Abstract
The immunomodulatory properties of mesenchymal stem cells (MSCs) make them attractive therapeutic agents for a wide range of diseases. However, the highly demanding cell doses used in MSC clinical trials (up to millions of cells/kg patient) currently require labor intensive methods and incur high reagent costs. Moreover, the use of xenogenic (xeno) serum-containing media represents a risk of contamination and raises safety concerns. Bioreactor systems in combination with novel xeno-free medium formulations represent a viable alternative to reproducibly achieve a safe and reliable MSC doses relevant for cell therapy. The main goal of the present study was to develop a complete xeno-free microcarrier-based culture system for the efficient expansion of human MSC from two different sources, human bone marrow (BM), and adipose tissue. After 14 days of culture in spinner flasks, BM MSC reached a maximum cell density of (2.0±0.2)×105 cells·mL−1 (18±1-fold increase), whereas adipose tissue-derived stem cells expanded to (1.4±0.5)×105 cells·mL−1 (14±7-fold increase). After the expansion, MSC expressed the characteristic markers CD73, CD90, and CD105, whereas negative for CD80 and human leukocyte antigen (HLA)-DR. Expanded cells maintained the ability to differentiate robustly into osteoblast, adipocyte, and chondroblast lineages upon directed differentiation. These results demonstrated the feasibility of expanding human MSC in a scalable microcarrier-based stirred culture system under xeno-free conditions and represent an important step forward for the implementation of a Good Manufacturing Practices–compliant large-scale production system of MSC for cellular therapy.
Introduction
The growing knowledge of the intrinsic immunologic properties and multilineage differentiation potential of human mesenchymal stem cells (MSCs) has intensified the research on their therapeutic applications.1 Recently, several clinical trials described the use of MSC in the field of cellular therapy, such as for the treatment of graft-versus-host disease,2 acute myocardial infarction,3 liver cirrhosis,4 and amyotrophic lateral sclerosis,5 and also to promote hematopoietic stem cell engraftment upon bone marrow (BM) transplantation.6
The large cell numbers required for MSC clinical applications (cell doses up to 5 million MSC/kg body weight7) will require a fast and reproducible ex vivo expansion protocol. However, the clinical-scale expansion of MSC has been traditionally performed under static conditions using culture flasks, which are limited in terms of cell productivity and culture monitoring, require extensive handling and relatively long cultivation times, and, consequently, multiple cell passages, which increases the risk of undesired genetic abnormalities.8 As an alternative, different dynamic systems have been developed to expand MSC at a laboratory-scale, either by using a rotary reactor9 or spinner flasks with microcarriers.10–14 Nevertheless, the cell numbers generated through these techniques are limited and most of these laboratory-scale systems targeted MSC differentiation toward the production of mature cells, namely, of osteoblastic or chondrogenic lineages, rather than the optimization of a reproducible, scalable process to produce nondifferentiated homogeneous MSC populations. Moreover, most of the studies focusing on such scale-up systems have used culture media supplemented with fetal bovine serum (FBS), which raises a major concern among clinicians, since it may be a source of animal proteins, bacteria, virus, or xenogeneic antibodies that might trigger an immune response upon MSC infusion.15,16 This can be a major hurdle to obtain the approval from the national and international regulatory agencies for a Good Manufacturing Practices (GMPs)–compliant process and transplant ready cells for therapy. In this context, recently developed clinical-grade medium formulations have been shown to support high MSC proliferation rates while maintaining immunophenotype and multipotency,17 which may greatly improve the safety of expanded MSC in clinical applications. Also, for other stem cell populations, namely, pluripotent stem cells, efforts have been made toward the delineation of xeno-free conditions for cell isolation, propagation, and differentiation.18,19
Our group has previously demonstrated the expansion of human BM MSC in a microcarrier-based stirred culture system, using a culture medium with reduced serum content (MesenPRO RS™, 2% FBS; Invitrogen),20 where Cultispher®-S (porcine gelatin; Sigma-Aldrich) microcarriers were coated with FBS to improve the initial cell adhesion, and, consequently, reduce the lag phase. In the present work, we hypothesized that MSC from other sources, such as adipose-derived stem cells (ASC), could be also efficiently expanded using this system. Moreover, considering the need to develop a scalable, GMP-compliant culture system for the fast clinical-grade expansion of MSC, our goal was to adapt our microcarrier-based culture system to xeno-free conditions, while maintaining the characteristic immunophenotype and multipotency differentiation potential of expanded BM MSC and ASC These results represent a major step toward the clinical-grade production of a safe and effective MSC for cellular therapy.
Materials and Methods
Human BM MSC cultures
BM aspirates were obtained from healthy donors after informed consent at Instituto Português de Oncologia Francisco Gentil, Lisboa, Portugal. MSCs were isolated according to the protocol described by Dos Santos et al.21 Cells from two different donors (average age of 36±11), at passages 5 and 8, were used.
Human ASC cultures
Human ASC were isolated and characterized as described previously in the literature.22 Cells from different donors (average age of 30±7), at passages 4 and 5, were used. The ASC were obtained from healthy donors after informed consent under a protocol reviewed and approved by the Pennington Biomedical Research Center Institutional Review Board.
Serum-free cultures under static conditions
Before cell inoculation in the spinner flasks, cryopreserved MSC were thawed and plated, at a cell density of 3000 cells/cm2, on CELLstart™ CTS™ (Invitrogen) pre-coated T-75 or T-175 flasks using StemPro® MSC SFM XenoFree (Invitrogen). At 70% cell confluence, MSC were detached from the flasks by adding TrypLE™ solution (Invitrogen) for 5 min at 37°C. Cell number and viability were determined using the Trypan Blue exclusion method.
Expansion of MSC in spinner flasks
In this work, Bellco® spinner flasks (Bellco Glass, Inc.) with a working volume of 80 mL, equipped with 90° paddles (normal paddles) and a magnetic stir bar, were used. The initial cell density used for both BM MSC and ASC was 5×104 cells/mL. Cultispher-S microcarriers were prepared as described in the literature23 and precoated with FBS.20 Nonporous plastic microcarriers (SoloHill Engineering, Inc.) were prepared according to manufacturer's instructions. Then, plastic microcarriers were coated with a CELLstart CTS solution (diluted 1:100 in PBS with Ca2+ and Mg2+) for 2 h at 37°C, with an intermittent agitation (1 min at 300 rpm, 10 min nonagitated) using a Thermomixer® confort (Eppendorf AG), and afterward equilibrated in prewarmed MesenPRO RS/StemPro MSC SFM XenoFree medium. Human MSC, previously expanded under xeno-free static conditions for two passages, were seeded on 20 g/L of pre-coated plastic microcarriers in 15 mL of the respective medium for 30 min, at 37°C and 5% CO2, with gentle agitation every 5 min. Then, prewarmed medium was added until reaching half of the final volume, and the cell suspension was transferred to the spinner flask. During the first day, an intermittent agitation regimen was set (15 min at 25 rpm followed by 2 h nonagitated). After the initial 24 h, agitation was set at 40 rpm. After day 3, 25% of the medium volume was renewed daily.
Cell counting on Cultispher-S microcarriers and nutrients/metabolites analyses were performed according to protocols described in the literature.23 For cell counting on plastic microcarriers, a microcarrier-cell suspension sample of 0.5 mL was taken from the homogeneous culture in the spinner flask. Microcarriers were washed with 2 mL of prewarmed PBS, and 1 mL of TrypLE Express (1×) was added. Microcarrier suspension was then incubated at 37°C for 5–7 min at 650 rpm using Thermomixer confort. Subsequently, 4 mL of the corresponding medium was added to stop enzymatic activity and the cell plus microcarrier suspension was filtered using a 100 mm Cell Strainer (BD Biosciences). Cell number and viability were determined using the Trypan Blue exclusion method.
BM MSC and ASC expansion in the spinner flask was also characterized by determining the specific growth and death rates (μ and kd [day−1]), as previously described.21
Telomere length analysis
The relative size of MSC telomeres before and after the expansion in spinner flasks was determined using the Telomere PNA Kit/FITC for Flow Cytometry (Dako) as previously described.24 Briefly, MSC samples were mixed with control cells (cell line 1301 [tetraploid]; Istituto Nazionale per la Ricerca sul Cancro c/o CBA) and a hybridization solution with or without a Telomere PNA Probe/FITC was added. After a 10 min cycle at 82°C, cell mixtures were kept in the dark at room temperature overnight. Then, samples were washed twice and a DNA Staining Solution was added. The analyses were then performed by flow cytometry (FACSCalibur™ equipment; Becton Dickinson and Company). The relative telomere length (RTL) of MSC was calculated by comparison to the telomere length of 1301 cells (control).
Immunophenotypic analysis
Before and after the expansion in the spinner flasks, cells were also analyzed by flow cytometry using a panel of mouse anti-human monoclonal antibodies (PE-conjugated) against: CD31 (Biolegend), CD73 (Becton Dickinson Immunocytometry Systems), CD80 (Biolegend), CD90 (R&D Systems), CD105 (Invitrogen), and human leukocyte antigen (HLA)-DR (Becton Dickinson Immunocytometry Systems). Cells were incubated with these monoclonal antibodies for 15 min in the dark at room temperature and then cells were washed in PBS and fixed with 1% paraformaldehyde (Sigma). Isotype controls were also prepared for every experiment. A minimum of 10,000 events was collected for each sample and the CellQuest software (Becton Dickinson) was used for acquisition and analysis.
Multilineage differentiation assays
Upon 14 days of culture in the spinner flask, BM MSC and ASC were retrieved from the microcarriers (as previously described).
Osteogenic differentiation
Expanded cells were plated at 3000 cells/cm2 on CELLstart CTS pre-coated 12-well plates using StemPro MSC SFM XenoFree. At 80% cell confluency, osteogenesis was induced using StemPro® Osteogenesis Differentation Kit (Invitrogen). The medium was changed twice a week for 14 days. After induction, cells were prepared for alkaline phosphatase (ALP) and von Kossa stainings. Briefly, cells were washed in cold PBS and fixed in 10% cold neutral-buffered formalin (Sigma) for 15 min. After fixing, cells were washed and kept in distilled water for 15 min. Cells were incubated with a 0.1 M solution of Tris-HCl (Sigma-Aldrich) containing Naphtol AS MX-PO4 (0.1 mg·mL−1) (Sigma) in dimethylformamide (Fischer Scientific) and 0.6 mg·mL−1 of Red Violet LB salt (Sigma) for 45 min and washed four times with distilled water. Cells were then observed under the microscope (Leica Microsystems) for ALP staining, as a result of osteogenic commitment. Cells were then stained with silver nitrate (2.5% w/v) (Sigma) for 30 min at room temperature for von Kossa staining to evaluate the deposits of calcium in the cultures.
Adipogenic differentiation
Cells retrieved from microcarriers were plated at 3000 cells/cm2 on CELLstart CTS-precoated 12-well plates using StemPro MSC SFM XenoFree. The adipogenic differentiation was induced at 80% cell confluence after culture for 14 days, using StemPro® Adipogenesis Differentiation Kit (Invitrogen). The medium was changed twice a week for 14 days. The assessment of differentiation toward an adipocytic phenotype was performed based on the accumulation of lipids, using Oil Red-O stain. Cells were washed with cold PBS and fixed in 2% formaldehyde for 30 min. After fixation, cells were then washed with distilled water and incubated with Oil Red-O solution (Sigma) (0.3% in isopropanol) at room temperature for 1 h.
Chondrogenic differentiation
Expanded BM MSC and ASC were plated as small droplets (5–10 μL) with high cell densities (∼2×107 cells·mL−1) on ultra low attachment culture plates (Corning). After 30 min, StemPro Chondrogenesis Differentation Kit (Invitrogen) was added. The medium was changed twice a week for 14 days. The assessment of differentiation toward a chondrocytic phenotype was performed based on the synthesis of proteoglycans by chondrocytes, using Alcian Blue stain. Cells were washed with cold PBS and fixed in 2% formaldehyde for 30 min. After fixation, cells were then washed with distilled water and incubated with 1% Alcian Blue solution (Sigma-Aldrich) at room temperature for 1 h.
RNA isolation
Cells were harvested for RNA isolation at the beginning and at the end of the spinner flask cultures as indicated before. Total RNA was collected using the High Pure RNA Isolation Kit (Roche) according to the manufacturer's instructions. RNA was quantified by UV spectrophotometry (NanoDrop Technologies) at 260 nm. Complementary DNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science) with anchored-oligo(dT)18 primers and 1 μg of RNA.
Quantitative real-time-polymerase chain reaction analysis
For real-time polymerase chain reaction (RT-PCR), a two-step PCR run was performed in a LightCycler® (Roche Diagnostics) using a SYBR® Green PCR master mix (Roche), containing 1 LightCycler FastStart® DNA Master Plus SYBR Green master mix, 4 mM MgCl2, 0.5 μM of each primer, and 2 μL of template resulting from the cDNA synthesis reaction in 20 μL of final volume. This two-step program consisted in an initial denaturation step at 95°C followed by 45 rounds of cycling between 10 s at 95°C, 10 s at the respective annealing temperature and 10 s at 72°C. The following primers for early differentiation cell markers were used: RGC32 (early osteocyte cell marker, 166 bp) (Fw) 5′-GCC ACT TCC ACT ACG AGG AG-3′, (Re) 5′-GCT GGG GTA GAG TCT GTT GG-3′; FABP4 (early adipocyte cell marker, 215 bp) (Fw) 5′-TCA TAC TGG GCC AGG AAT-3′, (Re) 5′-TCC CTT GGC TTA TGC TCT-3′ and SPP-1 (early chondrocyte cell marker, 229 bp) (Fw) 5′-CTC CAT TGA CTC GAA CGA CTC-3′, (Re) 5′-CAG GTC TGC GAA ACT TCT TAG AT-3′. Expression was normalized to the metabolic housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The specificity of the reactions was confirmed using gel electrophoresis. Control assays containing no templates were also performed.
Estimation of maximum shear stress under stirred conditions
Theoretical values of maximum shear stress under stirred conditions, τmax, as result of flow through Kolmogorov eddies, can be determined through Equation 1:
 |
(1) |
where ρ denotes fluid density and ν is the kinematic viscosity. The power dissipated per unit mass, ɛ, is defined by Equation 2:
 |
(2) |
in which VL is the vessel working volume; the power consumed, P, can be estimated by Equation 3:
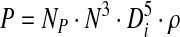 |
(3) |
where NP is the dimensionless power number, N the agitation rate used and Di the impeller diameter. NP was determined based on reported experimental data.
The Reynolds number (Re) can be calculated by Equation 4:
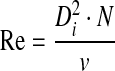 |
(4) |
For StemPro MSC SFM XenoFree, based on the literature, estimated values of 1.01 and 1×10−3 Pa.s for specific density and viscosity, respectively, were used.25
Statistical analysis
Results are presented as mean±standard error of mean (SEM) of the values obtained for human cell donors. The expansion of ASC using MesenPRO RS was replicated using cells from four different donors, whereas the expansion under xeno-free conditions was replicated using two donors of both BM MSC and ASC. Comparisons between experimental results were performed using the nonparametric Mann-Whitney U test. To assess statistical significance of the differences observed, a p-value<0.05 was considered.
Results
We have previously described the successful expansion of human BM MSC in a spinner flask system using gelatin-coated Cultispher-S microcarriers.20 Herein ASC were efficiently expanded using the previous established microcarrier-based culture system, using a low serum containing medium. Moreover, in the present studies BM MSC and ASC were also expanded using the microcarrier-based culture system under xeno-free conditions, namely, by replacing gelatin beads with plastic microcarriers and using culture medium and cell detachment solution of xeno-free origin.
MSC expansion using plastic microcarriers in MesenPRO RS medium
We started by comparing the performance of animal protein-free plastic microcarriers against Cultispher-S gelatin microcarriers for the expansion of both BM MSC and ASC. To maximize initial cell adhesion, a crucial step in a microcarrier-based expansion of MSC, the plastic microcarriers were pre-coated with CellStart CTS, a xeno-free substrate solution (replacing the FBS precoat on Cultispher-S microcarriers20). The initial cell adhesion was evaluated as the percentage of cells that adhered to the microcarriers after the first 24 h of dynamic culture. Although comparable values were obtained for BM MSC (90%±10% and 90%±7% for Cultispher-S and plastic microcarriers, respectively), a decrease in cell adhesion was observed for ASC with the plastic microcarriers (69%±14%) when compared to the gelatin microcarriers (88%±12%).
Since the two types of microcarriers have different values of surface area, cell growth was determined as number of cells per cm2. Throughout time in culture, using MesenPRO RS medium, cell expansion on plastic microcarriers displayed similar growth profiles for both BM MSC (Fig. 1A) and ASC (Fig. 1B) when compared to cell proliferation on Cultispher-S microcarriers. Moreover, after 9 days, higher cell productivities per surface area were obtained with plastic microcarriers, reaching densities of (1.4±0.1)×105 and (2.2±0.4)×105 cells/cm2 for BM MSC and ASC, respectively. However, the specific growth rate calculated for the expansion of ASC was significantly higher (p<0.05) with Cultispher-S microcarriers (0.70±0.07 day−1) than with plastic microcarriers (0.31±0.07 day−1), whereas no significant differences were observed for the specific death rate (0.05±0.02 and 0.02±0.02 day−1, respectively). Importantly, throughout time in culture, cell viability was always superior to 95%.
FIG. 1.
Ex vivo expansion of bone marrow mesenchymal stem cells (A) and ASC (B) in a microcarrier-based culture system in spinner flasks using serum-containing medium. Comparison of expansion performance of Cultispher®-S (black circles) and plastic microcarriers (white squares) under stirred conditions using MesenPRO RS™ Medium. Cell expansion was plotted as cell number/cm2. Values are represented as mean±SEM (n=4). ASC, adipose-derived stem cells; SEM, standard error of the mean.
Throughout the expansion, concentrations of nutrients (glucose and glutamine) and metabolites (lactate and ammonia) in the supernatant were measured (Fig. 2A) and specific consumption and production rates were calculated for ASC expanded using plastic microcarriers (Fig. 2B). BM MSC metabolic analysis displayed similar values compared to ASC (data not shown). In the first 4 days of culture, ASC displayed higher specific average consumption rates of glucose and glutamine (6.1±0.6 and 3.4±1.2 pmol·cell−1·day−1, respectively), whereas significant lower values were obtained between days 5 and 9 (3.3±0.8 and 1.0±0.4 pmol·cell−1·day−1, respectively) (Fig. 2B). Concomitantly, a sharp decrease in nutrient concentrations was observed until day 4, followed by slighter daily decreases until day 9, with glucose levels reaching a minimum value of 2.5 mM. On the other hand, glutamine concentrations did not reach values lower than 1.3 mM (Fig. 2A). In addition, throughout time in culture, the maximum concentration values measured for lactate and ammonia were, respectively, 5.9 and 2.5 mM (Fig. 2A). Accordingly, the average specific production rates of lactate and ammonia were also higher in the beginning of the culture (days 1–4) (13.3±1.6 and 3.0±1.0 pmol·cell−1·day−1, respectively) and decreased to lower values upon day 5 (6.2±1.5 and 1.0±0.5 pmol·cell−1·day−1, respectively) (Fig. 2B). Additionally, the apparent yield of lactate from glucose (Y′lactate/glucose) presented an average value of 2.0±0.2 mollactate·molglucose−1, with small variations throughout culture time (Fig. 2C).
FIG. 2.
Metabolic analyses of the expansion of adipose-derived stem cell (ASC) in spinner flasks, using plastic microcarriers and MesenPRO RS medium. Culture medium samples were analyzed to determine (A) concentration profiles, (B) specific consumption/production rates of nutrients and metabolites, and (C) the apparent yield of lactate from glucose. Values are represented as mean±SEM (n=4).
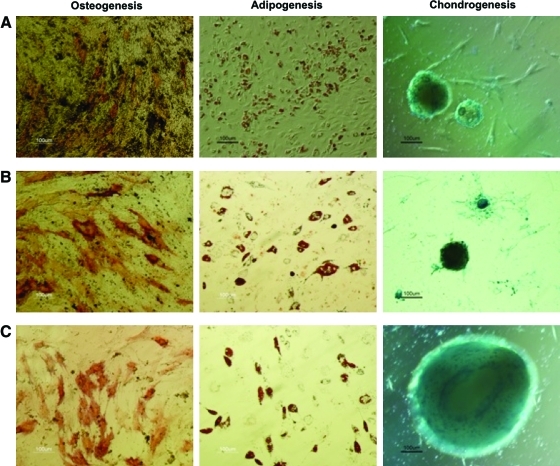
Before and after ASC expansion, immunophenotypic assays were performed to verify if MSC characteristic immunophenotype26 was affected by the spinner flask culture. In fact, although the expression of CD73 and CD90 was maintained above 90%, the expression of CD105 decreased to 85.9±7.9 and 86.7±2.4 for Cultispher-S and plastic microcarriers, respectively. The differentiative potential of expanded ASC was also assessed through the induction of multilineage differentiation (Fig. 3A). Expanded MSC were efficiently differentiated into cells of the three lineages studied (osteogenesis, adipogenesis and chondrogenesis). For instance, osteogenic differentiation was confirmed by ALP staining of early differentiating osteocytes and calcium deposits (dark Von Kossa staining), whereas lipid vacuoles were visible under adipogenic differentiating conditions (stained red with Oil Red-O). Chondrogenic differentiation was assessed by staining proteoglycans with Alcian Blue.
FIG. 3.
Multilineage differentiative potential of bone marrow mesenchymal stem cells (BM MSC) and ASC after spinner flask expansion. Cell differentiation was induced for 14 days and was assessed by staining for osteogenesis (alkaline phosphatase and von Kossa), adipogenesis (Oil Red-O), and chondrogenesis (Alcian blue). (A) ASC expanded using MesenPRO RS Medium and plastic microcarriers, (B) BM MSC, and (C) ASC expanded with StemPro MSC SFM XenoFree medium. Color images available online at www.liebertonline.com/tec
Xeno-free culture of human MSC in spinner flasks
BM MSC and ASC were expanded using 80 mL spinner flasks in a fully xeno-free culture system. Under serum-free conditions (StemPro MSC SFM Xenofree), the initial cell adhesion to plastic microcarriers was seriously impaired when compared to the results obtained with the low serum-containing media previously described. After 24 h of culture, the percentage of adherent cells was 23%±3% for BM MSC and 22%±4% for ASC. However, even though the initial attachment efficiency was reduced, cells expanded at expected rates. After 14 days of culture, BM MSC reached a cell density of (2.0±0.2)×105 cells·mL−1, which corresponded to a fold increase in total cell number of 18±1, whereas ASC expanded to a density of (1.4±0.5)×105 cells·mL−1 (fold increase 14±7) (Fig. 4). Accordingly, a higher average specific growth rate was determined for BM MSC compared to ASC (0.40±0.09 and 0.34±0.01 day−1, respectively). To understand how cell expansion in the spinner flask affected cell proliferative potential, the RTL was also determined. At the end of the expansion, the RTL of BM MSC was reduced by 28% (n=2), whereas no considerable reduction was observed for ASC. Of notice, under xeno-free conditions, BM MSC and ASC viability was always higher than 95%.
FIG. 4.
Ex vivo expansion of BM MSC and ASC in a xeno-free microcarrier-based culture system in spinner flasks (agitation of 40 rpm). Cells were incubated at a cell density of 5×104 cells·mL−1 with 20 g/L of precoated plastic microcarriers in StemPro MSC SFM XenoFree medium. Values are represented as mean±SEM (n=2).
The metabolic analysis of xeno-free stirred culture presented similar trends to ones observed for MSC expansion with serum-containing medium (Fig. 5). BM MSC exhibited a roughly constant specific consumption rate of glucose, with values between 5 and 15 pmol·cell−1·day−1 (with exception of day 7) and an average value of 10±1 pmol·cell−1·day−1 (Fig. 5B). However, a higher specific production rate of lactate between days 3 and 5 (with values over 20 pmol·cell−1·day−1, average of 23±1 pmol·cell−1·day−1) was observed, followed by lower values for the remaining culture time (between 10 and 20 pmol·cell−1·day−1, average of 15±1 pmol·cell−1·day−1) (Fig. 5B). Consequently, the apparent yield of lactate from glucose (Y′lactate/glucose) presented values higher than theoretical value of 2 on days 3 and 4, though the average value for the entire culture time was 1.8±0.4 mollactate·molglucose−1 (Fig. 5C). Additionally, glucose was depleted to a minimum level of 1 mM on day 14, whereas lactate accumulated to a maximum value of 8.4 mM (Fig. 5A).
FIG. 5.
Metabolic analyses of the expansion of BM MSC (A, B, E) and ASC (C–E) in spinner flasks, using StemPRO MSC SFM XenoFree medium. Media samples were analyzed to determine (A, C) concentration profiles, (B, D) specific consumption/production rates of nutrients and lactate, and (E) the apparent yield of lactate from glucose. Values are represented as mean±SEM (n=2).
ASC displayed a specific glucose consumption rate with small variations throughout the culture time (between 10 and 20 pmol·cell−1·day−1), with an average value of 12±2 pmol·cell−1·day−1 (Fig. 5E). On the other hand, the lactate-specific production rate exhibited a trend similar to the one observed for the expansion with 2% MesenPRO RS Medium (Fig. 5E): between days 3 and 6 with a higher average value of 28±3 pmol·cell−1·day−1, followed by lower values after day 7 (average of 19±2 pmol·cell−1·day−1). A similar average value of Y′lactate/glucose was also determined (1.8±0.2 mollactate·molglucose−1) (Fig. 5F). Moreover, glucose level was maintained above 1.5 mM, whereas lactate reached a maximum value of 7.3 mM (Fig. 5D).
Immunophenotypic analysis performed after the spinner flask expansion confirmed that it had not significantly affected BM MSC and ASC characteristic immunophenotype (Table 1). Further, expanded cells retained their multilineage differentiative potential (Fig. 3B, C).
Table 1.
Immunophenotype Analysis of Bone Marrow Mesenchymal Stem Cells and Adipose-Derived Stem Cells Before and After the Xenofree Spinner Flask Culture
| BM MSC | Day 0 | Day 14 | ASC | Day 0 | Day 14 |
|---|---|---|---|---|---|
| CD 31 | 0.6±0.5 | 1.1±0.2 | CD 31 | 0.0 | 0.3±0.2 |
| CD 73 | 98.4±0.8 | 99.1±0.1 | CD 73 | 98.9±0.2 | 98.1±0.6 |
| CD 80 | 0.6±0.0 | 0.1±0.0 | CD 80 | 0.4±0.1 | 0.7±0.0 |
| CD 90 | 97.1±2.0 | 91.7±2.0 | CD 90 | 97.3±1.4 | 82.4±0.0 |
| CD 105 | 96.8±2.4 | 95.4±0.0 | CD 105 | 98.6±0.8 | 96.6±0.9 |
| HLA-DR | 0.9±0.7 | 0.6±0.2 | HLA-DR | 0.0±0.0 | 0.0 |
Both types of cells were able to maintain their characteristic immunophenotype after the 14-day expansion in stirred conditions. Values are represented as mean±SEM (n=2).
Under stirred culture conditions, cells are subjected to continuous shear stress, which have been shown to induce MSC differentiation and is normally exacerbated under serum-free conditions.27,28 To determine if microcarrier culture had an effect of priming BM MSC and ASC differentiation to particular lineage, quantitative RT-PCR analyses were performed for three early differentiation genes: RGC32, FABP4, and SPP-1, which are early cell markers for osteogenesis, adipogenesis, and chondrogenesis, respectively. According to the Equations (1)–(4), the maximum shear stress determined for this spinner flask culture was 2.4 dyn/cm2. After the expansion in the spinner flask, quantitative RT-PCR analysis showed an upregulation of early osteocyte (∼10- and 12-fold) and chondrocyte (∼3- and 8-fold) cell markers for both BM MSC and ASC, whereas no difference was observed for the expression of the early adipocyte cell marker.
Discussion
High cell doses required for MSC clinical trials point to the need for reliable, reproducible, and safe methods to achieve the required cell numbers. Conventional static culture protocols have serious limitations and the use of xenogeneic serum-containing media presents major concerns about cell product safety. The development of xeno-free bioreactor culture system for the expansion of human MSC represents major progress in the fields of regenerative medicine and tissue engineering. Based on the previous studies from our group,20 we further developed a microcarrier-based culture system for the expansion BM MSC and ASC under xeno-free conditions. An important step envisaging a xeno-free culture system was to find a feasible alternative for the previously used gelatin-based microcarriers.20 With that purpose, plastic microcarriers were tested, proving to be at least as efficient as Cultispher-S microcarriers for the expansion of BM MSC and ASC. We were able to efficiently expand MSC using a xeno-free culture system, while maintaining MSC immunophenotype and differentiative potential.
In fact, after 9 days of culture, ASC reached a higher cell density on plastic beads (coated with CELLStart) than on gelatin microcarriers (coated with FBS), despite the latter exhibiting a high percentage of initial cell adhesion and a high cell specific growth rate. ASC presented a similar metabolism to BM MSC, with higher specific consumption/production rates of glucose/lactate in the beginning of the culture, followed by lower values after day 6. It is worth noting that the optimized 25% daily feeding regimen20 was sufficient to avoid nutrient depletion or growth-inhibition by metabolites. Moreover, the expansion on plastic microcarriers did not affect either the multilineage differentiative potential or the immunophenotype of ASC. Therefore, plastic microcarriers were shown to be suitable for the expansion of MSC in stirred-culture systems, and were used in all subsequent experiments.
However, a safer expansion protocol for human MSC for clinical application requires a total absence of xenogeneic-origin materials. In addition to the use of StemPro® Xeno-free culture medium and plastic microcarriers (precoated with CELLstart CTS, a xeno-free attachment substrate); we used an animal-origin free cell dissociation agent (TrypLE Select). Thus, we were able to implement a completely xeno-free microcarrier-based culture system through out the process for the expansion of MSC.
In the absence of serum, the initial cell adhesion to microcarriers was substantially reduced for both cell sources, most likely due to lack of important cell adhesion-promoting proteins, such as fibronectin and vitronectin.29 Taking into consideration that plastic microcarriers were precoated with a xeno-free substrate before cell inoculation, our results indicate that dynamic conditions were not sufficient to support a high initial MSC adhesion (∼75% of the initial cell number was lost), such as reported for static conditions.17 As a result of low cell attachment, a lag growth phase was observed in xeno-free cultures, in contrast to cases where serum-supplemented medium was used, which led to longer culture times. An improvement of the initial cell adhesion step, either by a more efficient bead precoating protocol or the addition of attachment-promoting proteins to the medium, will represent a crucial advance toward a faster and more productive MSC expansion process.
Notwithstanding the decreased initial cell adhesion, the xeno-free microcarrier-based culture system was shown to efficiently expand both BM MSC and ASC in spinner flasks, with comparable results to those described in the literature, which use serum-supplemented media with microcarriers30 or other strategies such as needle-suspended scaffolds.31 Despite an initial lag growth phase associated with lower cell adhesion levels, specific growth rates for xeno-free dynamic expansions were higher than for MesenPRO RS cultures that contained reduced serum. The higher cell densities achieved with BM MSC were reflected in the reduction of the RTL by 28%, which was similar to prior results obtained for static cultures.21
In terms of metabolic performance, BM MSC presented lower average values for both specific glucose consumption and lactate production rates than ASC. Nonetheless, the trends of consumption rates were identical for both cell types, with higher values up to days 5 or 6 followed by lower values for the remaining culture time, which is in accordance with data obtained using serum-containing media by our group and others.30 This MSC metabolic profiling appears to be characteristic of microcarrier-based stirred cultures, since it was not observed for static expansion conditions,21 and may be related to the metabolic adaptation to dynamic culture systems. The average Y′lactate/glucose was similar for both cases, which is evidence that carbon source metabolism was similar for the two cell sources. Importantly, nutrient/metabolite-related cell growth inhibition was not observed since glucose and lactate concentration were kept within nonlimiting ranges.13,21
Additionally, MSC characteristic immunophenotype was well maintained by the xeno-free dynamic expansion in spinner flasks for both BM MSC and ASC. A slightly lower percentage of CD90-positive cells for ASC after the expansion may be attributed to longer enzymatic cell detachment times or to an agitation effect, which are known to affect cell surface markers expression.32
The upregulation of the expression of early osteocyte and chondrocyte cell markers upon MSC expansion in the spinner flasks confirmed that the shear stress associated with a microcarrier-based stirred culture system might have a priming effect on MSC differentiation. In fact, several authors described in the literature that higher values of shear stress induced an upregulation of osteogenesis-related genes and proteins28,33,34 and even had an effect on MSC proliferation.27 However, the multilineage differentiation of BM MSC and ASC after the spinner flask expansion was evidence that cells maintained their mesodermal progenitor properties intact. More significantly, expanded MSC in xeno-free stirred conditions retained their hematopoietic supportive activity in vitro in a co-culture system to expand umbilical cord blood derived progenitors35 (data not shown). Further studies are needed to optimize the agitation rates and mixing dynamics of the cultures to reduce shear stress and its potential effects.
In conclusion, these results demonstrate that our microcarrier-based culture system is suitable for the efficient expansion of MSC in xeno-free conditions from different sources. To our knowledge, this is the first report of a completely serum-free and xeno-free microcarrier-based stirred system for the expansion of BM MSC and ASC, while maintaining cell characteristic immunophenotype and differentiative potential. Moreover, the ease of scalability of microcarrier-based cultures combined with cell densities obtained in spinner flasks are encouraging factors to scale-up the xeno-free culture system. Large-scale fully controlled bioreactor studies are now in progress in our laboratory, aiming at the production of clinically relevant human MSC in a more efficient manner.
Acknowledgments
This work was financially supported by the MIT-Portugal Program, Associação Portuguesa Contra a Leucemia, Fundação para a Ciência e Tecnologia (FCT, Portugal)–Project PTDC/EQU-EQU/114231/2009, and Life Technologies Corporation Collaborative Research Compact Program. Grants SFRH/BD/38719/2007 and SFRH/BD/38720/2007 were awarded to Francisco dos Santos and Pedro Z. Andrade, respectively, from FCT. The authors acknowledge Joana S. Boura for the help with RT-PCR assays.
Disclosure Statement
No disclosure for Francisco dos Santos, Pedro Z. Andrade, Manuel M. Abecasis, Jeffrey M. Gimble, Cláudia Lobato da Silva, and Joaquim M.S. Cabral. Andrew M. Campbell, Lucas G. Chase, Shayne Boucher, and Mohan C. Vemuri are employees and stockholders of Life Technologies Corporation.
References
- 1.Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 2.Le Blanc K. Frassoni F. Ball L. Locatelli F. Roelofs H. Lewis I., et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 3.Hare J.M. Traverse J.H. Henry T.D. Dib N. Strumpf R.K. Schulman S.P., et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kharaziha P. Hellstrom P.M. Noorinayer B. Farzaneh F. Aghajani K. Jafari F., et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 5.Mazzini L. Ferrero I. Luparello V. Rustichelli D. Gunetti M. Mareschi K., et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp Neurol. 2010;223:229. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Macmillan M.L. Blazar B.R. DeFor T.E. Wagner J.E. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I-II clinical trial. Bone Marrow Transplant. 2009;43:447. doi: 10.1038/bmt.2008.348. [DOI] [PubMed] [Google Scholar]
- 7.Ringden O. Uzunel M. Rasmusson I. Remberger M. Sundberg B. Lonnies H., et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 8.Tarte K. Gaillard J. Lataillade J.J. Fouillard L. Becker M. Mossafa H., et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- 9.Chen X. Xu H. Wan C. McCaigue M. Li G. Bioreactor expansion of human adult bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2052. doi: 10.1634/stemcells.2005-0591. [DOI] [PubMed] [Google Scholar]
- 10.Frauenschuh S. Reichmann E. Ibold Y. Goetz P.M. Sittinger M. Ringe J. A microcarrier-based cultivation system for expansion of primary mesenchymal stem cells. Biotechnol Prog. 2007;23:187. doi: 10.1021/bp060155w. [DOI] [PubMed] [Google Scholar]
- 11.Sart S. Schneider Y.J. Agathos S.N. Ear mesenchymal stem cells: an efficient adult multipotent cell population fit for rapid and scalable expansion. J Biotechnol. 2009;139:291. doi: 10.1016/j.jbiotec.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Schop D. Janssen F.W. Borgart E. de Bruijn J.D. van Dijkhuizen-Radersma R. Expansion of mesenchymal stem cells using a microcarrier-based cultivation system: growth and metabolism. J Tissue Eng Regen Med. 2008;2:126. doi: 10.1002/term.73. [DOI] [PubMed] [Google Scholar]
- 13.Schop D. Janssen F.W. van Rijn L.D. Fernandes H. Bloem R.M. de Bruijn J.D., et al. Growth, metabolism, and growth inhibitors of mesenchymal stem cells. Tissue Eng Part A. 2009;15:1877. doi: 10.1089/ten.tea.2008.0345. [DOI] [PubMed] [Google Scholar]
- 14.Wu Q.F. Wu C.T. Dong B. Wang L.S. [Cultivation of human mesenchymal stem cells on macroporous CultiSpher G microcarriers] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2003;11:15. [PubMed] [Google Scholar]
- 15.Spees J.L. Gregory C.A. Singh H. Tucker H.A. Peister A. Lynch P.J., et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Sundin M. Ringden O. Sundberg B. Nava S. Gotherstrom C. Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92:1208. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- 17.Lindroos B. Boucher S. Chase L. Kuokkanen H. Huhtala H. Haataja R., et al. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11:958. doi: 10.3109/14653240903233081. [DOI] [PubMed] [Google Scholar]
- 18.Ross P.J. Suhr S.T. Rodriguez R.M. Chang E.A. Wang K. Siripattarapravat K., et al. Human-induced pluripotent stem cells produced under xeno-free conditions. Stem Cells Dev. 2010;19:1221. doi: 10.1089/scd.2009.0459. [DOI] [PubMed] [Google Scholar]
- 19.Vaajasaari H. Ilmarinen T. Juuti-Uusitalo K. Rajala K. Onnela N. Narkilahti S., et al. Toward the defined and xeno-free differentiation of functional human pluripotent stem cell-derived retinal pigment epithelial cells. Mol Vis. 2011;17:558. [PMC free article] [PubMed] [Google Scholar]
- 20.Eibes G. dos Santos F. Andrade P.Z. Boura J.S. Abecasis M.M. da Silva C.L., et al. Maximizing the ex vivo expansion of human mesenchymal stem cells using a microcarrier-based stirred culture system. J Biotechnol. 2010;146:194. doi: 10.1016/j.jbiotec.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Dos Santos F. Andrade P.Z. Boura J.S. Abecasis M.M. da Silva C.L. Cabral J.M. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 22.Gimble J. Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes A.M. Fernandes T.G. Diogo M.M. da Silva C.L. Henrique D. Cabral J.M. Mouse embryonic stem cell expansion in a microcarrier-based stirred culture system. J Biotechnol. 2007;132:227. doi: 10.1016/j.jbiotec.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Martens U.M. Brass V. Engelhardt M. Glaser S. Waller C.F. Lange W., et al. Measurement of telomere length in haematopoietic cells using in situ hybridization techniques. Biochem Soc Trans. 2000;28:245. doi: 10.1042/bst0280245. [DOI] [PubMed] [Google Scholar]
- 25.Croughan M.S. Hamel J.F. Wang D.I. Hydrodynamic effects on animal cells grown in microcarrier cultures 1987. Biotechnol Bioeng. 2006;95:295. doi: 10.1002/bit.21158. [DOI] [PubMed] [Google Scholar]
- 26.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 27.Potier E. Noailly J. Ito K. Directing bone marrow-derived stromal cell function with mechanics. J Biomech. 2010;43:807. doi: 10.1016/j.jbiomech.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Yi W. Sun Y. Wei X. Gu C. Dong X. Kang X., et al. Proteomic profiling of human bone marrow mesenchymal stem cells under shear stress. Mol Cell Biochem. 2010;341:9. doi: 10.1007/s11010-010-0432-7. [DOI] [PubMed] [Google Scholar]
- 29.Hayman E.G. Pierschbacher M.D. Suzuki S. Ruoslahti E. Vitronectin—a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985;160:245. doi: 10.1016/0014-4827(85)90173-9. [DOI] [PubMed] [Google Scholar]
- 30.Schop D. van Dijkhuizen-Radersma R. Borgart E. Janssen F.W. Rozemuller H. Prins H.J., et al. Expansion of human mesenchymal stromal cells on microcarriers: growth and metabolism. J Tissue Eng Regen Med. 2009;4:131. doi: 10.1002/term.224. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y. Liu T. Song K. Fan X. Ma X. Cui Z. Ex vivo expansion of adipose tissue-derived stem cells in spinner flasks. Biotechnol J. 2009;4:1198. doi: 10.1002/biot.200800130. [DOI] [PubMed] [Google Scholar]
- 32.McDowell C.L. Papoutsakis E.T. Increased agitation intensity increases CD13 receptor surface content and mRNA levels, and alters the metabolism of HL60 cells cultured in stirred tank bioreactors. Biotechnol Bioeng. 1998;60:239. [PubMed] [Google Scholar]
- 33.Knippenberg M. Helder M.N. Doulabi B.Z. Semeins C.M. Wuisman P.I. Klein-Nulend J. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11:1780. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- 34.Yourek G. McCormick S.M. Mao J.J. Reilly G.C. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med. 2010;5:713. doi: 10.2217/rme.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goncalves R. Lobato da Silva C. Cabral J.M.S. Zanjani E.D. Almeida-Porada G. A Stro-1+ human universal stromal feeder layer to expand/maintain human bone marrow hematopoietic stem/progenitor cells. Exp Hematol. 2006;34:1353. doi: 10.1016/j.exphem.2006.05.024. [DOI] [PubMed] [Google Scholar]