Abstract
OBJECTIVE:
Cancer patients frequently require admission to intensive care unit. However, there are a few data regarding predictive factors for mortality in this group of patients. The aim of this study was to evaluate whether arterial lactate or standard base deficit on admission and after 24 hours can predict mortality for patients with cancer.
METHODS:
We evaluated 1,129 patients with severe sepsis, septic shock, or postoperative after high-risk surgery. Lactate and standard base deficit collected at admission and after 24 hours were compared between survivors and non-survivors. We evaluated whether these perfusion markers are independent predictors of mortality.
RESULTS:
There were 854 hospital survivors (76.5%). 24 h lactate >1.9 mmol/L and standard base deficit < -2.3 were independent predictors of intensive care unit mortality. 24 h lactate >1.9 mmol/L and 24 h standard base deficit < -2.3 mmol/Lwere independent predictors of hospital death.
CONCLUSION:
Our findings suggest that lactate and standard base deficit measurement should be included in the routine assessment of patients with cancer admitted to the intensive care unit with sepsis, septic shock or after high-risk surgery. These markers may be useful in the adequate allocation of resources in this population.
Keywords: Lactate, Mortality, Cancer, Critical Care
INTRODUCTION
Over the last decades, management of cancer patients has changed significantly, resulting in significant improvements in survival rates.1 While admissions to intensive care units (ICU) are frequently required, admission criteria are often difficult to be defined.2,3
Due to the lack of definitive predictive factors for mortality in these patients, an ICU trial was proposed and conducted by Lecuyer and colleagues4 suggesting that treatment limitation decisions should be considered after at least six days of complete management, when organ dysfunction scores could differentiate the survivors from the non-survivors.
A multicenter European study, the SOAP study,3 found that patients with hematologic malignancies and solid tumors had a 29% hospital mortality rate. The most significant outcome predictors were higher Simplified Acute Physiology Score (SAPSII) and the need for mechanical ventilation. In a recent multicenter and prospective study with 717 patients with cancer admitted over a two-month period into Brazilian ICUs,1 Soares and colleagues found that mortality was associated with the length of hospital days before ICU admission, the degree of organ dysfunction, the status performance, the need for mechanical ventilation and recurrent or progressing active malignancy.1
Lactate and standard base deficit (SBD), measured at admission and within the first days of the ICU stay, are important outcome markers in critically ill patients.5-8 However, there have been no studies evaluating serum lactate levels and SBD as predictive factors of mortality in patients with cancer.
The aim of this study was to evaluate whether arterial lactate levels or SBD deficit taken on admission and after 24 h can predict ICU and hospital mortality for patients with cancer admitted to the ICU.
METHODS
Study Design
Following approval by the institutional Medical Ethics Committee, this prospective observational study was performed at the medical-surgical ICU of the Cancer Institute do Estado de Sao Paulo, an oncology reference hospital linked to the University of Sao Paulo. This is a closed 44-bed ICU that admits 1,500 to 2,000 patients per year, with approximately 60% postoperative and 40% medical patients, and a global ICU mortality rate of approximately 25%.9
We evaluated all patients admitted from August 2008 to July 2010. Patients were considered eligible for the study if they had a diagnosis of severe sepsis or septic shock according to the current definition,11 with less than 24 hrs of sepsis-related organ dysfunction or if they were at immediate postoperative of high risk surgery, defined as emergency surgical procedures or elective prolonged surgery (>3 hours).11,12 Patients were excluded if their ICU stay was less than 24 hours or if palliative care was defined at the time of admission. Data were prospectively collected daily until hospital discharge or death, including age, gender, medical/surgical status, oncologic diagnosis, status of neoplasm, comorbidities, diagnosis for ICU admission, and performance status (Karnofsky scale). Clinical and laboratory data for the SAPS II and for the Acute Physiology and Chronic Health Evaluation II score (APACHE II) were recorded using the worst value within the first 24 hours after ICU admission.13,14 The need for mechanical ventilation, renal replacement therapy (RRT) or vasopressor agents was recorded. Serum lactate and SBD were collected at admission, after 24 hours and after an intervention involving a fluid challenge, use of inotropic agents or vasopressors. The SBD and arterial lactate levels were obtained from sampled arterial blood collected through an arterial line at the same time of central venous blood samples. The central venous catheter positions were previously checked. All blood samples were analyzed in an AVL Omni Roche gas analyzer (Basel, Switzerland). Standard base deficit was calculated according to the Siggaard-Andersen (Van Slyke) equation. The serum lactate level and SBD collected at admission (0 h) and after 24 hours (24 h) were compared between survivors and non-survivors through mean values and subgroup analysis: lactate <2, 2 to 4 and >4 mmol/L and SBD <-10, -10 to -4 and >-4 mmol/L. The intensive treatment of patients was done according to the Surviving Sepsis Campaign guidelines.10 We evaluated whether arterial lactate and SBD were independent predictors of ICU and hospital mortality.
Statistical Analysis
All data were analyzed using SPSS version 18 (SPSS, Inc., Chicago, IL, USA). Data were considered normal using the Kolmogorov-Smirnov test. Results are expressed as means with 95% confidence intervals (CIs) or medians with interquartile ranges (IQRs). Single medians between the groups were compared using the Mann-Whitney U test, and categorical data were compared using a chi-square analysis. We used analysis of variance for repeated measures to analyze levels of lactate and base deficit at 0 h and 24 h between the survivor and non-survivor groups.
A forward multiple logistic regression analysis was performed to estimate predictive factors for ICU mortality. The variables which in the univariate anlaysis presented p≤0.10 were used to build the model.
A multiple Cox proportional hazards model was performed to estimate predictive factors for hospital mortality. Receiver operating characteristic (ROC) curves were built for each variable to analyze its discriminating power in predicting hospital mortality. The areas under the ROC curves (±SE) were determined.
In logistic regression model for other significant variables, we built curves for each significant variable related to hospital mortality.
A p-value of less than 0.05 was considered to indicate statistical significance, and all tests were two-tailed.
RESULTS
Intensive Care Unit Mortality
Of a total of 2,653 patients admitted into the ICU during the study period, 1,129 patients fulfilled the inclusion criteria. Their characteristics are described in Table 1. There were 854 ICU survivors (76.5%) and 265 ICU non-survivors (23.5%) during the hospital stay. There were no differences between the survivor and non-survivor groups regarding age or gender. As expected, the non-survivor group presented significantly higher APACHE II and SAPS II scores compared to the survivor group.
Table 1.
Demographic and clinical characteristics of the patients and their outcomes.
| Variable | All patients | Survivors | Non-Survivors | p-value |
| (N = 1129) | (N = 864) | (N = 265) | ||
| Age (years), mean (95%CI) | 61 (60 to 62) | 61 (60 to 62) | 61 (59 to 63) | 0.708 |
| Male gender | 615 (54.5%) | 474 (54.9%) | 141 (53.2%) | 0.636 |
| APACHE II score | 15 (11 to 23) | 12 (9 to 18) | 24 (18 to 28) | <0.001 |
| SAPS II score, median (IQR) | 38 (24 to 53) | 30 (21 to 40) | 57 (43 to 78) | <0.001 |
| Type of Admission | ||||
| Medical | 625 (55.4%) | 394 (45.6%) | 231 (87.2%) | <0.001 |
| Sepsis | 400 (64%) | 294 (74.6%) | 106 (45.9%) | <0.001 |
| Septic shock | 225 (36%) | 100 (25.4%) | 125 (54.1%) | |
| Surgical | 504 (44.6%) | 470 (54.4%) | 34 (12.8%) | |
| Gastrointestinal | 162 (32.1%) | 144 (30.6%) | 18 (52.9%) | 0.007 |
| Thoracic | 34 (6.7%) | 29 (6.2%) | 5 (14.7%) | 0.055 |
| Urologic | 117 (23.2%) | 111 (23.6%) | 6 (17.6%) | 0.426 |
| Gynecologic | 63 (12.5%) | 62 (13.2%) | 1 (2.9%) | 0.081 |
| Other | 128 (25.4%) | 124 (26.4%) | 4 (11.8%) | 0.059 |
| Type of cancer | ||||
| Locoregional solid tumor | 777 (68.8%) | 628 (72.7%) | 149 (56.2%) | <0.001 |
| Metastatic solid tumor | 210 (18.6%) | 146 (16.9%) | 64 (24.2%) | |
| Haematological malignancies | 142 (12.6%) | 90 (10.4%) | 52 (19.6%) | |
| Karnofski, median (IQR) | 80 (65 to 90) | 80 (70 to 90) | 80 (60 to 90) | 0.612 |
| Co-morbidities and therapies on admission | ||||
| COPD | 260 (23%) | 197 (22.8%) | 63 (23.8%) | 0.742 |
| Chronic renal failure | 40 (3.5%) | 34 (3.9%) | 6 (2.3%) | 0.198 |
| Diabetes | 178 (15.8%) | 145 (16.8%) | 33 (12.5%) | 0.091 |
| Liver disease | 48 (4.3%) | 34 (3.9%) | 14 (5.3%) | 0.341 |
| Chemotherapy | 72 (6.4%) | 48 (5.6%) | 24 (9.1%) | 0.041 |
| Radiotherapy | 4 (0.4%) | 3 (0.3%) | 1 (0.4%) | 0.942 |
| Mechanical Ventilation on ICU admission | 388 (34.4%) | 251 (29.1%) | 137 (51.7%) | <0.001 |
| Dialysis on ICU admission | 45 (4%) | 18 (2.1%) | 27 (10.2%) | <0.001 |
| Vasopressors on ICU admission | 341 (30.2%) | 195 (22.6%) | 146 (55.1%) | <0.001 |
| ICU stay (days), median (IQR) | 4 (3 to 8) | 3 (2 to 6) | 6 (2 to 10) | <0,001 |
Abbreviations: 95% CI, 95% confidence interval; IQR, interquartile range; APACHE, Acute Physiology and Chronic Health disease; SAPS, Simplified Acute Physiology Score; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit. Parenthesis: % or CI or IQR.
A medical complication was the main reason for ICU admission. Non-survivors were more likely to have a medical diagnosis. There were 987 patients with solid tumors (87.4%) while 142 patients (12.6%) had hematological malignancies. The studied patients had a Karnofsky score of 80 (65-90). Non-survivors were more likely to have received chemotherapy in the last 30 days and in need of organ support at admission. They also had longer ICU stay durations.
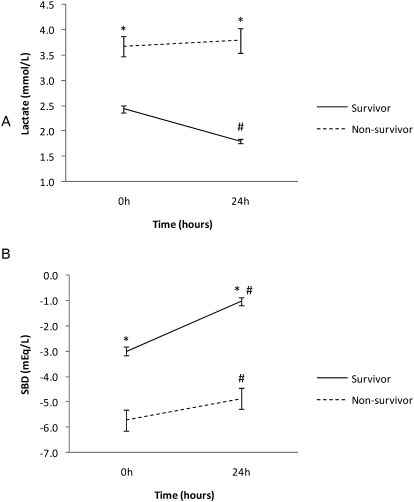
As shown in Figure 1, at the time of ICU admission (0 h), the mean (CI) initial lactate concentrations were 2.4 [95% CI, 2.3 to 2.6] mmol/L in the survivors and 3.7 [95% CI, 3.3 to 4.1] mmol/L in the non-survivors (p<.001). After 24 h, the lactate concentrations decreased to 1.8 [95% CI, 1.7 to 1.9] mmol/L in the survivors but remained at 3.8 [95% CI, 3.3 to 4.3] mmol/L in the non-survivors (differences p<0.001).
Figure 1.
Comparison between survivor and non-survivor groups of A) lactate (mmol/L) and B) standard base deficit (SBD in mmol/L) in two time points (0 and 24h). Mean +− SEM (*) p<0.05 survivors versus non-survivors (#) p<0.05.
Likewise, at baseline, the SBD was -3 [95% CI, -3.3 to -2.7] mmol/L in the survivors and -5.7 [95% CI, -6.6 to -4.9] mmol/L in the non-survivors (p<0.001). After 24 h, the SBD increased to 2 mmol/L in the survivors while it increased to 0.8 mmol/L in the non-survivors (p<0.001). (Figure 1).
In a multivariate model, medical admission, RRT or vasopressor therapy on ICU admission, 24 h lactate >1.9 mmol/L and SBD between –10 and -4 mmol/L were predictors of ICU mortality (Table 2).
Table 2.
Multiple logistic regression to determine predictive factors for ICU mortality.
| Variable | Odds ratio | 95%CI | p-value | |
| Medical admission | 4.216 | 2.761 | 6.436 | <0.001 |
| RRT on ICU admission | 4.212 | 1.848 | 9.602 | 0.001 |
| Vasopressors on ICU admission | 3.049 | 2.077 | 4.477 | <0.001 |
| SBD (mmol/L) - 24 h | <0.001 | |||
| <-2.3 | 2.400 | 1.635 | 3.523 | <0.001 |
| >-2.3 | Reference | <0.001 | ||
| Lactate (mmol/L) - 24 h | <0.001 | |||
| <1.9 | Reference | |||
| >1.9 | 4.018 | 2.704 | 5.972 | <0.001 |
Abbreviations: ICU, intensive care unit; SBD, Standard base deficit in mmol/L; RRT, renal replacement therapy.
Hospital Mortality
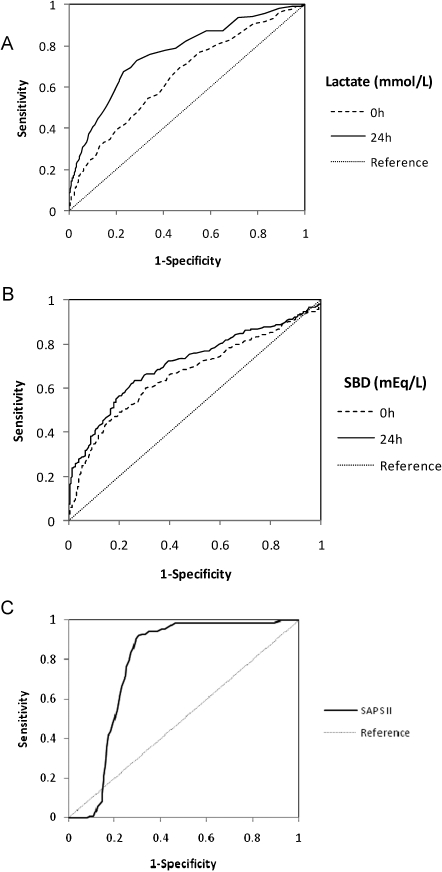
Of the 1,129 patients, 324 (28.7%) died during their hospital stay. In an univariate analysis, higher APACHE II and SAPS II scores, medical admissions, metastatic solid tumors, hematological malignancies, mechanical ventilation at ICU admission, and vasopressors at ICU admission were associated with hospital mortality. Also, higher levels of lactate at 0 h and 24 h and lower levels of SBD at 0 h and 24 h were associated with death during the hospital stay (Figure 2 and Table 3).
Figure 2.
Receiver Operating Characteristic (ROC) curve comparing the ability of A) lactate (mmol/L), B) standard base deficit (SBD in mmol/L) at baseline and after 24 and C) SAPS II SCORE after 24h in predicting ICU mortality.
Table 3.
Univariate Cox proportional hazard analysis with hospital mortality as the dependent variable.
| Variable | HR | 95%CI | p-value | |
| Lactate (mmol/L) | ||||
| 0 h | <0.001 | |||
| <2.3 (mmol/L) | reference | |||
| >2.3 (mmol/L) | 2.030 | 1.615 | 2.552 | <0.001 |
| 24 h | <0.001 | |||
| <1.9 (mmol/L) | reference | |||
| >1.9 (mmol/L) | 3.262 | 2.502 | 4.252 | <0.001 |
| SBD (mmol/L) | ||||
| 0 h | <0.001 | |||
| <-3.6 | 2.170 | 1.727 | 2.726 | <0.001 |
| >-3.6 | reference | |||
| 24 h | <0.001 | |||
| <-2.3 | 2.448 | 1.909 | 3.138 | <0.001 |
| >-2.3 | reference | |||
Abbreviations: ICU, intensive care unit; SBD, Standard base deficit in mmol/L.
Abbreviations: ICU, intensive care unit; SBD, Standard base deficit iLactate values at 0 h and at 24 h that were higher than 2.3 mmol/L and 1.9 mmol/L, respectively, were observed to increase hospital mortality. Also, SBD values at 0 h and at 24 h that were lower than -3.6 mmol/L and -2.3 mmol/L was observed to increase hospital mortality (Table 3).
From the ROC curves, the best predictors of hospital mortality in critically ill cancer patients were lactate values of greater than 2.3 mmol/L and a BD values lower than – 3.7 mmol/L at the time of admission. After 24 h, the best predictors of ICU mortality were lactate values greater than 1.9 mmol/L and a BD values lower than – 2.3 mmol/L.
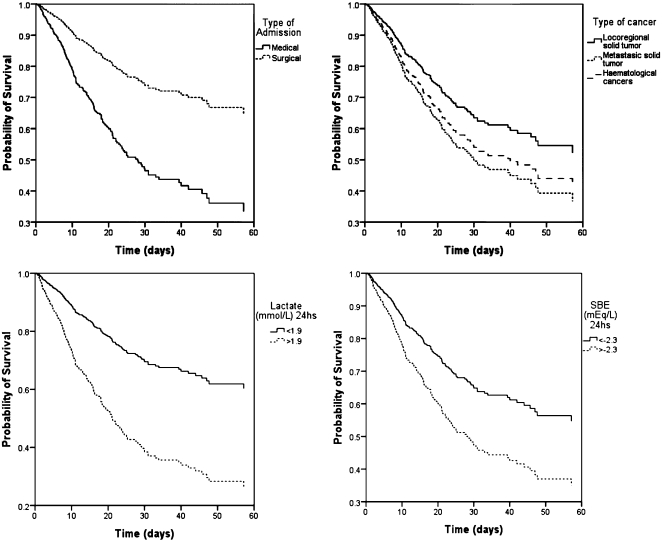
We graphically represented hospital survival curves for each parameter (Figure 3).
Figure 3.
Survival curves for type of admission, type of cancer and levels of lactate (mmol/L) and SBD (mmol/L) at 24h.
In a multivariate model, medical admission, metastatic solid tumor or hematological malignancies, 24 h lactate values higher than 1.9 mmol/L and 24 h SBD values lower than -2.3 mmol/L were the independent predictors of hospital death (Table 4).
Table 4.
Multivariate Cox proportional hazard analysis with hospital mortality as the dependent variable.
| Variable | HR | 95% CI | p-value | |
| Medical admission | 2.180 | 1.562 | 3.041 | <0.001 |
| Type of cancer | 0.014 | |||
| Locoregional solid tumor | Reference | |||
| Metastatic solid tumor | 1.495 | 1.114 | 2.007 | 0.007 |
| Haematological malignancies | 1.424 | 1.017 | 1.994 | 0.040 |
| Lactate (mmol/L) 24 h | <0.001 | |||
| <1.9 | Reference | |||
| >1.9 | 2.630 | 1.992 | 3.473 | <0.001 |
| SBD (mmol/L) 24 hs | <0.001 | |||
| <-2.3 | 1.738 | 1.331 | 2.270 | <0.001 |
| >-2.3 | Reference | |||
Abbreviations: ICU, intensive care unit; SBD, Standard base deficit in mmol/L.
DISCUSSION
This is the first study showing that lactate and standard base deficit levels are predictors of ICU and hospital mortality in patients with cancer admitted to intensive care units. In these patients with cancer, admission as well as 24 h lactate and SBD values can be used earlier and more accurately than other variables to identify patients who have a high risk for mortality.
Moreover, our data suggest that these variables are more sensible and specific in their capacity to predict mortality in a mixed population of medical and surgical patients with cancer, 24 h after initial admission to the ICU.
Recently, patients with cancer are experiencing increased survival rates, leading to increases in the number of ICU admissions.15 Such findings highlight the need to better characterize patients with cancers and to evaluate the prognoses at ICU admission.
Larché and colleagues described a third-day mortality of 65.5% in 84 patients with cancer and septic shock.2 In a report by Soares and colleagues, hospital mortality was 58% in cancer patients admitted due to medical complications and 37% in patients admitted after elective surgery.1 Our patients were severely ill, with a median SAPS II score of 38. Most were admitted due to sepsis or septic shock while a large percentage of patients had metastatic solid tumors or hematological cancers. Despite this, the overall ICU mortality rate in our study was 24%; 37% in medical patients and 7% in surgical patients. Compared to the survivors, non-survivors presented with higher lactate levels and lower base deficit levels at admission and at 24 h. These findings are similar to the results of previous studies assessing lactate, lactate clearance and base deficits in critically ill patients.16-18 A high single lactate level and limited lactate reduction during treatment have prognostic importance.
Smith and colleagues showed in 148 patients without cancer that both the base deficit and arterial lactate at admission have good prognostic abilities.17 The value of base deficit at the time of admission with the best predictive ability was a base deficit value of less than –4 mmol/L and a lactate value of greater than 1.5 mmol/L.
Nguyen and colleagues evaluated 111 septic patients and found that lactate values at admission are higher in non-survivors. Both lactate and lactate clearance are predictors of mortality in these patients.5 In a recent study, Khosravani and colleagues evaluated 11,581 patients and demonstrated that hyperlactatemia is common in both medical and surgical critically ill patients. Its presence at presentation or its development during ICU stay is associated with mortality.18 In our patients, lactate and SBD values at ICU admission were risk factors and 24 h lactate and SBD values were independent predictors of ICU and hospital mortality. These data suggest the addition of low cost markers to existing routine evaluations of cancer patients admitted to the ICU. Also, our findings highlight the importance of immediate and adequate treatments of these patients, as the serial improvement of these parameters is associated with a better prognosis.
As stated in previous studies, usual risk scores in critically ill patients such as SAPS II, APACHE II, SOFA, and MPM scores do not apply well in patients with cancer.2 These patients now live longer and present with higher rates of ICU admission, which reinforces the need for more sensible and specific parameters to evaluate patient prognosis.
In patients with cancer, it is important to emphasize that increased lactate levels may be due to causes other than tissue hypoxia liver disorders, lactate production by neoplastic cells, tumor lysis syndrome, or compromises in lactate clearance.19
A limitation of this study was that the study was performed in a single referral center, which could limit the generalizability of our findings. Another limitation is that we did not specifically address other causes for the high levels of lactate and the low levels of base deficit.
The addition of lactate and standard base deficit measurement at ICU admission may represent a new issue in the prognostic evaluation of cancer patients.
Our findings suggest the importance of the inclusion of lactate and base deficit measurements in the assessment of prognosis in cancer patients admitted to intensive care unit with sepsis, septic shock, or after high-risk surgery. More evidence is needed to clarify whether targeted therapy through lactate and base deficit level measurements can improve outcomes in cancer patients.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Soares M, Caruso P, Silva E, Teles JM, Lobo SM, Friedman G, et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med. 2010;38:9–15. doi: 10.1097/CCM.0b013e3181c0349e. 10.1097/CCM.0b013e3181c0349e [DOI] [PubMed] [Google Scholar]
- 2.Larche J, Azoulay E, Fieux F, Mesnard L, Moreau D, Thiery G, et al. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003;29:1688–95. doi: 10.1007/s00134-003-1957-y. 10.1007/s00134-003-1957-y [DOI] [PubMed] [Google Scholar]
- 3.Taccone FS, Artigas AA, Sprung CL, Moreno R, Sakr Y, Vincent JL. Characteristics and outcomes of cancer patients in European ICUs. Crit Care. 2009;13:R15. doi: 10.1186/cc7713. 10.1186/cc7713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lecuyer L, Chevret S, Thiery G, Darmon M, Schlemmer B, Azoulay E. The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 2007;35:808–14. doi: 10.1097/01.CCM.0000256846.27192.7A. 10.1097/01.CCM.0000256846.27192.7A [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Dufaye P, Berre J, Leeman M, Degaute JP, Kahn RJ. Serial lactate determinations during circulatory shock. Crit Care Med. 1983;11:449–51. doi: 10.1097/00003246-198306000-00012. 10.1097/00003246-198306000-00012 [DOI] [PubMed] [Google Scholar]
- 6.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–7. doi: 10.1097/CCM.0b013e31819fcf68. 10.1097/CCM.0b013e31819fcf68 [DOI] [PubMed] [Google Scholar]
- 7.Vernon C, LeTourneau JL. Lactic acidosis: recognition, kinetics, and associated prognosis. Crit Care Clin. 2010;26:255–283. doi: 10.1016/j.ccc.2009.12.007. 10.1016/j.ccc.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 8.Noritomi DT, Soriano FG, Kellum JA, Cappi SB, Biselli PJ, Libório AB, et al. Metabolic acidosis in patients with severe sepsis and septic shock: a longitudinal quantitative study. Crit Care Med. 2009;37:2733–2739. doi: 10.1097/ccm.0b013e3181a59165. 10.1097/CCM.0b013e3181a59165 [DOI] [PubMed] [Google Scholar]
- 9.Galas F, Hajjar L, Almeida JP, Trielli T, Vieira S, Bazan M, et al. Outcomes of 1,000 patients with cancer admitted to the intensive care unit: a Brazilian prospective study. Crit Care. 2010;14((suppl 1)):P425. 10.1186/cc8657 [Google Scholar]
- 10.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. 10.1097/01.CCM.0000298158.12101.41 [DOI] [PubMed] [Google Scholar]
- 11.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- 12.Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial. Crit Care. 2005;9:R687–R93. doi: 10.1186/cc3887. 10.1186/cc3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. 10.1001/jama.270.24.2957 [DOI] [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. 10.1097/00003246-198510000-00009 [PubMed] [Google Scholar]
- 15.Lamia B, Hellot MF, Girault C, Tamion F, Dachraoui F, Lenain P, et al. Changes in severity and organ failure scores as prognostic factors in onco-hematological malignancy patients admitted to the ICU. Intensive Care Med. 2006;32:1560–8. doi: 10.1007/s00134-006-0286-3. 10.1007/s00134-006-0286-3 [DOI] [PubMed] [Google Scholar]
- 16.Park M, Azevedo LC, Maciel AT, Pizzo VR, Noritomi DT, da Cruz Neto LM. Evolutive standard base excess and serum lactate level in severe sepsis and septic shock patients resuscitated with early goal-directed therapy: still outcome markers. Clinics. 2006;61:47–52. doi: 10.1590/s1807-59322006000100009. 10.1590/S1807-59322006000100009 [DOI] [PubMed] [Google Scholar]
- 17.Smith I, Kumar P, Molloy S, Rhodes A, Newman PJ, Grounds RM, et al. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive Care Med. 2001;27:74–83. doi: 10.1007/s001340051352. 10.1007/s001340051352 [DOI] [PubMed] [Google Scholar]
- 18.Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care. 2009;13:R90. doi: 10.1186/cc7918. 10.1186/cc7918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padilla GF, Garibay MA, Hummel HN, Avila R, Méndez A, Ramírez R. Fulminant non-Hodgkin lymphoma presenting as lactic acidosis and acute liver failure: case report and literature review. Acta Gastroenterol Latinoam. 2000;39:129–34. [PubMed] [Google Scholar]