Google Scholar]
- 83.Ido Y. Pyridine nucleotide redox abnormalities in diabetes. Antioxid Redox Signal. 2007;9:931–42 [DOI] [PubMed] [Google Scholar]
- 84.Kawasaki T, Akanuma H, Yamanouchi T. Increased fructose concentrations in blood and urine in patients with diabetes. Diabetes Care. 2002;25:353–7 [DOI] [PubMed] [Google Scholar]
- 85.Gillim SE, Paxton R, Cook GA, Harris RA. Activity state of the branched chain alpha-ketoacid dehydrogenase complex in heart, liver, and kidney of normal, fasted, diabetic, and protein-starved rats. Biochem Biophys Res Commun. 1983;111:74–81 [DOI] [PubMed] [Google Scholar]
- 86.Randle PJ. alpha-Ketoacid dehydrogenase complexes and respiratory fuel utilisation in diabetes. Diabetologia. 1985;28:479–84 [DOI] [PubMed] [Google Scholar]
- 87.Boyer B, Odessey R. Quantitative control analysis of branched-chain 2-oxo acid dehydrogenase complex activity by feedback inhibition. Biochem J. 1990;271:523–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boyer B, Odessey R. Kinetic characterization of branched chain ketoacid dehydrogenase. Arch Biochem Biophys. 1991;285:1–7 [DOI] [PubMed] [Google Scholar]
- 89.Williamson JR, Walajtys-Rode E, Coll KE. Effects of branched chain alpha-ketoacids on the metabolism of isolated rat liver cells. I. Regulation of branched chain alpha-ketoacid metabolism. J Biol Chem. 1979;254:11511–20 [PubMed] [Google Scholar]
- 90.Hu H, Jaskiewicz JA, Harris RA. Ethanol and oleate inhibition of alpha-ketoisovalerate and 3-hydroxyisobutyrate metabolism by isolated hepatocytes. Arch Biochem Biophys. 1992;299:57–62 [DOI] [PubMed] [Google Scholar]
- 91.Okita M, Watanabe A, Shiota T, Nagashima H. Plasma level of branched chain alpha-amino acids following its alpha-keto acid administration to rats. J Nutr Sci Vitaminol (Tokyo). 1986;32:83–91 [DOI] [PubMed] [Google Scholar]
- 92.Ciman M, Carignani G, Alexandre A, Siliprandi N. On the inhibition of -oxobutyrate utilization by fatty acids in rat liver mitochondria. Biochim Biophys Acta. 1971;253:24–8 [DOI] [PubMed] [Google Scholar]
- 93.Xue GP, Snoswell AM. Disturbance of methyl group metabolism in alloxan-diabetic sheep. Biochem Int. 1985;10:897–905 [PubMed] [Google Scholar]
- 94.Hargrove JL, Trotter JF, Ashline HC, Krishnamurti PV. Experimental diabetes increases the formation of sulfane by transsulfuration and inactivation of tyrosine aminotransferase in cytosols from rat liver. Metabolism. 1989;38:666–72 [DOI] [PubMed] [Google Scholar]
- 95.Wijekoon EP, Brosnan ME, Brosnan JT. Homocysteine metabolism in diabetes. Biochem Soc Trans. 2007;35:1175–9 [DOI] [PubMed] [Google Scholar]
- 96.Buckley WT, Milligan LP. Participation of cysteine and cystine in inactivation of tyrosine aminotransferase in rat liver homogenates. Biochem J. 1978;176:449–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hargrove JL, Wichman RD. A cystine-dependent inactivator of tyrosine aminotransferase co-purifies with gamma-cystathionase (cystine desulfurase). J Biol Chem. 1987;262:7351–7 [PubMed] [Google Scholar]
- 98.Hargrove JL. Persulfide generated from L-cysteine inactivates tyrosine aminotransferase. Requirement for a protein with cysteine oxidase activity and gamma-cystathionase. J Biol Chem. 1988;263:17262–9 [PubMed] [Google Scholar]
- 99.Reynolds RD. Vitamin B-6 requirement for irreversible inactivation of rat liver tyrosine aminotransferase. Arch Biochem Biophys. 1978;186:324–34 [DOI] [PubMed] [Google Scholar]
- 100.Sloger MS, Scholfield LG, Reynolds RD. Loss of in vitro inactivation of rat liver tyrosine aminotransferase with dietary vitamin B6 restriction. J Nutr. 1978;108:1355–60 [DOI] [PubMed] [Google Scholar]
- 101.Feng X, Chen Y, Zhao J, Tang C, Jiang Z, Geng B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem Biophys Res Commun. 2009;380:153–9 [DOI] [PubMed] [Google Scholar]
- 102.Jacobs RL, Stead LM, Brosnan ME, Brosnan JT. Hyperglucagonemia in rats results in decreased plasma homocysteine and increased flux through the transsulfuration pathway in liver. J Biol Chem. 2001;276:43740–7 [DOI] [PubMed] [Google Scholar]
- 103.Block KP, Heywood BW, Buse MG, Harper AE. Activation of rat liver branched-chain 2-oxo acid dehydrogenase in vivo by glucagon and adrenaline. Biochem J. 1985;232:593–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Block KP, Soemitro S, Heywood BW, Harper AE. Activation of liver branched-chain alpha-keto acid dehydrogenase in rats by excesses of dietary amino acids. J Nutr. 1985;115:1550–61 [DOI] [PubMed] [Google Scholar]
- 105.Aftring RP, Block KP, Buse MG. Leucine and isoleucine activate skeletal muscle branched-chain alpha-keto acid dehydrogenase in vivo. Am J Physiol. 1986;250:E599–604 [DOI] [PubMed] [Google Scholar]
- 106.Crowell PL, Block KP, Repa JJ, Torres N, Nawabi MD, Buse MG, Harper AE. High branched-chain alpha-keto acid intake, branched-chain alpha-keto acid dehydrogenase activity, and plasma and brain amino acid and plasma keto acid concentrations in rats. Am J Clin Nutr. 1990;52:313–9 [DOI] [PubMed] [Google Scholar]
- 107.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–60 [DOI] [PubMed] [Google Scholar]
- 108.Doi M, Yamaoka I, Fukunaga T, Nakayama M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem Biophys Res Commun. 2003;312:1111–7 [DOI] [PubMed] [Google Scholar]
- 109.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hinault C, Mothe-Satney I, Gautier N, Lawrence JC, Jr, Van Obberghen E. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J. 2004;18:1894–6 [DOI] [PubMed] [Google Scholar]
- 111.Hinault C, Van Obberghen E, Mothe-Satney I. Role of amino acids in insulin signaling in adipocytes and their potential to decrease insulin resistance of adipose tissue. J Nutr Biochem. 2006;17:374–8 [DOI] [PubMed] [Google Scholar]
- 112.Korsheninnikova E, van der Zon GC, Voshol PJ, Janssen GM, Havekes LM, Grefhorst A, Kuipers F, Reijngoud DJ, Romijn JA, Ouwens DM, et al. Sustained activation of the mammalian target of rapamycin nutrient sensing pathway is associated with hepatic insulin resistance, but not with steatosis, in mice. Diabetologia. 2006;49:3049–57 [DOI] [PubMed] [Google Scholar]
- 113.Noatsch A, Petzke KJ, Millrose MK, Klaus S. Body weight and energy homeostasis was not affected in C57BL/6 mice fed high whey protein or leucine-supplemented low-fat diets. Eur J Nutr. 2011;50:479–88 [DOI] [PubMed] [Google Scholar]
- 114.D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12:362–72 [DOI] [PubMed] [Google Scholar]
- 115.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J. 2000;14:1132–8 [PubMed] [Google Scholar]
- 116.Eller LK, Reimer RA. Dairy protein attenuates weight gain in obese rats better than whey or casein alone. Obesity (Silver Spring). 2010;18:704–11 [DOI] [PubMed] [Google Scholar]
- 117.Papakonstantinou E, Flatt WP, Huth PJ, Harris RB. High dietary calcium reduces body fat content, digestibility of fat, and serum vitamin D in rats. Obes Res. 2003;11:387–94 [DOI] [PubMed] [Google Scholar]
- 118.Pilvi TK, Korpela R, Huttunen M, Vapaatalo H, Mervaala EM. High-calcium diet with whey protein attenuates body-weight gain in high-fat-fed C57Bl/6J mice. Br J Nutr. 2007;98:900–7 [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–54 [DOI] [PubMed] [Google Scholar]
- 120.Parra P, Bruni G, Palou A, Serra F. Dietary calcium attenuation of body fat gain during high-fat feeding in mice. J Nutr Biochem. 2008;19:109–17 [DOI] [PubMed] [Google Scholar]
- 121.Shertzer HG, Woods SE, Krishan M, Genter MB, Pearson KJ. Dietary whey protein lowers the risk for metabolic disease in mice fed a high-fat diet. J Nutr. 2011;141:582–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kettelhut IC, Foss MC, Migliorini RH. Lipolysis and the antilipolytic effect of insulin in adipocytes from rats adapted to a high-protein diet. Metabolism. 1985;34:69–73 [DOI] [PubMed] [Google Scholar]
- 123.Guo K, Yu YH, Hou J, Zhang Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr Metab (Lond). 2010;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78:734–41 [DOI] [PubMed] [Google Scholar]
- 125.Nuttall FQ, Schweim K, Hoover H, Gannon MC. Effect of the LoBAG30 diet on blood glucose control in people with type 2 diabetes. Br J Nutr. 2008;99:511–9 [DOI] [PubMed] [Google Scholar]
- 126.Gannon MC, Hoover H, Nuttall FQ. Further decrease in glycated hemoglobin following ingestion of a LoBAG30 diet for 10 weeks compared to 5 weeks in people with untreated type 2 diabetes. Nutr Metab (Lond). 2010;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brinkworth GD, Noakes M, Parker B, Foster P, Clifton PM. Long-term effects of advice to consume a high-protein, low-fat diet, rather than a conventional weight-loss diet, in obese adults with type 2 diabetes: one-year follow-up of a randomised trial. Diabetologia. 2004;47:1677–86 [DOI] [PubMed] [Google Scholar]
- 128.Sargrad KR, Homko C, Mozzoli M, Boden G. Effect of high protein vs high carbohydrate intake on insulin sensitivity, body weight, hemoglobin A1c, and blood pressure in patients with type 2 diabetes mellitus. J Am Diet Assoc. 2005;105:573–80 [DOI] [PubMed] [Google Scholar]
- 129.Larsen RN, Mann NJ, Maclean E, Shaw JE. The effect of high-protein, low-carbohydrate diets in the treatment of type 2 diabetes: a 12 month randomised controlled trial. Diabetologia. 2011;54:731–40 [DOI] [PubMed] [Google Scholar]
- 130.Zemel MB, Sun X, Sobhani T, Wilson B. Effects of dairy compared with soy on oxidative and inflammatory stress in overweight and obese subjects. Am J Clin Nutr. 2010;91:16–22 [DOI] [PubMed] [Google Scholar]
- 131.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14 [DOI] [PubMed] [Google Scholar]
- 135.Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56:1592–9 [DOI] [PubMed] [Google Scholar]
- 136.Khatra BS, Chawla RK, Wadsworth AD, Rudman D. Effect of dietary branched-chain alpha-keto acids on hepatic branched-chain alpha-keto acid dehydrogenase in the rat. J Nutr. 1977;107:1528–36 [DOI] [PubMed] [Google Scholar]
- 137.Dixon JL, Harper AE. Effects on plasma amino acid concentrations and hepatic branched-chain alpha-keto acid dehydrogenase activity of feeding rats diets containing 9 or 50% casein. J Nutr. 1984;114:1025–34 [DOI] [PubMed] [Google Scholar]
- 138.Sears DD, Hsiao G, Hsiao A, Yu JG, Courtney CH, Ofrecio JM, Chapman J, Subramaniam S. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc Natl Acad Sci USA. 2009;106:18745–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hsiao G, Chapman J, Ofrecio JM, Wilkes J, Resnik JL, Thapar D, Subramaniam S, Sears DD. Multi-tissue, selective PPARgamma modulation of insulin sensitivity and metabolic pathways in obese rats. Am J Physiol Endocrinol Metab. 2011;300:E164–74 [DOI] [PMC free article] [PubMed] [Google Scholar]