Abstract
Background: In U.S. conventional poultry production, antimicrobials are used for therapeutic, prophylactic, and nontherapeutic purposes. Researchers have shown that this can select for antibiotic-resistant commensal and pathogenic bacteria on poultry farms and in poultry-derived products. However, no U.S. studies have investigated on-farm changes in resistance as conventional poultry farms transition to organic practices and cease using antibiotics.
Objective: We investigated the prevalence of antibiotic-resistant Enterococcus on U.S. conventional poultry farms that transitioned to organic practices.
Methods: Poultry litter, feed, and water samples were collected from 10 conventional and 10 newly organic poultry houses in 2008 and tested for Enterococcus. Enterococcus (n = 259) was identified using the Vitek® 2 Compact System and tested for susceptibility to 17 antimicrobials using the Sensititre™ microbroth dilution system. Data were analyzed using SAS software (version 9.2), and statistical associations were derived based on generalized linear mixed models.
Results: Litter, feed, and water samples were Enterococcus positive. The percentages of resistant Enterococcus faecalis and resistant Enterococcus faecium were significantly lower (p < 0.05) among isolates from newly organic versus conventional poultry houses for two (erythromycin and tylosin) and five (ciprofloxacin, gentamicin, nitrofurantoin, penicillin, and tetracycline) antimicrobials, respectively. Forty-two percent of E. faecalis isolates from conventional poultry houses were multidrug resistant (MDR; resistant to three or more antimicrobial classes), compared with 10% of isolates from newly organic poultry houses (p = 0.02); 84% of E. faecium isolates from conventional poultry houses were MDR, compared with 17% of isolates from newly organic poultry houses (p < 0.001).
Conclusions: Our findings suggest that the voluntary removal of antibiotics from large-scale U.S. poultry farms that transition to organic practices is associated with a lower prevalence of antibiotic-resistant and MDR Enterococcus.
Keywords: antibiotic resistance, antibiotic-resistant bacteria, antimicrobial growth promoter, Enterococcus, organic poultry
Antibiotic use in U.S. conventional poultry production poses potential public health concerns with regard to the selection of antibiotic-resistant foodborne bacteria (Institute of Medicine 1998; Levy and Marshall 2004; Silbergeld et al. 2008). In U.S. conventional poultry production, antibiotics are administered for therapeutic, prophylactic, and nontherapeutic purposes (Sapkota et al. 2007; Wegener 2003). Some researchers have estimated that use of antimicrobials in conventional U.S. poultry production (on a per bird basis) increased by 307% from 1985 to the late 1990s, with the use of nontherapeutic antimicrobial growth promoters (AGPs) accounting for a significant portion of this use (Mellon et al. 2001).
The use of AGPs in conventional poultry production selects for resistant bacterial populations in the production environment and retail poultry products (Aarestrup et al. 2000a; Hayes et al. 2001, 2005; Price et al. 2005; Witte 2000). Consequently, the amplification of resistant bacteria in poultry can result in possible increases in the risk of antibiotic-resistant bacterial infections in human populations (Aarestrup et al. 2000a; Hammerum et al. 2010; Levy 1998). Animal-derived antibiotic-resistant bacteria have been shown to spread from animals to humans through direct contact with animals and through the consumption of meat products (Donabedian et al. 2003; van Loo et al. 2007).
These findings are increasingly reported in mainstream news and have become one of the main drivers influencing consumer demand for organic poultry (Oberholtzer et al. 2006), which is perceived to be safer than conventional poultry (Crandall et al. 2009). This consumer demand has spurred increased production of organic poultry, making poultry one of the fastest growing segments of the U.S. organic products sector (Fanatico et al. 2009; Oberholtzer et al. 2006). Retail sales of organic poultry quadrupled between 2003 and 2006 and reached nearly $200 million in 2008 (Oberholtzer et al. 2006).
To accommodate increased consumer demand and to profit from the organic poultry niche, some conventional poultry growers are adopting organic practices and transitioning their conventional farms to certified organic poultry farms (Oberholtzer et al. 2006). These transitions—which include cessation in the use of all antibiotics and agrichemicals (Fanatico et al. 2009)—could result in changes in the prevalence of antibiotic-resistant bacteria on newly organic poultry farms and subsequent organic poultry products. European studies suggest that removing the nontherapeutic use of antibiotics from poultry farms can result in statistically significant reductions in antibiotic-resistant bacteria in animals and food products (Aarestrup et al. 2000b, 2001; Emborg et al. 2003; Hammerum et al. 2007; Heuer et al. 2002; Klare et al. 1999). Reductions in human carriage of resistant bacteria also have been documented in association with antibiotic withdrawals in European poultry production (Klare et al. 1999; van den Bogaard et al. 2000).
However, to date, the studies regarding this issue that have been conducted in the United States have been largely cross-sectional in nature (Han et al. 2009; Price et al. 2007). To the best of our knowledge, no prospective studies have been conducted in the United States to quantify on-farm, temporal changes in antibiotic resistance of foodborne bacteria when antibiotics are removed from U.S. poultry production environments. Voluntary transitions to organic practices among large-scale U.S. poultry producers provide an excellent opportunity to research this issue within the United States. Thus, the objective of this study was to prospectively evaluate the prevalence of antibiotic-resistant enterococci on large-scale conventional poultry farms that transitioned to organic practices. Here we describe the findings from the first year of this study.
Materials and Methods
Study sites. All of the poultry farms participating in this study were located in the Mid-Atlantic United States. Two types of poultry farms were included: large-scale conventional broiler farms that were maintaining conventional practices and using antibiotics (n = 5), and large-scale (previously conventional) broiler farms that had just received organic certification and were producing their first flock of certified organic broilers (n = 5). All participating farms were operating under the guidance of one feed mill that produced both conventional and certified organic poultry feed. Two individual poultry houses from each farm were included in the study, for a total of 20 poultry houses. Characteristics of the conventional and newly organic poultry houses are summarized in Table 1.
Table 1.
Characteristics of poultry houses at the time of sampling [mean (range)].
| Characteristic | Conventional (n = 10) | Organic (n = 10) | ||
|---|---|---|---|---|
| Months the farm practiced organic methods | 0 | 1.72 (0–3.6) | ||
| No. of antibiotics used in feed | 3 (2–4) | 0 | ||
| No. of antibiotics used in water | 0.18 (0–1) | 0 | ||
| Age of poultry house (years) | 15.7 (3–30) | 8.8 (3–15) | ||
| Length of poultry house (ft) | 407 (110–500) | 500 (500–500) | ||
| Width of poultry house (ft) | 44.5 (35–50) | 46.8 (44–48) | ||
| Months since complete poultry litter change | 1.2 (1–1.5) | 2.2 (1–3.6)a | ||
| No. of broiler chicks when flock arrived | 30,800 (30,800–30,800) | 22,608 (19,300–24,000) | ||
| Age of flock (days) | 36 (31–40) | 36 (29–41) | ||
| Cumulative mortality rate (%) | 2.51 (1.3–4.3) | 4.72 (3–7.5) | ||
| Minutes that broilers spent outdoors | 0 | 0b | ||
| aThe time since complete poultry litter change is greater than the time the farm practiced organic methods because the farms were not considered “organic” until the first birds to be produced under organic proctices arrived, and these first flocks did not always arrive immediately after the poultry litter cleanout. bThe organic birds did not go outside although they had the opportunity to do so. | ||||
All of the newly organic poultry houses were certified organic by a state agency accredited by the U.S. National Organic Program (NOP), which promulgates federal organic standards. An overview of common interpretations of the NOP standards that must be met before a poultry farm can be certified organic is provided in Appendix 1.
The specific antimicrobials that were used in feed in the conventional poultry houses were as follows: bacitracin (50 g/ton), virginiamycin (15 g/ton), roxarsone (45.35 g/ton), salinomycin (60 g/ton), nicarbazin (0.0125%), and decoquinate (27.2 g/ton). In addition, gentamicin (GEN) was used at the hatcheries that supplied chicks to conventional poultry houses, and bacitracin, virginiamycin, roxarsone, and salinomycin were used at the breeder facilities that supplied the initial eggs to the hatcheries.
Sample collection. From March to June 2008, poultry litter, water, and feed samples were aseptically collected [in sterile Whirl-Pak® collection bags (Nasco, Fort Atkinson, WI)] from the conventional and newly organic poultry houses. Litter samples (500 g) from the top 11–5 cm of litter were collected from three randomly selected areas of each poultry house. Two water samples (500 mL) were retrieved: a) one from raw source water before filtration or ultraviolet treatment, and b) one from finished water present in the waterlines. One poultry feed sample (300 g) was collected from the central feed hopper within each poultry house. All poultry litter, water, and feed samples were shipped overnight at 4°C and processed within 24 hr.
Isolation. Poultry litter and feed samples were enriched in a 1:10 weight-to-volume dilution of 100 mL Enterococcosel Broth (Becton Dickinson & Co., Franklin Lakes, NJ) for 24 hr at 41°C. We included positive and negative control broths for quality control and quality assurance. After 24 hr, 10 μL of the enrichment culture was streaked onto Enterococcosel Agar (EA; Becton Dickinson & Co.) and incubated overnight at 41°C. Presumptive colonies of Enterococcus spp.ranged in appearance from brown to black with a brown-black precipitate on EA. Three presumptive Enterococcus colonies from each litter and feed sample were streaked onto separate brain heart infusion (BHI) agar plates for purification and incubated at 41°C for 24 hr. A colony was collected from each BHI purification plate and archived at –80°C in Brucella broth with 20% glycerol.
Isolation of Enterococcus spp. from water samples was performed in accordance with U.S. Environmental Protection Agency (EPA) Method 1106.1 (U.S. EPA 2006). Briefly, 10-fold dilutions of each water sample were prepared in phosphate-buffered saline (U.S. EPA 2006), and 10 mL of each dilution was filtered through a 0.45-μm, 47-mm mixed cellulose ester filter (Millipore, Billerica, MA). Each filter was placed on a 60-mm plate containing EA, inverted, and incubated at 41°C for 24 hr. Resulting colonies typical of Enterococcus spp. were considered presumptive Enterococcus spp. Of the recovered presumptive Enterococcus spp., three isolates per water sample were purified on BHI and archived in Brucella broth with 20% glycerol at –80°C. Positive (Enterococcus faecalis ATCC 29212; ATCC, Manassas, VA) and negative (Escherichia coli ATCC 25922) controls were used throughout the isolation process.
Identification. All presumptive Enterococcus spp. were streaked from archived stocks onto tryptic soy agar amended with 5% sheep’s blood and incubated at 41°C for 24 hr. Presumptive identification of Enterococcus spp. was done by Gram staining and testing for catalase production and pyrrolidonyl arylamidase (PYR) activity. All gram-positive, catalase-negative, and PYR-positive isolates were confirmed and identified to the species level using the automated biochemical identification Vitek®2 Compact System (BioMérieux Inc., Hazelwood, MO) in accordance with the manufacturer’s specifications.
Antimicrobial susceptibility testing. We performed antimicrobial susceptibility testing (AST) on all confirmed Enterococcus isolates (n = 259) by microbroth dilution using the Sensititre™ system (Trek Diagnostic Systems, Westlake, OH) according to the manufacturer’s directions. Briefly, colonies from pure 18- to 24-hr cultures were transferred to tubes of sterile Sensititre demineralized water (Trek Diagnostic Systems) to achieve a turbidity equivalent to a 0.5 McFarland standard. Then, 50 μL of each suspension was added to sterile Sensititre cation-adjusted Mueller Hinton broth (Trek Diagnostic Systems), and 50 μL of the broth solution was then dispensed into microtiter, gram-positive 96-well plates embedded with 17 test antimicrobials [National Antimicrobial Resistance Monitoring System (NARMS) Enterococcus Plate Format; Trek Diagnostic Systems]. Plates were then incubated in the Automated Reading and Incubation System (ARIS; TREK Diagnostic Systems) at 37°C for 18 ± 1 hr. The first 100 plates were read both manually and via the ARIS system for quality assurance comparisons of minimal inhibitory concentration (MIC) determinations. After consistency between the two methods was determined, subsequent samples were read by the ARIS exclusively.
We used Clinical and Laboratory Standards Institute (CLSI) interpretive criteria for microbroth dilution methods (CLSI 2008) to evaluate resulting MICs where breakpoints were available, except for quinupristin/dalfopristin (SYN), for which we used the breakpoint from the European Committee on Antimicrobial Susceptibility Testing (EUCAST 2011). Otherwise, we used the provisional breakpoints used by NARMS (Food and Drug Administration 2009). The following specific antimicrobials (resistance breakpoints) were used: chloramphenicol (CHL; ≥ 32), ciprofloxacin (CIP; ≥ 4), daptomycin (DAP; no interpretive criteria available), erythromycin (ERY; ≥ 8), flavomycin (FLA; ≥ 32), gentamicin (GEN; > 500), kanamycin (KAN; ≥ 1,024), lincomycin (LIN; ≥ 8), linezolid (LZE; ≥ 8), nitrofurantoin (NIT; ≥ 128), penicillin (PEN; ≥ 16), streptomycin (STR; > 1,000), quinupristin/dalfopristin (SYN; ≥ 8), tetracycline (TET; ≥ 16), tigecycline (TIG; no interpretive criteria available), tylosin (TYL; ≥ 32), and vancomycin (VAN; ≥ 32). Multidrug resistance (MDR) was defined as acquired resistance to three or more antimicrobial classes. Enterococcus faecalis ATCC 29212, E. faecalis ATCC 51299, Staphylococcus aureus ATCC 29213, and Pseudomonas aeruginosa ATCC 27853 were used as quality control strains.
Statistical analysis. We used the generalized linear mixed model (GLMM) method to evaluate associations between the prevalence of antibiotic-resistant Enterococcus spp. and poultry production type (conventional or newly organic). The GLMM method was used to account for the clustered nature of the study design, which made it necessary to adjust for intra-poultry house and intra-poultry farm variability. Firth’s bias correction method was used when zero counts occurred for one group (Heinze and Puhr 2010). All statistical analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC).
Results
Prevalence of Enterococcus spp. Enterococcus spp. were isolated from 100% of all conventional and newly organic poultry houses. Poultry litter was the principal environmental media for the recovery of Enterococcus spp. from both farm types, with 100% of all litter samples testing positive; however, these microorganisms were also recovered from water and feed samples (Table 2).
Table 2.
Enterococcus spp. isolated from litter, feed, and water samples collected from conventional and newly organic poultry farms.
| Total Enterococcus isolates [n (%)] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farm type | E. durans | E. faecalis | E. faecium | E. gallinarum | E. hirae | Other | ||||||
| Conventional | ||||||||||||
| Litter (n = 90) | 1 (< 1) | 45 (34) | 42 (32) | 1 (< 1) | 1 (< 1) | 0 (0) | ||||||
| Feed (n = 29) | 0 (0) | 10 (7) | 15 (11) | 1 (< 1) | 3 (2) | 0 (0) | ||||||
| Source water (n = 1) | 1 (< 1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||
| Waterlines (n = 13) | 0 (0) | 0 (0) | 12 (9) | 0 (0) | 1 (< 1) | 0 (0) | ||||||
| Total conventional (n = 133) | 2 (1) | 55 (41) | 69 (52) | 2 (1) | 5 (4) | 0 (0) | ||||||
| Organic | ||||||||||||
| Litter (n = 95) | 6 (5) | 63 (50) | 18 (14) | 4 (3) | 3 (2) | 1 (< 1)a | ||||||
| Feed (n = 27) | 1 (< 1) | 0 (0) | 22 (17) | 0 (0) | 4 (3) | 0 (0) | ||||||
| Source water (n = 1) | 0 (0) | 0 (0) | 1 (< 1) | 0 (0) | 0 (0) | 0 (0) | ||||||
| Waterlines (n = 3) | 0 (0) | 0 (0) | 1 (< 1) | 1 (< 1) | 0 (0) | 1 (< 1)b | ||||||
| Total organic (n = 126) | 7 (6) | 63 (50) | 42 (33) | 5 (4) | 7 (6) | 2 (2) | ||||||
| aLow-discrimination E. gallinarum/faecium. bLow-discrimination E. durans/hirae. | ||||||||||||
Overall, 46% of Enterococcus spp. were identified as E. faecalis and 43% as Enterococcus faecium. Enterococcus durans, Enterococcus gallinarum, and Enterococcus hirae were also isolated from both types of poultry houses in several types of environmental media (Table 2). We found no significant differences in species prevalence between farm types.
MICs. MIC ranges, MIC50s (MIC for 50% of the bacteria are less than or equal to this MIC) and MIC90s (MIC for 90% of the bacteria are less than or equal to this MIC) for E. faecalis and E. faecium recovered from the poultry houses are shown in Table 3. For E. faecalis, 53% of MIC ranges (8 of 15 antibiotics, excluding LIN and SYN because of inherent resistance) differed depending on farm type; for E. faecium, 56% of MIC ranges (9 of 16 antibiotics, excluding FLA because of inherent resistance) differed depending on farm type (Table 3). Some MIC ranges differed depending on species (Table 3). Similarly, we also observed some differences in MIC50s and MIC90s between isolates recovered from different farm types, and between species (Table 3).
Table 3.
MIC range, MIC50, and MIC90 (µg/mL) for 17 antibiotics determined for E. faecalis and E. faecium recovered from conventional (CONV) and newly organic (ORG) poultry farms.
| E. faecalis | E. faecium | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | Farm type | MIC range | MIC50 | MIC90 | MIC range | MIC50 | MIC90 | |||||||
| CHL | CONV | 4 to ≥ 64 | 8 | 8 | 4 to 16 | 8 | 8 | |||||||
| ORG | 4 to 16 | 8 | 8 | ≤ 2 to 8 | 8 | 8 | ||||||||
| CIP | CONV | 0.5 to 4 | 1 | 2 | 0.25 to ≥ 8 | 4 | 4 | |||||||
| ORG | 1 to ≥ 8 | 1 | 2 | 0.5 to ≥ 8 | 2 | 4 | ||||||||
| DAP | CONV | ≤ 0.5 to 4 | 1 | 1 | ≤ 0.5 to 4 | 2 | 4 | |||||||
| ORG | ≤ 0.5 to 4 | 1 | 2 | ≤ 0.5 to 4 | 2 | 4 | ||||||||
| ERY | CONV | ≤ 0.5 to ≥ 16 | ≥ 16 | ≥ 16 | ≤ 0.5 to ≥ 16 | 1 | 8 | |||||||
| ORG | ≤ 0.5 to ≥ 16 | 1 | ≥ 16 | ≤ 0.5 to ≥ 16 | 2 | 4 | ||||||||
| FLAa | CONV | ≤ 1 to ≥ 32 | 2 | 8 | 2 to ≥ 32 | ≥ 32 | ≥ 32 | |||||||
| ORG | ≤ 1 to ≥ 32 | ≤ 1 | 2 | 2 to ≥ 32 | ≥ 32 | ≥ 32 | ||||||||
| GEN | CONV | ≤ 128 to ≥ 2,048 | ≤ 128 | ≤ 128 | ≤ 128 to ≥ 2,048 | ≤ 128 | 1,024 | |||||||
| ORG | ≤ 128 | ≤ 128 | ≤ 128 | ≤ 128 | ≤ 128 | ≤ 128 | ||||||||
| KAN | CONV | ≤ 128 to ≥ 2,048 | ≤ 128 | ≥ 2,048 | ≤ 128 to ≥ 2,048 | 256 | ≥ 2,048 | |||||||
| ORG | ≤ 128 to ≥ 2,048 | ≤ 128 | ≤ 128 | ≤ 128 to ≥ 2,048 | 256 | 256 | ||||||||
| LINb | CONV | 16 to ≥ 64 | ≥ 64 | ≥ 64 | ≤ 1 to ≥ 64 | ≥ 64 | ≥ 64 | |||||||
| ORG | 16 to ≥ 64 | ≥ 64 | ≥ 64 | ≤ 1 to ≥ 64 | 16 | ≥ 64 | ||||||||
| LZE | CONV | ≤ 0.5 to 2 | 1 | 2 | 1 to 4 | 2 | 2 | |||||||
| ORG | 1 to 4 | 2 | 4 | 1 to 4 | 2 | 4 | ||||||||
| NIT | CONV | 8 to ≥ 128 | 8 | 64 | 32 to ≥ 128 | ≥ 128 | ≥ 128 | |||||||
| ORG | 8 to ≥ 128 | 16 | 64 | 16 to ≥ 128 | 64 | ≥ 128 | ||||||||
| PEN | CONV | 2 to ≥ 32 | 4 | 8 | ≤ 0.5 to ≥ 32 | 16 | ≥ 32 | |||||||
| ORG | 2 to 8 | 4 | 8 | ≤ 0.5 to 16 | 4 | 8 | ||||||||
| STR | CONV | ≤ 512 to > 2,048 | ≤ 512 | 2,048 | ≤ 512 to > 2,048 | ≤ 512 | ≤ 512 | |||||||
| ORG | ≤ 512 to > 2,048 | ≤ 512 | 1,024 | ≤ 512 to > 2,048 | ≤ 512 | ≤ 512 | ||||||||
| SYNb | CONV | 2 to 32 | 4 | 16 | ≤ 1 to 32 | 4 | 8 | |||||||
| ORG | 2 to 8 | 8 | 8 | ≤ 1 to 16 | 2 | 4 | ||||||||
| TET | CONV | ≤ 4 to ≥ 64 | ≥ 64 | ≥ 64 | ≤ 4 to ≥ 64 | ≥ 64 | ≥ 64 | |||||||
| ORG | 32 to ≥ 64 | ≥ 64 | ≥ 64 | ≤ 4 to ≥ 64 | ≤ 4 | 32 | ||||||||
| TIG | CONV | 0.03 to 0.5 | 0.12 | 0.25 | 0.06 to 0.5 | 0.12 | 0.25 | |||||||
| ORG | 0.06 to 0.25 | 0.25 | 0.25 | 0.03 to 0.25 | 0.12 | 0.25 | ||||||||
| TYL | CONV | 1 to ≥ 64 | ≥ 64 | ≥ 64 | 1 to ≥ 64 | 4 | ≥ 64 | |||||||
| ORG | 1 to ≥ 64 | 4 | ≥ 64 | 2 to 16 | 4 | 8 | ||||||||
| VAN | CONV | ≤ 0.5 to 4 | 1 | 2 | ≤ 0.5 to 2 | ≤ 0.5 | 1 | |||||||
| ORG | 1 to 4 | 1 | 2 | ≤ 0.5 to 4 | 1 | 2 | ||||||||
| aE. faecium is intrinsically resistant to FLA. bE. faecalis is intrinsically resistant to LIN and streptogramin A (dalfopristin). | ||||||||||||||
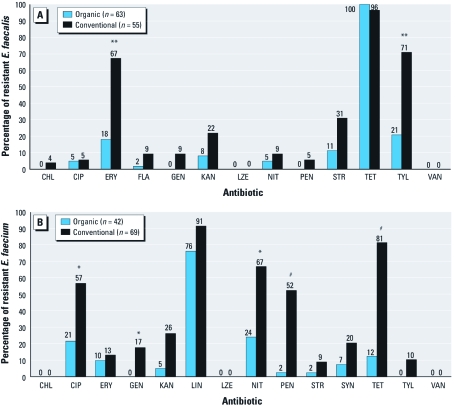
Acquired antibiotic resistance. Among E. faecalis isolates, acquired antibiotic resistance against nine antimicrobials (CHL, ERY, FLA, GEN, KAN, NIT, PEN, STR, and TYL) was lower among E. faecalis from newly organic poultry houses compared with conventional poultry houses (Figure 1A). The differences in percent resistance were statistically significant for ERY (p = 0.004) and TYL (p = 0.004) (Figure 1A).
Figure 1.
Percentage of E. faecalis (A) and E. faecium (B) from conventional and newly organic poultry houses expressing acquired resistance to a particular antibiotic. E. faecalis is intrinsically resistant to LIN and streptogramin A (dalfopristin) (Dina et al. 2003); E. faecium is intrinsically resistant to FLA. *p < 0.05, **p < 0.01, and #p < 0.001, compared with organic poultry houses.
Among E. faecalis, we observed acquired resistance to CHL, GEN, and PEN only among isolates recovered from conventional poultry houses (Figure 1A). GEN is one of the antibiotics used at the hatcheries that supplied chicks to the conventional poultry houses. We observed no resistance to LZE or VAN among any of the E. faecalis recovered from conventional or newly organic poultry houses (Figure 1A). The absence of VAN resistance is most likely attributed to the fact that glycopeptides have never been approved for use in U.S. animal agriculture.
Among E. faecium isolates, acquired antibiotic resistance against 11 antimicrobials (CIP, ERY, GEN, KAN, LIN, NIT, PEN, STR, SYN, TET, and TYL) was lower among E. faecium from newly organic poultry houses compared with conventional poultry houses (Figure 1B). The differences in percent resistance were statistically significant for CIP (p = 0.01), GEN (p = 0.047), NIT (p = 0.02), PEN (p < 0.001), and TET (p < 0.001) (Figure 1B).
Among E. faecium, we observed acquired resistance to GEN and TYL only among isolates recovered from conventional poultry houses (Figure 1B). We observed no resistance to CHL, LZE, or VAN among any of the E. faecium recovered from conventional or organic poultry houses (Figure 1B).
Sources of antibiotic-resistant bacteria. Most antibiotic-resistant E. faecalis were isolated from poultry litter samples (Table 4). E. faecalis isolated from conventional feed samples also expressed acquired resistance to eight antimicrobials (CHL, ERY, FLA, KAN, NIT, STR, TET, and TYL), indicating that conventional poultry feed could be a potential source of exposure to antibiotic-resistant E. faecalis among broilers (Table 4). No resistant E. faecalis were isolated from organic poultry feed or source water or waterline samples retrieved from either conventional or newly organic poultry houses.
Table 4.
Antibiotic-resistant E. faecalis and E. faecium isolated from different environmental sample types recovered from conventional (CONV) and newly organic (ORG) poultry farms.
| Resistant E. faecalis [n (%)] | Resistant E. faecium [n (%)] | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | Farm type | Litter | Feed | Source water | Water lines | Litter | Feed | Source water | Water lines | |||||||||
| CHL | CONV | 0 (0) | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||||||
| ORG | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| CIP | CONV | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 26 (62) | 8 (53) | 0 (0) | 5 (42) | |||||||||
| ORG | 3 (5) | 0 (0) | 0 (0) | 0 (0) | 9 (41) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| ERY | CONV | 27 (60) | 10 (100) | 0 (0) | 0 (0) | 4 (10) | 4 (27) | 0 (0) | 1 (0) | |||||||||
| ORG | 11 (17) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 3 (14) | 0 (0) | 0 (0) | ||||||||||
| FLAa | CONV | 4 (9) | 1 (10) | 0 (0) | 0 (0) | 21 (50) | 14 (93) | 0 (0) | 8 (67) | |||||||||
| ORG | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 14 (78) | 22 (100) | 0 (0) | 0 (0) | ||||||||||
| GEN | CONV | 5 (11) | 0 (0) | 0 (0) | 0 (0) | 9 (21) | 0 (0) | 0 (0) | 3 (25) | |||||||||
| ORG | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| KAN | CONV | 11 (24) | 1 (10) | 0 (0) | 0 (0) | 14 (33) | 1 (7) | 0 (0) | 3 (25) | |||||||||
| ORG | 5 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (9) | 0 (0) | 0 (0) | ||||||||||
| LINb | CONV | 45 (100) | 10 (100) | 0 (0) | 0 (0) | 40 (95) | 15 (100) | 0 (0) | 8 (67) | |||||||||
| ORG | 63 (100) | 0 (0) | 0 (0) | 0 (0) | 9 (50) | 22 (100) | 1 (100) | 0 (0) | ||||||||||
| NIT | CONV | 3 (7) | 2 (20) | 0 (0) | 0 (0) | 31 (74) | 7 (47) | 0 (0) | 8 (67) | |||||||||
| ORG | 3 (5) | 0 (0) | 0 (0) | 0 (0) | 7 (39) | 3 (14) | 0 (0) | 0 (0) | ||||||||||
| PEN | CONV | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 22 (52) | 5 (33) | 0 (0) | 9 (75) | |||||||||
| ORG | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| STR | CONV | 16 (36) | 1 (10) | 0 (0) | 0 (0) | 4 (10) | 1 (7) | 0 (0) | 1 (8) | |||||||||
| ORG | 7 (11) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| SYNb | CONV | 7 (16) | 10 (100) | 0 (0) | 0 (0) | 8 (19) | 5 (33) | 0 (0) | 1 (8) | |||||||||
| ORG | 38 (60) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 2 (9) | 0 (0) | 0 (0) | ||||||||||
| TET | CONV | 43 (96) | 10 (100) | 0 (0) | 0 (0) | 36 (86) | 11 (73) | 0 (0) | 9 (75) | |||||||||
| ORG | 63 (100) | 0 (0) | 0 (0) | 0 (0) | 4 (22) | 1 (5) | 0 (0) | 0 (0) | ||||||||||
| TYL | CONV | 29 (64) | 10 (100) | 0 (0) | 0 (0) | 3 (7) | 3 (20) | 0 (0) | 1 (8) | |||||||||
| ORG | 13 (21) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||||||||
| aE. faecium is intrinsically resistant to FLA. bE. faecalis is intrinsically resistant to LIN and streptogramin A (dalfopristin). | ||||||||||||||||||
Most antibiotic-resistant E. faecium also were isolated from poultry litter samples (Table 4). Antibiotic-resistant E. faecium were also recovered from feed and waterline samples from conventional poultry houses and from feed, source water, and waterline samples from newly organic poultry houses (Table 4). Conventional feed was contaminated with E. faecium that expressed acquired resistance to 10 antimicrobials (CIP, ERY, KAN, LIN, NIT, PEN, STR, SYN, TET, and TYL), whereas organic feed was contaminated with E. faecium that expressed acquired resistance to 6 antimicrobials (ERY, KAN, LIN, NIT, SYN, and TET) (Table 4). No conventional source water samples were contaminated with resistant E. faecium, whereas one organic source water sample was contaminated with one LIN-resistant E. faecium isolate. Conventional waterline samples were contaminated with E. faecium that expressed acquired resistance to 11 antimicrobials (CIP, ERY, GEN, KAN, LIN, NIT, PEN, STR, SYN, TET, and TYL), whereas organic waterline samples were not contaminated with antibiotic-resistant E. faecium (Table 4). The differences in waterline contamination between poultry house types could be attributed to the fact that conventional poultry houses, in general, were older than newly organic poultry houses (Table 1), allowing more time for contamination to occur.
Acquired MDR. The percentage of MDR E. faecalis was statistically significantly lower among isolates from newly organic poultry houses compared with isolates from conventional poultry houses (10% vs. 42%; p = 0.02; Figure 2). The percentage of MDR E. faecium also was statistically significantly lower among isolates from newly organic poultry houses compared with isolates from conventional poultry houses (17% vs. 84%; p < 0.001; Figure 2). Predominant MDR patterns are shown in Table 5.
Figure 2.
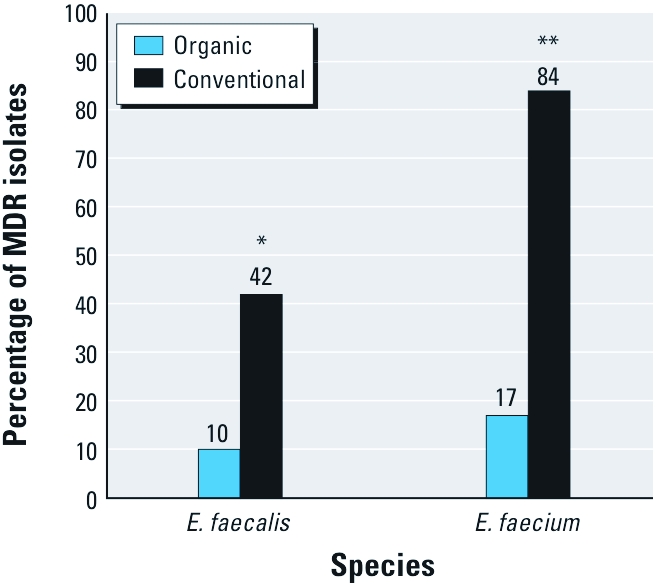
Percentages of MDR E. faecalis and E. faecium recovered from conventional and newly organic poultry houses *p = 0.02, and **p < 0.001.
Table 5.
Predominant (in > 2 isolates) acquired MDR patterns among E. faecalis and E. faecium isolated from conventional (CONV) and newly organic (ORG) poultry farms.a
| Species/farm type | MDR pattern | n (%) | ||
|---|---|---|---|---|
| E. faecalis | ||||
| CONV | ERY-GEN-KAN-STR-TET-TYL | 3 (5) | ||
| ERY-KAN-STR-TET-TYL | 4 (7) | |||
| ORG | ERY-KAN-STR-TET-TYL | 5 (8) | ||
| E. faecium | ||||
| CONV | CIP-GEN-KAN-LIN-TET-NIT | 4 (6) | ||
| CIP-LIN-PEN-TET-NIT | 10 (14) | |||
| CIP-LIN-TET-NIT | 5 (7) | |||
| LIN-PEN-TET-NIT | 3 (4) | |||
| LIN-SYN-TET-NIT | 5 (7) | |||
| ORG | CIP-LIN-NIT | 3 (7) | ||
| aE. faecalis is intrinsically resistant to LIN and streptogramin A (dalfopristin) (Dina et al. 2003), and E. faecium is intrinsically resistant to FLA; therefore, these species/drug combinations were excluded from the MDR analysis. | ||||
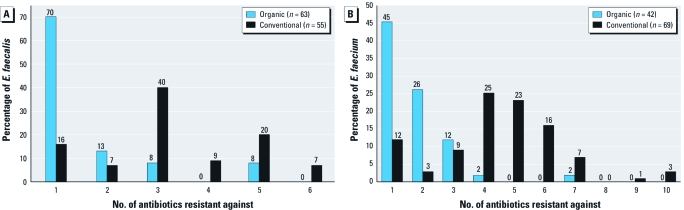
The mode number of antibiotics that E. faecalis expressed acquired resistance against was one and three, among isolates from newly organic houses and conventional houses, respectively (Figure 3A). The mode number of antibiotics that E. faecium expressed acquired resistance against was one and four, among isolates from newly organic houses and conventional houses, respectively (Figure 3B). These findings show that newly organic poultry houses are characterized by individual E. faecalis and E. faecium isolates that express resistance to fewer numbers of antibiotics compared with their conventional counterparts.
Figure 3.
Percentages of E. faecalis (A) and E. faecium (B) from conventional and newly organic poultry houses expressing acquired resistance to varying numbers of antibiotics.
Discussion
In this study, we observed a significantly lower prevalence of antibiotic-resistant and MDR E. faecalis and E. faecium on large-scale poultry farms that had just transitioned to organic practices compared with large-scale poultry farms that were maintaining conventional practices. To our knowledge, these are the first U.S. data to show immediate, on-farm changes in antibiotic resistance when antimicrobials are voluntarily withdrawn from large-scale U.S. poultry production.
These findings are in agreement with earlier European and Asian studies that have documented reductions in antibiotic-resistant Enterococcus spp. after governmental bans and/or voluntary withdrawals of the use of antibiotics in animal production (Aarestrup et al. 2001; Lauderdale et al. 2007). Using data from the Danish program for surveillance of antimicrobial resistance in bacteria recovered from animals, foods, and humans (Danish Integrated Antimicrobial Resistance Monitoring and Research Programme; DANMAP), Aarestrup et al. (2001) reported significant decreases in the percentages of E. faecalis and E. faecium resistant to avilamycin, ERY, avoparcin, and virginiamycin, four antibiotics banned by the Danish government for use as AGPs in the late 1990s. For example, from 1997 to 2000 the percentage of ERY-resistant E. faecium isolated from broilers decreased from 76.3% to 12.7%, and the percentage of virginiamycin-resistant E. faecium isolated from broilers decreased from 66.2% to 33.9% (Aarestrup et al. 2001). In the present study, we observed that the prevalence of E. faecium resistant to ERY was 13% and 10% among isolates from conventional and newly organic farms, respectively, whereas the prevalence of E. faecium resistant to SYN (a virginiamycin analogue) was 20% and 7% among isolates from conventional and newly organic farms, respectively (Figure 1B).
Reductions in percent resistance to ERY and other antibiotics observed among Enterococcus spp. from newly organic poultry farms in the present study may not be as dramatic as those observed by Aarestrup et al. (2001) and other European researchers because poultry houses in the present study were sampled during the production of the first flock of certified organic broilers. Although these poultry houses underwent extensive and comprehensive cleaning events before they could be certified as organic, reservoirs of resistant bacteria may have remained in the packed dirt floor and on fomites within the poultry houses, contributing to persistent low levels of antibiotic-resistant enterococci in newly organic poultry houses. Similarly, Heuer et al. (2002) demonstrated that VAN-resistant enterococci can persist in broiler flocks for > 5 years after antibiotic-selective pressures are removed from the production environment.
Two additional factors likely play significant roles in the persistence of low rates of antibiotic-resistant enterococci observed in newly organic poultry houses in this study. First, U.S. organic certification standards, promulgated through the NOP, apply starting on day 1 of a chick’s life (Fanatico et al. 2009). No organic certification standards need to be met before the first day of life. Thus, some breeder facilities that supply eggs to hatcheries, and hatcheries that ultimately produce “organic” chicks, do not have to meet any organic standards and can therefore use antibiotics among breeder stocks and inject antibiotics into eggs. These practices can result in exposures to antibiotics among “organic” broilers before the first day of life.
Second, organic broilers can be exposed to antibiotic-resistant bacteria through feed and water. Organic poultry feed is required by the NOP to be free of antibiotics, slaughter by-products, and genetically modified organisms (Fanatico et al. 2009). However, our data show that contamination of organic feed with antibiotic-resistant bacteria can occur (Table 4). The question remains as to whether feed is contaminated at the feed mill, during transport, and/or during storage at poultry houses via bioaerosols, insects, rodents, or other factors. Beyond feed, we observed that one source water sample from newly organic poultry houses was contaminated with one LIN-resistant E. faecium (Table 4) and one waterline sample from organic poultry houses was contaminated with one MDR E. gallinarum (data not shown).
We find it encouraging that the percentages of MDR E. faecalis and MDR E. faecium in the present study were significantly lower on newly organic poultry farms compared with farms that were maintaining conventional practices. E. faecalis recovered from newly organic and conventional farms expressed acquired resistance against a mode number of one and three antibiotics, respectively, and E. faecium from newly organic and conventional farms were resistant against a mode number of one and four antibiotics, respectively. These data are in agreement with a recent study by Miranda et al. (2007) that showed that rates of MDR Enterococcus spp. were significantly lower among isolates recovered from organic chicken and turkey products compared with conventional products.
As with all field-based studies, the present study had several limitations. As discussed above, we could not control for the fact that organic broilers may have been exposed to antibiotics before the first day of life. This could have influenced the rates of antibiotic resistance observed among Enterococcus spp. recovered from newly organic poultry houses; however, because we could not include a control farm that produced chicks that were known to have never been exposed to antibiotics, we could not estimate the contributions of these potential exposures to observed resistance rates. The study is also limited in terms of geographical location. All poultry farms included in this study are located in the Mid-Atlantic United States and under the advisement of one feed mill. Thus, it is unclear whether our results are generalizable across the United States and across the various large-scale contract growers that dominate the U.S. poultry industry. Larger-scale studies based in varying geographical areas at farms managed by different companies are necessary. Finally, this study is limited by the fact that separate conventional poultry farms served as control farms for the newly organic poultry farms. Although it would have been preferable to also sample the newly organic poultry farms before their conversion from conventional to organic practices, this was not possible.
Conclusions
This study provides the first on-farm U.S. data describing the impacts of eliminating antibiotics from large-scale U.S. poultry production on rates of antibiotic-resistant enterococci. The findings support the hypothesis that removing antibiotic use from large-scale U.S. poultry farms transitioning to organic practices can result in immediate and statistically significant reductions in on-farm antibiotic resistance.
Correction
In Table 1 of the manuscript originally published online, the value for months since complete poultry litter change was incorrect for organic broilers. It has been corrected here.
Acknowledgments
We thank the poultry growers for providing access to their farms, and D. Burns and T. Cravener for sampling the poultry houses. We thank A. Kim, N. Wang, Z. Chang, and J. Wang for performing laboratory and statistical analyses.
Appendix 1. Overview of Common Interpretations of U.S. NOP Standards for Broiler Production (Fanatico et al. 2009; U.S. Department of Agriculture 2010).
Producer must create and implement an organic system plan and manure management plan.
Broilers must be produced under continuous organic management starting “no later than the second day of life.
All feed components must be organically produced and contain no antibiotics, other animal drugs, slaughter by-products, or genetically modified organisms.
No antibiotics may be used for animal treatment.
Producer must establish preventative broiler health care practices, and diseases can be prevented with vaccines, biosecurity measures, prebiotics, and probiotics.
Maximum stocking densities of broilers is not specified by the NOP, but certifying agencies often require at least 0.14 m2 per bird.
An outdoor access area must be provided to ensure access to fresh air, exercise, and sunlight.
Clean and dry bedding must be provided in an indoor area.
Sanitizers and cleaners used on the property must be on approved products lists.
Agrichemicals cannot be used on the property
Footnotes
This research was funded by the Center for a Livable Future, Johns Hopkins Bloomberg School of Public Health.
The authors declare they have no actual or potential competing financial interests.
References
- Aarestrup FM, Agerso Y, Gerner-Smidt P, Madsen M, Jensen LB. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn Microbiol Infect Dis. 2000a;37:127–137. doi: 10.1016/s0732-8893(00)00130-9. [DOI] [PubMed] [Google Scholar]
- Aarestrup FM, Bager F, Andersen JS. Association between the use of avilamycin for growth promotion and the occurrence of resistance among Enterococcus faecium from broilers: epidemiological study and changes over time. Microb Drug Resist. 2000b;6:71–75. doi: 10.1089/mdr.2000.6.71. [DOI] [PubMed] [Google Scholar]
- Aarestrup FM, Seyfarth AM, Emborg HD, Pedersen K, Hendriksen RS, Bager F. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob Agents Chemother. 2001;45:2054–2059. doi: 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard—Third Edition. M31-A3; Vol. 28 No. 8. [Google Scholar]
- Crandall PG, Seideman S, Ricke SC, O’Bryan CA, Fanatico AF, Rainey R. Organic poultry: consumer perceptions, opportunities, and regulatory issues. J Appl Poult Res. 2009;18:795–802. [Google Scholar]
- Dina J, Malbruny B, Leclercq R. Nonsense mutations in the lsa-like gene in Enterococcus faecalis isolates susceptible to lincosamides and streptogramins A. Antimicrob Agents Chemother. 2003;47:2307–2309. doi: 10.1128/AAC.47.7.2307-2309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian SM, Thal LA, Hershberger E, Perri MB, Chow JW, Bartlett P, et al. Molecular characterization of gentamicin-resistant enterococci in the United States: evidence of spread from animals to humans through food. J Clin Microbiol. 2003;41:1109–1113. doi: 10.1128/JCM.41.3.1109-1113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg HD, Andersen JS, Seyfarth AM, Andersen SR, Boel J, Wegener HC. Relations between the occurrence of resistance to antimicrobial growth promoters among Enterococcus faecium isolated from broilers and broiler meat. Int J Food Microbiol. 2003;84:273–284. doi: 10.1016/s0168-1605(02)00426-9. [DOI] [PubMed] [Google Scholar]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing) Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 1.3, January 5, 2011. 2011. Available: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v1.3_pdf.pdf [accessed 27 April 2011]
- Fanatico AF, Owens CM, Emmert JL. Organic poultry production in the United States: Broilers. J Appl Poult Res. 2009;18:355–366. [Google Scholar]
- Food and Drug Administration. NARMS Retail Meat Annual Report, 2007. Rockville, MD:Food and Drug Administration. 2009. Available: http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm164662.htm [accessed 28 September 2011]
- Hammerum AM, Heuer OE, Emborg H, Baggesen DL, Bagger-Skjot L, Jensen VF, et al. Danish integrated antimicrobial resistance monitoring and research program. Emerg Infect Dis. 2007;13:1632–1639. doi: 10.3201/eid1311.070421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerum AM, Lester CH, Heuer OE. Antimicrobial-resistant enterococci in animals and meat: a human health hazard? Foodborne Pathog Dis. 2010;7:1137–1146. doi: 10.1089/fpd.2010.0552. [DOI] [PubMed] [Google Scholar]
- Han F, Lestari SI, Pu S, Ge B. Prevalence and antimicrobial resistance among Campylobacter spp. in Louisiana retail chickens after the enrofloxacin ban. Foodborne Pathog Dis. 2009;6:163–171. doi: 10.1089/fpd.2008.0171. [DOI] [PubMed] [Google Scholar]
- Hayes JR, McIntosh AC, Qaiyumi S, Johnson JA, English LL, Carr LE, et al. High-frequency recovery of quinupristin-dalfopristin-resistant Enterococcus faecium isolates from the poultry production environment. J Clin Microbiol. 2001;39:2298–2299. doi: 10.1128/JCM.39.6.2298-2299.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JR, Wagner DD, English LL, Carr LE, Joseph SW. Distribution of streptogramin resistance determinants among Enterococcus faecium from a poultry production environment of the USA. J Antimicrob Chemother. 2005;55:123–126. doi: 10.1093/jac/dkh491. [DOI] [PubMed] [Google Scholar]
- Heinze G, Puhr R. Bias-reduced and separation-proof conditional logistic regression with small or sparse data sets. Stat Med. 2010;29:770–777. doi: 10.1002/sim.3794. [DOI] [PubMed] [Google Scholar]
- Heuer OE, Pedersen K, Andersen JS, Madsen M. Vancomycin-resistant enterococci (VRE) in broiler flocks 5 years after the avoparcin ban. Microb Drug Resist. 2002;8:133–138. doi: 10.1089/107662902760190680. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Washington, DC: National Academy Press; 1998. Antimicrobial Resistance: Issues and Options. Workshop Report, Forum on Emerging Infections. [PubMed] [Google Scholar]
- Klare I, Badstubner D, Konstabel C, Bohme G, Claus H, Witte W. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb Drug Resist. 1999;5:45–52. doi: 10.1089/mdr.1999.5.45. [DOI] [PubMed] [Google Scholar]
- Lauderdale TL, Shiau YR, Wang HY, Lai JF, Huang IW, Chen PC, et al. Effect of banning vancomycin analogue avoparcin on vancomycin-resistant enterococci in chicken farms in Taiwan. Environ Microbiol. 2007;9:819–823. doi: 10.1111/j.1462-2920.2006.01189.x. [DOI] [PubMed] [Google Scholar]
- Levy SB. Multidrug resistance—a sign of the times. N Engl J Med. 1998;338:1376–1378. doi: 10.1056/NEJM199805073381909. [DOI] [PubMed] [Google Scholar]
- Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- Mellon M, Benbrook C, Benbrook KL. Cambridge, MA: Union of Concerned Scientists; 2001. Hogging It: Estimates of Antimicrobial Abuse in Livestock. [Google Scholar]
- Miranda JM, Guarddon M, Mondragon A, Vazquez BI, Fente CA, Cepeda A, et al. Antimicrobial resistance in Enterococcus spp. strains isolated from organic chicken, conventional chicken, and turkey meat: a comparative survey. J Food Prot. 2007;70:1021–1024. doi: 10.4315/0362-028x-70.4.1021. [DOI] [PubMed] [Google Scholar]
- Oberholtzer L, Greene C, Lopez E. Organic Poultry and Eggs Capture High Price Premiums and Growing Share of Specialty Markets. USDA Outlook Report from the Economic Research Service. LDP-M-150-01. 2006. Available: http://www.ers.usda.gov/Publications/LDP/2006/12Dec/LDPM15001/ldpm15001.pdf [accessed 23 November 2010]
- Price LB, Johnson E, Vailes R, Silbergeld E. Fluoroquinolone-resistant Campylobacter isolates from conventional and antibiotic-free chicken products. Environ Health Perspect. 2005;113:557–560. doi: 10.1289/ehp.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LB, Lackey LG, Vailes R, Silbergeld E. The persistence of fluoroquinolone-resistant Campylobacter in poultry production. Environ Health Perspect. 2007;115:1035–1039. doi: 10.1289/ehp.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota AR, Lefferts LY, McKenzie S, Walker P. What do we feed to food-production animals? A review of animal feed ingredients and their potential impacts on human health. Environ Health Perspect. 2007;115:663–670. doi: 10.1289/ehp.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. National Organic Program, Accreditation and Certification. 2010. Available: http://www.ams.usda.gov/AMSv1.0/ams.fetchTemplateData.do?template=TemplateN&navID=NationalOrganicProgram&leftNav=NationalOrganicProgram&page=NOPAccreditationandCertification&description=Accreditation%20and%20Certification&acct=nopgeninfo [accessed 23 November 2010]
- U.S. EPA (U.S. Environmental Protection Agency) Method 1106.1: Enterococci in Water by Membrane Filtration using Membrane-Enterococcus-Esculin Iron Agar (mE-EIA).Washington, DC:U.S. EPA. 2006. Available: http://www.epa.gov/microbes/1106_1sp02.pdf [accessed 28 September 2011]
- van den Bogaard AE, Bruinsma N, Stobberingh EE. The effect of banning avoparcin on VRE carriage in the Netherlands. J Antimicrob Chemother. 2000;46:146–148. doi: 10.1093/jac/46.1.146. [DOI] [PubMed] [Google Scholar]
- van Loo I, Huijsdens X, Tiemersma E, de Neeling A, van de Sande-Bruinsma N, Beaujean D, et al. Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg Infect Dis. 2007;13:1834–1839. doi: 10.3201/eid1312.070384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener HC. Antibiotics in animal feed and their role in resistance development. Curr Opin Microbiol. 2003;6:439–445. doi: 10.1016/j.mib.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Witte W. Selective pressure by antibiotic use in livestock. Int J Antimicrob Agents. 2000;16(suppl 1):S19–S24. doi: 10.1016/s0924-8579(00)00301-0. [DOI] [PubMed] [Google Scholar]