Abstract
A considerable part of the difficulty of determining nutrient requirements in pathologic states is the failure to understand the physiology unique to the specific condition. Here we take the specific example of burns in childhood and discuss the roles of the inflammatory and stress responses to the burn and the consequent transient increased bone resorption followed by osteoblast apoptosis and adynamic bone. This condition leads to a failure of the bone to take up and thus conserve the increased calcium liberated by the acutely increased bone resorption. On top of this mechanism, there is a cytokine-mediated upregulation in the parathyroid gland calcium-sensing receptor that results in hypocalcemic hypoparathyroidism and consequent urinary calcium wasting. As if that were not sufficient, the skin of the burned patient, both scarred area and normal-appearing adjacent skin, convert 7 dehydrocholesterol to pre-vitamin D3 at a rate that is 20–25% of normal skin and circulating levels of 25-hydroxyvitamin D are chronically low. Thus, burn injury gives rise to calcium wasting, failure of bone to take up excessive calcium, and vitamin D insufficiency to frank deficiency. These and other areas must be addressed before it can be determined how much vitamin D and calcium should be given to a patient with severe burn injury.
Anyone who has been involved with determining nutrient requirements in healthy individuals is aware that this is a very difficult task, often involving considerable disagreement among those who will issue the nutrient intake guidelines. Thus, one can only imagine the difficulties involved in attempting to determine nutrient requirements in various disease states or even in clinical conditions following trauma. The purpose of this review is to point out, using specific examples, how important it is to understand the unique pathophysiology of a given illness or specific trauma before it is possible to undertake the establishment of nutrient requirements in such a situation.
In this specific example, that of burns, we will examine the mechanisms at play that have been shown to affect calcium and vitamin D status as well as other mechanisms that can be postulated to play a role in calcium and vitamin D homeostasis.
Thus, in the case of calcium, we will address the pathogenesis of the acute state of hypocalcemia following burns, examine attempts to replace lost calcium, discuss the reasons replacement does not achieve a normocalcemic state, and add speculation as to whether additional newly discovered mechanisms may also contribute to this situation. We shall then go through the same exercise with vitamin D and examine conditions unique to burn injury that would adversely affect vitamin D sufficiency.
After examining these cases, it should be clear that the body’s adaptive responses, which are not unique to burn injury, may cause alteration of both calcium and vitamin D status and may provide clues with regard to the nutritional requirements of this traumatic condition.
Metabolic Response to Burn Injury
To start out with, it is highly unlikely that the human body evolved to this point to deal specifically with burn injury. This type of trauma is a relatively uncommon occurrence and the body’s responses are therefore an agglomeration of actions that in themselves are nonspecific and may have adverse consequences.
Among the responses are hypermetabolism, with a resting energy expenditure of 1.2–1.8 times the normal resting energy expenditure (1), negative nitrogen balance, sarcopenia (1, 2), and immobilization, especially acutely between surgical debridement and grafting procedures.
Some of these responses, namely sarcopenia and immobilization, could affect bone metabolism and, indirectly, calcium handling by the body. Thus, sarcopenia could decrease the secondary skeletal load and immobilization could lead to urinary calcium wasting and reduced bone formation, which would then lead to reduced bone calcification. The effect of decreased skeletal loading is similar to that of immobilization. Whether it is a key factor in calcium loss has not been studied in detail. On the other hand, preliminary data obtained from patients suggest that immobilization is not a factor in bone or calcium loss from the body (3).
What is known about calcium homeostasis in burned children is that despite receiving as much as 2–3 g of calcium/(m2 surface area burned ⋅ d) they still manifest continuous hypocalcemia (4). How this occurs is a function of 2 additional acute responses to burn injury: the stress response and the inflammatory response.
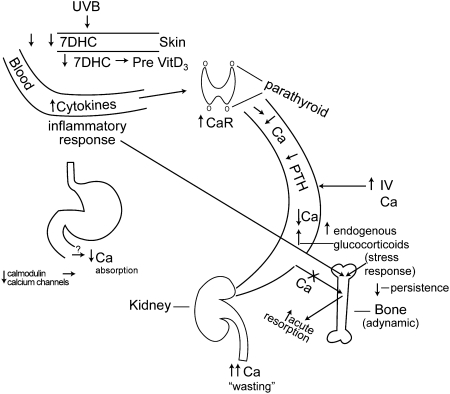
The stress response gives rise to the endogenous production of glucocorticoids and catecholamines for a period of up to 1 y. Of the 2 classes of compounds, the glucocorticoids are more consistently elevated and are produced in quantities that lead to urinary free cortisol excretion that is 3–8 times the upper limit of normal values in children depending on whether 50 or 125 μg/d is considered the upper limit of normal (5, 6). The endogenous glucocorticoids then have 2 effects on the bone. The first is acute stimulation of existing osteoblasts, or bone-forming cells, and presumably osteocytes as well, to produce the ligand for the activator of the nuclear receptor NF-κB (RANKL3). This stimulation causes RANKL to signal the bone marrow stem cells to increasingly differentiate into osteoclasts, leading to an increase in acute bone resorption. This increase in bone resorption leads to an increase in free calcium that the body cannot conserve, as will be explained below. If this stress response is sustained and there is a continuous high output production of corticosteroids by the body, the osteoblasts and osteocytes will become apoptotic, usually at ∼2 wk postburn. Although apoptosis has not been specifically documented, it is assumed due to the lack of osteoblasts on the bone surface as seen on biopsies and the reduced differentiation markers of marrow stromal cells into osteoblasts (6). Two consequences result. The first is that there will be a dramatic fall in the production of RANKL, the result of which will be a reduction in osteoclasts (5) and in bone resorption (5). The other is that there will be a major reduction in bone formation (5), with both disappearance of osteoblasts from the bone surface (6) and the reduced differentiation of marrow stromal cells into osteoblasts as evidenced by reduced stromal cell production of biochemical markers of osteoblast differentiation, such as type I collagen, alkaline phosphatase, core protein binding factor α-1 (also known as runx 2), and bone morphogenetic protein-2 (6). Thus, the reduction of both bone resorption and bone formation leads to a condition the nephrologists term “adynamic bone.” Adynamic bone, then, would not be conducive to mineralization and, one could speculate, would not be a mechanism by which the body could conserve calcium in contrast to a normal individual in whom the bones serve as a storehouse for 98% of the body’s calcium (Fig. 1).
Figure 1.
This diagram depicts the mechanisms discussed in postburn bone loss. UVB radiation to the skin acts on a diminished amount of vitamin D precursor, 7DHC and an abnormally reduced percentage of 7DHC is converted to pre-vitamin D3. In addition the level of cytokines in the blood increase secondary to the inflammatory response and these elevated cytokines act on the parathyroid gland to upregulate the membrane-bound CaR. This leads to reduced circulating calcium and reduced PTH secretion and results in renal calcium wasting. Additionally, the inflammatory response acts directly on the bone synergistically with the stress response to cause both proinflammatory cytokines and endogenous glucocorticoids to stimulate osteoblast RANK ligand production to increase acute bone resorption. However, with the persistence of the stress response, the osteoblasts and likely the osteocytes become apoptotic and bone turnover is markedly reduced, leading to adynamic bone and a consequent inability of bone to take up calcium from the blood. Finally, potential zinc deficiency might lead to conformational changes in calmodulin and/or the calcium channels, resulting in reduced gastrointestinal calcium absorption. CaR, calcium-sensing receptor; 7DHC, 7-dehydrocholesterol; PTH, parathyroid hormone.
The other adaptive response that occurs simultaneously with the stress response is the inflammatory response. Because the skin, normally the body’s main barrier to entry for micro-organisms, is destroyed, all burn victims are considered septic on admission to the hospital. The inflammatory response involves more than the stimulation of acute phase reactants, thus increasing such proteins as α-1-antitrypsin and decreasing constitutive proteins such as albumin (5); it also involves the increased production of cytokines by the mononuclear inflammatory cells. Among these cytokines, IL-1β and IL-6 are present in serum in concentrations of 3- to 125-fold above normal, respectively (5). These particular cytokines, and perhaps others, will add to the acute effects of the increased endogenous glucocorticoid production of the stress response in stimulating the osteoblasts to produce RANKL and ultimately in increasing bone resorption. However, when the adynamic state is produced in the bone, generally by 2 wk postburn, the circulating cytokines remain very high, as mentioned above. In contrast, these cytokines cannot increase bone resorption, because they work through the osteoblast production of RANKL and at this point in time there are minimal functional osteoblasts in the bone. However, these cytokines likely play another role in body calcium loss.
The in vitro work on bovine parathyroid cells by Nielsen et al. (7) in Boston and in equine parathyroid by Toribio et al. (8) in Columbus and Canaff et al. (9) in Montreal has shown us that incubation of parathyroid cells with IL-1 β results in an upregulation of the parathyroid calcium-sensing receptor (7, 8) and that i.p. injection of IL-6 into the rat resulted in an increase in calcium sensing receptor in the parathyroid as well as the thyroid and kidney (9).
Genetic mutations of the calcium-sensing receptor in humans have shown that an upregulation of the receptor results in a decreased set point for calcium suppression of PTH secretion. In practical terms, it would take a lower amount of circulating calcium, even to hypocalcemic levels, to suppress PTH secretion by the parathyroids. The consequence of that action would therefore be that the cytokine-induced hypocalcemia would still be sufficient to suppress PTH secretion. The resultant hypoparathyroidism would lead to urinary calcium wasting and would provide a reasonable explanation for the failure of the burned patient to conserve calcium.
It would therefore be incumbent to explore what happens to calcium homeostasis in a burned patient. Among the observations we have made in burned children, urinary calcium excretion at 2 wk postburn is ~8 mg/(kg⋅24 h), more than twice the normal quantity of 3 mg/(kg⋅24 h) (4). Thus, we have urinary calcium wasting. Moreover, a nomogram relating normal pediatric PTH response to various levels of ionized calcium showed that the majority of burned children studied were hypocalcemic and the majority of those who were hypocalcemic had PTH responses below the 99% CI, indicating that they were both hypocalcemic and hypoparathyroid (4). Additionally, s.c. administration of PTH resulted in a blunted renal response in terms of urinary phosphate and cyclic AMP excretion (4). Because magnesium depletion could also produce a failure of response to PTH and our patients were all acutely magnesium depleted because their resuscitation fluid, Ringer’s Lactate, contains no magnesium (4), we were able to replete only one-half of them by the end of their acute hospitalization and again plot their responses to serum ionized calcium on the PTH nomogram. Unfortunately, it made absolutely no difference whether patients were magnesium replete or depleted. All remained hypocalcemic and hypoparathyroid (10). Therefore, all these data pointed away from an effect of Mg depletion and toward a cytokine-mediated upregulation of the parathyroid calcium-sensing receptor.
To demonstrate that this was indeed the case, a sheep model of burn injury was utilized. Sheep given a controlled 40% total body surface area 3rd-degree flame burn under halothane anesthesia and controls undergoing sham burn were killed and parathyroid was extracted 48 h postburn. The parathyroids were identified and subjected to Northern blotting and the calcium sensing receptor mRNA proved to be upregulated by 50% by gel densitometry (11). To demonstrate an upregulation of the calcium-sensing receptor membrane protein as well, samples of parathyroid from both burned and sham-burned sheep were immunoperoxidase stained and, in a semiquantitative analysis, there was substantially more membrane-bound calcium-sensing receptor protein in the parathyroid of the burned sheep compared to sham-burned control (11).
Thus, the urinary calcium wasting by means of inflammatory upregulation of the parathyroid calcium-sensing receptor and the failure of adynamic bone to act as a sump for excess calcium can in combination become a significant source of calcium wasting from the body, regardless of how much exogenous calcium is given either orally or i.v.
In addition to the mechanisms just described, there are others that are not as intensively studied, the effects of which may also contribute to loss of calcium. These include inflammation-induced downregulation of the PHEX gene (phosphate regulating gene with homologies to endopeptidases on the X chromosome), inflammation-mediated downregulation of intestinal and renal calcium and phosphate transport, and downregulation of 1,25-dihydroxycholecalciferol (l) (cholecalciferol), which would also decrease both calcium and phosphate absorption. It is important to note that none of the mechanisms related to PHEX have been studied with respect to burn injury. However, if they are normally present and burn injury occurs in previously normal children, they are likely to play a role in reduced circulating calcium and, to a lesser extent, reduced circulating phosphorus, which we see in severe burn injury. We will now examine these putative mechanisms for calcium loss in more detail.
The PHEX gene itself inhibits renal tubular phosphate reabsorption (12). Its downregulation by cytokines such as TNF would therefore contribute to renal tubular calcium wasting. In addition, there are a series of trans-epithelial calcium transporters; in particular TRPV5 and TRPV6 would be key factors. TRPV5 is primarily seen in the distal convoluted renal tubules and TRPV6 is expressed in the duodenal brush border. Reduction of both TRPV5 and V6 mRNA levels is seen in the presence of inflammation (12), although not specifically burn-associated inflammation. Thus, calcium absorption in the small intestine and calcium reabsorption by the kidney may be adversely affected in the inflammatory state.
Another important factor in intestinal calcium absorption is 1,25-dihydroxycholecalciferol. This metabolically active form of vitamin D is generally normal 6 mo postburn (13), but its status is difficult to determine following the acute burn injury because serum albumin and Vitamin D Binding Protein are low and the assay for free 1,25-dihydroxycholecalciferol is no longer performed. Thus, during at least the first 6 mo following a burn injury, it is difficult to know whether low circulating levels of 1,25-dihydroxycholecalciferol (14) reflect normal or abnormal function with respect to intestinal calcium absorption.
These latter mechanisms must be interpreted with caution, because whether and to what degree they are operative in burn injury has not been studied. We already have adequate explanation for calcium wasting from the body by mechanisms that have been studied. Nonetheless, we really do not know how many mechanisms there are for accomplishing the same function.
Thus, when determining the amount of calcium required by a burn-injured patient, one must take into account the leakage of calcium from the body as well as the failure of the skeleton to take it up. Furthermore, before arriving at any plausible amount of calcium as a requirement for burned patients, it is necessary to realize that some of the pathophysiology must be corrected before any amount of administered calcium will be retained.
Next, we will address the problem of chronic vitamin D deficiency following burn injury, the nature of the problem, mechanisms that contribute to the deficiency, and possible implications with regard to setting requirements.
Vitamin D
Low serum levels of 25(OH)D were first noticed in ambulatory pediatric burn patients returning to follow-up clinics at 2 y and then again at 7 y postburn. Peak values for these children reached 20 ng/ml (15), whereas today it is recognized that normal serum levels of 25(OH)D are more likely to be 30 ng/ml (13). The minimum adequate level of circulating 25(OH)D is still a subject of much discussion. Although peak levels of 25(OH)D have reached the minimum level recommended by the 2011 DRI (16), it is uncertain if this level is optimal to restore the depleted calcium stores of bone in this setting. At each time point, i.e. at both 2 and 7 y postburn, 12 children were studied and in both cases the same thing was found: low serum levels of 25(OH)D. It is thought that this deficiency is progressive with time because at 2 y postburn, all the serum levels of cholecalciferol were normal. However, by 7 y postburn, at least 50% of the children manifested low circulating levels of 1,25-dihydroxycholecalciferol (15). The mechanism(s) leading to the low serum levels of 25 (OH)D were not immediately apparent.
What we do know is that the significance of low serum 25 (OH)D levels following acute burn injury cannot be assessed with reliability. The low circulating levels of both Vitamin D Binding Protein (14) and albumin (14) are part of the acute phase inflammatory response and it is unknown at present whether the free 25(OH) D level is “normal,” because this assay is not currently performed. Therefore, we must proceed cautiously by stating that albumin levels, which can return to normal at the earliest by 6 mo postburn (13), can serve as an indicator as to whether serum 25(OH)D levels can be reliably assessed. The failure of what was at the time a “standard” vitamin D supplement of 400 iu (10 μg)/d to raise serum 25(OH)D levels to normal suggests that in burned children this quantity is inadequate to produce vitamin D sufficiency. Efficacy of the currently recommended intake of 600 IU (15 μg)/d (16) has not been evaluated in this population.
Why should burned patients have such a high requirement for vitamin D? This is unknown, but one possibility would include burn-associated damage to skin as a factor in the inability of the body to synthesize vitamin D. What evidence is there that this is the case? There is one study performed by our group (17) that addresses this issue. Skin biopsies from burned children a mean of 14 mo postburn, at a time when serum levels of 25(OH) D were low, were analyzed for the amount of 7DHC precursor as well as for the percentage of 7DHC converted by a standard UV B radiation exposure to pre-vitamin D3. Not only was the percentage conversion of 7DHC to pre-vitamin D3 significantly reduced in the biopsies obtained from burned scar tissue compared to normal age-matched controls but so was the percentage of conversion of 7DHC to pre-vitamin D3 in the biopsies taken from normal-appearing skin adjacent to the burn scar. The reduction in the adjacent normal-appearing skin was not significantly different from the reduction of conversion in the burn scar samples obtained. Thus, the area of skin unable to synthesize normal quantities of vitamin D following sun exposure is even greater than the percentage body surface area burn.
What was even more surprising was that the absolute quantity of 7DHC in the skin of the burn scar biopsies and the normal-appearing adjacent skin was significantly depressed compared to normal and again to a degree not different between burn scar and adjacent normal-appearing skin. Thus, the precursor of vitamin D was significantly reduced in skin following burn injury, preventing normal synthesis of vitamin D on sunlight exposure. Furthermore, this finding can allow us to speculate that the surviving or regenerated epithelial cells are biochemically abnormal, specifically in achieving normal cholesterol biosynthesis. The implications of this finding are not presently clear. To date, this is the only known burn-associated mechanism that can explain the progressive vitamin D deficiency that occurs in the absence of adequate supplementation. What is also unknown, however, is whether vitamin D status differs in children whose bone loss has been prevented by use of a bisphosphonate compared to those who never received such treatment (18).
It should also be noted that the provision of a vitamin D supplement to burned children on discharge from the hospital is not standard practice. Furthermore, these children may develop heat intolerance if their sweat glands have been destroyed and exposure of the burn scar to excessive sunlight may produce hyperpigmentation. For all these reasons, most burn victims do not spend much time in the sun, nor do they receive adequate amounts of vitamin D as a nutritional supplement.
Are Any Other Mechanisms Involved in Loss of Calcium or Inability to Synthesize Vitamin D?
Another mechanism that commonly comes to mind is immobilization. Immobilization is mediated by the β receptor of the sympathetic nervous system (19), receptors for which have been shown to be on osteoblasts (19). Blocking the β receptor by use of pharmacologic doses of propranolol should eliminate the effects of immobilization. When burned children were given propranolol for at least 6 mo at a dose titrated to reduce their heart rate by 20%, total body bone mineral content was examined at the end of the ensuing 6-mo period and no difference was found in the percentage change in bone mineral content from hospital discharge (3). This finding effectively rules out immobilization as a contributor to bone loss following burn injury.
There may well be other mechanisms involved in the calcium wasting and failure of vitamin D synthesis that to date remain undiscovered. However, constructing the entire scenario of causes of calcium wasting and failure to synthesize vitamin D takes time.
On the interdependence of nutrients
Calcium and vitamin D are only 2 nutrients that have been singled out for the purpose of this review. However, they do not exist in a vacuum and it is quite possible that although we have identified some mechanisms by which these 2 nutrients are either lost or not synthesized, the role of other nutrients in calcium and vitamin D homeostasis have not been extensively explored. Among these nutrients, zinc and copper are 2 which have been studied to a limited degree, but their effects on calcium and vitamin D metabolism remain undefined. Moreover, although we know that the provision of these 2 micronutrients is currently inadequate, the requirements for them following burn injury remain controversial as well (20) and the effects of their deficiencies on calcium homeostasis at best are hypothetical at present.
One consideration for zinc is its possible alteration of conformational effects on the N-terminal domain of calmodulin as a result of its binding or lack of binding to this molecule (21) as well as possible conformational effects on various calcium channels related to Zn binding or failure to bind to the channel proteins (22). These effects could potentially alter the efficacy of calcium absorption or calcium transport. However, these considerations remain theoretical at the present time.
Summary and Conclusions
There are currently no answers regarding the requirements for either calcium or vitamin D following burn injury in either children or adults. We are, however, making progress in understanding the natural course of calcium and vitamin D metabolism as consequences of the adaptive responses of the body to a severe burn injury and, by understanding at least some of the mechanisms that produce deficiency of these 2 nutrients (Fig. 1), we can begin to address possible ways of supplying them in adequate quantities. What is required are ways to either prevent the adaptive responses to burn injury from producing calcium wasting, such as early action to prevent bone resorption, including consideration of the acute i.v. administration of a bisphosphonate compound. Preliminary data along these lines look very promising (18, 23). Moreover, perhaps with further knowledge as to the cause of urinary calcium wasting with upregulation of the calcium sensing receptor, we may be able to look for an agent to target this parathyroid receptor in order to minimize this specific receptor upregulation. Preliminary studies of so-called calcilytic agents have been unsuccessful and these are withdrawn from commercial production at the present time.
Data obtained from studies of adequacy of nutrient supply will vary with our success in changing or preventing the underlying adaptive mechanisms from causing the problems they currently do. Such studies should, at this stage of our knowledge, be oriented toward supplying sufficient calcium and vitamin D to make up for the deficiencies currently caused by the wasting of one and the synthetic failure of the other.
Acknowledgments
The sole author is responsible for all aspects of the final manuscript.
Footnotes
Supported by funds from the NIH (NIGMS 1 P50 GM 60338) and by grants from the Shriners Hospitals for Children.
Author disclosures: G. L. Klein, no confilicts of interest.
Abbreviations used: 7DHC, 7 dehydrocholesterol; 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone; RANKL, ligand of the receptor activator of NF-κB; TRP, transient receptor potential.
Literature Cited
- 1.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–902 [DOI] [PubMed] [Google Scholar]
- 2.Hart DW, Herndon DN, Klein G, Lee SB, Celis M, Mohan S, Chinkes DL, Wolf SE. Attenuation of post-traumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg. 2001;233:827–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez N, Herndon DN, Klein GL. Evidence against immobilization as a cause of post-burn bone loss. Bone. 2011;48 Suppl 2:S190 [Google Scholar]
- 4.Klein GL, Nicolai M, Langman CB, Cuneo BF, Sailer DE, Herndon DN. Dysregulation of calcium homeostasis after severe burn injury in children: possible role of magnesium depletion. J Pediatr. 1997;131:246–51 [DOI] [PubMed] [Google Scholar]
- 5.Klein GL, Herndon DN, Goodman WG, Langman CB, Phillips WA, Dickson IR, Eastell R, Naylor KE, Maloney NA, Desai M, et al. Histomorphometric and biochemical characterization of bone following severe burns in children. Bone. 1995;17:455–60 [DOI] [PubMed] [Google Scholar]
- 6.Klein GL, Bi LX, Sherrard DJ, Beavan SR, Ireland D, Compston JE, Williams WG, Herndon DN. Evidence supporting a role of glucocorticoids in short-term bone loss in burned children. Osteoporos Int. 2004;15:468–74 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen PK, Rasmussen AK, Butters R, Feldt-Rasmussen U, Bendtzen K, Diaz R, Brown EM, Olgaard K. Inhibition of PTH secretion by interleukin-1 beta in bovine parathyroid glands in vitro is associated with up-regulation of the calcium-sensing receptor mRNA. Biochem Biophys Res Commun. 1997;238:880–5 [DOI] [PubMed] [Google Scholar]
- 8.Toribio RE, Kohn CW, Capen CC, Rosol TJ. Parathyroid hormone (PTH) secretion, PTH mRNA and calcium-sensing receptor mRNA expression in equine parathyroid cells and effects of interleukin (IL)-1, IL-6 and tumor necrosis factor alpha on equine parathyroid cell function. J Mol Endocrinol. 2003;31:609–20 [DOI] [PubMed] [Google Scholar]
- 9.Canaff L, Zhou X, Hendy GN. The proinflammatory cytokine interleukin-6 up-regulates calcium-sensing receptor gene transcription via Stat 1/3 and Sp 1/3. J Biol Chem. 2008;283:13586–600 [DOI] [PubMed] [Google Scholar]
- 10.Klein GL, Langman CB, Herndon DN. Persistent hypoparathyroidism following magnesium repletion in burn-injured children. Pediatr Nephrol. 2000;14:301–4 [DOI] [PubMed] [Google Scholar]
- 11.Murphey ED, Chattopadhyay N, Bai M, Kifor O, Harper D, Traber DL, Hawkins HK, Brown EM, Klein GL. Up-regulation of the parathyroid calcium-sensing receptor after burn injury in sheep: a potential contributory factor to postburn hypocalcemia. Crit Care Med. 2000;28:3885–90 [DOI] [PubMed] [Google Scholar]
- 12.Huybers S, Apostolaki N, van der Eerden BCJ, Kollias G, Naber TH, Bindels RJ, Hoenderop JG. Murine TNF (Delta ARE) Crohn’s disease model displays diminished expression of intestinal Ca 2+ transporters. Inflamm Bowel Dis. 2008;14:803–11 [DOI] [PubMed] [Google Scholar]
- 13.Klein GL, Herndon DN, Chen TC, Kulp G, Holick MF. Standard multivitamin supplementation does not improve vitamin D insufficiency after burns. J Bone Miner Metab. 2009;27:502–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein GL, Herndon DN, Rutan TC, Sherrard DJ, Coburn JW, Langman CB, Thomas ML, Haddad JG, Jr, Cooper CW, Miller NL, et al. Bone disease in burn patients. J Bone Miner Res. 1993;8:337–45 [DOI] [PubMed] [Google Scholar]
- 15.Klein GL, Langman CB, Herndon DN. Vitamin D depletion following burn injury in children: a possible factor in post-burn osteopenia. J Trauma. 2002;52:346–50 [DOI] [PubMed] [Google Scholar]
- 16.Institute of Medicine. Report on dietary reference intakes for calcium and vitamin D, released 30 November 2010 [cited 8 July 2011;]. Available from: www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-calcium-and-vitaminD/Report-Brief.aspx.
- 17.Klein GL, Chen TC, Holick MF, Langman CB, Price H, Celis MM, Herndon DN. Synthesis of vitamin D in skin after burns. Lancet. 2004;363:291–2 [DOI] [PubMed] [Google Scholar]
- 18.Przkora R, Herndon DN, Sherrard DJ, Chinkes DL, Klein GL. Pamidronate preserves bone mass for up to two years following acute administration for pediatric burn injury. Bone. 2007;41:297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Oury F, Yadav VK, Wess J, Liu SX, Guo XE, Murshed M, Karsenty G. Signaling through the M(3) muscarinic receptor favors bone mass accrual by decreasing sympathetic activity. Cell Metab. 2010;11:231–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voruganti VS, Klein GL, Lu H-X, Thomas S, Freeland-Graves JH, Herndon DN. Impaired zinc and copper status in children with burn injuries: need to reassess nutritional requirements. Burns. 2005;31:711–6 [DOI] [PubMed] [Google Scholar]
- 21.Warren JT, Guo Q, Tang W-J. A 1.3 Ao structure of zinc-bound N-terminal domain of calmodulin elucidates potential early ion-bonding step. J Mol Biol. 2007;374:517–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H-S, Hui K, Lee DWK, Feng Z-P. Zn 2+ sensitivity of high-and low-voltage activated calcium channels. Biophys J. 2007;93:1175–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein GL, Wimalawansa SJ, Kulkarni G, Sherrard DJ, Sanford AP, Herndon DN. The efficacy of acute administration of pamidronate on the conservation of bone mass following severe burn injury in children: a double blind, randomized, controlled study. Osteoporos Int. 2005;16:631–5 [DOI] [PubMed] [Google Scholar]