Abstract
Background: Assessment of soil arsenic (As) bioavailability may profoundly affect the extent of remediation required at contaminated sites by improving human exposure estimates. Because small adjustments in soil As bioavailability estimates can significantly alter risk assessments and remediation goals, convenient, rapid, reliable, and inexpensive tools are needed to determine soil As bioavailability.
Objectives: We evaluated inexpensive methods for assessing As bioavailability in soil as a means to improve human exposure estimates and potentially reduce remediation costs.
Methods: Nine soils from residential sites affected by mining or smelting activity and two National Institute of Standards and Technology standard reference materials were evaluated for As bioavailability, bioaccessibility, and speciation. Arsenic bioavailability was determined using an in vivo mouse model, and As bioaccessibility was determined using the Solubility/Bioavailability Research Consortium in vitro assay. Arsenic speciation in soil and selected soil physicochemical properties were also evaluated to determine whether these parameters could be used as predictors of As bioavailability and bioaccessibility.
Results: In the mouse assay, we compared bioavailabilities of As in soils with that for sodium arsenate. Relative bioavailabilities (RBAs) of soil As ranged from 11% to 53% (mean, 33%). In vitro soil As bioaccessibility values were strongly correlated with soil As RBAs (R2 = 0.92). Among physicochemical properties, combined concentrations of iron and aluminum accounted for 80% and 62% of the variability in estimates of RBA and bioaccessibility, respectively.
Conclusion: The multifaceted approach described here yielded congruent estimates of As bioavailability and evidence of interrelations among physicochemical properties and bioavailability estimates.
Keywords: arsenic, bioaccessibility, bioavailability, gastrointestinal, human health, human health risk assessment, metalloid, soil physicochemical properties, speciation
The metalloid arsenic (As), a group 1 human carcinogen (International Agency for Research on Cancer 2004), is the second most common inorganic contaminant at Superfund sites [U.S. Environmental Protection Agency (EPA) 2001]. Hence, cancer risk associated with ingestion of As-contaminated soils (Calabrese et al. 1996; Davis et al. 1991; Dudka and Miller 1999) often drives risk assessments for human exposure to metal contaminants at Superfund sites (U.S. EPA 2007c). With increasing urbanization, exposure to As-contaminated soils grows more likely as residential areas extend into the vicinity or, in some cases, intrude onto Superfund sites (Scheckel et al. 2009). Reliable analysis of human health risks from ingestion of As-contaminated soil depends on estimating the bioavailability of As in the soil (U.S. EPA 1989). Current exposure estimates from ingestion of As-contaminated soils often do not consider differences between the bioavailability of As in water and soil (Ehlers and Luthy 2003). The use of default values that assume equivalent bioavailabilities for As in the two matrices can overestimate risk associated with ingestion of As-contaminated soil (Bradham and Wentsel 2010; U.S. EPA 2007b, 2007c). Speciation of As in soil, concentrations of other metals or metalloids, and other soil properties (e.g., pH and mineralogy) can affect the bioavailability of soil As and the amount available for systemic disposition [Kelly et al. 2002; National Research Council (NRC) 2003; U.S. EPA 2007b]. Because even small adjustments in soil As bioavailability estimates can significantly affect estimated risk and cleanup goals (U.S. EPA 2007c), methods are needed that quickly and inexpensively provide accurate and reliable data that can be applied to cleanups of As-contaminated sites worldwide.
Studies of soil As bioavailability have used species as diverse as rodents, swine, and monkeys (Casteel et al. 1997; Freeman et al. 1995; Lorenzana et al. 1996; Nagar et al. 2009; Ng et al. 1998; Pascoe et al. 1994; Rees et al. 2009; Roberts et al. 2002). Time and cost considerations may limit use of some species in bioavailability assays (U.S. EPA 2007a, 2007b). In the present study, we chose the mouse as the test species because of low purchase and husbandry costs, ease of handling, improved predictive value of data because of the feasibility of an increased sample size in assays, and the potential for widespread use of a mouse-based assay in many laboratories. Mice are well characterized physiologically and can be manipulated experimentally (e.g., altered dietary components, altered genotype) to determine the effects of biological variation on the gastrointestinal absorption of metals and metalloids. Extant data on gastrointestinal absorption of ingested arsenicals facilitate use of the mouse as a test species in assays of soil As bioavailability (Hughes et al. 2003, 2005, 2008). Although mice and humans differ in metabolism and disposition of arsenicals (Vahter 1999), similarities are sufficient to permit use of mouse data to create physiologically based pharmacokinetic models that can be scaled for humans (El-Masri and Kenyon 2008; Evans et al. 2008; Gentry et al. 2004a, 2004b; Hughes et al. 1999).
Use of complementary experimental approaches to assess bioavailability has been advocated as a strategy to develop models that reduce uncertainty in risk assessment (NRC 2003). In this study, we linked in vivo and in vitro assays with physicochemical characterization of soils in a unified approach to develop accurate and reliable methods for risk assessment of As-contaminated soils. Results for test soils and standard reference materials (SRMs) suggest that concerted use of in vivo and in vitro methods combined with physicochemical characterization of soils provides a stronger scientific basis for the refinement of risk assessments for As-contaminated soils. In addition, correlations between physicochemical properties of soils and estimates of As bioavailability and bioaccessibility indicate that use of physicochemical properties could profitably inform the refinement of both animal-based and in vitro assays.
Materials and Methods
Soil origin, processing, and physicochemical characterization. For full description of soil origin, processing, and physicochemical characterization, see Supplemental Material (http://dx.doi.org/10.1289/ehp.1003352). Soils used in this study were collected from sites affected by mining and smelter activities. Physicochemical properties were determined in duplicate samples of each soil.
Arsenic speciation in soils was examined using the Materials Research Collaborative Access Team’s beamline 10-ID (Sector 10, Advanced Photon Source, Argonne National Laboratory, Argonne, IL). A principal component analysis coupled with linear combination fitting was used to identify the major As species in the samples. Linear combination fits were performed using X-ray absorption spectroscopy k2 space spectra from reference standards to As phases in the soil samples.
Arsenic concentrations in all soil and biological samples were determined by Instrumental Neutron Activation Analysis (INAA) at the Department of Nuclear Engineering, North Carolina State University (Raleigh, NC; mean As mass detection limit, 0.035 μg). All bioavailability and bioaccessibility calculations were based on INAA values.
Mouse bioavailability assay. The Institutional Animal Care and Use Committee of the U.S. EPA National Health and Environmental Effects Research Laboratory approved the protocol for mouse use, which assured humane treatment and alleviation of suffering. Female C57BL/6 mice 4–6 weeks of age (Charles River Laboratory, Raleigh, NC) were acclimated in groups of three in a 12/12-hr light/dark photocycle at 20–22°C. Mice had free access to rodent diet (TestDiet, Richmond, IN) and tap water that contained < 11 μg/L As (Kenyon et al. 2008). Composition of AIN-93G purified rodent diet (Reeves et al. 1993) obtained from Dyets (Bethlehem, PA) is given in Supplemental Material, Table 1 (http://dx.doi.org/10.1289/ehp.1003352). Soil-amended diets were prepared by thorough mixing of test soil with powdered AIN-93G purified rodent diet to a 1% (wt/wt) soil:diet ratio. Arsenate (AsV)-amended diet prepared by addition of sodium arsenate heptahydrate (Sigma, St. Louis, MO) to powdered AIN-93G purified rodent diet was used to determine the bioavailability of a freely soluble As salt. Diets were stored at 4°C until used.
Table 1.
Description, elemental composition, and As speciation in test soils.a
| Arsenic speciationb | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil sourcec | Soil properties | AsV | AsIII | Reduced chi squaredg | ||||||||||||||||||
| Soil ID | Asd (mg/kg) | Fee,f (g/kg) | Mne,f (g/kg) | Ale,f (g/kg) | pHf | Sorbed AsV (%) | Scorodite (%) | Realgar (%) | Arsenopyrite (%) | |||||||||||||
| 1 | Urban residential | 990 | 20.9 | 0.5 | 11.8 | 6.1 | 52.0 | 21.2 | 26.8 | — | 0.004 | |||||||||||
| 2 | Urban residential | 829 | 20.5 | 0.7 | 9.4 | 6.3 | 96.7 | 3.3 | — | — | 0.004 | |||||||||||
| 3 | Urban residential | 379 | 18.9 | 0.2 | 9.0 | 5.0 | 53.1 | 15.2 | 31.7 | — | 0.003 | |||||||||||
| 4 | Smelter slag | 837 | 294.4 | 2.7 | 13.2 | 7.2 | 18.7 | 1.6 | 47.7 | 32.1 | 0.001 | |||||||||||
| 5 | Residential | 244 | 46.0 | 0.8 | 21.7 | 7.3 | 96.2 | 3.8 | — | — | 0.002 | |||||||||||
| 6 | Residential | 173 | 63.4 | 0.7 | 20.9 | 6.6 | 66.8 | 33.2 | — | — | 0.002 | |||||||||||
| 7 | Smelter slag | 6,899 | 144.5 | 0.9 | 15.0 | 5.2 | 18.3 | 47.1 | — | 34.6 | 0.001 | |||||||||||
| 8 | Residential | 280 | 72.3 | 0.0 | 3.9 | 2.1 | 79.5 | 20.5 | — | — | 0.007 | |||||||||||
| 9 | Smelter slag | 4,495 | 120.1 | 0.4 | 12.3 | 2.6 | 67.6 | 32.4 | — | — | 0.011 | |||||||||||
| 10 | NIST 2710 | 601 | 29.2 | 8.5 | 17.2 | 5.0 | 95.0 | 5.0 | — | — | 0.007 | |||||||||||
| 11 | NIST 2710a | 1,513 | 34.0 | 1.7 | 10.0 | 4.0 | 66.8 | 23.2 | 9.9 | — | 0.01 | |||||||||||
| aThe < 250 μm particle size fraction was used for all analyses. bDetermined by linear combination of As X-ray absorption spectroscopy. cSource of As-contaminated soil. dDetermined by INAA. eExtracted using U.S. EPA Method 3051A (U.S. EPA 2007d) and analyzed using U.S. EPA Method 6010C (U.S. EPA 2007e) by ICP-OES. fData represent the mean of duplicate analyses. gReduced chi-square values = (data – fit)2/data2. | ||||||||||||||||||||||
At the start of an assay, three mice housed together during acclimation were transferred as a group to a metabolic cage that separated urine and feces (Nalgene, Rochester, NY). Twelve mice in four metabolic cages constituted an experimental run. Metabolic cages were maintained for 10 days under environmental conditions given above with unlimited access to test diet and drinking water. For sample collection and data analysis, the unit of observation was the cage and the standard assay for a soil had a sample size of four (except soil 9, which had a sample size of three). To examine assay variability and reproducibility, bioavailability of As in soils 4 and 10 were assayed two and three times, respectively, over a 2-year period.
Daily food consumption for each cage was calculated as the difference between the weight of the food hopper immediately after each morning’s filling and before replenishment the next morning. Cumulative food consumption for each cage was the sum of daily food consumption. Urine and feces were collected each morning from each metabolic cage. Combined body weights of the three mice in each metabolic cage were determined immediately before initial transfer into the metabolic cage and at termination. Mice were euthanized by carbon dioxide (CO2) anesthesia on day 10.
Daily urine or feces collections for each cage were stored at –20°C until processed to produce a single cumulative urine sample and single cumulative feces sample. After thorough mixing, multiple aliquots of the cumulative urine sample for each cage were taken for determination of As concentration by INAA. Cumulative urinary excretion of As was calculated as the product of As concentration in the cumulative urine sample and the volume of the cumulative urine sample. Cumulative feces samples were homogenized with a freezer/mill (model 6850; Spex CertiPrep, Metuchen, NJ). Multiple aliquots of cumulative feces sample were taken for determination of As concentration by INAA. Cumulative fecal excretion of As was calculated as the product of As concentration in the cumulative feces sample and the mass of the cumulative feces sample.
Absolute bioavailability (ABA) of As from ingestion of a soil- or AsV-amended diet was calculated as the ratio of cumulative excretion of As in urine and cumulative dietary intake of As (NRC 2003; U.S. EPA 2007c). ABA is commonly calculated and expressed on a percentage basis:
%ABA = (cumulative As excreted in urine ÷ cumulative As consumed) × 100, [1]
with As measured in micrograms. Relative bioavailability (RBA) was calculated as the ratio of the ABA for As in a specific soil-amended diet to the ABA for As in a diet containing sodium arsenate (NRC 2003; U.S. EPA 2007c). RBA is commonly expressed on a percentage basis:
%RBA = (ABA of As in a specific diet ÷ ABA of As in sodium arsenate) × 100. [2]
Bioaccessibility assays. For a full description of bioaccessibility assays, see Supplemental Material (http://dx.doi.org/10.1289/ehp.1003352). Bioaccessible As was determined using an in vitro method developed by the Solubility/Bioavailability Research Consortium (SBRC) assay (Kelly et al. 2002). In vitro assays were performed in triplicate for each soil and included addition of 1 g test soil to 100 mL gastric fluid consisting of 0.4 M glycine at pH 1.5 in a 125-mL high-density polyethylene bottle and rotating end over end in a water bath at 37°C for 1 hr. All soils tested in the bioaccessibility protocol were identical to those administered to mice in the in vivo studies and used in the mineralogy studies described above. All in vitro extraction solutions were refrigerated at 4°C for preservation and subsequent analysis by Inductively Coupled Plasma–Optical Emission Spectroscopy (ICP-OES) (U.S. EPA 2007e).
In vitro bioaccessibility (IVBA) was calculated and expressed on a percentage basis:
%IVBA = (in vitro extractable mg As/kg soil ÷ total contaminant mg As/kg soil) × 100. [3]
Statistical analysis. Simple linear regression was used to evaluate the relationship between in vivo As RBA data and IVBA data and to examine the effect of selected soil physicochemical properties on As RBA and bioaccessibility. All analyses were performed using R software (version 2.9.1; R Development Core Team, Vienna, Austria), and figures were created using GraphPad Prism (version 5.0; GraphPad, San Diego, CA).
Results
Soil characterization. Table 1 summarizes selected characteristics of test soils. Total As concentration in test soils ranged from 173 to 6,899 /mg/kg. Arsenic speciation by oxidation state varied among soils [see Supplemental Material, Figure 1 (http://dx.doi.org/10.1289/ehp.1003352)]. Soils 1, 3, 4, 7, and 11 had varying ratios of arsenite (AsIII) to AsV species; soils 2, 5, 6, 8, 9, and 10 contained only AsV. We identified realgar in soils 1, 3, 4, and 11 and arsenopyrite in soils 4 and 7. Sorbed AsV and scorodite are common As species in soil environments and often result from the oxidation of As ore materials such as realgar or arsenopyrite. Concentration ranges of iron (Fe), manganese (Mn), and aluminum (Al) in soils were 18.9–294.4 g/kg, 0–8.5 g/kg, and 3.9–21.7 g/kg, respectively. Soil pH ranged from 2.1 to 7.3.
Figure 1.
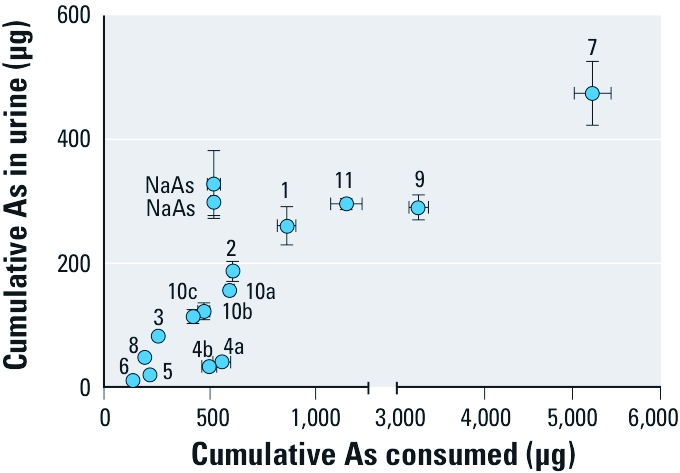
Relationship between cumulative As intake and cumulative urinary As excretion (mean ± SD). For soil numbers, see Table 1. Replicate assays are shown for soil 4 (4a, 4b) and soil 10 (10a, 10b, 10c). NaAs, sodium arsenate–amended diet.
Mouse bioavailability assay. The gross clinical condition of mice was unaffected by ingestion of any of the amended diets; amendment of diet with soil or sodium arsenate did not significantly affect cumulative diet consumption (data not shown). Thus, amendment of AIN-93G rodent diet with 1% (wt/wt) soil or AsV did not affect diet palatability for mice. Mean cumulative consumption of As strongly correlated with the concentration of As in the diet [see Supplemental Material, Figure 2 (http://dx.doi.org/10.1289/ehp.1003352)]. We evaluated mouse assay performance by determining the percentage of cumulative As intake recovered in cumulative urine and feces collections. Arsenic recoveries in excreta averaged 83.7% (range, 67–96%) for sodium arsenate–amended or soil-amended diets. For all dietary additives, percentage recovery and dietary As concentration were not correlated (R2 = 0.227; p = 0.398, Pearson product moment correlation).
Figure 2.
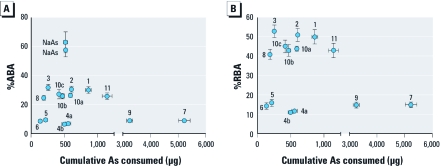
%ABA (A) and %RBA (B) of As from amended diets as a function of cumulative As intake (mean ± SD). Replicate assays are shown for soil 4 (4a, 4b) and soil 10 (10a, 10b, 10c); NaAs, sodium arsenate–amended diet.
Increasing cumulative ingestion of As from amended diets was associated with increasing cumulative urinary excretion of As (Figure 1). Figure 2A shows As ABA estimates from diets amended with AsV, test soils, or SRMs. Duplicate assays with AsV-amended diet yielded an As ABA of approximately 60%. Arsenic ABA estimates for test soils ranged widely from approximately 7% to approximately 33%. Duplicate assays with diets amended with soil 4 (4a, 4b) yielded As ABA estimates of 6.7% and 7.1%. Triplicate assays with diets amended with National Institute of Standards and Technology’s NIST-2710, Montana Soil SRM (soils 10a, 10b, 10c), yielded As ABA estimates ranging from 25.9% to 27.2%. For comparison, NIST-2710a SRM-amended diets (soil 11) dosed at multiple levels yielded an As ABA of approximately 26% for each dose level [see Supplemental Material, Figure 2 (http://dx.doi.org/10.1289/ehp.1003352)]. Figure 2B shows As RBA estimates for test soils and SRMs. Relative to AsV bioavailability, As RBA estimates for test soils ranged from 11% to 53%. Arsenic RBA estimates for NIST 2710–amended diet (soil 10) and NIST 2710a–amended diet (soil 11) were approximately 44%. Supplemental Material, Table 2, summarizes data from mouse assays.
Table 2.
Results of linear regression analyses to explore the influence of select soil properties on As RBA and IVBA.
| RBA | IVBA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Equation | R2 | p-Value | Equation | R2 | p-Value | ||||||
| Sorbed AsV (%) | 0.2x + 17.1 | 0.14 | 0.26 | 0.3x + 18.4 | 0.11 | 0.31 | ||||||
| Scorodite (%) | –0.4x + 38.9 | 0.10 | 0.35 | –0.7x + 50.9 | 0.16 | 0.22 | ||||||
| Realgar (%) | 0.1x + 31.1 | 0.01 | 0.80 | 0.2x + 36.1 | 0.01 | 0.73 | ||||||
| Arsenopyrite (%) | –0.7x + 36.2 | 0.28 | 0.09* | –0.7x + 42.5 | 0.16 | 0.23 | ||||||
| AsV (%) | 0.2x + 19.0 | 0.05 | 0.50 | 0.1x + 26.9 | 0.02 | 0.70 | ||||||
| AsIII (%) | –0.2x + 34.7 | 0.05 | 0.50 | –0.1x + 40.2 | 0.02 | 0.70 | ||||||
| As (mg/kg) | x + 37.3 | 0.17 | 0.21 | x + 45.2 | 0.15 | 0.23 | ||||||
| Fe (g/kg) | –0.1x + 43.5 | 0.48 | 0.02** | –0.2x + 51.4 | 0.32 | 0.07* | ||||||
| Al (g/kg) | –1.9x + 57.3 | 0.34 | 0.06* | –2.7x + 73.3 | 0.32 | 0.07* | ||||||
| Mn (g/kg) | 0.7x + 31.0 | 0.01 | 0.77 | 1.1x + 36.3 | 0.01 | 0.76 | ||||||
| pH | –2.2x + 43.3 | 0.05 | 0.52 | –1.2x + 44.0 | 0.01 | 0.82 | ||||||
| Fe+Al (mol/kg) | –8.8x + 48.7 | 0.58 | 0.01# | –10.5x + 57.9 | 0.40 | 0.04** | ||||||
| Log(Fe+Al) (mol/kg) | –53.1x + 41.6 | 0.80 | 0.00# | –67.5x + 50.1 | 0.62 | 0.00# | ||||||
| *p ≤ 0.10, **p ≤ 0.05, and #p ≤ 0.01. | ||||||||||||
Correlations among estimates of bioaccessibility and bioavailability and physicochemical properties. IVBA values ranged from 6.8% to 67% (SD were 0–3%). We extracted NIST SRMs (soils 10 and 11) multiple times over the course of the study in accordance with the SBRC assay (SDs were 4.1 and 1.7, respectively). We used linear regression to assess predictability of As RBAs from bioaccessibility values derived from the SBRC assay. The derived regression model accounted for 92% of the variability in As bioavailability observed in the mouse assay (R2 = 0.92; Pearson correlation = 0.96; Figure 3).
Figure 3.
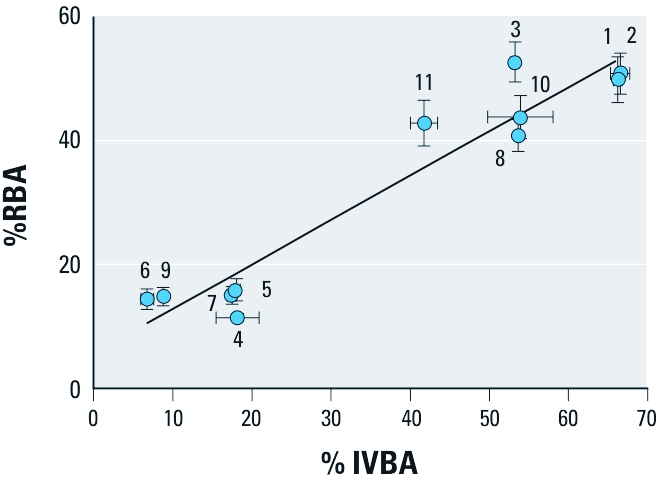
Correlation between estimates of As bioaccessibility and bioavailability (mean ± SD). %RBA = 0.72(%IVBA) + 5.64 (R2 = 0.92).
We examined predictability of As bioavailability or bioaccessibility from the physicochemical properties and speciation of As in soils by simple linear regression (Table 2). Physicochemical properties of soil that were significant predictors (p < 0.10) of As RBA estimates were also significant predictors of IVBA estimates, with the exception of percent arsenopyrite. Among predictors, sums of concentrations of extractable soil Fe and Al (Fe+Al) accounted for the largest amount of variation in RBA and IVBA estimates (R2 = 0.58 and 0.40, respectively). Log(Fe+Al) improved the predictive value of this term (R2 = 0.80 and 0.62 for RBA and IVBA, respectively). Although multivariable linear regression analysis has been used to estimate As bioavailability (Yang et al. 2002), application of this method in the present study did not materially improve predictions of As RBA or IVBA.
Discussion
The concordance of RBA and bioaccessibility estimates obtained in mouse and in vitro assays with common physicochemical characteristics of soils suggested that these approaches could be used in a complementary manner to reduce uncertainty in assessment of risk associated with exposure to As-contaminated soils.
The mouse assay proved adaptable for use with soils with a wide range of As concentrations and physicochemical properties. Amended diets were palatable, and as anticipated from earlier studies (Xie et al. 2004), mice remained in apparent good health throughout the experimental period. In this study, calculation of the As ABA used results from the mouse assay for a diet amended with 7 ppm As as sodium arsenate. This amendment produced As dose levels of 8.9 and 9.2 mg/kg in duplicate studies [see Supplemental Material, Tables 1 and 2 (http://dx.doi.org/10.1289/ehp.1003352)]. The dose levels for AsV-amended diets exceeded those for contaminated soils 3, 5, 6, 8, and 10b; approximately equaled (i.e., with overlapping standard deviations) those for soils 4a, 4b, 10a, and 10c; and was lower than those for soils 1, 2, 7, 9, and 11. Hence, for most soils tested, the concentration of AsV added to the diet equaled or exceeded that present in diet after soil amendment. Although additional studies with AsV-amended diets are needed to confirm that estimates of bioavailability of AsV or As in soil are unaffected by As concentration in amended diets, studies in AsV-treated laboratory mice suggest that dose level does not affect the rate of urinary clearance of As (Hughes and Thompson 1996; Hughes et al. 1994; Kenyon et al. 2008). Similarities in the pattern and extent of urinary clearance of As in mice that have received sodium arsenate over a wide range of dose levels suggest that dose level does not influence uptake of AsV across the gastrointestinal barrier or its clearance into urine. In the absence of a change in the rate of urinary clearance of As over a wide dose range, it is likely that mice ingesting diets amended with AsV or As-containing soils will reach whole-body steady-state body burden during the experimental period used in this study (Hughes et al. 2003).
Similar estimates of As bioavailability obtained for soils 4 and 10 in assays over a 2-year period indicated that assay performance was stable (Figure 2A,B). In adult female mice receiving repeated daily oral doses of sodium arsenate, the body burden of As reaches steady state after 8 or 9 days of dosing (Hughes et al. 2003, 2010). Under steady-state conditions, concentrations of As in tissues and outputs of As in urine and feces will reach plateau values that will remain unchanged throughout the dosing interval. Although concentrations of As in urine and feces are both good indicators of current exposure, the predominance of urine as the route for As clearance after oral administration of inorganic As (Hughes et al. 2003) makes it ideal for estimating the extent of absorption of dietary As. Summing amounts of As excreted in urine and feces during the experimental period can be used to approximate recovery of As in the mouse assay. For the materials evaluated in the mouse assay, recoveries of ingested As in excreta ranged from 67% to 96%. However, these values should be regarded as minimal estimates because they do not include As that is retained in tissues of mice.
The mouse assay can be further refined by examining the role of dietary composition on the estimates of soil As bioavailability obtained with this model. Compared with AIN-93 purified diets, the human diet common in developed countries derives more calories from fat, contains less fiber, and may not be optimal in terms of mineral and vitamin composition. These differences in dietary composition could affect the bioavailability of As in two ways. First, the elemental composition of the diet can affect As uptake across the gastrointestinal barrier. For example, an increasing concentration of phosphate reduces in vitro uptake of AsV by Caco-2 intestinal cells derived from human colonic adenocarcinoma cells (Calatayud et al. 2010) and gastrointestinal uptake of As in rats dosed orally with AsV (Gonzalez et al. 1995). Second, in humanized gnotobiotic mice the microbiota of the gastrointestinal tract is quickly altered by consumption of a diet with a high fat and high sugar content (Turnbaugh et al. 2009). Alteration of the microbiota of the gastrointestinal tract produced by changes in dietary composition could alter gastrointestinal uptake of ingested AsV. Recent studies show that the anaerobic microbiota from the mouse cecum extensively metabolize AsV to produce inorganic thioarsenicals and methylated oxy- and thioarsenicals (Pinyayev et al. 2011). The mouse model can readily be adapted to examine effects of dietary composition of diets on the bioavailability of As in soils.
Soil As RBA estimates obtained in juvenile swine and monkeys have ranged from 0% to 52% (Casteel et al. 1997; Freeman et al. 1995; Lorenzana et al. 1996; Rees et al. 2009; Roberts et al. 2002; Rodriguez et al. 1999). Comparisons of As RBA data obtained in mice and juvenile swine are problematic because of differences in experimental design and dosing levels. However, four soils have been evaluated in both species. For three soils (soils 9, 10, and 11 in this study), As RBA estimates from mouse and juvenile swine differed by 4%, 0%, and 1%, respectively (U.S. EPA 2009). For the fourth soil (soil 8 in this study), As RBA estimates differed by 19.1% (with estimates of 40.9% for mouse and 60% for juvenile swine. Differences in As RBAs for mouse and juvenile swine may reflect physiological differences between species. Additional soils should be evaluated in both species to identify possible sources of variability and permit a detailed comparison of the assays.
A recent NRC report has recommended development and validation of in vitro assays that can replace in vivo assays and can provide reliable and accurate data that reduce uncertainty in risk assessment (NRC 2007). This recommendation prompted development of bioaccessibility assays that reflect processes that control As bioavailability in the human gastrointestinal tract (Basta et al. 2007; Juhasz et al. 2007; Kelly et al. 2002; Rodriguez et al. 1999; Ruby et al. 1999). High correlation (R2 = 0.92, Pearson correlation = 0.96) between the As bioaccessibility data from the SBRC assay and As RBA estimates from the mouse assay is consistent with the high correlation of estimates of As RBA from juvenile swine with As bioaccessibility estimates from the SBRC assay (R2 = 0.75, Pearson correlation = 0.87) (Juhasz et al. 2009). The correlation of findings from the SBRC assay and the mouse assay suggests that the bioaccessibility assay provides useful information about the characteristics of As-containing soils that influence As RBA as measured in the mouse assay. In addition, strong agreement of estimates from the SBRC in vitro assay and the mouse assay suggest that the mouse assay can be used to validate performance of bioaccessibility assays.
Metal speciation and the concentrations of Fe, Al, and Mn are known to affect solubilities and bioavailabilities of metals in soils (Bradham et al. 2006; Kelly et al. 2002; NRC 2003; Scheckel et al. 2009). In this study, we evaluated the effects of As speciation and metal concentrations on estimates of soil As RBA and bioaccessibility obtained in the mouse assay and SBRC assay by linear regression analyses. We found significant inverse correlations between concentrations of extractable Fe and Al in soils and estimates of soil As RBA and bioaccessibility. For example, the log-transformed sum of Fe+Al accounted for 80% and 62% of the variability in estimates of As RBA and bioaccessibility, respectively. The high predictive value of log(Fe+Al) suggests that sorption of As to Fe and Al oxides reduces As solubilization and thereby reduces As RBA and bioaccessibility. Beak et al. (2006a, 2006b) found similar results for As bioaccessibility using a modified Rodriguez et al. (1999) in vitro method, which investigated As sorption on ferrihydrite [Fe3+5O3(OH)9] and corundum (Al2O3). Thus, determination of the concentrations and forms of Fe and Al in soils may be useful in assessing As bioavailability. Several clay minerals contain ferrous and ferric iron that, upon release via weathering, will form iron oxides and hydroxides in soil environments (Bowell 1994). Similar processes are also identified for aluminum and manganese oxides in soils (Jenne 1968; McKeague et al. 1971). Lower As RBA estimates for soils containing sulfide forms of As (realgar or arsenopyrite) may reflect slow dissolution kinetics of these mineral species. Although arsenopyrite was present in only two of the test soils, its presence significantly reduced As bioavailability estimates (p < 0.10). This finding is consistent with reports showing that As in arsenopyrite is bound tightly; therefore, As bioavailability is likely to be low (Roberts et al. 2007). Additional studies would be useful to identify other metals and metalloids in soils that are potential modifiers of As bioavailability and bioaccessibility and to determine concentration dependencies of these interactions.
Conclusions
A multifaceted approach combining in vivo assays, in vitro assays, and physicochemical characterization of soils yielded comparable estimates of As bioavailability and provided evidence of interrelations among physicochemical properties and estimates of As bioavailability. The range of As RBA estimates in this study (11–53%) implies that use of a default value of 100% for As bioavailability in human health risk assessments may overestimate risk associated with exposure to As-contaminated soils. Further studies with the mouse assay and the in vitro assay coordinated with physiochemical characterization of test soils can confirm and extend the results obtained in this study and identify refinements in experimental design and data analysis that can improve the accuracy and reliability of estimates of bioaccessibility and bioavailability.
Supplemental Material
Acknowledgments
We thank K. Herbin-Davis and B. Edwards for excellent technical assistance and the EPA Region 9 Superfund program for their support. Materials Research Collaborative Access Team operations at Argonne National Laboratory are supported by the U.S. Department of Energy and institutional members.
Footnotes
The U.S. Environmental Protection Agency through its Office of Research and Development partially funded and collaborated in this research.
The research described in this article has been subjected to agency review and approved for publication.
The authors declare they have no actual or potential competing financial interests.
References
- Basta NT, Foster JN, Dayton EA, Rodriguez RR, Casteel SW 2007. The effect of dosing vehicle on arsenic bioaccessibility in smelter-contaminated soils. J Environ Sci Health A Tox Hazard Subst Environ Eng 42:1275–1281. [DOI] [PubMed]
- Beak DG, Basta NT, Scheckel KG, Traina SJ. Bioaccessibility of arsenic bound to corundum using a simulated gastrointestinal system. Environ Chem. 2006a;3:208–214. doi: 10.1021/es0516413. [DOI] [PubMed] [Google Scholar]
- Beak DG, Basta NT, Scheckel KG, Traina SJ. Bioaccessibility of arsenic (V) bound to ferrihydrite using a simulated gastrointestinal system. Environ Sci Technol. 2006b;40:1364–1370. doi: 10.1021/es0516413. [DOI] [PubMed] [Google Scholar]
- Bowell RJ. Sorption of arsenic by iron oxides and oxyhydroxides in soils. Appl Geochem. 1994;9:279–286. [Google Scholar]
- Bradham K, Dayton E, Basta N, Schroder J, Payton M, Lanno R. Effect of soil properties on lead bioavailability and toxicity to earthworms. Environ Toxicol Chem. 2006;25:769–775. doi: 10.1897/04-552r.1. [DOI] [PubMed] [Google Scholar]
- Bradham K, Wentsel R. Scientific issues in the U.S. EPA framework for metals risk assessment. J Toxicol Environ Health. 2010;73:1–4. doi: 10.1080/15287390903337084. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Stanek E, Barnes R. Methodology to estimate the amount and particle size of soil ingested by children: implications for exposure assessment at waste sites. Regul Toxicol Pharmacol. 1996;24:264–268. doi: 10.1006/rtph.1996.0139. [DOI] [PubMed] [Google Scholar]
- Calatayud M, Gimeno J, Vélez D, Devesa V, Montoro R. Characterization of the intestinal absorption of arsenate, monomethylarsonic acid, and dimethylarsinic acid using the Caco-2 cell line. Chem Res Toxicol. 2010;23:547–556. doi: 10.1021/tx900279e. [DOI] [PubMed] [Google Scholar]
- Casteel SW, Brown LD, Dunsmore ME, Weis CP, Henningsen GM, Hoffman E, et al. Relative Bioavailability of Arsenic in Mining Wastes. Denver, CO:U.S. Environmental Protection Agency. 1997. Available: http://www.epa.gov/region8/r8risk/pdf/r8_asrba1997.pdf [accessed 2 March 2011]
- Davis A, Ruby MV, Bergstrom PD. Bioavailability of arsenic and lead in soils from the Butte, Montana mining district. Environ Sci Technol. 1991;26:461–468. [Google Scholar]
- Dudka S, Miller WP. Permissible concentrations of arsenic and lead in soils based on risk assessment. Water Air Soil Pollut. 1999;113:127–132. [Google Scholar]
- Ehlers LJ, Luthy RG. Contaminant bioavailability in soils and sediments. Environ Sci Technol. 2003;37:295A–302A. doi: 10.1021/es032524f. [DOI] [PubMed] [Google Scholar]
- El-Masri HA, Kenyon EM. Development of a human physiologically based pharmacokinetic (PBPK) model for inorganic arsenic and its mono- and di-methylated metabolites. J Pharmacokinet Pharmacodyn. 2008;35:31–68. doi: 10.1007/s10928-007-9075-z. [DOI] [PubMed] [Google Scholar]
- Evans MV, Dowd SM, Kenyon EM, Hughes MF, El-Masri HA. A physiologically based pharmacokinetic model for intravenous and ingested dimethylarsinic acid in mice. Toxicol Sci. 2008;104:250–260. doi: 10.1093/toxsci/kfn080. [DOI] [PubMed] [Google Scholar]
- Freeman GB, Schoof RA, Ruby MV, Davis AO, Dill JA, Liao SC, et al. Bioavailability of arsenic in soil and house dust impacted by smelter activities following oral administration in cynomolgus monkeys. Fundam Appl Toxicol. 1995;28:215–222. doi: 10.1006/faat.1995.1162. [DOI] [PubMed] [Google Scholar]
- Gentry PR, Covington TR, Mann S, Shipp AM, Yager JW, Clewell HJ., III Physiologically based pharmacokinetic modeling of arsenic in the mouse. J Toxicol Environ Health A. 2004a;67:43–71. doi: 10.1080/15287390490253660. [DOI] [PubMed] [Google Scholar]
- Gentry PR, Haber LT, McDonald TB, Zhao Q, Covington T, Nance P, et al. Data for physiologically based pharmacokinetic modeling in neonatal animals: physiological parameters in mice and Sprague-Dawley rats. J Child Health. 2004b;2:363–411. [Google Scholar]
- Gonzalez MJ, Aguilar MV, Martinez Para MC. Gastrointestinal absorption of inorganic arsenic (V): the effect of concentration and interactions with phosphate and dichromate. Vet Hum Toxicol. 1995;37:131–136. [PubMed] [Google Scholar]
- Hughes MF, Devesa V, Adair BM, Conklin SD, Creed JT, Styblo M, et al. Tissue dosimetry, metabolism and excretion of pentavalent and trivalent dimethylated arsenic in mice after oral administration. Toxicol Appl Pharmacol. 2008;227:26–35. doi: 10.1016/j.taap.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF, Devesa V, Adair BM, Styblo M, Kenyon EM, Thomas DJ. Tissue dosimetry, metabolism and excretion of pentavalent and trivalent monomethylated arsenic in mice after oral administration. Toxicol Appl Pharmacol. 2005;208:186–197. doi: 10.1016/j.taap.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF, Edwards BC, Herbin-Davis KM, Saunders J, Styblo M, Thomas DJ. Arsenic (+3 oxidation state) methyltransferase genotype affects steady-state distribution and clearance of arsenic in arsenate-treated mice. Toxicol Appl Pharmacol. 2010;249:217–223. doi: 10.1016/j.taap.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Kenyon EM, Edwards BC, Mitchell CT, Del Razo LM, Thomas DJ. Accumulation and metabolism of arsenic in mice after repeated oral administration of arsenate. Toxicol Appl Pharmacol. 2003;191:202–210. doi: 10.1016/s0041-008x(03)00249-7. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Kenyon EM, Edwards BC, Mitchell CT, Thomas DJ. Strain-dependent disposition of inorganic arsenic in the mouse. Toxicology. 1999;137:95–108. doi: 10.1016/s0300-483x(99)00068-2. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Menache M, Thompson DJ. Dose-dependent disposition of sodium arsenate in mice following acute oral exposure. Fundam Appl Toxicol. 1994;22:80–89. doi: 10.1006/faat.1994.1011. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Thompson DJ. Subchronic dispositional and toxicological effects of arsenate administered in drinking water to mice. J Toxicol Environ Health. 1996;49:177–196. doi: 10.1080/009841096160916. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risks Hum. 2004;84:1–477. [PMC free article] [PubMed] [Google Scholar]
- Jenne EA. Controls on Mn, Fe, Co, Ni, Cu, and Zn concentrations in soils and water: the significant role of hydrous Mn and Fe oxides. Trace Inorg Water. 1968;73:337–387. [Google Scholar]
- Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, et al. Comparison of in vivo and in vitro methodologies for the assessment of arsenic bioavailability in contaminated soils. Chemosphere. 2007;69:961–966. doi: 10.1016/j.chemosphere.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Juhasz AL, Weber J, Smith E, Naidu R, Rees M, Rofe A, et al. Assessment of four commonly employed in vitro assays for predicting in vivo relative arsenic bioavailability in contaminated soils. Environ Sci Technol. 2009;43:9487–9494. doi: 10.1021/es902427y. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Brauning SE, Schoof RA, Ruby MV. Columbus, OH: Battelle Press; 2002. Assessing Oral Bioavailability of Metals in Soil. [Google Scholar]
- Kenyon EM, Hughes MF, Adair BM, Highfill JH, Crecelius EA, Clewell HJ, et al. Tissue distribution and urinary excretion of inorganic arsenic and its methylated metabolites in C57BL6 mice following subchronic exposure to arsenate in drinking water. Toxicol Appl Pharmacol. 2008;232:448–455. doi: 10.1016/j.taap.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Lorenzana RM, Duncan B, Ketterer M, Lowry J, Simon J, Dawson M, et al. Seattle, WA: U.S. Environmental Protection Agency; 1996. Bioavailability of Arsenic and Lead in Environmental Substrates. EPA 910/R-96-002. [Google Scholar]
- McKeague JA, Brydon JE, Miles MN. Differentiation of forms of extractable iron and aluminum in soils 1. Soil Sci Soc Am J. 1971;35:33–38. [Google Scholar]
- Nagar R, Sarkar D, Makris KC, Datta R, Sylvia V. Bioavailability and bioaccessibility of arsenic in a soil amended with drinking water treatment residuals. Arch Environ Contam Toxicol. 2009;57:755–766. doi: 10.1007/s00244-009-9318-7. [DOI] [PubMed] [Google Scholar]
- Ng JC, Kratzmann SM, Qi L, Crawley H, Chiswell B, Moore MR. Speciation and absolute bioavailability: risk assessment of arsenic-contaminated sites in a residential suburb in Canberra. Analyst. 1998;123:889–892. doi: 10.1039/a707728i. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) Washington, DC: National Academy Press; 2003. Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications. [Google Scholar]
- NRC (National Research Council) Washington, DC: National Academies Press; 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. [Google Scholar]
- Pascoe GA, Blanchet RJ, Linder G. Bioavailability of metals and arsenic to small mammals at a mining waste-contaminated wetland. Arch Environ Contam Toxicol. 1994;1:44–50. doi: 10.1007/BF00203886. [DOI] [PubMed] [Google Scholar]
- Pinyayev TS, Kohan MJ, Herbin-Davis K, Creed JT, Thomas DJ. Preabsorptive metabolism of sodium arsenate by anaerobic microbiota of mouse cecum forms a variety of methylated and thiolated arsenicals. Chem Res Toxicol. 2011;24(4):475–477. doi: 10.1021/tx200040w. [DOI] [PubMed] [Google Scholar]
- Rees M, Sansom L, Rofe A, Juhasz A, Smith E, Weber J, et al. Principles and application of an in vivo swine assay for the determination of arsenic bioavailability in contaminated matrices. Environ Geochem Health. 2009;31:167–177. doi: 10.1007/s10653-008-9237-y. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Roberts SM, Munson JW, Lowney YW, Ruby MV. Relative oral bioavailability of arsenic from contaminated soils measured in the cynomolgus monkey. Toxicol Sci. 2007;95:281–288. doi: 10.1093/toxsci/kfl117. [DOI] [PubMed] [Google Scholar]
- Roberts SM, Weimar WR, Vinson JR, Munson JW, Bergeron RJ. Measurement of arsenic bioavailability in soil using a primate model. Toxicol Sci. 2002;67:303–310. doi: 10.1093/toxsci/67.2.303. [DOI] [PubMed] [Google Scholar]
- Rodriguez RR, Basta NT, Casteel SW, Pace LW. An in vitro gastrointestinal method to estimate bioavailable arsenic in contaminated soils and solid media. Environ Sci Technol. 1999;33:642–649. [Google Scholar]
- Ruby MV, Schoof R, Brattin W, Goldade M, Post G, Harnois M, et al. Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environ Sci Technol. 1999;33:3697–3705. [Google Scholar]
- Scheckel KG, Chaney RL, Basta NT, Ryan JA. Advances in assessing bioavailability of metal(loids) in contaminated soils. Adv Agron. 2009;104:1–52. [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI 2009. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1(6):6ra14; doi:10.1126/scitranslmed.3000322 [Online 11 November 2009]. [DOI] [PMC free article] [PubMed]
- U.S. EPA (U.S. Environmental Protection Agency) Washington, DC: U.S. EPA, Office of Emergency and Remedial Response; 1989. Risk Assessment Guidance for Superfund Volume 1: Human Health Evaluation Manual (Part A). EPA/540/1-89/002. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) Washington, DC: U.S. EPA, Office of Solid Waste and Emergency Response; 2001. CERCLIS 3 Database. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) Washington, DC: U.S. EPA, Office of Solid Waste and Emergency Response; 2007a. Estimation of Relative Bioavailability of Lead in Soil and Soil-like Materials Using in Vivo and in Vitro Methods. OSWER 9285.7-77. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) Washington, DC: U.S. EPA; 2007b. Framework for Metals Risk Assessment. EPA 120/R-07/001. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) Washington, DC: U.S. EPA, Office of Solid Waste and Emergency Response; 2007c. Guidance for Evaluating the Oral Bioavailability of Metals in Soils for Use in Human Health Risk Assessment. OSWER 9285.7-80. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils. Washington, DC:U.S. EPA, Office of Solid Waste. 2007d. Available: http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/3051a.pdf [accessed 2 March 2011)
- U.S. EPA (U.S. Environmental Protection Agency) Method 6010C: Inductively Coupled Plasma-Atomic Emission Spectrometry. Washington, DC:U.S. EPA, Office of Solid Waste. 2007e. Available: http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/6010c.pdf [accessed 2 March 2011]
- U.S. EPA (U.S. Environmental Protection Agency) Washington, DC: U.S. EPA, Office of Superfund Remediation Technology Innovation; 2009. Relative bioavailability of arsenic in NIST 2710 (Montana soil) (Internal Report) [Google Scholar]
- Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog. 1999;82:69–88. doi: 10.1177/003685049908200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Liu J, Liu Y, Klaassen CD, Waalkes MP. Toxicokinetic and genomic analysis of chronic arsenic exposure in multidrug-resistance mdr1a/1b(-/-) double knockout mice. Mol Cell Biochem. 2004;255:11–18. doi: 10.1023/b:mcbi.0000007256.44450.8c. [DOI] [PubMed] [Google Scholar]
- Yang J, Barnett MO, Jardine PM, Basta NT, Casteel SW. Adsorption, sequestration, and bioaccessibility of As(V) in soils. Environ Sci Technol. 2002;36:4562–4569. doi: 10.1021/es011507s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.