Abstract
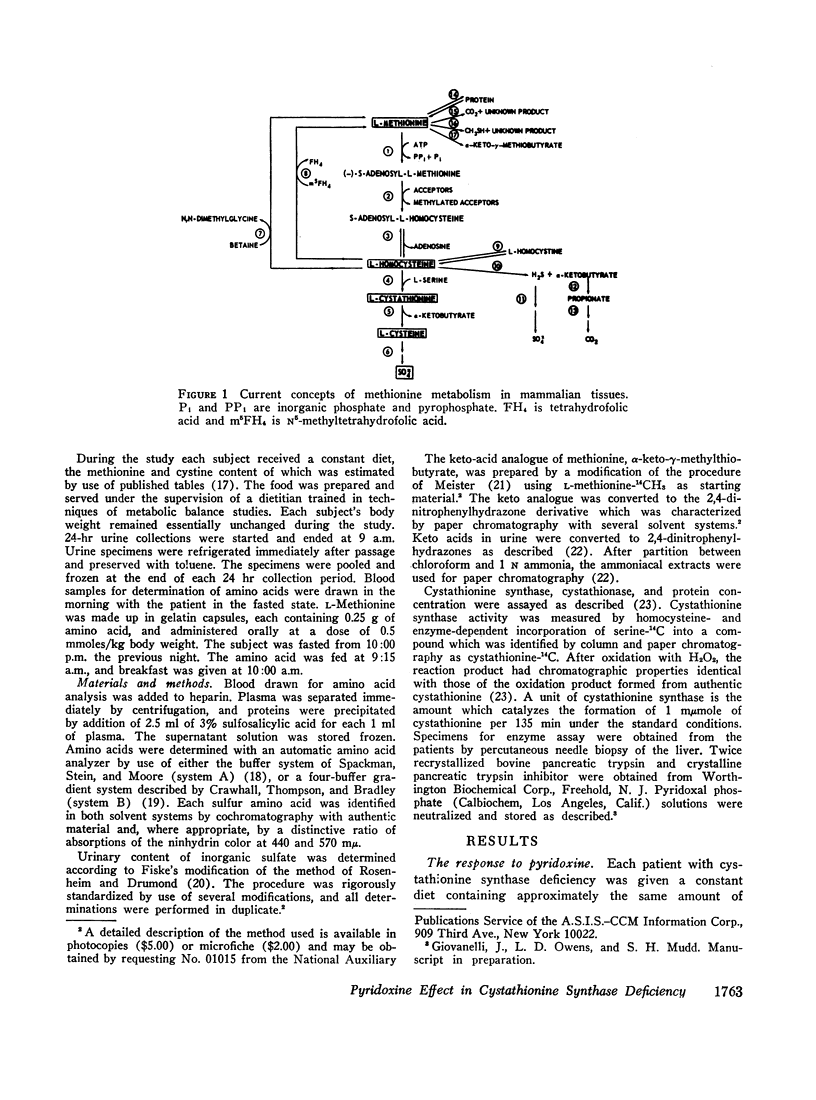
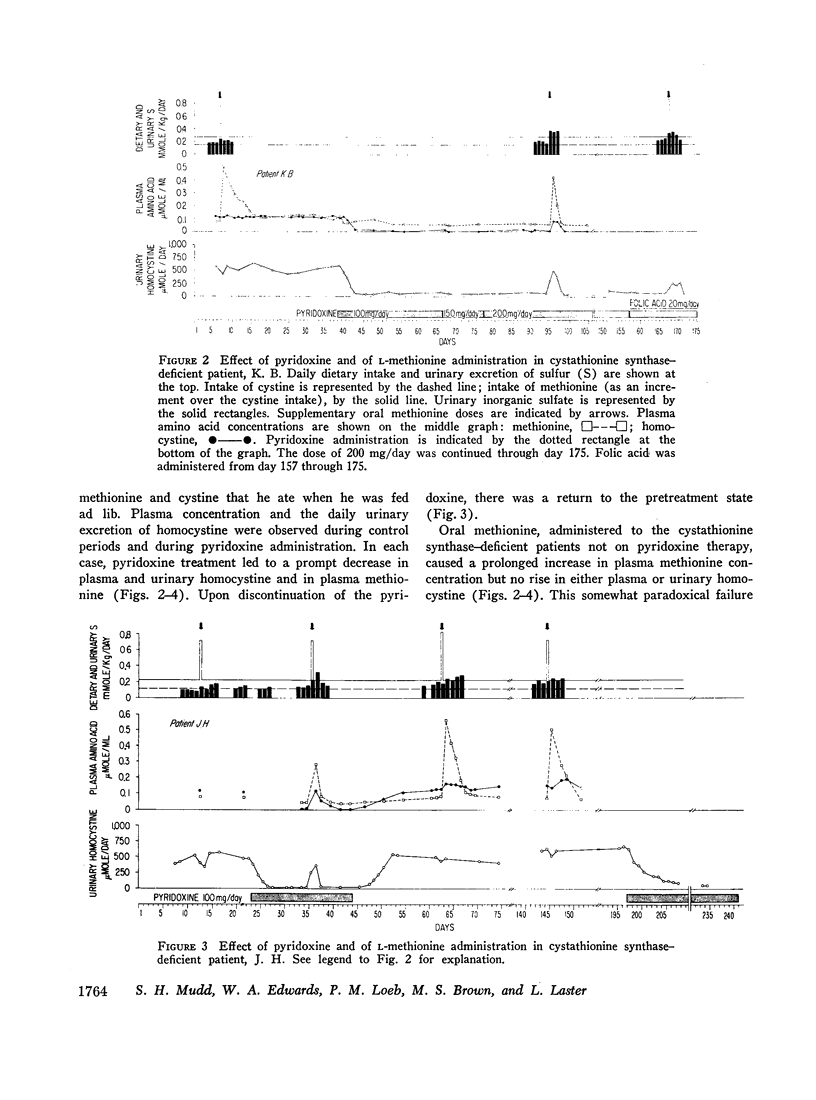
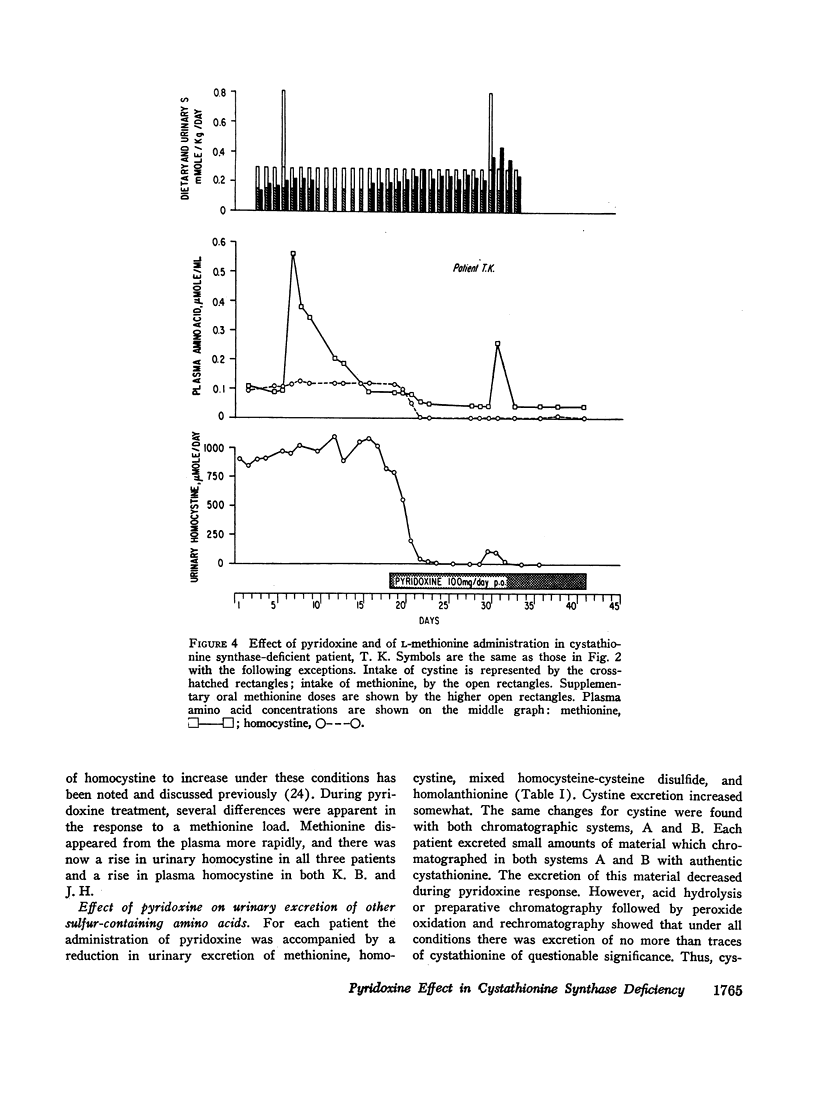
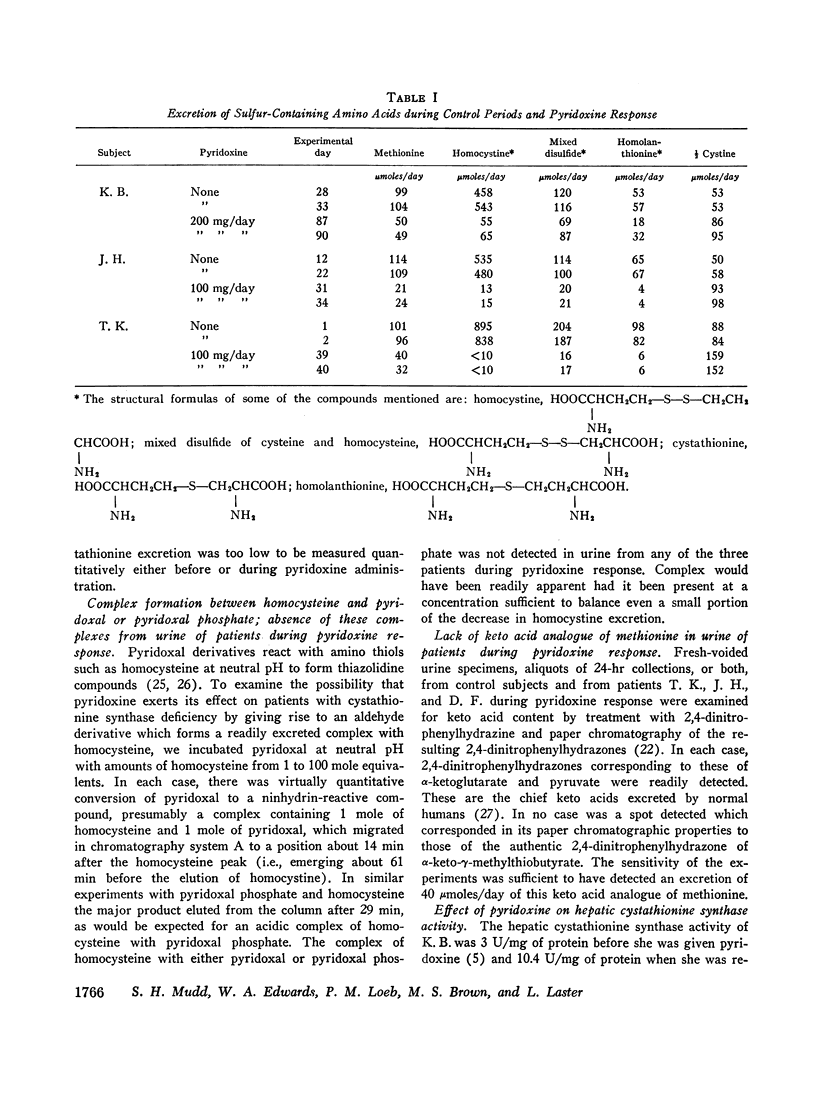
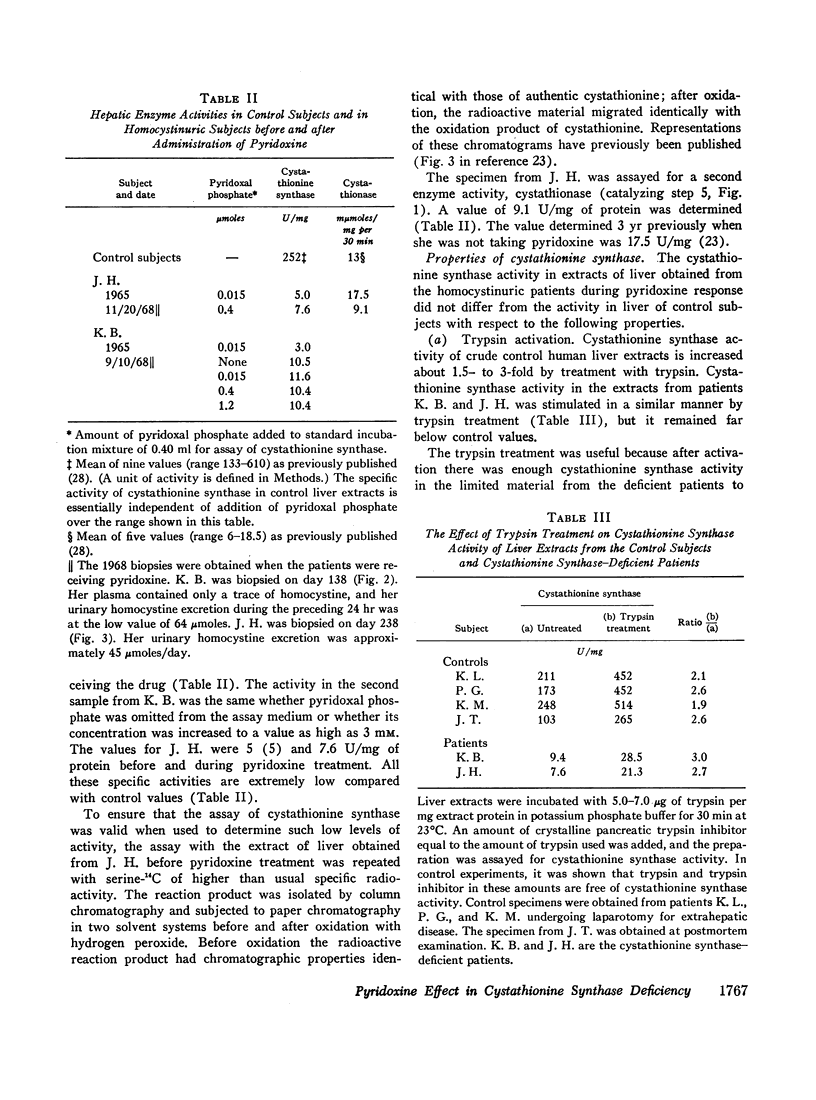
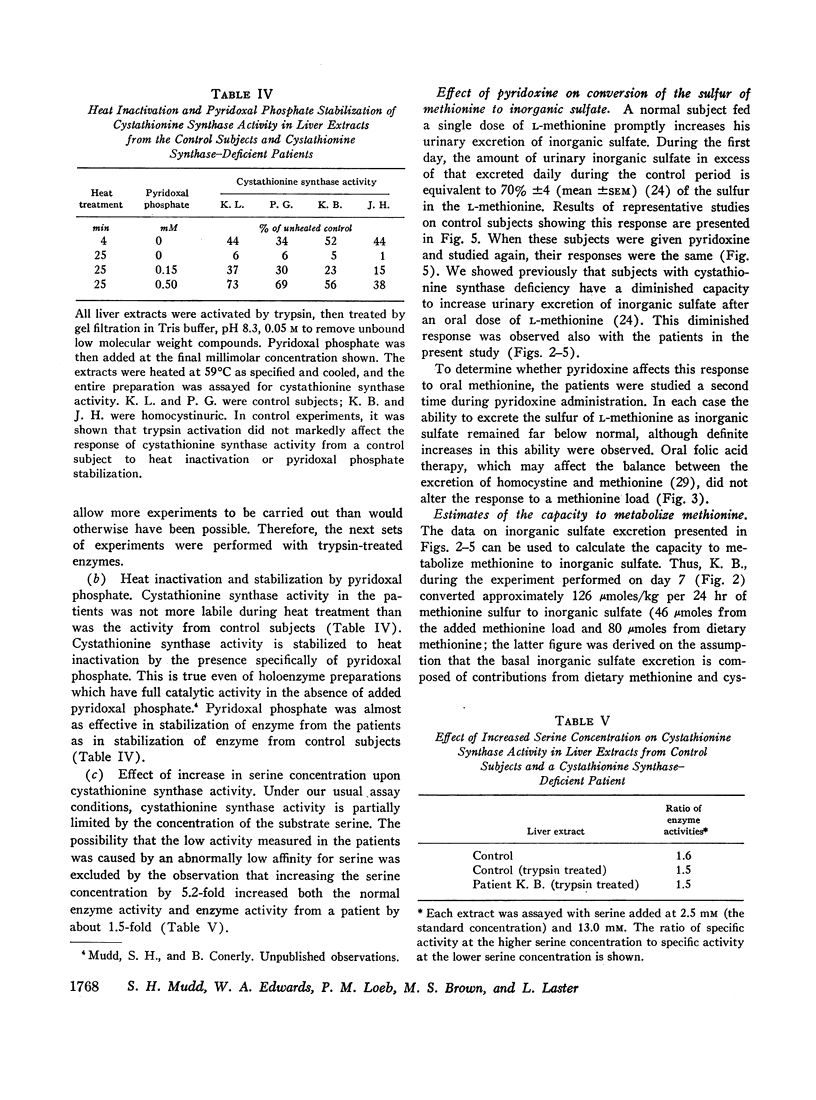
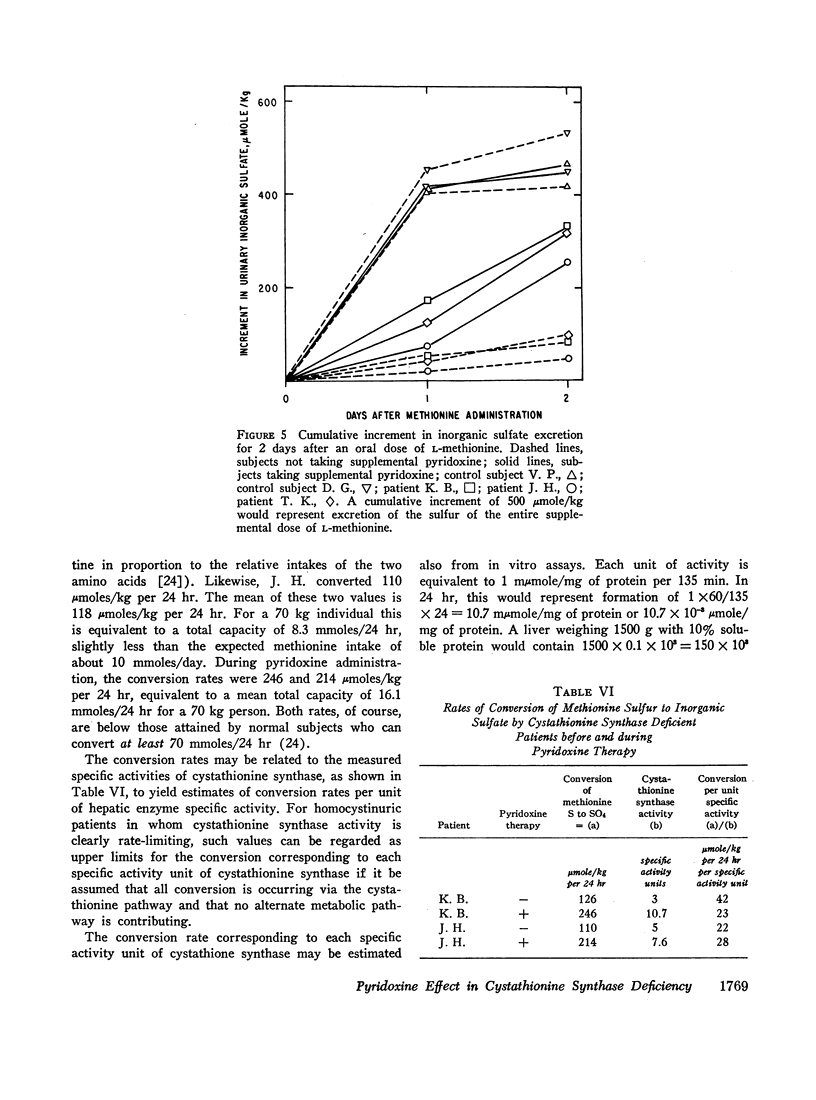
We investigated the effect of pyridoxine administration in three patients with homocystinuria due to cystathionine synthase deficiency. The drug decreased the plasma concentration and urinary excretion of methionine and homocystine and the urinary excretion of homolanthionine and the homocysteine-cysteine mixed disulfide. Urinary cystine rose somewhat. Oral methionine tolerance tests before and during the patients' response to pyridoxine indicated that during response they remained deficient in their capacity to convert the sulfur of methionine to inorganic sulfate, although this capacity increased somewhat. During pyridoxine response only, the methionine loads caused increased homocystinuria. There was no indication that pyridoxine stimulated an alternate pathway of metabolism. The values for specific activity of cystathionine synthase in liver biopsy specimens from two patients in pyridoxine response were 3 and 4% of the mean control value. When these patients were not receiving pyridoxine, comparable values were 2 and 1%, respectively. The hepatic enzyme activity of the mutant patients was similar to normal enzyme activity with respect to trypsin activation, heat inactivation, and stabilization by pyridoxal phosphate. Approximate estimates were made of the relation between total body capacity to metabolize methionine and hepatic cystathionine synthase activity. These estimates suggested that because of the large normal reserve capacity of cystathionine synthase, a few per cent residual activity is sufficient to metabolize the normal dietary load of methionine. Thus, small increases in residual capacity may be of major physiological importance. However, many liver biopsies would be required to establish unequivocally that such changes were due to the administration of a particular therapeutic agent rather than to biological variation. All the data in the present study are consistent with the interpretation that pyridoxine does act by causing an increase in residual cystathionine synthase activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber G. W., Spaeth G. L. The successful treatment of homocystinuria with pyridoxine. J Pediatr. 1969 Sep;75(3):463–478. doi: 10.1016/s0022-3476(69)80274-x. [DOI] [PubMed] [Google Scholar]
- CARSON N. A., CUSWORTH D. C., DENT C. E., FIELD C. M., NEILL D. W., WESTALL R. G. HOMOCYSTINURIA: A NEW INBORN ERROR OF METABOLISM ASSOCIATED WITH MENTAL DEFICIENCY. Arch Dis Child. 1963 Oct;38:425–436. doi: 10.1136/adc.38.201.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVALLINI D., FRONTALI N. Quantitative determination of keto-acids by paper partition chromatography. Biochim Biophys Acta. 1954 Mar;13(3):439–445. doi: 10.1016/0006-3002(54)90351-0. [DOI] [PubMed] [Google Scholar]
- CAVALLINI D., FRONTALI N., TOSCHI G. Keto-acid content of human blood and urine. Nature. 1949 Nov 5;164(4175):792–792. doi: 10.1038/164792b0. [DOI] [PubMed] [Google Scholar]
- Carson N. A., Carré I. J. Treatment of homocystinuria with pyridoxine. A preliminary study. Arch Dis Child. 1969 Jun;44(235):387–392. doi: 10.1136/adc.44.235.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawhall J. C., Thompson C. J., Bradley K. H. Separation of cystine, penicillamine disulfide and cysteine-penicillamine mixed disulfide by automatic amino acid analysis. Anal Biochem. 1966 Mar;14(3):405–413. doi: 10.1016/0003-2697(66)90282-x. [DOI] [PubMed] [Google Scholar]
- Cusworth D. C., Dent C. E. Homocystinuria. Br Med Bull. 1969 Jan;25(1):42–47. doi: 10.1093/oxfordjournals.bmb.a070668. [DOI] [PubMed] [Google Scholar]
- GERRITSEN T., WAISMAN H. A. HOMOCYSTINURIA, AN ERROR IN THE METABOLISM OF METHIONINE. Pediatrics. 1964 Mar;33:413–420. [PubMed] [Google Scholar]
- GREENGARD O., GORDON M. THE COFACTOR-MEDIATED REGULATION OF APOENZYME LEVELS IN ANIMAL TISSUES. I. THE PYRIDOXINE-INDUCED RISE OF RAT LIVER TYROSINE TRANSAMINASE LEVEL IN VIVO. J Biol Chem. 1963 Nov;238:3708–3710. [PubMed] [Google Scholar]
- Gaull G. E., Rassin D. K., Sturman J. A. Pyridoxine-dependency in homocystinuria. Lancet. 1968 Dec 14;2(7581):1302–1302. doi: 10.1016/s0140-6736(68)91797-2. [DOI] [PubMed] [Google Scholar]
- Hambraeus L., Wranne L., Lorentsson R. Biochemical and therapeutic studies in two cases of homocystinuria. Clin Sci. 1968 Dec;35(3):457–466. [PubMed] [Google Scholar]
- Hollowell J. G., Jr, Coryell M. E., Hall W. K., Findley J. K., Thevaos T. G. Homocystinuria as affected by pyridoxine, folic acid, and vitamin B12. Proc Soc Exp Biol Med. 1968 Nov;129(2):327–333. doi: 10.3181/00379727-129-33314. [DOI] [PubMed] [Google Scholar]
- Holten D., Wicks W. D., Kenney F. T. Studies on the role of vitamin B6 derivatives in regulating tyrosine alpha-ketoglutarate transaminase activity in vitro and in vivo. J Biol Chem. 1967 Mar 10;242(5):1053–1059. [PubMed] [Google Scholar]
- Homocystinuria due to cystathionine synthase deficiency. Ann Intern Med. 1965 Dec;63(6):1117–1142. doi: 10.7326/0003-4819-63-6-1117. [DOI] [PubMed] [Google Scholar]
- Kelly S., Copeland W. A hypothesis on the homocystinuric's response to pyridoxine. Metabolism. 1968 Sep;17(9):794–795. doi: 10.1016/0026-0495(68)90029-2. [DOI] [PubMed] [Google Scholar]
- Laster L., Mudd S. H., Finkelstein J. D., Irreverre F. Homocystinuria due to cystathionine synthase deficiency: the metabolism of L-methionine. J Clin Invest. 1965 Oct;44(10):1708–1719. doi: 10.1172/JCI105278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISTER A. Enzymatic preparation of alpha-keto acids. J Biol Chem. 1952 May;197(1):309–317. [PubMed] [Google Scholar]
- MUDD S. H., FINKELSTEIN J. D., IRREVERRE F., LASTER L. HOMOCYSTINURIA: AN ENZYMATIC DEFECT. Science. 1964 Mar 27;143(3613):1443–1445. doi: 10.1126/science.143.3613.1443. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Finkelstein J. D., Irreverre F., Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem. 1965 Nov;240(11):4382–4392. [PubMed] [Google Scholar]
- Mudd S. H., Irreverre F., Laster L. Sulfite oxidase deficiency in man: demonstration of the enzymatic defect. Science. 1967 Jun 23;156(3782):1599–1602. doi: 10.1126/science.156.3782.1599. [DOI] [PubMed] [Google Scholar]
- Roisin M. P., Chatagner F. Purification et étude de quelques propriétés de l'homocystéine désulfhydrase du foie de rat. Identification a la cystathionase. Bull Soc Chim Biol (Paris) 1969 Jul 25;51(3):481–493. [PubMed] [Google Scholar]
- Uhlendorf B. W., Mudd S. H. Cystathionine synthase in tissue culture derived from human skin: enzyme defect in homocystinuria. Science. 1968 May 31;160(3831):1007–1009. doi: 10.1126/science.160.3831.1007. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Tada K., Yokoyama Y., Arakawa T. Homocystinuria of vitamin B6 dependent type. Tohoku J Exp Med. 1968 Nov;96(3):235–242. doi: 10.1620/tjem.96.235. [DOI] [PubMed] [Google Scholar]




