Abstract
Intact rat diaphragms were exposed in vitro to varying CO2 tensions and bicarbonate concentrations, and the steady-state citrate content of diaphragm muscle was measured to investigate the relationship between metabolism and extracellular pH, PCO2, and (HCO3-). In addition, rat hemidiaphragms were incubated with 1,5-citrate-14C under different acid-base conditions, and 14CO2 production was determined as a measure of citrate oxidation.
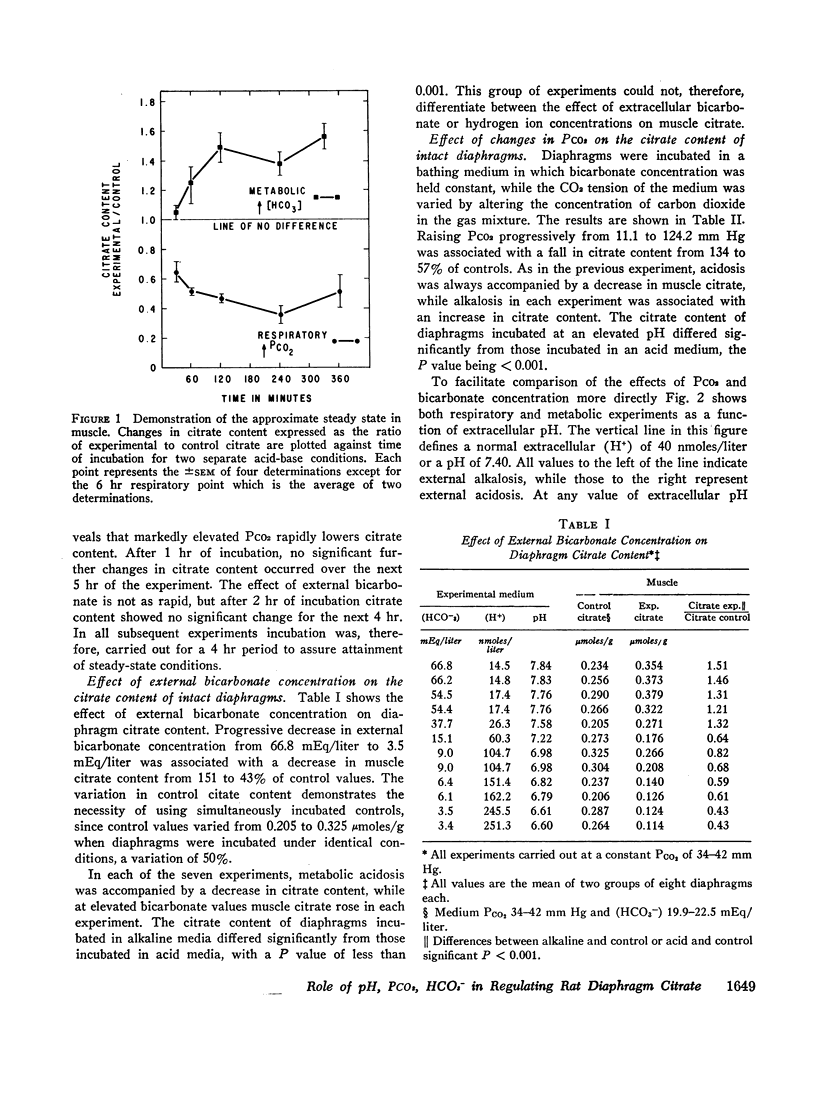
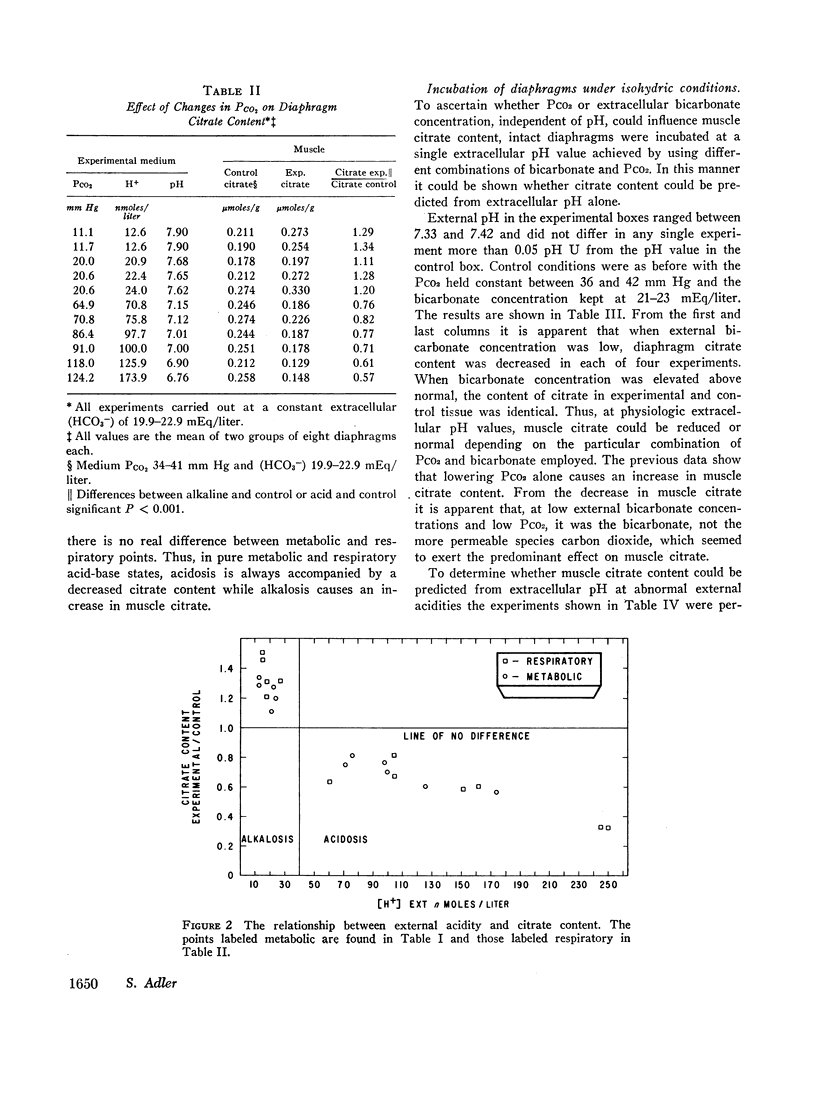
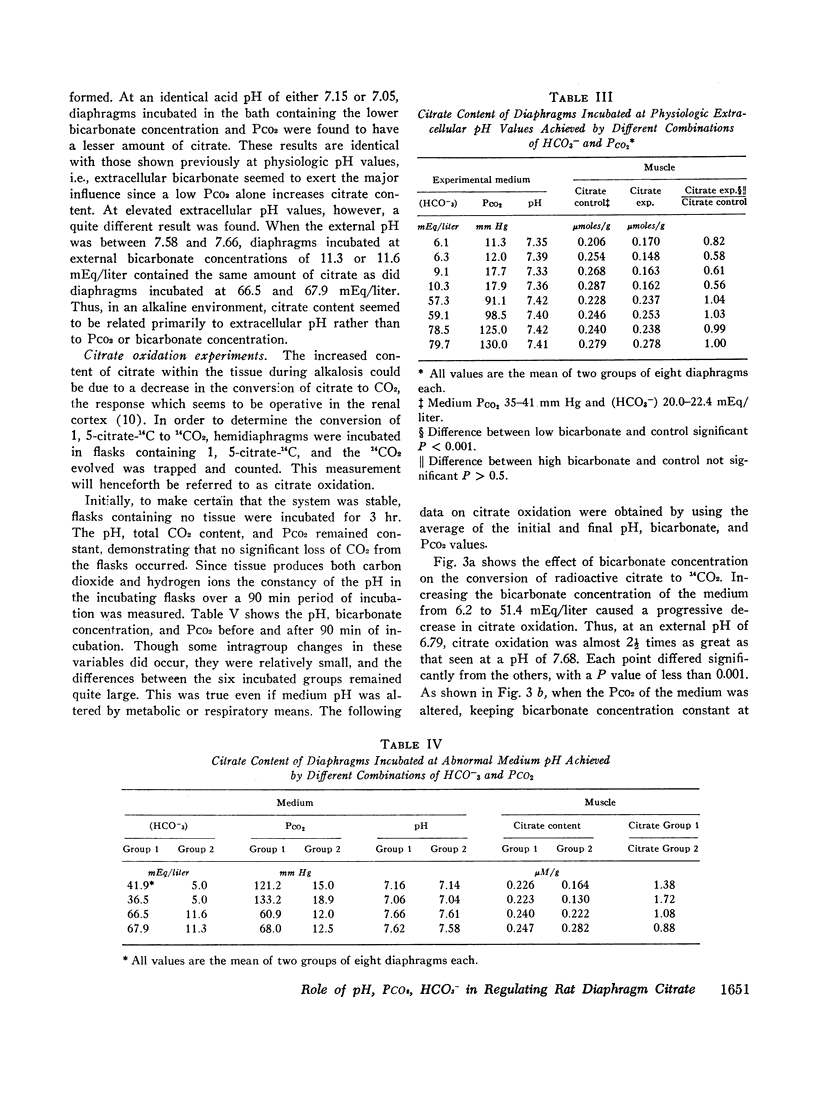
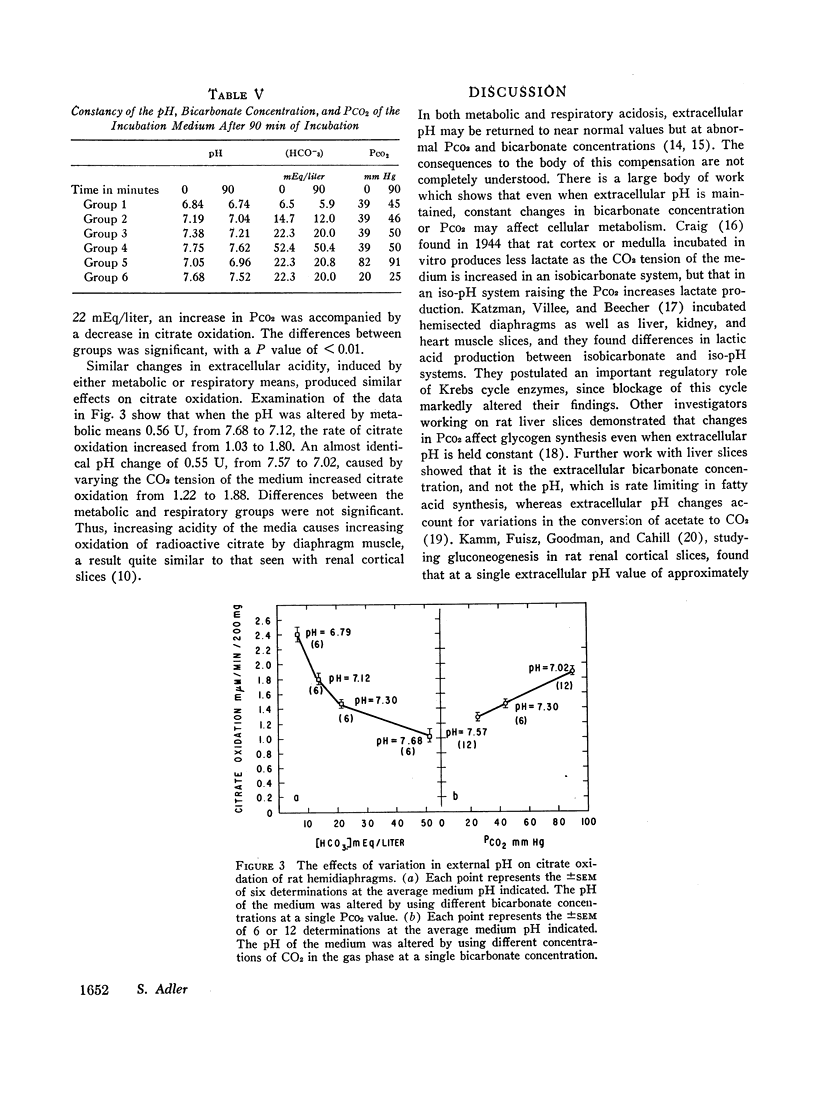
Acidification of the bathing medium achieved by raising CO2 tension or lowering (HCO3-) was associated with a decrease in muscle citrate content. On the other hand, alkalinization of the medium induced by lowering CO2 tension or raising (HCO3-) caused tissue citrate content to rise. At a physiologic extracellular pH value of approximately 7.40, citrate content was decreased or normal depending on the CO2/HCO3- combination employed to attain the pH. Under low bicarbonate and low PCO2 conditions, citrate content was reduced. A similar result was found at external pH values of 7.15, implying that at these two extracellular pH levels (HCO3-) primarily determines citrate content. When changes in citrate content were compared with intracellular pH data reported earlier using the same intact diaphragm preparation, no simple relation between citrate content and intracellular pH was found. The effect of acidity on citrate content seems related to a change in citrate oxidation since the latter increased progressively with increasing degrees of medium acidity.
These results show that cellular metabolism is not a simple function of extracellular pH but is dependent on the particular combination of PCO2 and bicarbonate employed to achieve the pH value. These studies also suggest that accumulation or disposal of organic acids, such as citric acid, helps to regulate cellular acidity thereby contributing to the cells' defense against external acid-base disorders.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. I. THE RESPONSE OF MUSCLE CELLS TO CHANGES IN CO2 TENSION OR EXTRACELLULAR BICARBONATE CONCENTRATION. J Clin Invest. 1965 Jan;44:8–20. doi: 10.1172/JCI105129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. II. THE INTERACTION BETWEEN CO-2 TENSION AND EXTRACELLULAR BICARBONATE IN THE DETERMINATION OF MUSCLE CELL PH. J Clin Invest. 1965 Jan;44:21–30. doi: 10.1172/JCI105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S. An extrarenal action of aldosterone on mammalian skeletal muscle. Am J Physiol. 1970 Mar;218(3):616–621. doi: 10.1152/ajplegacy.1970.218.3.616. [DOI] [PubMed] [Google Scholar]
- Burnell J. M. In vivo response of muscle to changes in CO2 tension or extracellular bicarbonate. Am J Physiol. 1968 Dec;215(6):1376–1383. doi: 10.1152/ajplegacy.1968.215.6.1376. [DOI] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J., NEWSHOLME E. A. CITRATE AS AN INTERMEDIARY IN THE INHIBITION OF PHOSPHOFRUCTOKINASE IN RAT HEART MUSCLE BY FATTY ACIDS, KETONE BODIES, PYRUVATE, DIABETES, AND STARVATION. Nature. 1963 Oct 12;200:169–170. doi: 10.1038/200169a0. [DOI] [PubMed] [Google Scholar]
- HUDSON J. B., RELMAN A. S. Effects of potassium and rubidium on muscle cell bicarbonate. Am J Physiol. 1962 Jul;203:209–214. doi: 10.1152/ajplegacy.1962.203.1.209. [DOI] [PubMed] [Google Scholar]
- KATZMAN R., VILLEE C. A., BEECHER H. K. Effect of increased carbon dioxide concentrations on fixed acid production in vitro. Am J Physiol. 1953 Feb;172(2):317–323. doi: 10.1152/ajplegacy.1953.172.2.317. [DOI] [PubMed] [Google Scholar]
- KIBLER R. F., O'NEILL R. P., ROBIN E. D. INTRACELLULAR ACID-BASE RELATIONS OF DOG BRAIN WITH REFERENCE TO THE BRAIN EXTRACELLULAR VOLUME. J Clin Invest. 1964 Mar;43:431–443. doi: 10.1172/JCI104928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm D. E., Fuisz R. E., Goodman A. D., Cahill G. F., Jr Acid-base alterations and renal gluconeogenesis: effect of pH, bicarbonate concentration, and PCO2. J Clin Invest. 1967 Jul;46(7):1172–1177. doi: 10.1172/JCI105610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGMORE W. J., HASTINGS A. B., HARRISON E. S. THE EFFECT OF PHYSIOLOGICAL VARIATIONS IN PH AND CO2 CONCENTRATIONS ON ACETATE-1-C14 METABOLISM. Proc Natl Acad Sci U S A. 1964 Oct;52:1040–1044. doi: 10.1073/pnas.52.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGMORE W. J., HASTINGS A. B., MAHOWALD T. A. EFFECT OF ENVIRONMENTAL CO2 AND PH ON GLYCEROL METABOLISM BY RAT LIVER IN VITRO. J Biol Chem. 1964 Jun;239:1700–1704. [PubMed] [Google Scholar]
- Lennon E. J., Lemann J., Jr Defense of hydrogen ion concentration in chronic metabolic acidosis. A new evaluation of an old approach. Ann Intern Med. 1966 Aug;65(2):265–274. doi: 10.7326/0003-4819-65-2-265. [DOI] [PubMed] [Google Scholar]
- Longmore W. J., Landau B. R., Baker E. S., Hastings A. B., Lum D. M., Williams H. R. Effect of pH and CO2 concentration on glucose metabolism by rat adipose tissue in vitro. Am J Physiol. 1968 Sep;215(3):582–586. doi: 10.1152/ajplegacy.1968.215.3.582. [DOI] [PubMed] [Google Scholar]
- MILNE M. D., SCRIBNER B. H., CRAWFORD M. A. Non-ionic diffusion and the excretion of weak acids and bases. Am J Med. 1958 May;24(5):709–729. doi: 10.1016/0002-9343(58)90376-0. [DOI] [PubMed] [Google Scholar]
- Moellering H., Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966 Dec;17(3):369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- RELMAN A. S., GORHAM G. W., LEVINSKY N. G. The relation between external potassium concentration and the electrolyte content of isolated rat muscle in the steady state. J Clin Invest. 1961 Feb;40:386–393. doi: 10.1172/JCI104265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ W. B., BRACKETT N. C., Jr, COHEN J. J. THE RESPONSE OF EXTRACELLULAR HYDROGEN ION CONCENTRATION TO GRADED DEGREES OF CHRONIC HYPERCAPNIA: THE PHYSIOLOGIC LIMITS OF THE DEFENSE OF PH. J Clin Invest. 1965 Feb;44:291–301. doi: 10.1172/JCI105143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer J., Berry M. N. Effect of alkalosis on glycolysis in the isolated rat heart. Am J Physiol. 1967 Nov;213(5):1143–1148. doi: 10.1152/ajplegacy.1967.213.5.1143. [DOI] [PubMed] [Google Scholar]
- Schwartz W. B., Kassirer J. P. Medical management of chronic renal failure. Am J Med. 1968 May;44(5):786–802. doi: 10.1016/0002-9343(68)90259-3. [DOI] [PubMed] [Google Scholar]
- Simpson D. P. Regulation of renal citrate metabolism by bicarbonate ion and pH: observations in tissue slices and mitochondria. J Clin Invest. 1967 Feb;46(2):225–238. doi: 10.1172/JCI105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOBIN R. B. Plasma, extracellular and muscle electrolyte responses to acute metabolic acidosis. Am J Physiol. 1956 Jul;186(1):131–138. doi: 10.1152/ajplegacy.1956.186.1.131. [DOI] [PubMed] [Google Scholar]
- Trivedi B., Danforth W. H. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966 Sep 10;241(17):4110–4112. [PubMed] [Google Scholar]
- UTTER M. F. The role of CO2 fixation in carbohydrate utilization and synthesis. Ann N Y Acad Sci. 1959 Feb 6;72(12):451–461. doi: 10.1111/j.1749-6632.1959.tb44173.x. [DOI] [PubMed] [Google Scholar]
- Ui M. A role of phosphofructokinase in pH-dependent regulation of glycolysis. Biochim Biophys Acta. 1966 Aug 24;124(2):310–322. doi: 10.1016/0304-4165(66)90194-2. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]



