Abstract
Background
In the Old World, sandfly species of the genus Phlebotomus are known vectors of Leishmania, Bartonella and several viruses. Recent sandfly catches and autochthonous cases of leishmaniasis hint on spreading tendencies of the vectors towards Central Europe. However, studies addressing potential future distribution of sandflies in the light of a changing European climate are missing.
Methodology
Here, we modelled bioclimatic envelopes using MaxEnt for five species with proven or assumed vector competence for Leishmania infantum, which are either predominantly located in (south-) western (Phlebotomus ariasi, P. mascittii and P. perniciosus) or south-eastern Europe (P. neglectus and P. perfiliewi). The determined bioclimatic envelopes were transferred to two climate change scenarios (A1B and B1) for Central Europe (Austria, Germany and Switzerland) using data of the regional climate model COSMO-CLM. We detected the most likely way of natural dispersal (“least-cost path”) for each species and hence determined the accessibility of potential future climatically suitable habitats by integrating landscape features, projected changes in climatic suitability and wind speed.
Results and Relevance
Results indicate that the Central European climate will become increasingly suitable especially for those vector species with a current south-western focus of distribution. In general, the highest suitability of Central Europe is projected for all species in the second half of the 21st century, except for P. perfiliewi. Nevertheless, we show that sandflies will hardly be able to occupy their climatically suitable habitats entirely, due to their limited natural dispersal ability. A northward spread of species with south-eastern focus of distribution may be constrained but not completely avoided by the Alps. Our results can be used to install specific monitoring systems to the projected risk zones of potential sandfly establishment. This is urgently needed for adaptation and coping strategies against the emerging spread of sandfly-borne diseases.
Author Summary
Growing evidence exists on the emergence of sandfly-borne diseases in the light of climate change. Determining the principle responses of phlebotomine sandflies to climatic changes supports our understanding of future regions that will be threatened by new-establishments of this important group of disease vectors. The aim of this paper is to combine projected climatic suitability for five Phlebotomus species in Central Europe (Austria, Germany and Switzerland) for different time-periods during the 21st century with their potential spreading capacity to disperse to climatically suitable areas. We indicate that the Central European climate will develop toward the preferred bioclimatic niche of the species, especially from mid-century onwards. Nevertheless, we also elucidate within this study that sandflies will hardly be able to occupy the whole areas that will provide suitable climatic conditions due to their limited natural dispersal ability. Our approach provides a framework to combine statistical modelling techniques with expert knowledge on species ecology. Indications of future occurrences of disease vectors may help to initiate surveillance systems in specific regions at an early stage of risk exposure. Hence, the threat of the climate-driven spatial extension of disease vectors and consequently of potentially emerging vector-borne diseases can be counteracted.
Introduction
Globally, the number of vector-borne infections in humans and animals increases rapidly, meanwhile causing almost one third of all cases of emerging infectious diseases [1]. In the Old World, sandfly species of the genus Phlebotomus serve as vectors for sandfly-borne pathogens such as Leishmania, Bartonella and several viruses (e.g. Phlebovirus, Vesiculovirus and Orbivirus) [2]–[4]. Sandfly-borne diseases and in particular visceral leishmaniasis are a main public health concern [5], which demands more attention in science and policy [6]. While the spatial distribution of leishmaniasis seems to expand in southern parts of Europe [7], [8], first cases of autochthonous origin are recently reported from Central Europe [9]–[11], where this disease was not endemic in the past.
The presence of sandflies as vectors is mainly regulated by the species' climatic requirements on temperature and humidity or soil moisture, respectively [3], [12]–[15]. Temperature and humidity are also the main factors impacting the altitudinal structure of sandfly occurrences [16]. It is known that sandflies react very sensitive to wind speed and prefer breeding sites sheltered from wind [17]–[20]. Beyond that, high wind speed decreases or even excludes flight activity [17], [21]. For the purpose of inferring geographic distribution for sandflies, the advantages of ecological niche models have been demonstrated on the example of Lutzomyia species (Lutzomyia spp.) in the New World [22]. For the first time, Peterson and Shaw [23] integrated climate change scenarios in order to project future distribution of Lutzomyia spp. in Brazil. Recently, range expansions for sandflies of the genus Lutzomyia have also been projected for North America in the face of climate change [24].
For Europe, surprisingly, only few studies estimated the risk of potential range expansions of sandflies in the face of climate change (e.g. [25], [26]). The need for such studies is supported by the first sandflies catches in Central Europe. P. mascittii has been caught in Austria on the frontier to Slovenia [27]. Furthermore, P. mascittii is reported from the “Upper Rhine Valley” in the outermost southwest of Germany near the French border [28]. P. perniciosus seems to be established in the German state of “Rhineland-Palatinate” [29]. These findings may either indicate spreading tendencies from Mediterranean regions or range expansion from small Central European refugial areas, which may have already been occupied by the species during the Holocene climate optimum about 6,500 years ago [30]. Possibly, sandflies has occupied more areas in the past than it was noticed. For Austria, establishment of sandflies in formerly non-endemic areas can be expected already by moderately increasing temperatures in the 21st century [25]. Recently, Fischer et al. [26] estimated potential temperature-derived establishment of sandflies in Germany by transferring the required temperature during their activity phase and annual mean temperature for persistence to the expected future climate conditions in Germany using data of a spatio-temporal highly resolved regional climate model. But up to now, projections of the current and climate-driven potential future distribution of Phlebotomus spp. which additionally consider species-specific dispersal ability are missing. As climate is expected to change rapidly in the 21st century, sandflies are forced to react promptly. Here, we close this gap of knowledge and hypothesise:
Climatic suitability for Phlebotomus spp. in Central Europe will generally increase within the 21st century. Expectedly, the climatic requirements for sandflies with current (south-) western European regions of distribution are supposed to be fulfilled in the south-westernmost parts of Central Europe in the 21st century. Instead, species with a south-eastern focus of distribution are thought to find favourable conditions in the south-easternmost Central European regions.
Species with current (south-) western focus of distribution will spread north-eastwards as they are not hampered by natural dispersal barriers. Instead, the Alps will restrict a direct range expansion for species that are currently distributed in the (south-) east of Europe.
Materials and Methods
Bioclimatic envelope modelling for sandfly species
Species presence records and climatic variables
Documented presence records of Phlebotomus spp. were taken from literature. Most of the occurrence data were provided by Artemiev and Neronov [31]. This was done by digitising their analogue maps of presence records. Additional presence records were taken from peer-reviewed articles by searching within the literature databases ISI Web of Science, MEDLINE and BIOSIS Previews from 1984 onwards. The number of presence records is listed in Table 1.
Table 1. Number of species presence records, AUC-values, and training gain for the selected bioclimatic variables.
| P. ariasi | P. mascittii | P. perniciosus | P. neglectus | P. perfiliewi | |
| Presence records | 79 | 66 | 273 | 90 | 124 |
| AUC (Training data) | 0.94 | 0.97 | 0.92 | 0.92 | 0.96 |
| AUC (Test data) | 0.93 | 0.93 | 0.90 | 0.89 | 0.95 |
AUC-values are a threshold-independent model quality criterion and range from 0 to 1 (perfect fit). Useful models yielded in AUC-values above 0.7, where excellent models achieve at least AUC-scores above 0.9. Training gains are determined by Jackknife test for the selected variables by using only this variable for the model (upper value) and if the specific variable is removed for the rest of the variable set (lower value). For the species with current (south-) western focus of distribution (P. ariasi, P. mascittii and P. perniciosus) BIO 11 (Mean temperature of the coldest quarter) represents the most important variable. This is indicated by the highest training gain of the model by using only this variable and the lowest training when this variable is removed from the set of variables. The drop of BIO 10 (Mean temperature of the warmest quarter) from the set of variables instead seems to lower training gain most for the species with (south-) eastern focus of distribution. BIO 13 (Precipitation of the wettest month) is identified as most important variable when used in isolation for P. neglectus, while BIO 11 seems to be most influencing factor in isolation regarding the occurrences of P. perfiliewi.
Current bioclimatic data were taken from http://www.worldclim.org [32] in 5 Arcmin resolution (approximately 10 km grid size for Central Europe). Higher spatial resolution would not correspond to the spatial accuracy of occurrence data for sandfly species. We defined all regions that contain our presence records (Europe, northern Africa and the Middle East) as climatic background, where our models were trained. Selection of the most important bioclimatic variables was done via Jackknife test. We considered results of the Jackknife tests for the model training gain for all variables in isolation and for the remaining set of variables when the isolated variable is dropped from the set [33]. To reduce collinearity in the data set [34] those variables that had a Pearson correlation coefficient r>0.7 with any other higher-ranking variable in the results of the Jackknife test variables were removed. We applied the variable selection procedure separately for each species.
The high-resolution regional climate model COSMO-CLM (CCLM) was applied for future projections in Europe. This dynamically downscaled model is nested into the global model ECHAM5 [35]. In contrast to their driving global models, regional climate models integrate topography and can project climate change at a much higher spatial resolution which enhances the quality of studies on climate change impacts [36]. Our future projections refer to the IPCC A1B and B1 emission scenarios for greenhouse gases [37]. In short, the A1B scenario is characterised by a rapid economic growth in an integrated world with a balanced technological emphasis on fossil and non-fossil energy sources. The B1 scenario is based on the same economic growth as in A1B but with a more rapid change towards a service and information economy. Climatic data were averaged over time periods 2011–2040, 2041–2070 and 2071–2100 for each scenario separately. Bioclimatic variables for modelling future climate projections were calculated in the same way as they are provided by http://www.worldclim.org [32] for current conditions.
Non-analogue climatic conditions are a problematic issue in projections [38]. We used a Multivariate Environmental Similarity Surface (MESS) analysis introduced by Elith et al. [39] to detect regions where projections are inappropriate due to dissimilarity in values of the used variables for training and projecting the model [40]. The MESS-analysis measures the similarity between those environments used to train the model and the new projected environments for any grid cell [39], [40]. Occurrence records and climatic data were prepared in ArcGIS 10.0, correlation analysis was performed in PASW Statistics 18, Jackknife test to measure the variable's importance is implemented in MaxEnt 3.3.3e.
Model runs
All models were generated using the maximum entropy algorithm. Maximum entropy basically is a machine-learning technique combining species occurrence data with detailed climatic and environmental datasets [41], [42]. This algorithm implemented in MaxEnt software computes a probability distribution covering the study area that satisfies a set of constraints which are derived from environmental conditions at species presence records. The algorithm then chooses a distribution with maximum entropy within all possible distributions [41]. MaxEnt generally performs better than other presence-only or pseudo-presence-only models [41], [43], [44], which becomes especially apparent by using small numbers of species occurrence records [45]–[47]. Furthermore, the influence of spatial errors in species occurrences on model performance of MaxEnt due to e.g. inaccurate georeferences is less severe in comparison to other algorithms [48].
The MaxEnt-models settings have been adapted from a previous study concerning the projections of climatic suitability for Aedes albopictus in Europe [49]. We randomly selected 70% of the occurrence data to train each model and used the remainder to test each model in order to evaluate model accuracy (e.g. [50]). Models were replicated 100 times for each species and the results were finally averaged. We used both, threshold-dependent as well as threshold-independent quality criteria. Eleven binary omission rates were calculated as the proportion of test respective training points that were not predicted at a threshold probability that equalled the minimum probability on any pixel containing an occurrence point [42]. Those were tested using one-sided p-values for the null hypothesis that test points are predicted no better than by a random prediction with the same fractional predicted area. This was practiced previously for the evaluation of model results for Lutzomyia spp. [24]. Furthermore, model performance was evaluated using area under the receiver operator curve (AUC) statistics, which compares how likely a random presence site will have a higher predicted value in the model than a random absence [43]. The receiver operator curves appeals to be independent on a user-defined threshold for determining presence versus absence. We limited the study area to the geographic extent of the sampling distribution (see chapter Species presence records and climatic variables) in order to avoid inflated AUC-scores that are associated with geographical extents that go beyond the presence environmental domain [51], [52]. All models were built in the latest available version (MaxEnt 3.3.3e).
Least-cost path for species dispersal
It is well known that species dispersal ability is dependent on the environment and varies strongly with landscape structure [53]. In order to make projections more realistic according to spatial characteristics, we used a least-cost path analysis based on graph theory [54]–[56] to determine the most likely way for Phlebotomus spp. to move across a spatio-temporally changing landscape. The path function indicates the least efforts (“costs”) for a species in moving through any particular cell in the respective landscape [57]–[59]. Least-cost path analyses are frequently used to determine potential dispersal pathways for mammals [60]–[63] but have also been applied to insects [58].
Definition of cost surfaces and calculation of distances and backlinks
Our aim was to identify the species-specific least-cost pathway for potential movement of the five Phlebotomus spp. to each of the modelled climatically suitable habitats during the 21st century for the selected time-periods and for each scenario, respectively. Therefore, we created three different species-specific cost surfaces. The first cost-surface was generated for costs arising for the species movement to climatically suitable habitats of the upcoming time-period (2011–2040). The second one was built for the movement up to mid-century (2041–2070) and finally to the end of the century (2071–2100). The respective cost factors are listed in Table 2. Each cost surface includes as well temporarily stable as varying environmental landscape features.
Table 2. Cost factors within the defined cost surfaces for Phlebotomus species.
| Landscape feature | Value or area | Cost factor |
| Elevation | 0–800 | 0 |
| 801–1200 | 1 | |
| >1200 | 2 | |
| Further landscape features | River valleys | 0 |
| Non-valleys | 4 | |
| Sea | - | |
| Climatic suitability (taken from MaxEnt-models, averaged over two subsequent time-periods) | 0.81–1.0 | 0 |
| 0.61–0.8 | 1 | |
| 0.41–0.6 | 2 | |
| 0.21–0.4 | 3 | |
| 0.01–0.2 | 4 | |
| Wind speed one metre above the ground(in m/s, averaged over two subsequent time-periods) | 0.01–1.5 | 0 |
| 1.51–2.5 | 1 | |
| 2.51–3.5 | 2 | |
| >3.5 | 3 |
Factors are surfaces were generated by considering both, spatio-temporal stable and variable environmental conditions within the 21st century. Two cost factors are stable within the 21st century: River valleys are considered as the preferred breeding sites for sandflies [2]. Hence, regions which include river valleys are attributed with costs. Sea as absolute barrier cannot be crossed. Beyond, an altitudinal cost structure was developed in accordance to the preferred elevation of sandflies [2]. Two factors vary in the 21st century: MaxEnt-values of climatic suitability range theoretically from 0 (unfavourable conditions) to 1 (perfect conditions). These values are classified and attributed with costs in accordance to the suitability; the lower the suitability the higher the costs. This factor is species-specific. Cost factors for wind speeds are related to the observations concerning wind-speed dependent flight activity. They are taken from findings of Lane [94] concerning the highest flight activity up to 1.5 m/s; from Quate [95] who observed a reduced flight activity between 1.5–2.5 m/s and Roberts [96] who noticed no flight activity above wind speeds of 3.5 m/s. Climatic suitability and wind speed were averaged over two subsequent time-periods.
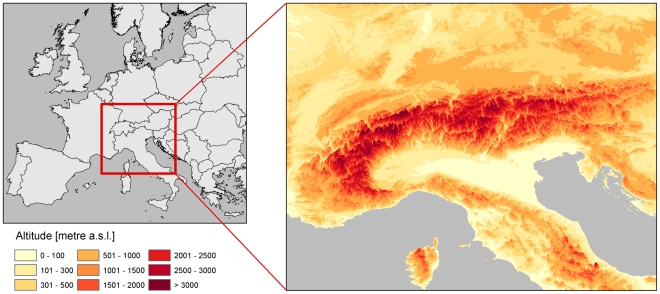
a) Environmental landscape features that do not to change in the 21st century were consequently considered as constant (stable) cost factors in all created cost surfaces: River valleys provide the preferred breeding sites due to high temperatures and moist and humid soils [64], [65] and can hence be considered as preferred dispersal corridors. We buffered this features with a distance of ten kilometres for consistency with the climatic data and attributed it without costs. Only the surrounding landscape was addressed with costs. Additionally, increasing elevation was attributed with rising costs [2]. The topographical structure of Europe is presented in Figure 1. Sea surfaces and high mountains were considered as “absolute barriers” which cannot be crossed. These costs were the same in all cost surfaces throughout the 21st century and for all species.
Figure 1. Location and topography of the Alps.
Elevation is visualised in tenfold vertical exaggeration. The Alps are the highest mountain range in the continental interior of Europe separating the Mediterranean region from Central Europe and extending from France (West) through Switzerland and Italy (South) into Austria, Germany (North) Slovenia, and Croatia (East). Alpine regions are considered as the main natural barrier for natural sandfly dispersal.
b) Within the cost surfaces temporally varying environmental landscape features in the 21st century (due to climatic effects) were integrated: The species-specific changing climatic suitability of an area between two subsequent time-steps (current-2011/2040, 2011/2040–2041/2070, 2041/2070–2071/2100) was integrated as a further cost factor in the respective cost surfaces. For this purpose the projected climatic suitability was averaged over two subsequent time-periods. The values of climatic suitability were taken from MaxEnt-models. Regions that have to be overcome between two time-periods but would persist to remain outside of the preferred bioclimatic niche were attributed with higher costs. We furthermore integrated wind speed in the cost surface as sandflies react very sensitive to high wind speed by reducing flight activity [17], [21]. Data of current and projected wind speed (for A1B and B1 scenario) are taken from CCLM. Data were averaged for the equivalent time-periods, which were already used to model climatic suitability for the single sandfly species (2011–2040, 2041–2070 and 2071–2100). It is realised that sandflies predominantly prefer to be active near the soil surface (up to one metre above the ground) and usually do not exceed two metres above the ground [64]. The provided data of wind speed, however, are given for a height of ten metres above the ground. In consequence, we applied the wind profile power law that is derived from the logarithmic wind profile equation for the lower atmosphere in order to relate wind speeds given at one height to another [66]. The equation to calculate wind speed in one metre was calculated for each time-period by:
with V1m representing wind speed in one metre, V10m representing the (given) wind speed in ten metre above the ground, h1m/h10m = 0.1 (height of one metre, divided by the height of ten metre - where the velocity is given by data of CCLM) and RF (roughness factor). Land cover decreases the near surface wind speed due to the roughness of the landscape features. The RF should not be considered as spatially constant as it varies for different surface obstacles, which must be taken into account [67]. Therefore, we integrated land cover data provided by http://earth.esa.int [68] for the calculation of the near-surface wind speed. In our study, land cover is considered not to change during the 21st century. We reclassified the provided map of land cover in Europe into three classes (with different RF) proposed by Kleemann and Meliss [69]:
Low RF ( = 0.16): cropland, grassland vegetation, bare areas
Mid RF ( = 0.28): different types of forests, vegetation dominated by trees and rural communities
High RF ( = 0.40): urban areas
Then, the wind speeds were averaged over two subsequent time-periods of species movement as it was done for the modelled climatic suitability. For all species, the development in wind speed was attributed with equal costs (Table 2). Due to these temporal changing factors (species climatic suitability and wind speed) different cost surfaces were generated.
Determining the least-cost path based on the generated cost surfaces
Determining the least-cost path requires cost distance and cost backlink calculations as inputs which are both assigned on the basis of the defined cost surfaces. Cost distances were calculated in order to account for the minimal accumulated travel costs that accrue by travelling with increasing distance from the source to the target area [63]. The cost backlinks indicate the direction for each grid cell to which direction the costs are cheapest. Details on calculations of cost distance and assignment of cost backlink can be found elsewhere (for review see [70]). Our initial source grid for species occurrences as starting points included all areas with documented current European presence records. As destination area we defined Central Europe (Austria, Germany and Switzerland) for all species throughout the 21st century. The natural dispersal ability of sandflies is limited. Generally, the flight range around their breeding area is about one kilometre [71]. The flight range for P. ariasi, however, can reach two kilometres [17]. In Mediterranean areas, sandflies are able to establish up to three generation each year [2]. Therefore, we limited the maximal natural range expansion for P. ariasi to six kilometres per year (180 km/30 year time interval) and for the other sandfly species to three kilometres per year (90 km/30 year time interval) for moving through any particular cost surface.
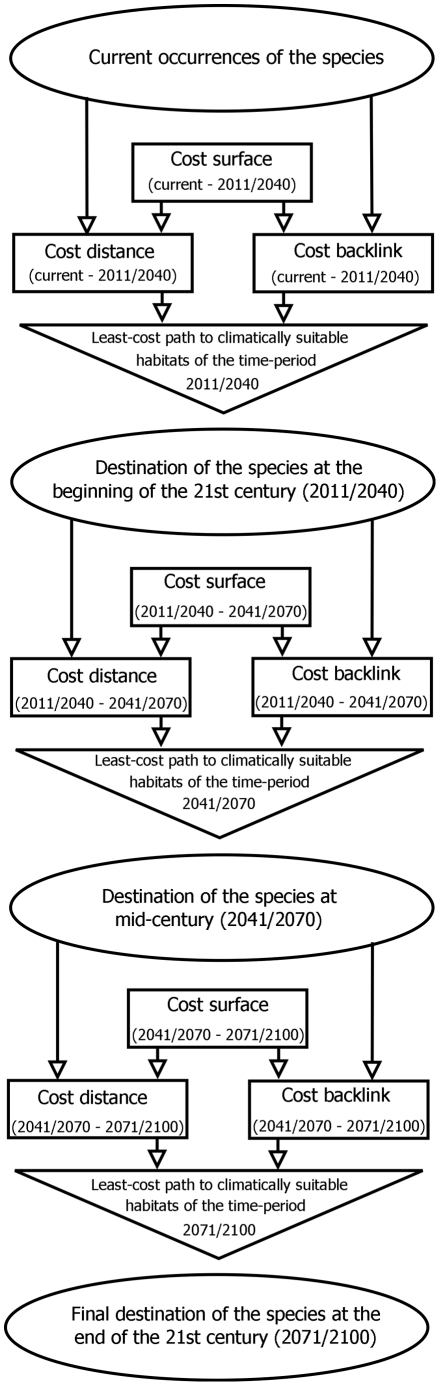
In our study, species are assumed to be able to establish in climatically suitable habitats indicated by values higher than 0.5 of the MaxEnt-models. This typically corresponds to values of the climatic suitability on the respective presence records [42]. Therefore, those regions were indicated as species occurrences of the subsequent time period (2011–2040) that overtop the threshold of suitability and that are connected via the initial least-cost path. Those areas were defined as new starting points for the least-cost path to the expected climatically suitable habitats at mid-century (2041–2070). This procedure was repeated for a third time in order to determine reachable location of the sandfly species at the end of the 21st century (2071–2100). The principle is illustrated in Figure 2. Least-cost analysis was performed using distance functions within the “Spatial Analyst Tool” implemented in ArcGIS 10.0.
Figure 2. Principle of the least-cost analysis.
Least-cost analysis was used to determine the most likely way of natural dispersal sandfly species in the 21st century.
Results
Model results for species climatic suitability
AUC-values yielded in high scores for five species (Table 1). Binominal tests indicated that tests points are predicted better by the model than a random prediction with the same factional predicted area at the significance level p<0.01. Similarity between current and projected climate is analysed by MESS-analysis. Highest similarity in projections is indicated for southern parts of Germany, the northernmost regions of Switzerland as well as for eastern and north-eastern parts of Austria. Instead, lowest similarity exists for alpine regions and northern Germany. However, our projections seem not to be biased by non-analogue climate.
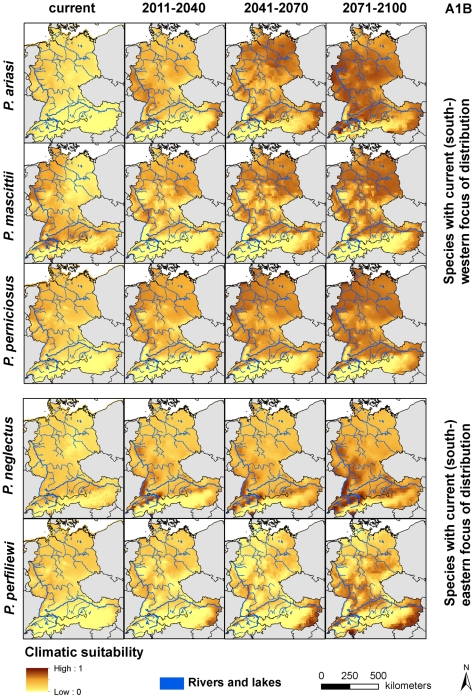
In general, climatic suitability can be expected to increase for all species in the 21st century (Figure 3 and S1, Table 3 and S1). This is in accordance with the first part of our first hypothesis assuming increasing climatic suitability for the species in Central Europe. Nevertheless, we cannot completely verify the second part of our first hypothesis of more favourable conditions in the (south-) westernmost parts of Central Europe for species with current (south-) western focus of European distribution and the opposite for species which are currently distributed in (south-) eastern parts of Europe. Overall, projections based on the A1B scenario (Table 3) represent higher suitability for species in comparison to projection of the B1 scenario (Table S1). Nevertheless, the spatial patterns of potential climatically suitable habitats remain to be the same for both scenarios. The detailed annotation of climatic suitability in the following refers to the A1B scenario.
Figure 3. Current and projected climatic suitability for five Phlebotomus species.
Values of climatic suitability range theoretically from 0 (unfavourable conditions) to 1 (perfect conditions). Projections refer to the A1B scenario.
Table 3. Current and projected climatic suitability for Phlebotomus species in Central Europe.
| Central Europe | Austria | Germany | Switzerland | |||||||||||||
| current | 2011–2040 | 2041–2070 | 2071–2100 | current | 2011–2040 | 2041–2070 | 2071–2100 | current | 2011–2040 | 2041–2070 | 2071–2100 | current | 2011–2040 | 2041–2070 | 2071–2100 | |
| P. ariasi | 0.14 (+/−0.10) | 0.32 (+/−0.13) | 0.38 (+/−0.17) | 0.54 (+/−0.17) | 0.02 (+/−0.02) | 0.17 (+/−0.22) | 0.29 (+/−0.19) | 0.39 (+/−0.21) | 0.17 (+/−0.08) | 0.36 (+/−0.11) | 0.43 (+/−0.11) | 0.60 (+/−0.10) | 0.07 (+/−0.08) | 0.18 (+/−0.24) | 0.11 (+/−0.15) | 0.39 (+/−0.29) |
| P. mascittii | 0.28 (+/−0.19) | 0.30 (+/−0.20) | 0.40 (+/−0.23) | 0.47 (+/−0.25) | 0.26 (+/−0.17) | 0.22 (+/−0.19) | 0.28 (+/−0.24) | 0.28 (+/−0.23) | 0.32 (+/−0.18) | 0.36 (+/−0.16) | 0.48 (+/−0.18) | 0.55 (+/−0.18) | 0.21 (+/−0.18) | 0.12 (+/−0.14) | 0.14 (+/−0.16) | 0.15 (+/−0.19) |
| P. perniciosus | 0.25 (+/−0.14) | 0.35 (+/−0.19) | 0.46 (+/−0.20) | 0.52 (+/−0.22) | 0.09 (+/−0.10) | 0.17 (+/−0.20) | 0.26 (+/−0.23) | 0.33 (+/−0.26) | 0.31 (+/−0.10) | 0.42 (+/−0.14) | 0.53 (+/−0.12) | 0.60 (+/−0.12) | 0.13 (+/−0.15) | 0.10 (+/−0.17) | 0.15 (+/−0.23) | 0.19 (+/−0.26) |
| P. neglectus | 0.23 (+/−0.10) | 0.31 (+/−0.15) | 0.38 (+/−0.14) | 0.49 (+/−0.16) | 0.18 (+/−0.13) | 0.23 (+/−0.20) | 0.36 (+/−0.20) | 0.46 (+/−0.20) | 0.24 (+/−0.08) | 0.34 (+/−0.11) | 0.38 (+/−0.09) | 0.49 (+/−0.12) | 0.23 (+/−0.17) | 0.30 (+/−0.30) | 0.40 (+/−0.28) | 0.53 (+/−0.31) |
| P. perfiliewi | 0.10 (+/−0.06) | 0.10 (+/−0.13) | 0.19 (+/−0.17) | 0.33 (+/−0.23) | 0.04 (+/−0.03) | 0.22 (+/−0.22) | 0.28 (+/−0.28) | 0.36 (+/−0.32) | 0.12 (+/−0.05) | 0.07 (+/−0.06) | 0.17 (+/−0.12) | 0.34 (+/−0.20) | 0.05 (+/−0.04) | 0.17 (+/−0.26) | 0.17 (+/−0.20) | 0.29 (+/−0.30) |
Noted are mean values and standard deviation in brackets. Projections refer to the A1B scenario.
Climatic suitability for species with current (south-) western focus of distribution
Results for species with current (south-) western focus of distribution (P.ariasi, P. mascittii and P. perniciosus) show - regardless of slight differences - a comparable tendency in spatial patterns of projected climatic suitability for the upcoming time-period (Figure 3 and S1). Expectedly, these species achieve highest values of current and projected climatic suitability in the westernmost parts of Germany and Switzerland. Projections for the conditions from mid-century onwards, however, indicate increasing suitability for the eastern parts of the countries. Interestingly, moderate suitability is indicated for P. perniciosus on western parts of Germany and the coast of the North Sea for the current climatic conditions. In those regions no presence of the species is documented up to now. Favourable conditions for P. mascittii and P. perniciosus can be expected in north-eastern Germany at the end of the century and in less extent also for P. ariasi. Instead, P. ariasi will achieve highest values of climatic suitability in Switzerland. Nevertheless it is worth mentioning that favourable conditions can be expected for all species in certain river valleys in the northern and north-eastern parts of Switzerland on the border to France and Germany and along the “Danube valley”. This becomes especially apparent regarding the projections for the end of the 21st century. For P. mascittii climatic suitability will persist in eastern (including south-eastern and north-eastern parts) of Austria. For P. ariasi and P. perniciosus suitability can be expected to increase in those regions. Austrian alpine regions remain to persist outside the preferred niche of all three species throughout the 21st century.
Climatic suitability for species with current (south-) eastern focus of distribution
The results of the current and projected climatic suitability for the two species with (south-) eastern European focus of distribution (P. neglectus and P. perfiliewi) differ remarkably. For P. neglectus, habitats in Upper Austria along the the “Lake Constance” regions, located in the southern parts of Germany and northern parts of Switzerland, are detected to be adequate under current conditions. For the upcoming time-period, it can be expected that especially the “Upper Rhine Valley” in the southwest of Germany will provide suitable climatic conditions. Starting at mid-century, almost all regions in Germany will provide favourable conditions for species establishment. At the end of the century, additionally, northern and southern parts of Switzerland will achieve favourable climatic conditions. Then, only the highest Alpine regions are expected to remain climatically unsuitable the establishment of P. neglectus. The current and projected values of climatic suitability for P. perfiliewi differ spatially from those that are calculated for P. neglectus. Currently, climatic requirements of P. perfiliewi will not be fulfilled in Central Europe. Favourable conditions can be expected for the up-coming time period and mid-century higher in spatially limited areas for southernmost parts of Switzerland - canton Ticino - and (south-) eastern parts of Austria. Germany remains unfavourable for the establishment of P. perfiliewi until mid-century. At the end of the 21st century, the river valley of the “Rhine” and “Danube” will provide preferable climatic conditions for P. perfiliewi.
Least-cost path analysis
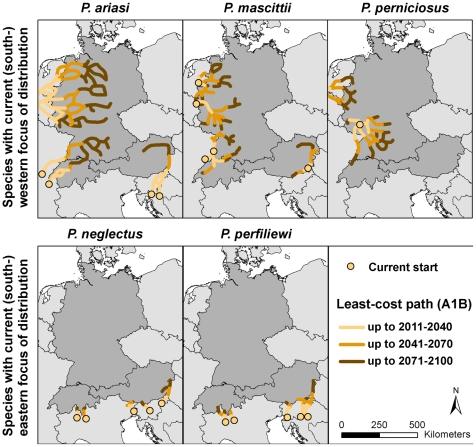
In general, projections hint on spreading tendencies for all studied Phlebotomus spp. to areas where they have not occurred so far in both scenarios (Figure 4 and S2). Nevertheless, sandfly species will not be able to become established in all climatically suitable areas of Central Europe according to the limited natural dispersal ability. The detected dispersal pathways show some differences between the two applied scenarios, in contrast to the modelled climatic suitability, where just temporal but no spatial variations are pointed out.
Figure 4. Least-cost paths for Phlebotomus species.
The detected pathways indicate direction of spread in the 21st century. Spatio-temporal varying climatic suitability and wind speed included in the cost surface that must be crossed by species in the 21st century refer to the A1B scenario.
The first part of our second hypothesis that species with current (south-) western focus of distribution are likely to disperse eastwards can be affirmed only for P. perniciosus but not for P. ariasi and for P. mascittii. We cannot confirm the part of the second hypothesis that the Alps will prohibit completely a northward spread for the species which are currently distributed in (south-) eastern European regions. However, it is very likely that the Alps will decelerate the range expansion.
Least-cost path for species with current (south-) western focus of distribution
In contrast to the similar tendency in climatically suitable habitats, dispersal pathways for species with current (south-) western focus of distribution (P. ariasi, P. mascittii and P. perniciosus) will differ. P. ariasi is characterised by the highest dispersal ability and seems to spread to Switzerland and Germany from recent occurrences in France. Consequently, the western parts of these countries can be expected to be occupied already in the upcoming time-period. From then on, the species seems to spread along the border of Germany and Switzerland to Bavaria in the southeast of Germany until the end of the century. Additionally, P. ariasi will spread to north-eastern parts of Germany until the end of the century in A1B but not in the B1 scenario. A further dispersal pathway is detected starting from Croatian and Slovenian occurrences directed to the (south-) eastern parts of Austria. Up to the end of the century, P. ariasi will also be able to occupy eastern and north-eastern parts of Austria. Two pathways are detected for P. mascittii starting from the “Upper Rhine Valley” in the southwest of Germany, which are either directed northwards or southwards (to the northeast of Switzerland). Additionally, a direct spread from France to Switzerland is identified. As two pathways are directed to northern parts of Switzerland, this region seems to be especially endangered regarding species establishment, expectedly from mid-century onwards. The north-eastern expansion for P. mascittii in Germany is solely indicated when applying the A1B scenario. Additionally, two pathways are detected from “Carinthia” in Austria. A northward spread to the river valley of the “Danube” and a westward spread along the Slovenian and Italian boarder can be expected in both scenarios. From the recent occurrences of P. perniciosus in the southwest of Germany, potential pathways are determined mainly to southern and eastern directions. Along the southernmost parts of Germany P. perniciosus will disperse to the southeast of Germany (Bavaria) until the end of the 21st century. Beyond that, western parts of Germany will be reached from an expected movement from French regions across Belgium. This range expansion is more pronounced when applying the A1B scenario. Switzerland and Austria seem not to be exposed to a direct northward spread from the species across the Alps.
Least-cost path for species with current (south-) eastern focus of distribution
In comparison to species with current (south-) western focus of distribution, potential range expansions for Phlebotomus spp. with current (south-) eastern focus of distribution (P. neglectus and P. perfiliewi) are more restricted. The detected least-cost paths for P. neglectus are rather diverse. For this species, several pathways indicate a northward spread to locations in Switzerland (from Italy) and Austria (from Slovenia). Expectedly at mid-century, the southern parts of Switzerland and Austria will be achieved by the specimen. Only in A1B scenario, P. neglectus is expected to be able to spread to eastern regions of Austria and establish permanent populations there. There is no evidence that this species may move further northwards to Germany. P. perfiliewi is currently established in northern Italy. This species will reach southern areas of Switzerland and Austria within the period 2011–2040. Slight range expansions to eastern Austria can be expected until mid-century, but these will become more pronounced towards the end of the century. Further dispersal of P. perfiliewi in Switzerland is unlikely to happen. Germany seems not to become a part of this species' range during this century.
Discussion
Relevance and generality of the study
Our aim was to determine future occurrences of five Phlebotomus spp. with spreading tendencies in the face of a changing climate. These sandflies serve as proven or assumed (P. mascittii) vectors of Leishmania infantum causing the leishmaniasis. Knowledge concerning the potential future presence of disease vectors is a first step towards an accurate and efficient risk assessment of vector-borne diseases [72]. Conventional static bioclimatic niche modelling can be extended by novel avenues for instance regarding species-specific abilities to disperse [73]. Therefore, we integrated species-specific dispersal pathways to the detected climatically suitable habitats. Within this study, we focus on active and natural dispersal of the species and excluded potential human assistance for range expansions, for instance via the transport of subtropical plants containing eggs or larvae in the moist substrate. However, these effects are not clearly understood and hence not included in this analysis. The results of this study represent the minimum range expansion of Phlebotomus spp. that is only related to active and natural movement in a changing environment without potential human-assistance.
Our results suggest that the development of Central European climate will increasingly support suitable habitats for phlebotomine sandflies. This general trend will become even more pronounced in the second half of the 21st century. We project sandfly establishment in formerly non-endemic areas. This will additionally increase the risk of emerging sandfly-borne diseases in Central Europe such as leishmaniasis. Nevertheless, it is unlikely that sandflies will reach and occupy the provided climatically suitable habitats entirely. During the upcoming years, a spatial focus of surveillance regarding potential new-establishment of Phlebotomus spp. with current south-(western) focus of distribution should be directed for western parts of the German state “North Rhine-Westphalia”. These species may additionally occupy the regions around the “Lake Constance” (southern part of Germany and northern part of Switzerland). Furthermore, Switzerland must be aware on potential sandfly occurrences around the river valley of the “Aare” and the “Lac dé Neuchâtel’ already within some years. In Austria, especially the south-eastern states “Carinthia’, “Styria’ and “Burgenland’ seem to be at risk by new-infestations due to the spreading tendencies of P. mascittii and P. ariasi. In the case of sandfly species with current south (-eastern) focus of distribution (P. neglectus and P. perfiliewi), the canton “Ticino” in southern Switzerland must be alert. According to our results, especially the regions around “Lago Maggiore” and “Lago di Lugano” should be monitored systematically. Furthermore, the Austrian regions neighbouring Slovenia and Hungary should be prepared for the establishment of these sandfly species in the near future.
Limitations
The main limitation in our projections refers to the accuracy of georeferencing maps of sandfly distribution. We have chosen an algorithm that is capable to cope with this source of uncertainty [48]. Expectedly, improved model projections would arise with geographically more accurate point data. However, the intension of this paper is rather to provide a methodological approach. Recently, it has been pointed out that pixel values of predictor variables in close proximity will be highly correlated, which would reduce the effect of inaccuracy in spatial data set of species occurrences [74]. Furthermore, when comparing the reports for cases of autochthonous leishmaniasis in regions that were considered as being non-endemic (e.g. [9]) with documented presence records of sandflies leads to the assumption that sandflies may be wider distributed than realised. However, as it is unknown which species acted in such regions as vectors, only documented presence records at the species level of the Phlebotomus spp. can reasonably be integrated.
Assuming climate is generally considered to be suitable for the permanent establishment of populations, the presence of Phlebotomus spp. is additionally dependent on land cover e.g. forest, agriculture and urban areas [75], [76]. In this study, we integrated altitudinal structures such as river valleys and mountain ridges in least-cost path analysis. In order to recalculate wind speed we additionally integrated land cover data as surface roughness to decelerate near-surface wind speed. Climate change may contribute to alterations in land use and cover, due to warming, changes of precipitation regimes and increases of climatic extreme events such as droughts or floods. This is likely to affect the spatial structure of agricultural systems. In addition to direct climatic impacts, these changes of land cover will additionally affect the spread and distribution of sandflies. However, land cover and land use changes depend on complex processes of decision making under specific political and economical conditions [77] and are hence difficult to project. Therefore land use and cover were considered to remain constant in this study. In general, biotic interactions such as predation or competition are crucial for species distribution [78]. The modifications of the ecological links or networks of an organism by climate change can substantially alter the realised niche of species population [79]. In Germany, P. perniciosus and P. mascittii do not co-exist at the same locations [29]. This can be a result of diverging invasion pathways or of competitive exclusion in the respective regions. Unfortunately, knowledge on biotic interactions of sandflies is scarce. Furthermore, one has to bear in mind that presence of phlebotomine sandflies is dependent on humans and their social factors, for instance living conditions [80], [81]. However, all these factors become more important for species distribution on smaller spatial scales than applied in this study [82], [83].
Concerning the least-cost analysis for species movement it has to be noted that the attributed costs are based on assumptions and/or preliminary observations and hence may not include all of the relevant factors [55], [61]. For instance, it is questionable whether humans assist in the spread of sandflies. Nevertheless, in comparison to mosquitoes, direct human effects on dispersal are of minor importance. Furthermore, the species movement behaviour must not necessarily be optimal or well adapted in human-modified landscapes [53]. Especially dispersal behaviour of individuals between populations may differ from the general tendency at the metapopulation level [58].
Besides the effects of changes in long-term climatic conditions used in this study, extreme weather events are expected to increase in Europe [84]. This will influence organisms and ecosystems remarkably [85], [86]. It has been shown that climatic variability in general [87] and extreme weather events such as floods particularly affect sandfly occurrences [88]. In order to integrate weather extremes in a satisfactory quality within climatic projections, a further downscaling of their spatial resolution to the local scale is required [89]. This is the only way to account for the contribution of weather extremes on disease vectors in risk analysis.
Strengths
Bioclimatic envelope models are powerful tools to envisage potential responses in species distribution to climate change from regional to global scales [82], [90]. They can be seen as a useful first filter for approximations of the impact of climate change on the species distribution [82], [91]. A well-adapted modelling approach is required to project climate change effects on species [44]. Therefore, we selected MaxEnt as algorithm, due to better performances in comparison with further presence-only and (pseudo-) presence-only algorithms (see chapter Model runs for details). Results yielded in high model quality criteria, emphasised by threshold-dependent and independent criteria for Phlebotomus spp.
In order to cope with the general uncertainty in species distribution modelling regarding the climatic evolvement [78], we projected the climatic suitability based on two IPCC [37] scenarios (A1B and B1) that best illustrate the respective storyline. We choose bioclimatic variables that are considered to be biologically meaningful variables for model input. Our projections of future climatic suitability refer to data of the European regional climate model CCLM, which is nested into the well-established global climate model ECHAM5 [35]. In comparison to their driving global models, regional patterns of climate change are projected more precisely, which enhances the quality of climate impact studies [36]. For instance, global climate models fail particularly in replication of observed wind speeds. Obviously, projections of changes in wind speed profit from downscaling to the regional level [92]. Furthermore, potential regions with non-analogue climatic conditions and where hence projections are inappropriate were excluded via MESS-analysis.
Evidently, the consideration of the dispersal capacity of insects in a changing climate improves the quality of projections of species distribution [93]. Hence, we combined projected climatic suitability in the 21st century with dispersal ability for five Phlebotomus spp. We practiced least-cost analysis for future movement patterns by including temporarily stable (elevation, landscape features) and variable factors (wind speed and development of climatic suitability). This allows integrating and combining expert knowledge on sandfly ecology and biology with statistical methods. In doing so, potential species-specific dispersal pathways can be pointed out. This offers the opportunity to distinguish between climatically suitable habitats that can be reached by invasive species and those that are not accessible to them.
The proposed method for the detection of dispersal pathways can be applied to other invasive and mobile disease vectors in the face of climate change. For this purpose, however, species-specific cost surfaces have to be generated. The Alps, for instance, may not be seen as crucial natural barrier for the mainly human-assisted spread of invasive mosquitoes such as the Asian tiger mosquito (Aedes albopictus). However, they are an efficient natural barrier for other species.
Conclusions
Here, we provide a powerful methodological approach to extend conventional bioclimatic envelope modelling of disease vectors by species specific dispersal ability. Our findings promise more realistic projections concerning the vector species future distributions. We identify those Central European regions that are especially exposed to the emerging threat of disease vectors in the light climate change. For the modelling of hitherto neglected vector-connected risks, expertise from various scientific disciplines has been taken into account. Proactive monitoring activities and development of feasible adaptation strategies are required before the establishment of disease vectors may take place. Hence, those analyses help to focus control programmes on specific areas at risk on a regional scale. Due to the identification of spatial hot-spots for such activities, cost- and time-efficient surveillance strategies can be developed. This enables target-orientated counteractions directed against the suggested spread of disease vectors in time. Consequently, the risk of disease transmission in formerly non-endemic areas can be reduced. Once disease vectors such as sandflies are established, vector control and disease prevention have proven to be limited.
Supporting Information
Current and projected climatic suitability for five Phlebotomus species. Values of climatic suitability range theoretically from 0 (unfavourable conditions) to 1 (perfect conditions). Projections refer to the B1 scenario.
(TIF)
Least-cost paths for Phlebotomus species. The detected pathways indicate direction of spread in the 21st century. Spatio-temporal varying climatic suitability and wind speed included in the cost surface that must be crossed by species in the 21st century refer to the B1 scenario.
(TIF)
Current and projected climatic suitability for Phlebotomus species in Central Europe. Noted are mean values and standard deviation in brackets. Projections refer to the B1 scenario.
(DOC)
Acknowledgments
The authors would like to thank the “Bavarian Health and Food Safety Authority” for the coordination of the Co-operative Project VICCI (“Vector-borne Infectious Diseases in Climate Change Investigations”). Three anonymous reviewers gave very helpful comments which improved this paper.
Footnotes
The authors have declared that no competing interests exist.
The study was funded by the Bavarian State Ministry of the Environment and Public Health (http://www.stmug.bayern.de/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jones KE, Patel NG, Levy MA, Storegard A, Balk D, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindgren E, Naucke TJ. Leishmaniasis: Influences of climate and climate change: Epidemiology, ecology and adaption measures. In: Menne B, Ebi KL, editors. Climate change and adaptation strategies for human health. Darmstadt: Springer; 2006. pp. 131–156. [Google Scholar]
- 3.Aspöck H, Gerersdorfer T, Formayer H, Walochnik J. Sandflies and sandfly-borne infections of humans in Central Europe in the light of climate change. Wien Klin Wochen. 2008;120(Suppl. 4):24–29. doi: 10.1007/s00508-008-1072-8. [DOI] [PubMed] [Google Scholar]
- 4.Depaquit J, Grandadam M, Fouque F, Andry PE, Peyrefitte C. Arthropod-borne viruses transmitted by phlebotomine sandflies in Europe: a review. Eurosurveillance. 2010;15:19507. [PubMed] [Google Scholar]
- 5.Camargo LB, Langoni H. Impact of leishmaniasis on public health. J Venom Anim Toxins Trop Dis. 2006;12:527–548. [Google Scholar]
- 6.Dujardin JC, Campino L, Canavate C, Dete JP, Gradoni L, et al. Spread of vector-borne diseases and neglect of leishmaniasis, Europe. Emerg Infect Dis. 2008;14:1013–1018. doi: 10.3201/eid1407.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maroli M, Rossi L, Baldelli R, Capelli G, Ferroglio E, et al. The northward spread of leishmaniasis in Italy: evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Trop Med Int Health. 2008;13:256–264. doi: 10.1111/j.1365-3156.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- 8.Morosetti G, Bongiorno G, Beran B, Scalone A, Moser J, et al. Risk assessment for canine leishmaniasis spreading in the north of Italy. Geospatial Health. 2009;4:115–127. doi: 10.4081/gh.2009.214. [DOI] [PubMed] [Google Scholar]
- 9.Bogdan C, Schonian G, Banuls AL, Hide M, Pratlong F, et al. Visceral leishmaniasis in a german child who had never entered a known endemic area: Case report and review of the literature. Clin Infect Dis. 2001;32:302–306. doi: 10.1086/318476. [DOI] [PubMed] [Google Scholar]
- 10.Koehler K, Stechele M, Hetzel U, Domingo M, Schonian G, et al. Cutaneous leishmaniosis in a horse in southern Germany caused by Leishmania infantum. Vet Parasitol. 2002;109:9–17. doi: 10.1016/s0304-4017(02)00246-7. [DOI] [PubMed] [Google Scholar]
- 11.Müller N, Welle M, Lobsiger L, Stoffel MH, Boghenbor KK, et al. Occurrence of Leishmania sp in cutaneous lesions of horses in Central Europe. Vet Parasitol. 2009;166:346–351. doi: 10.1016/j.vetpar.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Cross ER, Hyams KC. The potential effect of global warming on the geographic and seasonal distribution of Phlebotomus papatasi in Southwest Asia. Environ Health Perspect. 1996;104:724–727. doi: 10.1289/ehp.96104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rispail P, Dereure J, Jarry D. Risk zones of human leishmaniases in the Western Mediterranean basin. Correlations between vector sand flies, bioclimatology and phytosociology. Mem Inst Oswaldo Cruz. 2002;97:477–483. doi: 10.1590/s0074-02762002000400004. [DOI] [PubMed] [Google Scholar]
- 14.Gebre-Michael T, Malone JB, Balkew M, Ali A, Berhe N, et al. Mapping the potential distribution of Phlebotomus martini and P.orientalis (Diptera: Psychodidae), vectors of kala-azar in East Africa by use of geographic information systems. Acta Trop. 2004;90:73–86. doi: 10.1016/j.actatropica.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Oshagi MA, Ravasan NM, Javadian E, Rassi Y, Sadraei J, et al. Application of predictive degree day model for field development of sandfly vectors of visceral leishmaniasis in northwest of Iran. J Vector Borne Dis. 2009;46:247–254. [PubMed] [Google Scholar]
- 16.Aransay AM, Testa JM, Morillas-Marquez F, Lucientes J, Ready PD. Distribution of sandfly species in relation to canine leishmaniasis from the Ebro Valley to Valencia, northeastern Spain. Parasitol Res. 2004;94:416–420. doi: 10.1007/s00436-004-1231-4. [DOI] [PubMed] [Google Scholar]
- 17.Killick-Kendrick R, Wilkes TJ, Bailly M, Bailly I, Righton LA. Preliminary field observations on the flight speed of a phlebotomine sandfly. Trans R Soc Trop Med Hyg. 1986;80:138–142. doi: 10.1016/0035-9203(86)90213-0. [DOI] [PubMed] [Google Scholar]
- 18.Ximenes M deFF deM, Castellon EG, de Souza M deF, Lara Menezes AA, Queiroz JW, et al. Effect of abiotic factors on seasonal population dynamics of Lutzomyia longipalpis (Diptera: Psychodidae) in northeastern Brazil. J Med Entomol. 2006;43:990–995. [PubMed] [Google Scholar]
- 19.Galvez R, Descalzo MA, Miro G, Jimenez MI, Martin O, et al. Seasonal trends and spatial relations between environmental/meteorological factors and leishmaniosis sand fly vector abundances in Central Spain. Acta Trop. 2010;115:95–102. doi: 10.1016/j.actatropica.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Orshan L, Szekely D, Khalfa Z, Bitton S. Distribution and seasonality of Phlebotomus sand flies in cutaneous leishmaniasis foci, Judean Desert, Israel. J Med Entomol. 2010;47:319–328. doi: 10.1603/me09096. [DOI] [PubMed] [Google Scholar]
- 21.Sawalha SS, Shtayeh MS, Khanfar HM, Warburg A, Abdeen ZA. Phlebotomine sand flies (Dipteria, Psychodidae) of the Palestinian West Bank: Potential vectors of leishmaniasis. J Med Entomol. 2003;40:321–328. doi: 10.1603/0022-2585-40.3.321. [DOI] [PubMed] [Google Scholar]
- 22.Peterson AT, Pereira RS, Fonseca de Camargo Neves V. Using epidemiological survey data to infer geographic distributions of leishmaniasis vector species. Rev Soc Bras Med Trop. 2004;37:10–14. doi: 10.1590/s0037-86822004000100003. [DOI] [PubMed] [Google Scholar]
- 23.Peterson AT, Shaw J. Lutzomyia vectors for cutaneous leishmaniasis in Southern Brazil: ecological niche models, predicted geographic distributions, and climate change effects. Int J Parasitol. 2003;33:919–931. doi: 10.1016/s0020-7519(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 24.Gonzales C, Wang O, Strutz SE, Gonzáles-Salazar C, Sanchez-Cordero Sarkar S. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:e585. doi: 10.1371/journal.pntd.0000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aspöck H, Walochnik J, Geresdorfer T, Formayer H. Risiko-Profil für das autochthone Auftreten von Leishmaniosen in Österrreich - Startclim 2006 B. 2007. www.austroclim.at/startclim, accessed: April- 2- 2010.
- 26.Fischer D, Thomas SM, Beierkuhnlein C. Temperature-derived potential for the establishement of phlebotomine sandflies and visceral leishmaniasis in Germany. Geospatial Health. 2010;5:59–69. doi: 10.4081/gh.2010.187. [DOI] [PubMed] [Google Scholar]
- 27.Naucke TJ, Lorentz S, Rauchenwald F, Aspöck H. Phlebotomus (Transphlebotomus) mascittii Grassi, 1908 in Carinthia: first record of the occurrence of sandflies in Austria (Diptera: Psychodidae: Phlebotominae). Parasitol Res. 2011;109:1161–1164. doi: 10.1007/s00436-011-2361-0. [DOI] [PubMed] [Google Scholar]
- 28.Naucke TJ, Pesson B. Presence of Phlebotomus (Transphlebotomus) mascittii Grassi, 1908 (Diptera: Psychodidae) in Germany. Parasitol Res. 2000;86:335–336. doi: 10.1007/s004360050053. [DOI] [PubMed] [Google Scholar]
- 29.Naucke TJ, Menn B, Massberg D, Lorentz S. Sandflies and leishmaniasis in Germany. Parasitol Res. 2008;103(Suppl. 1):65–68. doi: 10.1007/s00436-008-1052-y. [DOI] [PubMed] [Google Scholar]
- 30.Aspoeck H. Postglacial formation and fluctuations of the biodiversity of Central Europe in the light of climate change. Parasitol Res. 2008;103(Suppl. 1):7–10. doi: 10.1007/s00436-008-1057-6. [DOI] [PubMed] [Google Scholar]
- 31.Artemiev MM, Neronov VM. Distribution and ecology of sandflies of the Old World (genus Phlebotomus) Moscow: The USSR Committee for the Unesco Programme on Man and the Biosphere (MAB), Institute of Evolutionary Morphology and Animal Ecology, USSR Academy of Science; 1984. 375 [Google Scholar]
- 32.Hijamns RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. http://www.worldclim.org, accessed: September-2-2010. [Google Scholar]
- 33.Yost AC, Peterson SL, Gregg M, Miller R. Predictive modelling and mapping sagegrouse (Centrocercus urophasianus) nesting habitat using Maximum Entropy and a long-term dataset from Southern Oregon. Ecol Inform. 2008;3:375–386. [Google Scholar]
- 34.Dormann CF, Purschke O, Garcia Marquez JR, Lautenbach S, Schroeder B. Components of uncertainty in species distribution analysis: a case study of the Great Grey Shrike. Ecology. 2008;89:3371–3386. doi: 10.1890/07-1772.1. [DOI] [PubMed] [Google Scholar]
- 35.Rockel B, Will A, Hense A. The Regional Climate Model COSMO-CLM (CCLM). Meteorol Z. 2008;17:347–348. [Google Scholar]
- 36.Jacob D. Short communication on regional climate change scenarios and their possible use for impact studies on vector-borne diseases. Parasitol Res. 2008;103(Suppl. 1):3–6. doi: 10.1007/s00436-008-1099-9. [DOI] [PubMed] [Google Scholar]
- 37.Intergovernmental Panel on Climate Change (IPCC) Climate Change 2007: Synthesis Report. 2007. http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr.pdf, accessed: July-4-2010.
- 38.Fitzpatrick MC, Hargrove WW. The projection of species distribution models and the problem of non-analog climate. Biodivers Conserv. 2009;18:2255–2261. [Google Scholar]
- 39.Elith J, Kearney M, Phillips S. The art of modelling range-shifting species. Methods in Ecology and Evolution. 2010;1:330–342. [Google Scholar]
- 40.Elith J, Phillips SJ, Hasti T, Dudik M, Chee YE, et al. A statistical explanation of MaxEnt for ecologists. Divers Distrib. 2011;17:43–57. [Google Scholar]
- 41.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distribution. Ecol Model. 2006;190:231–259. [Google Scholar]
- 42.Phillips SJ, Dudik M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31:161–175. [Google Scholar]
- 43.Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 44.Hijmans RJ, Graham CH. The ability of climate envelope models to predict the effect of climate change on species distributions. Global Change Biol. 2006;12:2272–2281. [Google Scholar]
- 45.Hernandez PA, Graham CH, Master LL, Albert DL. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography. 2006;29:773–785. [Google Scholar]
- 46.Pearson RG, Raxworthy CJ, Nakumara M, Peterson AT. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr. 2007;34:102–117. [Google Scholar]
- 47.Wisz MS, Hijmans RJ, Li J, Peterson AT, Graham CH, Guisan A. Effects of sample size on the performance of species distribution models. Divers Distrib. 2008;14:763–773. [Google Scholar]
- 48.Graham CH, Elith J, Hijmans RJ, Guisan A, Peterson AT, et al. The influence of spatial errors in species occurrence data used in distribution models. J Appl Ecol. 2008;45:239–247. [Google Scholar]
- 49.Fischer D, Thomas SM, Niemitz F, Reineking B, Beierkuhnlein C. Projection of climatic suitability for Aedes albopictus Skuse (Culicidae) in Europe under climate change conditions. Glob Planet Change. 2011;78:54–64. [Google Scholar]
- 50.Araujo MB, Perason RG, Thuiller W, Erhardt M. Validation of species-climate impact models under climate change. Glob Ecol Biogeogr. 2005;11:1504–1513. [Google Scholar]
- 51.Lobo JM, Jimenez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr. 2008;17:145–151. [Google Scholar]
- 52.Peterson AT, Papes M, Soberon J. Rethinking receiver operating characteristic analysis applications in ecological niche modelling. Ecol Model. 2008;213:63–72. [Google Scholar]
- 53.Fahrig L. Non-optimal animal movement in human-altered landscapes. Funct Ecol. 2007;21:1003–1015. [Google Scholar]
- 54.Douglas DH. Least-cost path in GIS using an accumulated weighted surface and slope lines. Cartographica. 1994;31:37–51. [Google Scholar]
- 55.Bunn AG, Urban DL, Keitt TH. Landscape connectivity: A conservation application of graph theory. J Environ Manage. 2000;59:265–278. [Google Scholar]
- 56.Pinto N, Keitt TH. Beyond the least-cost path: evaluating corridor redundancy using a graph-theoretic approach. Landsc Ecol. 2009;24:253–266. [Google Scholar]
- 57.Adriaensen F, Chardon JP, de Blust G, Swinnen E, Villalba S, et al. The application of ‘least-cost’ modelling as a functional landscape model. Landsc Urban Plan. 2003;64:233–247. [Google Scholar]
- 58.Sutcliffe OL, Bakkestuen V, Fry G, Stabbetorp OE. Modelling the benefits of farmland restoration: methodology and application to butterfly movement. Lands Urban Plan. 2003;63:15–31. [Google Scholar]
- 59.Foltete JC, Berthier K, Cosson JF. Cost distance defined by a topological function of landscape. Ecol Model. 2008;210:104–114. [Google Scholar]
- 60.Larkin JL, Maehr DS, Hoctor TS, Orlando MA, Whitney K. Landscape linkages and conservation planning for the black bear in west-central Florida. Anim Conserv. 2004;7:23–34. [Google Scholar]
- 61.LaRue MA, Nielsen CK. Modelling potential dispersal corridors for cougars in midwestern North America using least-cost path methods. Ecol Model. 2008;212:372–381. [Google Scholar]
- 62.Wang IJ, Savage WK, Shaffer HB. Landscape genetics and least-cost path analysis reveal unexpected dispersal routes in the California tiger salamander (Ambystoma californiense). Mol Ecol. 2009;18:1365–1374. doi: 10.1111/j.1365-294X.2009.04122.x. [DOI] [PubMed] [Google Scholar]
- 63.Li HL, Li DH, Li T, Qiao Q, Yang Y, Zhang HM. Application of least-cost path model to identify a giant panda dispersal corridor network after the Wenchuan earthquake - Case study of Wolong Nature Reserve in China. Ecol Model. 2010;221:944–952. [Google Scholar]
- 64.Naucke TJ. Leishmaniosis, a tropical disease and its vectors (Diptera Psychodidae, Phlebotominae) in Central Europe. Denisia. 2002;6:163–178. [Google Scholar]
- 65.Feliciangeli MD. Natural breeding places of phlebotomine sandflies. Med Vet Entomol. 2004;18:71–80. doi: 10.1111/j.0269-283x.2004.0487.x. [DOI] [PubMed] [Google Scholar]
- 66.Peterson EW, Hennessey JP., Jr On the use of power laws for estimates of wind power potential. J Appl Meteorol. 1978;17:390–394. [Google Scholar]
- 67.Touma JS. Dependence of the wind profile power law on stability for various locations. J Air Pollut Contr Assoc. 1977;27:863–866. [Google Scholar]
- 68. http://earth.esa.int, accessed: October-4-2010.
- 69.Kleemann M, Meliss M. Regenerative Energiequellen. Berlin: Springer; 1993. 315 [Google Scholar]
- 70.Fischer D, Thomas SM, Beierkuhnlein C. Modelling climatic suitability and dispersal of disease vectors: the example of a phlebotomine sandfly in Europe. Procedia Environmental Sciences. 2011;7:164–169. [Google Scholar]
- 71.Alexander B, Young DG. Dispersal of phlebotomine sand flies (Diptera: Psychodidae) in a Colombian focus of Leishmania (Viannia) braziliensis. Mem Inst Oswaldo Cruz. 1992;87:397–403. doi: 10.1590/s0074-02761992000300010. [DOI] [PubMed] [Google Scholar]
- 72.Fischer D, Thomas S, Beierkuhnlein C. Climate change effects on vector-borne diseases in Europe. Nova Acta Leopoldina. 2010;384:99–107. [Google Scholar]
- 73.Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 74.Naimi B, Skidmore AK, Groen TA, Hamm NAS. : Spatial autocorrelation in predictors reduces the impact of positional uncertainty in occurrence data on species. J Biogeogr. 2011;38:1497–1509. [Google Scholar]
- 75.Rossi E, Rinaldi L, Musella V, Veneziano V, Carbone S, et al. Mapping the main Leishmania phlebotomine vector in the endemic focus of the Mt. Vesuvius in southern Italy. Geospatial Health. 2007;1:191–198. doi: 10.4081/gh.2007.267. [DOI] [PubMed] [Google Scholar]
- 76.Colacicco-Mayhugh MG, Masuoka PM, Grieco JP. Ecological niche model of Phlebotomus alexandri and P. papatasi (Diptera: Psychodidae) in the Middle East. Int J Health Geogr. 2010;9:2. doi: 10.1186/1476-072X-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parker DC, Manson SM, Jannsen MA, Hoffman MJ, Deadman P. Multi-agent systems for the simulation of land-use and land-cover change: a review. A Assoc Am Geog. 2003;93:314–347. [Google Scholar]
- 78.Thuiller W. Patterns and uncertainties of species' range shifts under climate change. Global Change Biol. 2004;10:2020–2027. doi: 10.1111/gcb.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Araujo MB, Luoto M. The importance of biotic interactions for modelling species distributions under climate change. Glob Ecol Biogeogr. 2007;16:743–753. [Google Scholar]
- 80.Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999;17:279–289. doi: 10.1016/s0738-081x(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 81.Chaves LF, Cohen JM, Pascual M, Wilson ML. Social exclusion modifies climate and deforestation impacts on a vector-borne disease. PloS Neglect Trop Dis. 2008;2:e176. doi: 10.1371/journal.pntd.0000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob Ecol Biogeogr. 2003;12:361–371. [Google Scholar]
- 83.Luoto M, Virkkala R, Heikkinen RK. The role of land cover in bioclimatic models depends on spatial resolution. Glob Ecol Biogeogr. 2007;16:34–42. [Google Scholar]
- 84.Beniston M, Stephenson DB, Christensen OB, Ferro CAT, Frei C, et al. Future extreme events in European climate: an exploration of regional climate model projections. Clim Change. 2007;81:71–95. [Google Scholar]
- 85.Jentsch A, Kreyling J, Beierkuhnlein C. A new generation of climate change experiments: events not trends. Front Ecol Environ. 2007;5:365–374. [Google Scholar]
- 86.Jentsch A, Beierkuhnlein C. Research frontiers in climate change: effects of extreme meteorological events on ecosystems. C R Geosci. 2008;340:621–628. [Google Scholar]
- 87.Chaves LF, Pascual M. Climate cycles and forecasts of cutaneous leishmaniasis, a nonstationary vector-borne disease. PLoS Med. 2006;3:1320–1328. doi: 10.1371/journal.pmed.0030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emch M. Relationships between flood control, kala-azar, and diarrheal disease in Bangladesh. Environ Planning A. 2000;32:1051–1063. [Google Scholar]
- 89.Knote C, Heinemann G, Rockel B. Changes in weather extremes: Assessment of return values using high resolution climate simulations at convection-resolving scale. Meteorol Z. 2010;19:11–23. [Google Scholar]
- 90.Wiens JA, Stralberg D, Jongsomjit D, Howell CA, Snyder MA. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc Natl Acad Sci USA. 2009;106:19729–19736. doi: 10.1073/pnas.0901639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heikkinen RK, Luoto M, Araujo MB, Virkkala R, Thuiller W, et al. Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog Phys Geogr. 2006;30:751–777. [Google Scholar]
- 92.Pryor SC, Schoof JT, Barthelmie RJ. Winds of change? Projections of near-surface winds under climate change scenarios. Geophys Res Lett. 2006;33:L11702. [Google Scholar]
- 93.Walters RJ, Hassal M, Telfer MG, Hewitt GM, Palutikof JP. Modelling dispersal of a temperate insect in a changing climate. Proc R Soc A. 2007;273:2017–2023. doi: 10.1098/rspb.2006.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lane RP. Sandflies (Phlebotominae) In: Lane RP, Crosskey RW, editors. Medical insects and arachnids. London et al.: Chapman & Hall; 1993. pp. 78–119. [Google Scholar]
- 95.Quate LW. Phlebotomus sandflies from the Paloich area in the Sudan (Dipter, Psychodidae). J Med Entomol. 1964;1:213–268. doi: 10.1093/jmedent/1.3.213. [DOI] [PubMed] [Google Scholar]
- 96.Roberts DM. Arabian sand flies (Diptera: Psychoidae) prefer the hottest nights? Med Vet Entemol. 1994;8:194–198. doi: 10.1111/j.1365-2915.1994.tb00163.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Current and projected climatic suitability for five Phlebotomus species. Values of climatic suitability range theoretically from 0 (unfavourable conditions) to 1 (perfect conditions). Projections refer to the B1 scenario.
(TIF)
Least-cost paths for Phlebotomus species. The detected pathways indicate direction of spread in the 21st century. Spatio-temporal varying climatic suitability and wind speed included in the cost surface that must be crossed by species in the 21st century refer to the B1 scenario.
(TIF)
Current and projected climatic suitability for Phlebotomus species in Central Europe. Noted are mean values and standard deviation in brackets. Projections refer to the B1 scenario.
(DOC)