Hook2 partitions between the Golgi apparatus and the centrosome, and its depletion hinders ciliogenesis after mother centriole maturation without Golgi breakdown. Hook2 interacts with PCM1 and Rab8a, and Hook2-depleted cells can be forced to grow primary cilia by overexpressing GFP::Rab8a, indicating that Rab8a acts downstream of Hook2 and PCM1.
Abstract
Primary cilia originate from the centrosome and play essential roles in several cellular, developmental, and pathological processes, but the underlying mechanisms of ciliogenesis are not fully understood. Given the involvement of the adaptor protein Hook2 in centrosomal homeostasis and protein transport to pericentrosomal aggresomes, we explored its role in ciliogenesis. We found that in human retinal epithelial cells, Hook2 localizes at the Golgi apparatus and centrosome/basal body, a strategic partitioning for ciliogenesis. Of importance, Hook2 depletion disrupts ciliogenesis at a stage before the formation of the ciliary vesicle at the distal tip of the mother centriole. Using two hybrid and immunoprecipitation assays and a small interfering RNA strategy, we found that Hook2 interacts with and stabilizes pericentriolar material protein 1 (PCM1), which was reported to be essential for the recruitment of Rab8a, a GTPase that is believed to be crucial for membrane transport to the primary cilium. Of interest, GFP::Rab8a coimmunoprecipitates with endogenous Hook2 and PCM1. Finally, GFP::Rab8a can overcome Hook2 depletion, demonstrating a functional interaction between Hook2 and these two important regulators of ciliogenesis. The data indicate that Hook2 interacts with PCM1 in a complex that also contains Rab8a and regulates a limiting step required for further initiation of ciliogenesis after centriole maturation.
INTRODUCTION
Primary cilia are microtubule-based, hair-like extracytoplasmic organelles present on the surface of many growth-arrested cells. They are nonmotile and consist of a basal body and a membrane-surrounded ciliary axoneme comprising nine doublets of microtubules. Primary cilia play sensory functions in compartmentalizing receptors and signaling machinery, including effectors of olfaction, phototransduction, and mechanotransduction. They also play a crucial role in regulating vertebrate developmental pathways and general tissue homeostasis. Defects in genes involved in primary cilium assembly or function have been associated with numerous disorders (ciliopathies), including retinal degeneration, obesity, diabetes, polycystic kidney disease, left–right asymmetry defects, hydrocephalus, and Bardet–Biedl syndrome (BBS) (Pazour and Rosenbaum, 2002; Gerdes et al., 2009).
Electron microscopic analyses of epithelial cells, fibroblasts, and smooth muscle cells led to a model for the initial steps of primary cilium assembly (Sorokin, 1962; Alieva and Vorobjev, 2004). These steps go from the docking of a Golgi-derived vesicle to the distal end of the mother centriole, to the formation of a growing sheath surrounding the elongating axoneme by assembly of microtubule doublets, and, finally, to the fusion of the sheath with the plasma membrane (Pazour and Rosenbaum, 2002; Pedersen and Rosenbaum, 2008). Studies of the molecular mechanisms that regulate the initiation of ciliogenesis revealed an important role of several proteins, among them cenexin/OFD2 and centriolar protein 170 (CEP170), which are proteins of the distal appendages of the mother centriole (Guarguaglini et al., 2005; Ishikawa et al., 2005). Other centrosomal proteins, such as pericentrin and PCM1, have also been implicated in the initiation of ciliogenesis (Graser et al., 2007; Mikule et al., 2007). PCM1 is required for efficient targeting to the primary cilium of Rab8a, which is believed to regulate membrane transport to the primary cilium, allowing the formation of the ciliary sheath (Nachury et al., 2007; Kim et al., 2008). In the retina, interference with trafficking to the cilium by disruption of Rab8a function prevents rhodopsin-carrying post-Golgi vesicles from reaching the outer segment of retinal photoreceptor via the connecting cilium, leading to retinal degeneration (Moritz et al., 2001). This suggests that post-Golgi trafficking is involved in ciliary membrane formation. Beyond these findings, it remains unclear how the transport, targeting, and attachment of post-Golgi vesicles to the mother centriole are initiated and regulated at the onset of ciliogenesis.
The proteins of the mammalian Hook family are adaptor-like proteins composed of a conserved NH2-terminal (N-term) domain that is believed to facilitate interaction with microtubules, a common central coiled-coil domain possibly involved in homodimerization (Xu et al., 2008), and a more divergent C-terminal (C-term) domain that has been implicated in the binding of each of the Hook proteins to different classes of organelles (Walenta et al., 2001; Szebenyi et al., 2007). Hook proteins seem to be potential regulators of cargo loading for microtubule-based transport and act as anchors for organelles (Linstedt, 2004). Consistent with this possibility, Hook2 was shown to promote the dynein1-dependent, microtubule-based delivery of protein aggregates to pericentriolar aggresomes. Hook2 was also identified in a multiprotein complex with its mammalian paralogues Hook1 and Hook3, the protein Fused Toes (FTS), and the FTS and Hook interacting protein (FHIP) (Xu et al., 2008). FHIP contains a domain with similarity with the GRAB domain that facilitates localization to Golgi structures (Gillingham et al., 2004). The multiprotein complex FTS/Hook/FHIP interacts with components of the homotypic vesicular protein sorting (HOPS) complex that promotes vesicle trafficking and fusion (Xu et al., 2008). Hook2 was recently shown to interact directly with the centromere protein F, a microtubule-associated protein at the centrosome (Moynihan et al., 2009) that binds the soluble N-ethylmaleimide–sensitive factor attachment protein receptor protein syntaxin 4 and plays a role in the regulation of microtubule-driven vesicle trafficking to the centrosome (Pooley et al., 2006, 2008). In addition to a role of Hook2 during vesicle transport, Hook2 was reported to localize to the centrosome through all phases of the cell cycle, its C-term domain directly binds to centriolin/CEP110, and it was demonstrated to contribute to the establishment and maintenance of centrosomal structure and function (Szebenyi et al., 2007).
Given the involvement of Hook2 in both protein transport and centrosomal homeostasis, two important processes underlying ciliogenesis, we sought to determine whether Hook2 could play a role in the regulation of ciliogenesis. We show that Hook2 localizes to the Golgi apparatus and the centrosome/basal body. Hook2 depletion prevents ciliogenesis initiation, and this defect can be overcome by overexpression of murine Hook2 or Rab8a. Hook2 also interacts with and regulates the expression of PCM1 that controls Rab8a recruitment to the primary cilium. Our data highlight the existence of a functional complex containing Rab8a and Hook2 in association with PCM1, where Hook2 could play a role as an adaptor in the attachment of post-Golgi vesicles to the mother centriole during the formation of the ciliary sheath.
RESULTS
Hook2 localizes in the Golgi–primary cilium vicinity
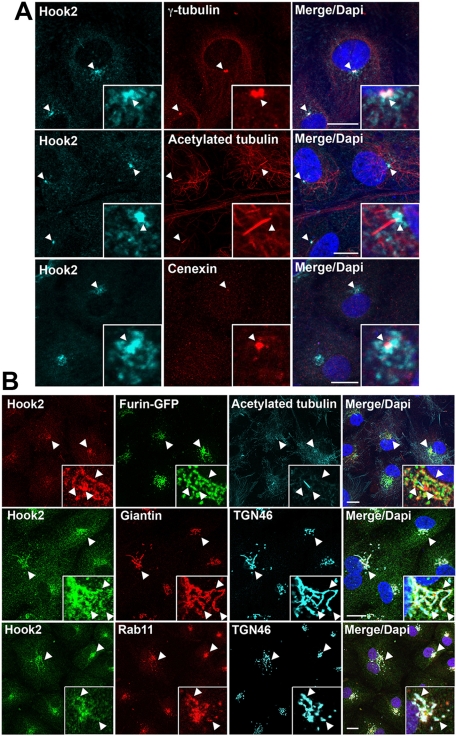
It was demonstrated that arising retinal pigmented epithelial cells 19 (ARPE19) comprise a spontaneously arising human RPE cell line with normal karyology that forms polarized epithelial monolayers on porous filter supports (Dunn et al., 1996). This cell line is not transformed, keeps characteristics of RPE cells in vivo, and undergoes spontaneous morphogenesis of a primary cilium without serum starvation, as previously described (den Hollander et al., 2007). By transmission electron microscopy (TEM), we were able to record the main steps of ciliogenesis in 75% of the cells after 4 d in culture (unpublished data). Figure 1A shows results for ciliated ARPE19 cells that were fixed in methanol to extract most cell organelle membranes, dehydrate the cells, and precipitate soluble proteins on the cellular architecture (Melan, 1994; Smith-Clerc and Hinz, 2010). In these conditions, Hook2 mainly localized to the centrosome or the basal body (γ-tubulin staining) at the base of the primary cilium (acetylated tubulin staining), including the mother centriole (cenexin staining). This centrosomal localization of Hook2 is reminiscent to the previously reported localization of Hook2 in the mouse B lymphocyte Raw cell line after methanol fixation (Szebenyi et al., 2007). Also consistent with that report, Hook2 localization was less sharp than with γ-tubulin or cenexin.
FIGURE 1:
Hook2 localizes to the centrosome, the basal body, and the Golgi apparatus. (A, B) Seven-day cultured ARPE19 cells were simultaneously fixed and permeabilized with methanol (A) or sequentially fixed in PFA and permeabilized with Tx100 (B). In A, the cells were then costained with antibodies raised against Hook2 and proteins localized either at the centrosome/basal body (γ-tubulin, top), the mother centriole (cenexin, bottom), or the primary cilium (acetylated tubulin, middle). In B, top, ARPE19 cells stably transfected with furin-GFP that localizes at the TGN were costained with antibodies staining Hook2 and acetylated tubulin. In B, middle and bottom, ARPE19 cells were costained with antibodies staining Hook2, as well as proteins that localize either in cis-medial Golgi compartments (giantin, middle) or in the TGN, the recycling endosomes, and the ciliary base (Rab11, bottom). Arrowheads indicate colocalizing markers. Bar, 10 μm; inset magnification, ×15. See also Supplemental Figure S1.
Because methanol is not the best fixation to preserve cellular organelles and morphology, paraformaldehyde (PFA) fixation was also tested to study Hook2 localization. PFA is used as cross-linking reagent, generating a network of proteins/antigens through free amino groups. Lipids are also fixed and preserved. To allow antigen accessibility, the cell membranes need to be permeabilized using detergents (Melan, 1994; Smith-Clerc and Hinz, 2010). In Figure 1B, ciliated ARPE19 cells were fixed in PFA and permeabilized with Triton. In these conditions, Hook2 colocalized with the trans-Golgi network (TGN) markers TGN46 and furin as well as with the TGN-recycling endosome-ciliary base marker Rab11. In addition, Hook2 partially colocalized with the cis-medial Golgi marker giantin (Figure 1B and Supplemental Movies S1–S3). This Golgi localization of Hook2 is reminiscent of the localization of Hook2 in human laryngeal carcinoma Hep2 cells (Walenta et al., 2001). In contrast, Hook2 colocalized neither with the early endosomal marker protein 1 (EEA1) nor with the late endosomal-lysosomal marker lysosomal-associated membrane protein (Lamp1; Supplemental Figure S1A). These data suggest the existence of centrosome- and Golgi-associated pools of Hook2 in ARPE19 cells that were revealed by means of two different methods of fixation. We thus suggest that the Golgi-associated pool of Hook2 revealed in PFA-fixed cells was extracted by methanol fixation. Consistent with this centrosome/Golgi partitioning, an N-term deletion mutant of Hook2 (C-term::Myc, amino acids [aa] 641–720) colocalized with TGN46 (Supplemental Figure S1B, top), whereas the C-term deletion mutant of Hook2 (N-term::Myc, aa 1–533) localized to the cytoplasm as previously described (Szebenyi et al., 2007) but also to spots reminiscent of the centrosome (Supplemental Figure S1B, bottom).
To determine whether Hook2 would also localize in situ in epithelia that display motile cilia, we used CD1 new-born mouse heads that were fixed in PFA and permeabilized with Triton. We found that Hook2 localized at the base of the cilia of multiciliated cells of the tracheonasal tract (Supplemental Figure S1C, left and middle) and colocalized with TGN46 (Supplemental Figure S1C, right). Taken together, these data indicate a dual localization of Hook2 at the Golgi apparatus and the centrosome/basal body, at the base of primary and motile cilia.
Hook2 is specifically required for ciliogenesis
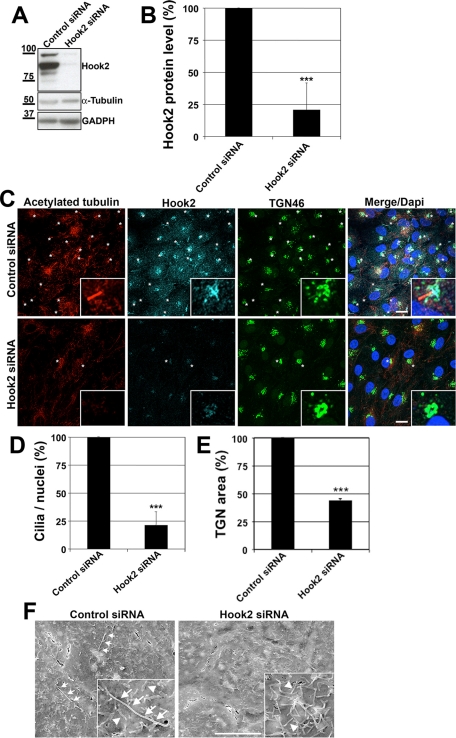
Because our data indicate that Hook2 partitions between the centrosome/basal body and the Golgi apparatus, two organelles that are potentially involved in ciliogenesis, we further tested a role of Hook2 during ciliogenesis. We used a small interfering RNA (siRNA) strategy with three different siRNAs to transiently deplete Hook2 in ARPE19 cells. After 7 d in culture, Hook2 protein level was decreased by more than 75% in Hook2-depleted cells compared with control cells (Figure 2, A and B). Immunofluorescence staining and confocal microscopy analysis of Hook2-depleted cells showed a drastic inhibition of ciliogenesis as compared with control siRNA treated cells (Figure 2C, acetylated tubulin staining, and quantification, Figure 2D). We noticed that Hook2-depleted cells presented an intact but more compact Golgi apparatus compared with control cells (Figure 2C, TGN46 staining, and quantification, Figure 2E). No cilium was found in the absence of Hook2 when we used individually each siRNA or a mix of four different siRNAs that do not overlap the positions of the first set of three siRNAs that we used, but also when imaging a second cilium marker, Arl13::green fluorescent protein (GFP), stably transfected in ARPE19 cells (unpublished data). The inhibition of ciliogenesis was further confirmed in Hook2 siRNA–depleted cells by scanning electron microscopy (SEM). Consistent with the immunostaining data, Hook2-depleted cells were completely devoid of cilia at their surface as compared with the control cells (Figure 2F). The inhibition of ciliogenesis induced by Hook2 depletion was also observed in a human renal epithelial cell line, HK-2, that was transiently transfected with Hook2 siRNA (unpublished data), indicating a general role for Hook2 in the formation of the primary cilium.
FIGURE 2:
Hook2 depletion hinders ciliogenesis without Golgi breakdown in ARPE 19 cells. (A) A mix of 3 siRNAs (H1, H2, and H3) targeting human Hook2 mRNA was used to transiently decrease Hook2 expression for 7 d as measured by WB analysis of Hook2 levels in ARPE19 total cell lysates treated either with Hook2 siRNAs or luciferase siRNA (control siRNA). Antibodies against α-tubulin and GAPDH were used as loading controls. Molecular weight (MW) markers are indicated on the left in kilodaltons. (B) Quantification of Hook2 depletion. Error bars, SD; n = 20 and p < 0001. (C) SiRNA Hook2-depleted cells were fixed in PFA and compared with control siRNA-treated cells for their ability to promote ciliogenesis (acetylated tubulin staining) and maintain Golgi architecture (TGN46 staining) by immunofluorescence and confocal microscopy analysis. Bar, 10 μm; inset magnification, ×15. Asterisks indicate cilia. Note that Hook2 siRNA depletion does not induce breakdown but rather a compaction of the Golgi apparatus. (D) Quantification of ciliogenesis inhibition by Hook2 depletion from three independent experiments. Error bars, SD; n = 200 cells per condition and p < 0001. (E) Quantification of Golgi compaction by Hook2 depletion from three independent experiments. Error bars, SD; n = 100 cells per condition and p < 0001. (F) SEM analysis of control and Hook2 siRNA transiently depleted ARPE19 cells. Bar, 5 μm; inset magnification, ×2. Arrows indicate cilia in the control cells, and arrowheads mark some microvilli in Hook2-depleted cells. See also Supplemental Figure S2.
Our SEM study (Figure 2F) suggested that Hook2 is necessary for the early stages of ciliogenesis, since we did not detect any shorter or malformed primary cilium. It was suggested that ciliogenesis begins when the mother centriole undergoes maturation (Sorokin, 1962; Alieva and Vorobjev, 2004) marked by the recruitment of several proteins, including Cenexin1 and CEP170. We therefore asked whether Hook2 depletion affected cenexin1 and CEP170 recruitment to the mother centriole. We found that cenexin and CEP170 were still localized to the mother centriole after Hook2 depletion, indicating that at least this maturation step of the mother centriole was not affected by Hook2 depletion (Supplemental Figure S2, A and B). However, we noticed in Hook2 siRNA–depleted cells a remaining small Hook2 population associated with the mother centriole (Supplemental Figure S2A). This stable population of Hook2 may be sufficient to support mother centriole maturation but not for the subsequent steps of ciliogenesis to go to completion.
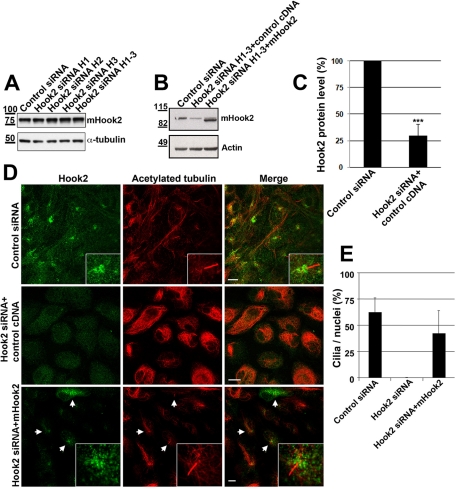
To assay the specificity of the ciliogenesis disruption caused by Hook2 depletion, we performed a transient rescue experiment with a mouse Hook2 cDNA. We first verified that in the mouse cell line 4T1, Hook2 was not significantly depleted after 7 d in culture by the three different siRNAs targeting human Hook2 (Figure 3A). A positive control for siRNA depletion of mHook2 in the murine cell line is lacking, although we have tried a pool of four validated siRNAs specifically targeting mouse Hook2, but it did not deplete murine Hook2 (unpublished data). Szebenyi et al. (2007) also reported that their attempts to knock down the expression of Hook2 in RAW cells by RNA interference–based methods were unsuccessful. We also verified that the overexpression of mouse Hook2 cDNA in ARPE19 cells was not inhibited by siRNAs targeting human Hook2 (Figure 3B, and quantification, Figure 3C). Our immunofluorescence staining and confocal microscopy analysis revealed that transient heterologous expression of mouse Hook2 restored ciliogenesis in ARPE19 cells that were Hook2 depleted (Figure 3D, and quantification, Figure 3E). These data indicate a role of Hook2 subsequent to cenexin and CEP170 recruitment to the mother centriole at the onset of ciliogenesis.
FIGURE 3:
Exogenous mouse Hook2 rescues defect in ciliogenesis by Hook2 depletion in human ARPE19 cells. (A) 4T1 mouse cells were transiently transfected with control or different human Hook2 siRNA as indicated. After 7 d of incubation, the cells were lysed and tested by WB for Hook2 and α-tubulin production. Molecular weights are indicated on the left in kilodaltons. Note that human Hook2 siRNAs do not deplete mouse Hook2. (B–E) ARPE19 Hook2 siRNA transiently transfected cells were further transfected with an empty vector or mouse Hook2 cDNA and compared with control cells. After 7 d, cells were either processed for WB analysis of Hook2 and actin (B, and quantification of human Hook2 depletion in C) or fixed in PFA and compared by immunofluorescence and confocal microscopy analysis for their ability to promote ciliogenesis (D). Bar, 10 μm; inset magnification, ×15. Arrows indicate cells that express mouse Hook2 cDNA. (E) Quantification of ciliogenesis rescue. Bars represent the percentage of identified cilia per nuclei in each condition. Error bars, SD.
Hook2 is involved in the formation of the pericentriolar vesicle during the initiation of ciliogenesis
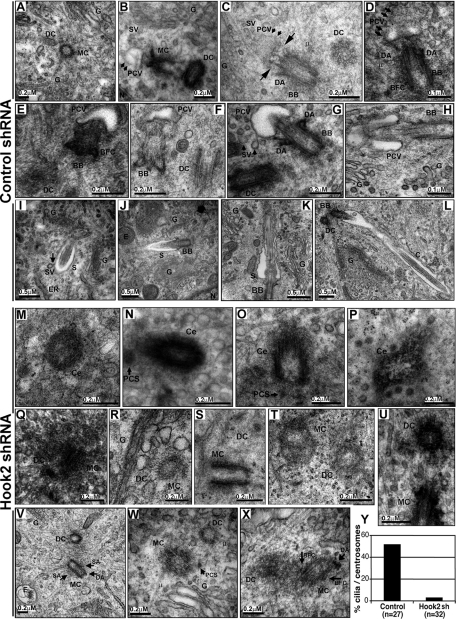
Beyond centriole maturation, early ciliogenesis is believed to comprise fission of post-Golgi vesicles from the TGN, transport and docking of these vesicles to the mother centriole, attachment of one vesicle to the mother centriole distal tip to form the periciliary vesicle, and fusion of secondary vesicles to the forming sheath (Sorokin, 1962; Alieva and Vorobjev, 2004; Pedersen and Rosenbaum, 2008; Nachury et al., 2010). We used TEM analysis to verify this model in ARPE19 cells and to test whether one of these characteristic steps was affected in Hook2-depleted cells. To answer this question using a homogeneous population of permanently Hook2-depleted cells, we generated two Hook2 small hairpin RNA (shRNA)–expressing plasmids that encode H1 or H2 siRNA targeting sequence and that also encode GFP. Efficiency of these shRNAs was tested in transiently transfected ARPE19 cells. We confirmed the inhibition of ciliogenesis in Hook2 shRNA–depleted cells compared with control shRNA-transfected cells (Supplemental Figure S3A). Using these constructs, we obtained stably transfected clones and tested them for Hook2 levels by Western blot (WB) and for inhibition of ciliogenesis by immunofluorescence staining and confocal microscopy analyses. Our data show that 70 ± 20% depletion of Hook2 levels was sufficient to inhibit ciliogenesis using shRNA (Supplemental Figure S3B). Because of their respective levels of expression and the associated ciliogenesis phenotypes, clones 2.3 and 2.5 were chosen as representative control and Hook2-depleted clones, respectively (Supplemental Figure S3, C and D). Ciliogenesis was compared for both clones using TEM (Figure 4, A–X). In control cells, we confirmed that all the characteristic stages of ciliogenesis from centriole duplication (Figure 4A), vesicle attachment (Figure 4B), vesicle flattening and axoneme growth (Figure 4, C and D), sheath growth with secondary vesicle fusion (Figure 4, E–H), and ciliary elongation to ciliary maturation (Figure 4, I–L) occurred as previously reported (Sorokin, 1962; Alieva and Vorobjev, 2004; Pedersen and Rosenbaum, 2008; Nachury et al., 2010). In contrast, Hook2-depleted cells did not show any stage of ciliogenesis beyond mother centriole maturation (Figure 4, M–X, and quantification in Figure 4Y). In Hook2-depleted cells, we observed subdistal and distal appendages (SA and DA, Figure 4V), periciliary satellites (PCS, Figure 4W), and even basal feet and caps (BFC, Figure 4X) on mother centrioles. These data suggest that Hook2 is required in a critical step beyond centriole maturation for the appropriate formation of the ciliary sheath.
FIGURE 4:
Hook2 is involved before the formation of the pericentriolar vesicle during the initiation of ciliogenesis. (A–X) Both control shRNA 2.3 and Hook2 shRNA 2.5 clones were assayed for primary cilium formation after 7 d in culture by TEM analysis. The different steps of ciliogenesis are shown for the control cells: (A) centriole duplication, (B) pericentriolar vesicle attachment, (C, D) vesicle flattening and axoneme growth, (E–H) sheath growth, (I, J) ciliary elongation, and (K, L) ciliary maturation. Only single centrioles (M–P), as well as centriole duplication (Q–U) and maturation (V–X), were observed for the Hook2-depleted cells. BB, basal body; BFC, basal foot and cap; C, cilium; Ce, centriole; DA, distal appendages; DC, daughter centriole; E, endosome; ER, endoplasmic reticulum; MC, mother centriole; G, Golgi apparatus; N, nucleus; PCS, pericentriolar satellite; PCV, pericentriolar vesicle; S, sheath; SA, subdistal appendages; SV, secondary vesicle; μ, microtubule. Long arrows in C depict a growing axoneme that flattens a pericentriolar vesicle to form the sheath at the distal tip of the mother centriole. The shorter arrows in G and I point at a secondary vesicle (SV) fusing with the ciliary sheath. (Y) Percentage of centrosomes that were associated with a pericentriolar vesicle or a cilium in control and Hook2-depleted cells. See also Supplemental Figure S3.
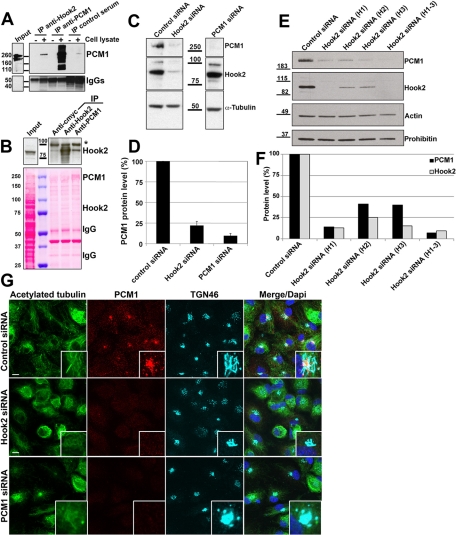
Hook2 interacts with PCM1
Hook3 interacts with PCM1 to regulate pericentriolar material assembly (Ge et al., 2010). PCM1 is also implicated in the initiation of ciliogenesis (Graser et al., 2007; Mikule et al., 2007). We therefore tested whether Hook2 and PCM1 might also interact, using a yeast two-hybrid approach and immunoprecipitation (IP) assays. The C-term part of Hook2 was used as bait to screen a human colon cDNA library. Seventy-five positive clones were sequenced, and PCM1 was found to be a putative candidate for direct interaction with Hook2. This putative interaction was further strengthened by co-IP in human embryonic kidney (HEK) cell lysates of endogenous Hook2 and PCM1 with an anti-Hook2 antibody (Figure 5A) and with an anti-PCM1 antibody (Figure 5B). We then searched for a functional interaction between both proteins by comparing their respective level of expression after siRNA depletion in ARPE19 cells by WB and immunofluorescence analyses. Hook2 depletion by the pool of three Hook2 siRNAs dramatically reduced PCM1 protein levels, whereas PCM1 depletion did not affect Hook2 levels of expression (Figure 5C, and quantification, Figure 5D). To check whether this side effect of Hook2 depletion on PCM1 level of expression was due to an off-target effect of one of the siRNAs included in the pool, we verified that each Hook2 siRNA of the targeting pool reduced PCM1 level of expression (Figure 5E, and quantification, Figure 5F). It is noteworthy that PCM1, like Hook2 depletion, induced a compaction of the Golgi apparatus (Figure 5G and Supplemental Figure S4, A and B, TGN46 staining), a ciliogenesis defect (Figures 5G) as previously described (Nachury et al., 2007), and did not affect cenexin and CEP170 recruitment to the mother centriole (Supplemental Figure S4, A and B, respectively). Collectively, these data demonstrate that Hook2 is in a complex with PCM1 and highlights the importance of Hook2 in the appropriate recruitment and stabilization of PCM1 to the pericentriolar region.
FIGURE 5:
Hook2 interacts with and regulates PCM1. (A) Lysis buffer (−) or HEK cell lysate (+) were incubated overnight with rabbit anti-Hook2 antibodies, rabbit anti-PCM1 antibodies (positive control), or rabbit preimmune serum (negative control). The immune complexes were precipitated with protein A/G agarose bead mix, and the immunoprecipitates were analyzed by WB with anti-PCM1 (top) or goat anti-rabbit antibodies (bottom). Molecular weight markers are indicated in the middle in kilodaltons. IgGs, heavy chains of immunoglobulins. (B) The reverse experiment was repeated with HEK cell lysate (input) that was incubated with rabbit anti–c-Myc (negative control), rabbit anti-Hook2 (positive control), or rabbit anti-PCM1 antibodies for 12 h. The immune complexes were precipitated with protein A/G agarose bead mix, and the immunoprecipitates were analyzed by WB with anti-Hook2 antibodies (top). Molecular weight (MW) markers are indicated in the middle in kilodaltons. The Ponceau red staining of the membrane was sufficient to detect PCM1 in the IP PCM1, Hook2 in the Hook2 IP, and equal quantities of IgGs in each IP lane. The asterisk marks a 100-kDa cross-reacting band. (C, D) Control, Hook2, or PCM1 siRNA-transfected cells were compared by WB analysis (C) and further quantification (D) for PCM1 production. MW markers are indicated in the middle in kilodaltons; n = 3, p < 0001. Note that PCM1 siRNAs do not affect Hook2 level of expression. (E, F) ARPE19 cells were transfected with individual or a mix of the three siRNAs targeting Hook2 and compared with control siRNA-transfected cells after processing for WB analysis (B) and further quantification (C) for the production of Hook2 and PCM1. Actin and prohibitin were used as loading controls. MW markers are indicated on the left in kilodaltons; n = 2. (G) SiRNA Hook2–depleted cells were fixed in PFA and compared with control siRNA-treated cells by immunofluorescence and confocal microscopy analysis for their ability to promote ciliogenesis (acetylated tubulin staining), express PCM1 in the pericentriolar area (PCM1 staining), and maintain Golgi architecture (TGN46 staining). Bar, 10 μm; inset magnification, ×15. Note that Hook2 depletion highly decreases PCM1 levels of expression. See also Supplemental Figure S4.
Distinct functions of Hook isoforms during ciliogenesis
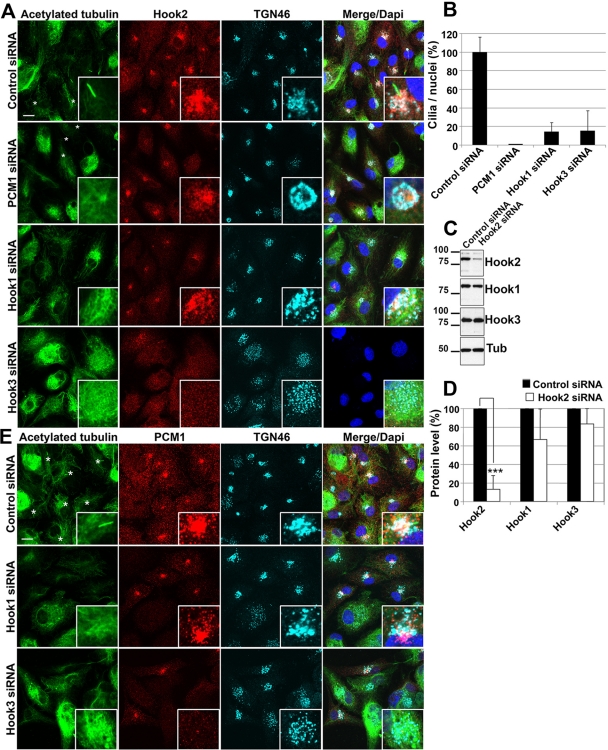
Results from yeast two-hybrid analysis and tissue culture cells indicate that mammalian Hook isoforms (Hook1, 2, and 3) form homodimers and heterodimers in a complex that also contains the proteins FTS and FHIP (Xu et al., 2008). In this study, it was suggested that the complex is an obligate multimer, with Hook proteins assembling interchangeably to form a complex (Xu et al., 2008). It was also reported that mouse Hook3 associates with the Golgi apparatus and participates in the architecture and localization of this organelle (Walenta et al., 2001). It was further shown that mouse Hook3 also localizes to the centriolar satellites, interacts with PCM1 in yeast two-hybrid screen, and is necessary for proper recruitment of PCM1-containing satellites to the pericentrosomal area (Ge et al., 2010). These data suggested the existence of a complex of proteins among which Hook isoforms could have interchangeable roles during the formation of pericentriolar satellites and ciliogenesis. To test this possibility, we studied the respective effect of Hook1 and Hook3 depletion on ciliogenesis in ARPE19 cells. We found that, like Hook2 or PCM1 depletion, Hook1 or Hook3 depletion also hindered ciliogenesis (Figures 6, A and E, acetylated tubulin staining, and quantification, Figure 6B). Of interest, we noticed that, compared with control cells, Hook1-depleted cells showed no effect on Hook2 localization and TGN morphology, whereas Hook3-depleted cells displayed a highly vesiculated TGN with a consequently vesiculated Hook2 staining (Figure 6A, Hook2 and TGN46 stainings). The dispersal of the Golgi complex was reported after interference with Hook3 function (Walenta et al., 2001). We further verified that Hook2 depletion did not affect Hook1 and Hook3 levels of expression by WB analyses (Figure 6C, and quantification, Figure 6D). This indicates that Hook3 depletion may induce a defect in ciliogenesis through a general dispersion of Golgi-associated proteins that are putatively involved in ciliogenesis (therefore including Hook2).
FIGURE 6:
Unlike Hook1 and Hook3, PCM1 phenocopies Hook2 depletion. (A) siRNA-treated cells were fixed in PFA after 7 d in culture and compared by immunofluorescence and confocal microscopy analysis for ciliogenesis (acetylated tubulin) and Hook2 localization as well as Golgi architecture (TGN46). Asterisks are next to cilia. Bar, 10 μm; inset magnification, ×15. Note that unlike PCM1 depletion, which phenocopies Hook2 depletion, Hook3 siRNA induces a Golgi breakdown, as well as a redistribution of Hook2 and consequently a ciliogenesis defect. (B) Quantification of the ciliogenesis defect induced by the depletion of Hook1 and Hook3 as compared with control cells or PCM1-depleted cells. (C, D) To confirm the specificity of Hook2 siRNA, ARPE19 cells were transfected with a mix of the three siRNAs targeting Hook2 and compared with control siRNA-transfected cells after processing for WB analysis (C) and further quantification (D) for the production of the three Hook isoforms. MW markers are indicated on the left in kilodaltons; n = 3, p < 0001. (E) siRNA-treated cells were fixed in PFA after 7 d in culture and compared by immunofluorescence and confocal microscopy analysis for ciliogenesis (acetylated tubulin) and PCM1 localization, as well as Golgi architecture (TGN46). Asterisks are next to cilia. Bar, 10 μm; inset magnification, ×15. See also Supplemental Figure S4.
A defect in PCM1-positive pericentriolar satellites formation was described after Hook3 depletion (Ge et al., 2010). As shown in Figure 6E, we confirmed a marked decrease in PCM1 expression in Hook3-depleted cells. We also showed that in contrast to Hook3, Hook1 depletion did not significantly affect PCM1 levels of expression and localization (Figure 6E). This further suggests that the maturation process of the centriole could also be affected by Hook3 depletion. To test this hypothesis, we analyzed the centriolar recruitment of cenexin and CEP170 and found that, unlike Hook1, Hook2, and PCM1 depletion, Hook3 depletion hinders the association of cenexin with the centrosome (Supplemental Figure S4A). However, CEP170 recruitment to the centrosome was unaffected by any of these depletions (Supplemental Figure S4B), indicating that cenexin and CEP170 might be recruited to the mother centriole through different processes. Taken together, these data indicate that each Hook isoform is involved in ciliogenesis, albeit at different stages of the process. This further demonstrates that the Hook paralogues are not interchangeable, at least during the initiation stages of ciliogenesis.
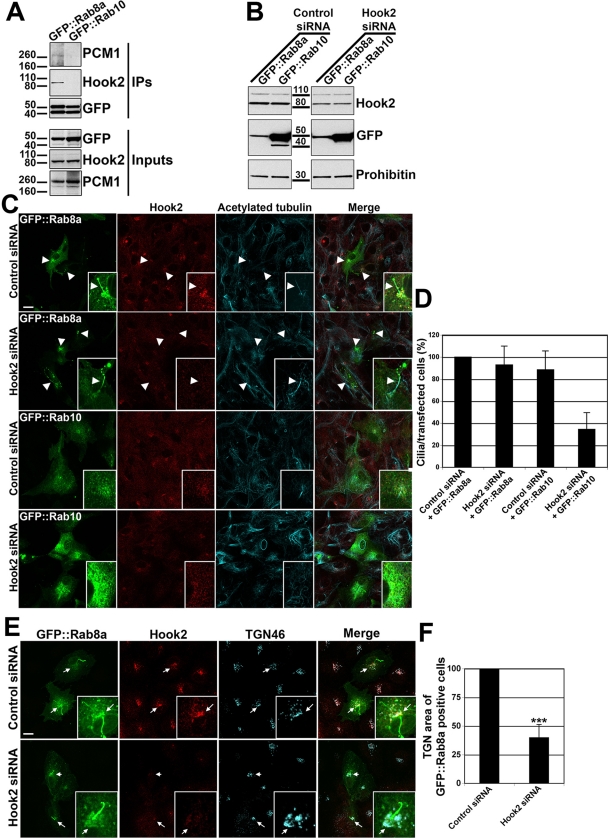
Hook2 and PCM1 are in a complex containing Rab8a
Kim et al. (2008) reported that PCM1 is required for efficient targeting of Rab8a to the primary cilium. This suggests that Hook2 could modulate PCM1 levels, which in turn regulates Rab8a targeting to the primary cilium. We therefore tested the hypothesis of an interaction of Hook2, PCM1, and Rab8a. We found that Hook2 and PCM1 specifically coimmunoprecipitate with GFP::Rab8a but not with GFP::Rab10 (Figure 7A). We then tested whether Rab8a is downstream of Hook2 and PCM1 in the pathway leading to the formation of the ciliary sheath. To address this possibility, we transiently expressed GFP::Rab8a or Rab10 and compared their ability to compensate for the observed Hook2-dependent defect in ciliogenesis (Figure 7B). In control cells that were fixed in PFA, GFP::Rab8a was localized at the plasma membrane and the primary cilium and, as previously described (Nachury et al., 2007), gave rise to an abnormally extended ciliary membrane around the axoneme (Figure 7C, top). On methanol fixation, GFP::Rab8a was observed at the plasma membrane and at the centrosome (unpublished data), indicating that the pool of GFP::Rab8a that accumulates at the primary cilium is extracted by an organic solvent like methanol. Of interest, in Hook2-depleted cells, primary cilia were formed when GFP::Rab8a was overexpressed, despite the depletion of Hook2 (Figure 7C, bottom, and quantification, Figure 7D), indicating that overexpressed GFP::Rab8a but not GFP::Rab10 can bypass the low levels of Hook2 and PCM1 and restore ciliogenesis. Nevertheless, we cannot rule out the possibility that the small fraction of Hook2 that resists depletion by siRNA could be sufficient to allow for the subsequent function of overexpressed Rab8a. We also noticed that Rab8a overexpression did not correct the compaction of the Golgi apparatus induced by Hook2 depletion (Figure 7E, and quantification, Figure 7F). This indicates that the lack of ciliogenesis in Hook2-depleted cells is not uniquely due to a consequence of Golgi compaction. We measured the ability of mouse Hook2 or GFP::Rab8a to rescue PCM1 protein levels after Hook2 depletion. Our results showed that neither mouse Hook2 cDNA nor GFP::Rab8a was able to significantly rescue PCM1 levels, suggesting that overexpressed Hook2 and Rab8a might act independently of PCM1 to restore ciliogenesis (Supplemental Figure S5A, and quantification, Supplemental Figure S5B).
FIGURE 7:
Hook2 and PCM1 are in a complex with Rab8a. (A) Lysates from HEK cells transfected with GFP::Rab8a or GFP::Rab10 were lysed and incubated with G protein–coated beads coupled to mouse anti-GFP antibodies and processed for WB analysis with rabbit antibodies anti-PCM1, anti-Hook2, and anti-GFP and immunoglobulin light chain–specific secondary antibodies. MW markers are indicated on the left in kilodaltons. Note that Hook2 and PCM1 were coimmunoprecipitated with GFP::Rab8a but not with GFP::Rab10. (B, C) Control or Hook2 siRNA (H1–3) transiently depleted cells were further transfected with GFP::Rab8a or GFP::Rab10 cDNA in addition to control or Hook2 siRNA. After 7 d, the cells were processed for WB analyses (B) with the mitochondrial marker prohibitin as a loading control. In parallel, the cells were fixed in PFA, permeabilized with Tx100, stained with antibodies raised against Hook2 and acetylated tubulin, and compared by immunofluorescence and confocal microscopy analysis (C). Bars, 10 μm; inset magnification, ×15. Arrowheads indicate GFP::Rab8a–positive cilia, even in the absence of Hook2 (siRNA Hook2 panels). (D) The number of transfected cells with a cilium in each siRNA condition is quantified and normalized to 100% for the control cells (n = 5). (E) Control or Hook2 siRNA (H1–3) transiently depleted cells were further transfected with GFP::Rab8a in addition to control or Hook2 siRNA as in B and C. After 7 d, the cells were processed for confocal microscopy analysis after immunofluorescence staining with antibodies raised against Hook2 and TGN46. Bars, 10 μm; inset magnification, ×15. Arrows indicate the TGN area of transfected cells with a cilium. (F) The volume of the TGN was measured in GFP::Rab8a–transfected cells in control and Hook2 siRNA conditions (n = 3, p < 0001). Note that GFP::Rab8a restored the ciliogenesis defect but not the Golgi compaction induced by Hook2 depletion. See also Supplemental Figures S5 and S6.
To confirm the data obtained with transient overexpression of GFP::Rab8a, we established a clonal ARPE19 cell line stably expressing GFP::Rab8a. This cell line was depleted for Hook2 and compared with nondepleted control cells after 7 d in culture (Supplemental Figure S6A). As observed in the transiently transfected cells (Figure 7, C and D), ciliogenesis was not inhibited, but the TGN was more compacted in the ARPE19-GFP::Rab8a stable cell line that was Hook2 depleted (Supplemental Figure S6, A and B, and quantification, Supplemental S6, C and D). Our data suggest that Hook2 acts upstream of Rab8a and, via its interaction with PCM1, might regulate Rab8a-dependent formation of the ciliary sheath.
DISCUSSION
Role of Hook2 in ciliogenesis
The molecular characterization of Hook2 in several contexts has so far suggested that Hook2 exclusively associates with and functions at the centrosome (Moynihan et al., 2009). Through its C-term domain, it has been shown to physically (Szebenyi et al., 2007) and functionally interact with CEP110/centriolin, a protein that was reported to localize to a specific domain of the centrosome associated with centrosome maturation (Ou et al., 2002) and more precisely to subdistal appendages found on maternal but not on daughter centrioles (Gromley et al., 2003). Hook2 was also reported to be a regulator of the establishment and maintenance of centrosomal structure and function, potentially through its role in assisting proteins that contribute to centrosomal function like CEP110 (Szebenyi et al., 2007). However, a molecular role for Hook2 specifically during ciliogenesis per se had not been demonstrated. Our data confirm the localization of Hook2 at the centrosome from which the primary cilium is generated, consistent with the previous report that overexpressed Hook2 accumulates at the mature centrosome in the region where ninein accumulates (Szebenyi et al., 2007).
In our study, using two different staining methods, we showed that in addition to its centrosomal localization, Hook2 is localized at the Golgi apparatus and colocalizes with the ciliary base marker Rab11 (Knodler et al., 2010). Consistent with this localization, our study underscores a critical role of Hook2 before or during the formation at the distal tip of the mother centriole of a pericentriolar vesicle that is believed to originate from the TGN and, significantly, demonstrates that this step is determinant for ciliogenesis to proceeding further. Because Hook proteins were proposed to act as potential regulators of cargo loading for microtubule-based transport and anchors for organelles (Linstedt, 2004), it is tempting to speculate that Hook2 might be involved in the formation or docking of the pericentriolar vesicle at the distal tip of the mother centriole, as Hook2 depletion results in a disruption in ciliogenesis at a step after centriole maturation, just before the formation of the pericentriolar vesicle that gives rise to the sheath. Alternatively, Hook might be involved as an adaptor in the anchoring of the nascent axonemal microtubules at the distal tip of the mother centriole, allowing the anchoring of the pericentriolar vesicle and the growth of the axoneme-sheath structure. We cannot rule out, however, that Hook2 may also be required for mother centriole maturation due to a presumably stable population of Hook2 around the mother centriole that is refractory to siRNA depletion and might be sufficient to drive this step up.
Our EM data clearly show that the depletion of Hook2 results in a strong defect in ciliogenesis at a very early stage when compared with profiles representing control cells at different stages of ciliogenesis. However, the pictures were obtained from populations of cells that were cultured for 7 d and therefore might have reached a quiescent stage. Therefore, as discussed by Sorokin (1962), we cannot rule out that, even though serial sections have been made to avoid plan artifacts, the distinct profiles shown in Figure 4, B–L, might correspond to different section planes of mature cilia rather than different stages of ciliogenesis. In any case, if the latter hypothesis was true, it would still emphasize the fact that in the samples from cells that were stably Hook2 depleted, we could not find any of these profiles beyond centriole maturation profiles. This would strengthen our hypothesis that Hook2 is involved after centriole maturation.
It was reported that endogenous Hook proteins display distinct localization and homodimerize but do not heterodimerize, which suggested separate functions for the three isoforms (Walenta et al., 2001). In contrast, another study demonstrated with yeast two-hybrid screen and tissue culture cells that mammalian Hook isoforms (Hook1, 2, and 3) form homodimers and heterodimers in the FTS/Hook/FHIP complex. In this study, it was suggested that the complex is an obligate multimer (Xu et al., 2008). Mouse Hook3 was also found to interact with PCM1 in a two-hybrid analysis and is necessary for proper recruitment of PCM1-containing satellites to the pericentrosomal area, since Hook3 siRNA caused a redistribution of PCM1 and inhibited pericentriolar satellite formation (Ge et al., 2010). Here we show that Hook2 also binds to PCM1, which supports the hypothesis that Hook proteins interact with each other in a multimeric complex with common partners and therefore putatively some interchangeable roles. However, the effects of Hook2 and Hook3 depletion on Golgi morphology and centrosomal maturation are not equivalent, demonstrating that, in addition to these overlapping roles, the three isoforms clearly have distinct nonredundant functions during ciliogenesis. Our data thus indicate that the three paralogues of Hook2 are involved in ciliogenesis but are not interchangeable throughout the different steps involved in this process.
Interconnection among Hook2, PCM1, and Rab8a during ciliogenesis
We also show that unlike Hook3 (Ge et al., 2010), Hook2 is crucial for PCM1 stabilization. Mammalian Hooks are adaptor-like proteins that are believed to serve as linkers that can associate with microtubules and organelles (Walenta et al., 2001). They are emerging as potential regulators of organelle positioning both as facilitators in the loading of cargos for microtubule-based retrograde transport and as anchors for organelles (Linstedt, 2004). We therefore suggest that Hook2 might associate with PCM1 in a complex that could be involved in the loading, transport, targeting, and/or anchoring of pericentriolar vesicles, as well as anchoring of the nascent axoneme during ciliogenesis initiation. If this is true, when Hook2 is depleted, the complex is destabilized and ciliogenesis is impaired. Pericentriolar satellites are believed to drive cargos to the centrosomes and basal bodies and play a role during ciliogenesis (Kubo et al., 1999; Kubo and Tsukita, 2003; Dammermann and Merdes, 2002). One hypothesis is that, through the dynamic movements of the centriolar satellites, the Hook2–PCM1 complex might regulate the loading of proteins that are necessary for ciliogenesis into cargos or the positioning of nascent pericentriolar vesicles to the centrosomes. In accordance to this hypothesis, PCM1 was reported to be necessary for Rab8a targeting to the base of primary cilium (Kim et al., 2008). Rab8a is a mother centriole/primary cilium protein since it interacts directly with cenexin/ODF2 and is also enriched in the primary cilium (Yoshimura et al., 2007). Rab8a also interacts specifically with the BBSome through Rabin8, which binds BBS1 (Nachury et al., 2007). Of interest, it was shown in Xenopus photoreceptors that Rab8a regulates the late stages of delivery of post-Golgi membranes containing rhodopsin to the plasma membrane near the base of the connecting cilium (Moritz et al., 2001). In mammalian cells, activated Rab8a promotes extension of the ciliary membrane (Nachury et al., 2007), and a recent study showed that a GFP::Rab8a accumulates in the assembling ciliary membrane during de novo formation of a primary cilium in serum-starved RPE cells (Westlake et al., 2011). Thus Rab8a is likely to play an early role in ciliary membrane assembly. Our observations that exogenous Rab8a can be pulled down in a complex containing Hook2 and PCM1 and that Rab8a overexpression can overcome the defect in ciliogenesis induced by Hook2 depletion are consistent with a Hook2-dependent function upstream of Rab8a and PCM1. This specific role for Hook2 and Rab8a in the early steps of cilium formation is supported by the observation that exogenous Rab8a can rescue the ciliogenesis defect but not the TGN compaction that occurs in Hook2-depleted cells, indicating that these events are independent. The interaction between a protein of a Hook family and Rab proteins has been reported in vivo, with the binding of the endocytic Rab proteins 7, 9, and 11 to Hook1 (Luiro et al., 2004). This Hook paralogue regulates endosomal clustering and spermatide head shape (Mendoza-Lujambio et al., 2002), and the Hook1 yeast orthologue Btn2 is involved in late endosome–Golgi protein sorting (Kama et al., 2007).
In conclusion, our data suggest a model in which Hook2 interacts with PCM1 in a complex containing Rab8a at the mother centriole to promote ciliary primary vesicle formation or docking. In the absence of Hook2 (and in consequence PCM1), overexpressed Rab8a could still initiate the formation of the ciliary sheath by binding directly to cenexin that is still present at the activated mother centriole, bypassing the Hook2–PCM1 pathway.
MATERIALS AND METHODS
Antibodies and reagents
Antibodies were raised in rabbits against the amino acids 427–719 of human Hook2, affinity purified as previously described (Walenta et al., 2001), and their specificity was further tested (Szebenyi et al., 2007). Anti-Hook2 antibodies were also raised in rats against the peptides 65 (CLGRLASLNLRPTDKH) and 66 (DTPDSLSPETYGNFDS) present in the human Hook2 C-term and N-term domains, respectively. The rat anti-Hook2 antibodies were used uniquely for WB and IP analyses and gave the same results as the rabbit anti-Hook2 antibodies. Antibodies were also raised in rabbits against mouse Hook1 and Hook3 and affinity purified as previously described (Walenta et al., 2001). Mouse monoclonal anti-cenexin antibody (clone CD1B4) was a gift from K. Gull (Lange and Gull, 1995). Rabbit anti-cenexin antibody was from Atlas Antibodies (St. Louis, MO). Mouse monoclonal anti-giantin antibody was a gift from H. P. Hauri (Linstedt and Hauri, 1993). Mouse monoclonal anti–α-tubulin (clone B-5-1-2), anti–γ-tubulin (clone GTU-88), and anti–acetylated α-tubulin (clone 6-11B-1) antibodies were from Sigma (Lyon, France). Mouse monoclonal anti-Rab11 (clone 47) and anti-EEA1 (clone 14) antibodies were from BD Biosciences PharMingen (Le Pont de Claix, France). Mouse monoclonal anti–c-Myc (clone 9E10) and anti-GFP (clone B-2) and rabbit polyclonal anti–glyceraldehyde-3-phosphate dehydrogenase (FL-335) antibodies were from Santa Cruz Biotechnology/Clinisciences (Montrouge, France). Mouse anti–Lamp1/CD107a (clone H4A3) antibody was from Biolegend/Ozyme (Saint Quentin en Yvelines, France). Sheep anti–human TGN46 and anti–mouse TGN38 antibodies were from AbDSerotec (Düsseldorf, Germany). Affinity-purified rabbit anti-CEP170 and anti-PCM1 antibodies were from Bethyl Laboratories (Montgomery, TX). Mouse anti-prohibitin antibody (II-14-10) was from Abcam (Cambridge, United Kingdom). Secondary antibodies were from Jackson ImmunoResearch (Immunotech, Marseille, France) or molecular probes (Invitrogen, Cergy Pontoise, France). Tris, bovine serum albumin (BSA), methanol, PFA, saponin, sucrose, and 4′,6-diamidino-2-phenylindole were from Sigma. Other reagents were as follows: glycine and coverslips (VWR, Fontenay, France), SDS and Tx100 (Euromedex, Mundolsheim, France), 1,4-diazabicyclo[2.2.2]octane (DABCO) and Mowiol (Calbiochem/Merck, Meudon, France), Tissue Matrix Optimal Cutting Temperature (OCT) and Superfrost Plus slides (Labonord, Templemars, France), phosphate-buffered saline (PBS; Eurobio, Les Ulis, France), glutaraldehyde EM grade 25%, osmium, and silver lacquer (Electron Microscopy Sciences, Ayguesvives, France), and protein A–Sepharose CL-4B and protein G–Sepharose 4 fast flow (GE Healthcare, La Penne sur Huveaune, France).
siRNA, shRNAs, plasmids, and yeast-two hybrid assay
Hook2 siRNA H1 (ID 20301: 5′ggagacucugauuuuauau3′), H2 (ID 20392: 5′ggaccacauccagagaauc3′), H3 (ID 20208: 5′ggucagcaaucugaagaug3′), and control siRNA against a sequence of luciferase (5′cguacgcggaauacuucga3′) were from Ambion/Applied Biosystems (Courtaboeuf, France). A mix of the three siRNAs targeting Hook2 was used throughout the study unless otherwise indicated. Control shRNA targeting luciferase sequence as well as Hook2 shRNA–expressing plasmids that encode H1 or H2 siRNA targeting sequence and that also encode GFP were generated from the pRNAi vector. ON-TARGETplus SMARTpools of 4 Hook2 siRNAs (L-020408-02) that do not overlap H1, H2, or H3 positions, four PCM1 siRNAs (L-005165-00), four Hook1 siRNAs (L-016845-01), and four Hook3 siRNAs (L-013558), as well as ON-TARGETplus nontargeting pool control siRNAs (D-001810-10), were from Dharmacon/Thermo Scientific (Lafayette, CO). Hook2 full-length cDNA clone (IRAVp968E0334D) expressing untagged mouse Hook2 was from ImaGenes (Berlin, Germany), and the control empty vector pcDNA3.1 was from Invitrogen. The plasmid pEGFPC::Rab8a (wild type) was from A. Echard (Institut Pasteur, Paris, France), pEGFPC::Rab10 was from D. M. Scidmore (Cornell University College of Veterinary Medicine, Ithaca, NY), and pEGFPN::Arl13b was from G. Pazour (University of Massachusetts Medical School, Worcester, MA). Yeast two-hybrid screens were performed as described (Thalappilly et al., 2008). All yeast media were prepared as described (Walhout and Vidal, 2001; Thalappilly et al., 2008). C-term Hook2 (BamH1 to end) inserted in the Gateway System (pZEO; Invitrogen) was used as bait to screen a human colon cDNA Library. The positive control on all phenotypes was pCL1 (full-length GAL4) with pPC86 (AD), and the negative control on all phenotypes was pPC97-CYH2 (DB) with pPC86 (AD). Seventy-five positive clones were sequenced.
Cell culture, transfections, and generation of clones
ARPE19 and HK-2 cells (American Type Culture Collection [ATCC], Manassas, VA) were cultured in DMEM/F12 (1:1) with l-glutamine containing 10% fetal bovine serum (Perbio Thermo Scientific, Brebières, France) and insulin/transferrin/selenium-A. For HK-2, the complete medium was supplemented with 10 μg/l epidermal growth factor, 36 μg/l hydrocortisone, and 4 μg/l 3′,3′,5-triiodo-l-thyronine sodium salt. The 4T1 cell (ATCC) complete medium was RPMI 1640 containing 2 mM l-glutamine, 10% fetal bovine serum, 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 1 mM sodium pyruvate. HEK 293F cells were cultured in DMEM with l-glutamine and 10% fetal bovine serum. For ciliogenesis, ARPE19, HK-2, and HEK 293F cells were cultured for 7 d in their complete medium without any serum starvation. ARPE19 cells were transfected with Fugene HD transfection reagent using the manufacturer's instructions (Roche, Indianapolis, IN). Transfected cells were selected to generate stable cell lines using genetin/G418 sulfate. All cell culture reagents were from Life Technologies/Invitrogen (Cergy-Pontoise, France), unless otherwise indicated.
Immunofluorescence staining and confocal microscopy
Cultured cells on glass coverslips were fixed either with 3% PFA for 20 min, rinsed, and permeabilized with 0.5% Tx100 for 10 min (except for Lamp-1 and EEA1 stainings) or with methanol for 4 min at −20°C. The cells were rinsed in PBS, quenched with 0.2% BSA and 0.05% saponin in PBS for ½ h, and stained with the indicated antibodies. P0 CD1 mice were fixed in 4% PFA for 1 h, washed three times in PBS, incubated in 20% sucrose overnight at 4°C, embedded in OCT, and frozen on dry ice. The 12 μM embedded head cryosections were dried on Superfrost Plus slides and permeabilized for 10 min with PBS containing 2% BSA, 0.05% saponin, and 0.5% Tx100. The cryosections were quenched with 2% BSA and 0.05% saponin in PBS for ½ h and immunostained. Coverslips and slides were mounted in DABCO/Mowiol and observed with a Zeiss Meta confocal microscope (Zeiss, Le Pecq, France) with a UV laser and 100× and 63× objectives for cells and 40× and 10× objectives for tissues. Confocal image analyses were performed using LSM5 Image Browser (Zeiss), ImageJ (National Institutes of Health, Bethesda, MD), and Photoshop (Adobe, San Jose, CA) softwares.
SEM
siRNA-treated cells cultured on glass coverslips, washed in PBS, fixed in 2.5% glutaraldehyde in PBS for 1h, washed in PBS, and postfixed in 1% osmium in PBS. The cells were dehydrated in a 25–100% graded series of ethanol and washed twice in alcohol/hexamethyldisilazane (1:1) for 2 h. The coverslips were pasted on SEM disk targets with silver lacquer and gold metalized in an Edwards S150B sputter coater (Boc Edwards, Guildford, United Kingdom). Cells were observed using a Leica S440 scanning electron microscope (Leica Microsystèmes SAS, Nanterre, France) under 30 kV.
TEM
Stable clones were cultured for 7 d on glass coverslips. The cells were incubated for 5 min with complete medium containing 2.5% glutaraldehyde, washed once in PBS, and fixed for 1 h in cacodylate sodium buffer 0.1 M, pH 7.2, containing 2.5% glutaraldehyde, 0.1% tannic acid, and 0.01% CaCl2. The samples were washed three times in cacodylate buffer, postfixed for 1 h in cacodylate buffer containing 1% OsO4, washed three times, dehydrated in a graded series of ethanol, impregnated in propylene oxide, and embedded in Epon resin. Ultrathin 80-nm sections were obtained using a Leica Ultracut UCT microtome (Leica, Wetzlar, Germany) equipped with a diamond knife (Drukker, Cuijk, Netherlands). Sections were collected on copper grids and stained with uranyl acetate and lead citrate (Reynolds, 1963). Centrosomal sections were selected, and observations were performed on an EM 912 electron microscope (Zeiss) at 100-kV acceleration equipped with a BioScan camera (Model 792; Gatan, Warrendale, PA). Images were acquired with the DigitalMicrograph software (Gatan).
WB and IP
For WB analyses, cells were resuspended either directly in Laemmli buffer or in lysis buffer A containing 50 mM Tris, pH 7.5, 150 mM NaCl, 50 mM NaF, 1 mM EDTA, 1% NP40, 1 mM orthovanadate, and protease inhibitor cocktail containing 1 μg/ml antipain, 1 μg/ml pepstatin, 15 μg/ml benzamidine, and 1 μg/ml leupeptin. The cell lysates were centrifuged, and the supernatants were completed with Laemmli buffer. The proteins were heat denaturated and processed by SDS–PAGE, transferred to nitrocellulose, stained with Ponceau red, and immunoblotted with the indicated antibodies. The proteins were detected with the Western Lightning Chemiluminescence Reagent Plus (PerkinElmer, Courtaboeuf, France). For co-IP of Hook2 and PCM1, HEK cells were lysed in buffer A and incubated overnight with control rabbit anti–c-Myc, rabbit preimmune serum, anti-Hook2, or anti-PCM1 antibodies. The immune complexes were precipitated with protein A/G agarose bead mix, and the immunoprecipitates were analyzed by WB with the indicated antibodies. For co-IP of Hook2 and PCM1 with GFP::Rab8a, HEK cells were transfected with GFP::Rab8a or GFP::Rab10, incubated for 48 h, lysed in buffer A, and incubated overnight with anti-GFP coupled to G protein/agarose beads. The immunoprecipitates were analyzed by WB as indicated.
Statistics
Quantifications were performed using Excel software (Microsoft, Redmond, WA). The error bars represent SDs with n experiments (n is the number of distinct experiments, unless otherwise indicated). Statistical difference p was calculated using Student's t test.
Supplementary Material
Acknowledgments
We thank members of the Le Bivic and Borg teams, G. Mottola, and R. Roy for helpful discussions and reading of the manuscript. We also thank P. Weber and D. Isnardon at IBDML and CRCM imaging facilities, respectively, and R. Grifone, C. Ricard, M. Sangiardi, and the CRCM computing facility for technical assistance. This work was supported by the Centre National de la Recherche Scientifique (UMR 6216), Université de la Méditerranée, Coordination Theme 1 (Health) of the European Community FP7, Grant Agreement HEALTH-F2-2008-200234, and the Agence Nationale de la Recherche (ANR) BLAN07-2-186738. A.L.B. was supported by the Ligue Nationale Contre le Cancer (LNCC; Label 2008). C.L.B.G. was a recipient of a fellowship from the Fondation pour la Recherche Médicale and received support through ANR BLAN07-2-186738, Marie Curie International Reintegration Grant 46575 Polarity, and European Consortium for Anticancer Antibody Development FP7 (Health-F5-2008-200755). E.P.P. was a recipient of a fellowship from the French Ministry for Research and Education and from the Fondation pour la Recherche Médicale. J.P.B. was supported by the LNCC (Label 2010) and the Infrastructures en Biologie Santé et Agronomie (Plateforme de Protéomique, Marseille). H.K. was supported by grants from the Welch Foundation (I‑1300) and the National Institutes of Health (EY10199). C.L.B.G. dedicates this work to Anaïs, Alicia, and P.H.
Abbreviations used:
- ARPE
arising retinal pigmented epithelial cell
- BBS
Bardet–Biedl syndrome
- CEP
centriolar protein
- EEA1
early endosomal protein 1
- FHIP
Fused Toes and Hook interacting protein
- FTS
Fused Toes
- HEK
human embryonic kidney cell
- HK-2
human kidney cell
- Lamp
lysosomal-associated membrane protein
- PCM1
pericentriolar material protein 1
- shRNA
small hairpin RNA
- siRNA
small interfering RNA
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-05-0405) on October 12, 2011.
REFERENCES
- Alieva IB, Vorobjev IA. Vertebrate primary cilia: a sensory part of centrosomal complex in tissue cells, but a “sleeping beauty” in cultured cells? Cell Biol Int. 2004;28:139–150. doi: 10.1016/j.cellbi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Dammermann A, Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J Cell Biol. 2002;159:255–266. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, et al. Mutations in LCA5, encoding the ciliary protein lebercilin, cause Leber congenital amaurosis. Nat Genet. 2007;39:889–895. doi: 10.1038/ng2066. [DOI] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Ge X, Frank CL, Calderon de Anda F, Tsai LH. Hook3 interacts with PCM1 to regulate pericentriolar material assembly and the timing of neurogenesis. Neuron. 2010;65:191–203. doi: 10.1016/j.neuron.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Tong AH, Boone C, Munro S. The GTPase Arf1p and the ER to Golgi cargo receptor Erv14p cooperate to recruit the golgin Rud3p to the cis-Golgi. J Cell Biol. 2004;167:281–292. doi: 10.1083/jcb.200407088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graser S, Stierhof YD, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol. 2007;179:321–330. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley A, Jurczyk A, Sillibourne J, Halilovic E, Mogensen M, Groisman I, Blomberg M, Doxsey S. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J Cell Biol. 2003;161:535–545. doi: 10.1083/jcb.200301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, Nigg EA. The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol Biol Cell. 2005;16:1095–1107. doi: 10.1091/mbc.E04-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Kubo A, Tsukita S, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol. 2005;7:517–524. doi: 10.1038/ncb1251. [DOI] [PubMed] [Google Scholar]
- Kama R, Robinson M, Gerst JE. Btn2, a Hook1 ortholog and potential Batten disease-related protein, mediates late endosome-Golgi protein sorting in yeast. Mol Cell Biol. 2007;27:605–621. doi: 10.1128/MCB.00699-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17:3796–3805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Sasaki H, Yuba-Kubo A, Tsukita S, Shiina N. Centriolar satellites: molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis. J Cell Biol. 1999;147:969–980. doi: 10.1083/jcb.147.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Tsukita S. Non-membranous granular organelle consisting of PCM-1: subcellular distribution and cell-cycle-dependent assembly/disassembly. J Cell Sci. 2003;116:919–928. doi: 10.1242/jcs.00282. [DOI] [PubMed] [Google Scholar]
- Lange BM, Gull K. A molecular marker for centriole maturation in the mammalian cell cycle. J Cell Biol. 1995;130:919–927. doi: 10.1083/jcb.130.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD. Positioning the Golgi apparatus. Cell. 2004;118:271–272. doi: 10.1016/j.cell.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Hauri HP. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiro K, Yliannala K, Ahtiainen L, Maunu H, Jarvela I, Kyttala A, Jalanko A. Interconnections of CLN3, Hook1 and Rab proteins link Batten disease to defects in the endocytic pathway. Hum Mol Genet. 2004;13:3017–3027. doi: 10.1093/hmg/ddh321. [DOI] [PubMed] [Google Scholar]
- Melan MA. Overview of cell fixation and permeabilization. Methods Mol Biol. 1994;34:55–66. doi: 10.1385/0-89603285-x:55. [DOI] [PubMed] [Google Scholar]
- Mendoza-Lujambio I, Burfeind P, Dixkens C, Meinhardt A, Hoyer-Fender S, Engel W, Neesen J. The Hook1 gene is non-functional in the abnormal spermatozoon head shape (azh) mutant mouse. Hum Mol Genet. 2002;11:1647–1658. doi: 10.1093/hmg/11.14.1647. [DOI] [PubMed] [Google Scholar]
- Mikule K, Delaval B, Kaldis P, Jurcyzk A, Hergert P, Doxsey S. Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat Cell Biol. 2007;9:160–170. doi: 10.1038/ncb1529. [DOI] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan KL, Pooley R, Miller PM, Kaverina I, Bader DM. Murine CENP-F regulates centrosomal microtubule nucleation and interacts with Hook2 at the centrosome. Mol Biol Cell. 2009;20:4790–4803. doi: 10.1091/mbc.E09-07-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier. Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou YY, Mack GJ, Zhang M, Rattner JB. CEP110 and ninein are located in a specific domain of the centrosome associated with centrosome maturation. J Cell Sci. 2002;115:1825–1835. doi: 10.1242/jcs.115.9.1825. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12:551–555. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- Pooley RD, Moynihan KL, Soukoulis V, Reddy S, Francis R, Lo C, Ma LJ, Bader DM. Murine CENPF interacts with syntaxin 4 in the regulation of vesicular transport. J Cell Sci. 2008;121:3413–3421. doi: 10.1242/jcs.032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley RD, Reddy S, Soukoulis V, Roland JT, Goldenring JR, Bader DM. CytLEK1 is a regulator of plasma membrane recycling through its interaction with SNAP-25. Mol Biol Cell. 2006;17:3176–3186. doi: 10.1091/mbc.E05-12-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Clerc J, Hinz B. Immunofluorescence detection of the cytoskeleton and extracellular matrix in tissue and cultured cells. Methods Mol Biol. 2010;611:43–57. doi: 10.1007/978-1-60327-345-9_4. [DOI] [PubMed] [Google Scholar]
- Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebenyi G, Hall B, Yu R, Hashim AI, Kramer H. Hook2 localizes to the centrosome, binds directly to centriolin/CEP110 and contributes to centrosomal function. Traffic. 2007;8:32–46. doi: 10.1111/j.1600-0854.2006.00511.x. [DOI] [PubMed] [Google Scholar]
- Thalappilly S, Suliman M, Gayet O, Soubeyran P, Hermant A, Lecine P, Iovanna JL, Dusetti NJ. Identification of multi-SH3 domain-containing protein interactome in pancreatic cancer: a yeast two-hybrid approach. Proteomics. 2008;8:3071–3081. doi: 10.1002/pmic.200701157. [DOI] [PubMed] [Google Scholar]
- Walenta JH, Didier AJ, Liu X, Kramer H. The Golgi-associated hook3 protein is a member of a novel family of microtubule-binding proteins. J Cell Biol. 2001;152:923–934. doi: 10.1083/jcb.152.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhout AJ, Vidal M. High-throughput yeast two-hybrid assays for large-scale protein interaction mapping. Methods. 2001;24:297–306. doi: 10.1006/meth.2001.1190. [DOI] [PubMed] [Google Scholar]
- Westlake CJ, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci USA. 2011;108:2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Sowa ME, Chen J, Li X, Gygi SP, Harper JW. An FTS/Hook/p107(FHIP) complex interacts with and promotes endosomal clustering by the homotypic vacuolar protein sorting complex. Mol Biol Cell. 2008;19:5059–5071. doi: 10.1091/mbc.E08-05-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.