Abstract
A radioimmunoassay for human plasma retinol-binding protein (RBP) has been developed utilizing a double antibody precipitation technique. RBP was purified 1500- to 2000-fold by procedures described previously. A specific anti-human RBP antiserum was prepared in rabbits by three once-weekly injections of purified RBP emulsified with Freund's adjuvant. RBP was iodinated with 131I and the RBP-131I was purified by gel filtration on Sephadex G-100 after complex formation with human plasma prealbumin. The RBP-131I was completely (> 95%) immunoprecipitable in the presence of an excess of specific antiserum, it was not (< 5%) immunoprecipitable in the absence of specific antiserum, and it could be completely displaced from antibody by excess unlabeled RBP. The standard curve obtained in the immunoassay with normal plasma was identical to that with pure RBP. Duplicate samples differed from their mean by 5 ±5% (±SD). There was a quantitative recovery of pure RBP added in varying amounts to normal plasma. The immunoassay accurately measured RBP in amounts of 10-100 ng per assay tube. There was no significant difference in the immunoreactivity of apo-RBP as compared to holo-RBP. The mean plasma values (±SEM) for a group of 76 normal subjects were 47.2 ±1.6 μg/ml for males and 41.6 ±1.6 μg/ml for females. Plasma RBP levels were markedly depressed (15 ±2.3 μg/ml) in 14 patients with acute viral hepatitis. There was a highly significant correlation between the plasma levels of RBP and of vitamin A in both normal subjects and patients with hepatitis. In all subjects plasma RBP was generally saturated with retinol. The data suggest that under normal circumstances RBP circulates almost exclusively as the holoprotein.
Full text
PDF

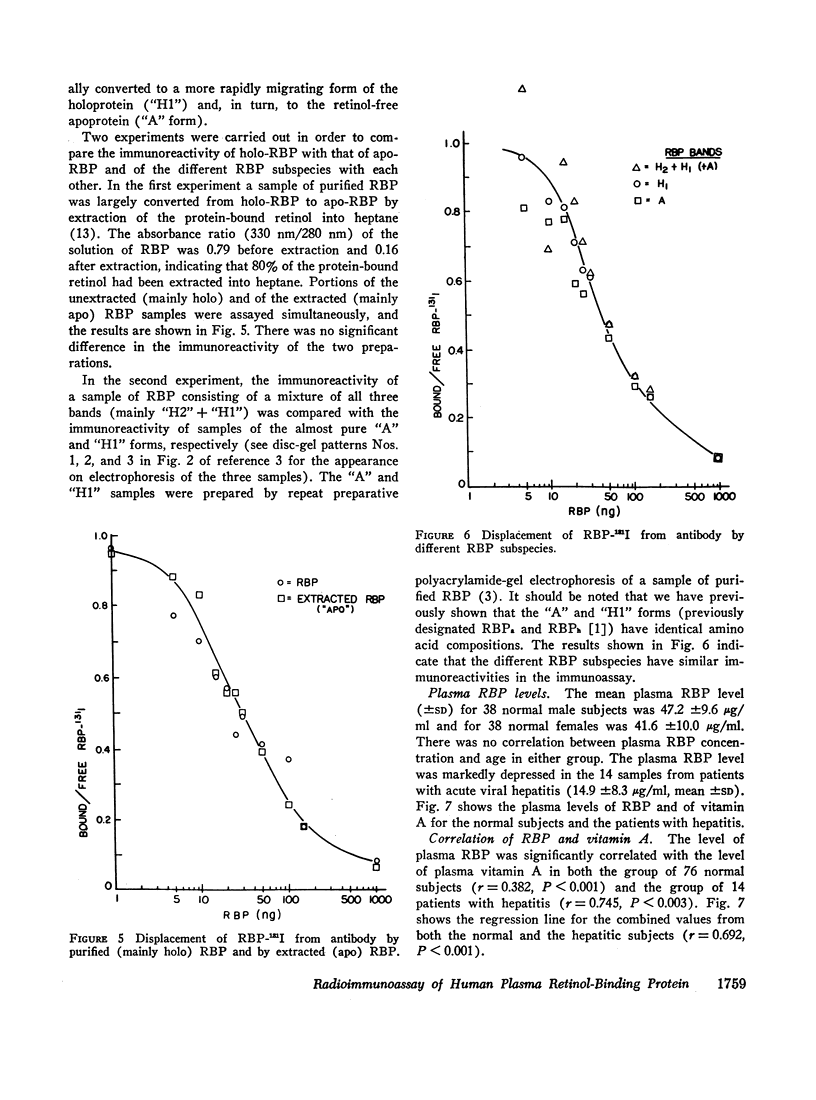
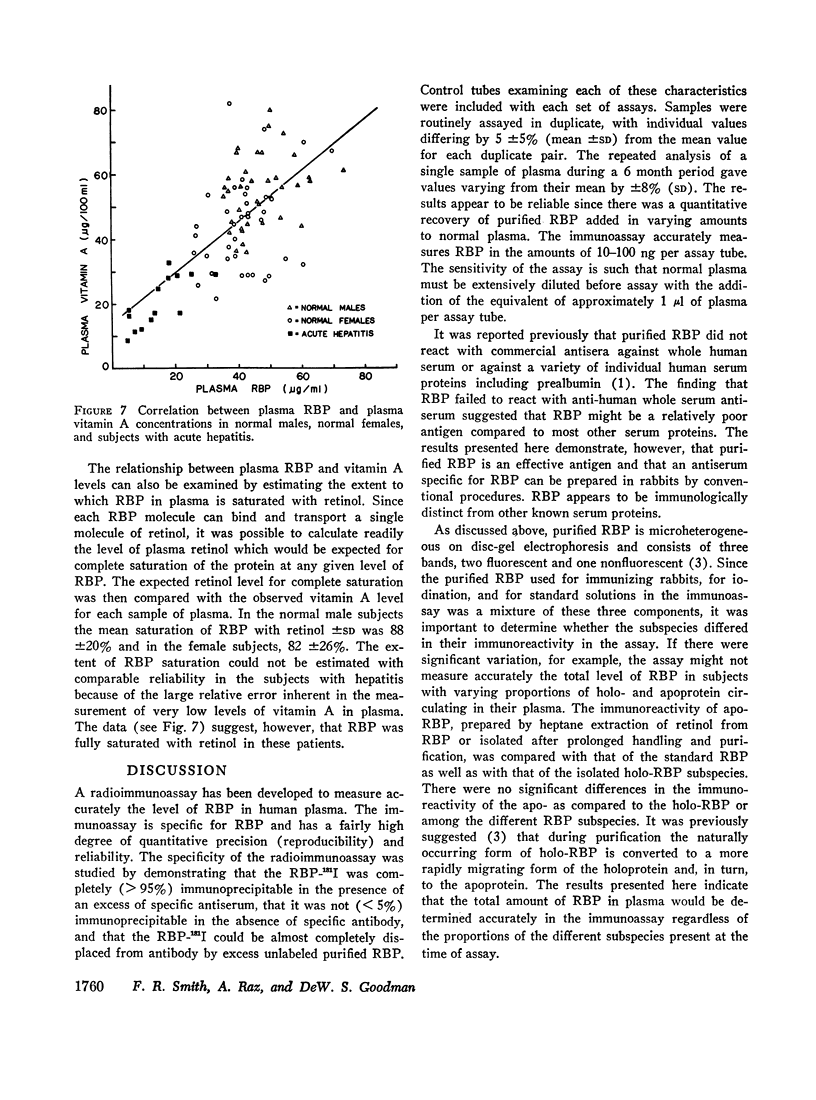

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GRABAR P., WILLIAMS C. A., Jr Méthode immuno-électrophorétique d'analyse de mélanges de substances antigéniques. Biochim Biophys Acta. 1955 May;17(1):67–74. doi: 10.1016/0006-3002(55)90320-6. [DOI] [PubMed] [Google Scholar]
- Goodman D. S. Retinol transport in human plasma. Am J Clin Nutr. 1969 Jul;22(7):911–912. doi: 10.1093/ajcn/22.7.911. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M., Raz A., Goodman D. S. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968 Sep;47(9):2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Goodman D. S. The interaction of thyroxine with human plasma prealbumin and with the prealbumin-retinol-binding protein complex. J Biol Chem. 1969 Jun 25;244(12):3230–3237. [PubMed] [Google Scholar]
- Raz A., Shiratori T., Goodman D. S. Studies on the protein-protein and protein-ligand interactions involved in retinol transport in plasma. J Biol Chem. 1970 Apr 25;245(8):1903–1912. [PubMed] [Google Scholar]