Abstract
OBJECTIVES:
Cytotoxic agents and steroids are used to treat lymphoid malignancies, but these compounds may exacerbate chronic viral hepatitis. For patients with multiple myeloma, the impact of preexisting hepatitis virus infection is unclear. The aim of this study is to explore the characteristics and outcomes of myeloma patients with chronic hepatitis virus infection.
METHODS:
From 2003 to 2008, 155 myeloma patients were examined to determine their chronic hepatitis virus infection statuses using serologic tests for the hepatitis B (HBV) and C viruses (HCV). Clinical parameters and outcome variables were retrieved via a medical chart review.
RESULTS:
The estimated prevalences of chronic HBV and HCV infections were 11.0% (n = 17) and 9.0% (n = 14), respectively. The characteristics of patients who were hepatitis virus carriers and those who were not were similar. However, carrier patients had a higher prevalence of conventional cytogenetic abnormalities (64.3% vs. 25.0%). The cumulative incidences of grade 3-4 elevation of the level of alanine transaminase, 30.0% vs. 12.0%, and hyperbilirubinemia, 20.0% vs. 1.6%, were higher in carriers as well. In a Kaplan-Meier analysis, carrier patients had worse overall survival (median: 16.0 vs. 42.4 months). The prognostic value of carrier status was not statistically significant in the multivariate analysis, but an age of more than 65 years old, the presence of cytogenetic abnormalities, a beta-2-microglobulin level of more than 3.5 mg/L, and a serum creatinine level of more than 2 mg/dL were independent factors associated with poor prognosis.
CONCLUSION:
Myeloma patients with chronic hepatitis virus infections might be a distinct subgroup, and close monitoring of hepatic adverse events should be mandatory.
Keywords: Hepatitis B virus, Hepatitis C virus, Multiple myeloma, Cytogenetic abnormalities, Adverse events
INTRODUCTION
Multiple myeloma is a B-cell malignancy characterized by the proliferation of clonal plasma cells in the bone marrow. Clinically, it often presents with hypercalcemia, renal dysfunction, anemia, and bone disability.1 Since the introduction of combination chemotherapy consisting of melphalan and prednisolone (MP) in the 1960s, steroids have become the backbone of treatment for multiple myeloma. Over the past decade, there has been a dramatic increase in therapeutic options for treating multiple myeloma. These novel agents enhance efficacy and improve survival.2 Nevertheless, steroids remain a major component in these novel regimens. However, cytotoxic agents and immunosuppressive therapy, such as the use of steroids, may lead to uncontrolled replication of hepatitis viruses, followed by an exaggerated immunological response to virus-infected hepatocytes, which can trigger the reactivation or acute exacerbation of chronic hepatitis virus infection.3 In fact, early studies have shown that even the use of steroids alone can have a deleterious effect on patients with chronic hepatitis virus infections.4
Lymphoma patients experience a greater frequency of hepatitis B virus (HBV) reactivation during treatment with steroids and cytotoxic agents; such reactivation can be a fatal complication.5 Takai et al. reported that, in 601 patients with hematological malignancies, the incidence of post-chemotherapy liver injury among patients with chronic HBV infection, i.e., HBV carriers, was significantly higher than that among non-carriers, suggesting that chronic hepatitis virus infection might interfere with chemotherapy and affect the outcomes of these patients.6 Another study that investigated chronic HBV infections in diffuse large B-cell lymphoma patients showed that the overall survival of carrier patients with hepatic dysfunction was significantly shorter than that of patients without liver dysfunction.7 In contrast, significant hepatic dysfunction and reactivation of the hepatitis C virus (HCV) are less common among HCV-infected patients treated with chemotherapy for hematological malignancies.3,8 Even so, HCV seropositivity has been reported to be a significant risk factor for non-relapse mortality after allogeneic hematopoietic stem cell transplantation (SCT).9 Despite the abundant research investigating the impact of hepatitis in lymphoid malignancies, previous studies have included only a small number of patients with multiple myeloma, and reports on the impact of chronic hepatitis virus infection in patients with multiple myeloma are lacking.
Taiwan is an endemic area for HBV and HCV, with prevalences of 17.3% and 4.4%, respectively.10 We have previously reported a higher chronic HBV infection rate (23.5%) in patients with non-Hodgkin's lymphoma, whereas the prevalence of chronic HCV infection was similar to that in the general population (4.8%).11 The aims of the current study were to assess the prevalence of chronic hepatitis virus infections and to investigate their characteristics and prognostic significance in a consecutive series of myeloma patients.
MATERIALS AND METHODS
Patients
From January 2003 to December 2008, 222 consecutive patients with multiple myeloma were diagnosed at Taipei Veterans General Hospital, a tertiary medical center in Taiwan. After excluding cases without serologic test results for hepatitis viruses, 155 patients (69.8%) were enrolled for analysis. The diagnosis of multiple myeloma was based on the criteria proposed by the International Myeloma Working Group,12 and all patients were staged at diagnosis according to the International Staging System. Patients diagnosed with monoclonal gammopathy of undetermined significance or POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes) syndrome were excluded from this study. Variables regarding clinical characteristics, laboratory data, and pathology reports were retrieved from the hospitalization database via a medical chart review. This study was approved by the Institutional Review Board of Taipei Veterans General Hospital.
Cytogenetic analysis
A conventional cytogenetic analysis was performed using bone marrow samples collected at diagnosis using the Giemsa-banding staining technique. At least 20 metaphase cells were examined. A patient was considered to have cytogenetic abnormality if either one of the following was observed: 1) a minimum of two mitotic cells with a gain of the same chromosome or with the same structural abnormality, or 2) three mitotic cells with the loss of the same chromosome. If diagnostic aspiration yielded fewer than 20 metaphase cells, then a repeat aspiration was usually indicated to fulfill the requirement.
Treatment
The general treatment strategy in this study adhered to the suggestions for multiple myeloma in international treatment guidelines.13 Briefly, for patients who were transplant eligible, induction chemotherapy consisting of vincristine, doxorubicin, and dexamethasone (VAD) and thalidomide-based regimens were the most commonly used front-line therapies. Hematopoietic stem cells were collected after four to six cycles of induction therapy, and then autologous transplantations proceeded with melphalan-based conditioning regimens. For those patients who were transplant ineligible, chemotherapy consisting of MP with or without thalidomide was the preferred regimen. Thalidomide was also used in relapsed or refractory settings, either alone or in combination with chemotherapy. Taiwan National Health Insurance has reimbursed the cost of bortezomib use in relapsed or refractory myeloma patients since 2007. Bortezomib was found to be commonly given as a single agent or in combination with dexamethasone. Lenalidomide was not available in Taiwan throughout our study period. Radiotherapy and adjunctive treatments, such as bisphosphonate, were given as clinically indicated.
Serology tests for chronic hepatitis virus infection and the definition of carriers
HBsAg and antibodies to the hepatitis C virus (anti-HCV) were detected serologically using microparticle enzyme immunoassays (IMx-Abbott Laboratories, Abbott Park, IL, USA, and MEIA, Abbott IMx HCV version 3.0, USA).11 Carrier patients were defined as individuals who had positive serology tests for HBsAg or anti-HCV. The existence of antibodies to surface (anti-HBs) or core (anti-HBc) antigens of HBV and the viral loads of HBV and HCV were not routinely checked in all patients. Such checks were performed at the discretion of the treating physicians. The grading of adverse events related to the hepatobiliary system was based on the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Laboratory data on alanine transaminase (ALT), aspartate transaminase (AST), and total bilirubin levels were obtained at diagnosis and throughout the treatment course.
Statistical analysis
The correlations between variables and hepatitis virus carriage were assessed using Fisher's exact test, a χ2 test, or the Mann-Whitney U test, as appropriate. The date of the first adverse hepatic event after the diagnosis of multiple myeloma in each patient was recorded. The numbers of hepatic adverse events of each grade between the two groups were compared using Fisher's exact test or a χ2 test, as appropriate. Cumulative incidence curves of the first hepatic adverse events were plotted, and the time to the first event was compared based on the carrier status. The survival end-point was overall survival (OS) and was measured from the date of diagnosis to the date of death or the last follow-up. Survival was estimated using the Kaplan-Meier method, and the log-rank test was used to compare the survival curves between each variable. The Cox proportional hazards model was used in univariate and multivariate analyses to determine the influence of variables on OS. Variables with p<0.10 in the univariate analysis were included in the multivariate analysis. A p-value<0.05 in a two-tailed test was considered statistically significant. SPSS (version 17.0, SPSS, Chicago, IL, USA) was used for all statistical analyses.
RESULTS
Among 155 patients with multiple myeloma, 30 were chronic hepatitis carriers, and the estimated prevalence was 19.4% (95% confidence interval [CI], 13.0–25.7%). Among these carriers, 17 were HBsAg positive (11.0%, 95% CI, 5.9–16.0%), 14 were anti-HCV positive (9.0%, 95% CI, 4.4–13.6%), and one patient was co-infected with HBV and HCV. The median age of all 155 patients was 69.0 years old (range, 29–91 years), including 91 patients (58.7%) 65 years old or older. The median follow-up was 19.3 months (range, 0.2–83.9 months). According to the ISS definition, 54.2% (84/155) of the patients were classified as stage 3. Table 1 shows that the baseline characteristics, including age, sex, M-protein, presence of plasmacytoma, number of bone lesions, ISS stage, and laboratory parameters, were similar for patients regardless of carrier status. Approximately one-fourth of the patients was heavily treated, but the number of received therapies, the use of thalidomide and bortezomib, and the number of patients receiving stem cell transplantation did not differ between the two groups.
Table 1.
Characteristics of 155 patients with multiple myeloma according to their viral hepatitis carrier status.
| No. of patients (%) | p-value* | ||||
| Non-carriers(n = 125) | Carriers(n = 30) | ||||
| Age ≥65 years | 74 | (59.2) | 17 | (56.7) | 0.800 |
| Male sex | 90 | (72.0) | 23 | (76.7) | 0.606 |
| Serum M-protein | 0.382 | ||||
| IgG | 64 | (51.2) | 12 | (40.0) | |
| IgA | 38 | (30.4) | 13 | (43.3) | |
| Light chain disease | 19 | (15.2) | 3 | (10.0) | |
| Others† | 4 | (3.2) | 2 | (6.7) | |
| Presence of plasmacytoma | 15 | (12.0) | 5 | (16.7) | 0.494 |
| Bone lesion(s) | 0.642 | ||||
| None | 40 | (32.0) | 7 | (23.3) | |
| 1 | 16 | (12.8) | 4 | (13.3) | |
| ≥2 | 69 | (55.2) | 19 | (63.3) | |
| ISS stage | 0.805 | ||||
| 1 | 35 | (28.0) | 9 | (30.0) | |
| 2 | 23 | (18.4) | 4 | (13.3) | |
| 3 | 67 | (53.6) | 17 | (56.7) | |
| Baseline laboratory parameters | |||||
| β2m ≥3.5 mg/L | 94 | (75.2) | 22 | (73.3) | 0.832 |
| Albumin <3.5 g/L | 56 | (44.8) | 13 | (43.3) | 0.885 |
| Hemoglobin <10 g/dL | 75 | (60.0) | 18 | (60.0) | 1.00 |
| Platelets <100×109/L | 23 | (18.4) | 6 | (20.0) | 0.840 |
| Serum calcium ≥12.0 mg/dL | 11 | (8.8) | 5 | (16.7) | 0.203 |
| Serum creatinine ≥2.0 mg/dL | 36 | (28.8) | 13 | (43.3) | 0.124 |
| Serum ALT >upper normal limit | 16 | (12.9) | 4 | (13.3) | 0.950 |
| Number of therapies | 0.428 | ||||
| 0-1 | 56 | (44.8) | 17 | (56.7) | |
| 2 | 33 | (26.4) | 5 | (16.7) | |
| ≥3 | 36 | (28.8) | 8 | (26.7) | |
| Use of thalidomide | 69 | (55.2) | 11 | (36.7) | 0.068 |
| Use of bortezomib | 19 | (15.2) | 5 | (16.7) | 0.842 |
| Stem cell transplantation | 30 | (24.0) | 5 | (16.7) | 0.388 |
Fisher's exact test or χ2 test as appropriate.
Including IgM, IgD, IgE, and non-secretory myeloma.
ALT, alanine transaminase; β2m, beta-2-microglobulin; ISS, International Staging System.
Of the 94 patients with cytogenetic data available, cytogenetic abnormalities, all of which were complex karyotypes, were reported in 30.9% (29/94) of patients (Table 2). Abnormal karyotypes were noted in 9 of 14 carrier patients, and the incidence of abnormal karyotypes was significantly higher among carriers than among non-carriers (64.3% vs. 25.0%, p = 0.003). Hypodiploidy, chromosome 13 deletion, and chromosome 11q abnormalities, which have been previously reported as high-risk cytogenetic alterations,14-16 were found in 15 (16.0%), 14 (14.9%), and 5 (5.3%) patients, respectively. Carrier patients harbored a substantial number of these adverse cytogenetic abnormalities as well (21.4%, 21.4%, and 7.1%, respectively).
Table 2.
Cytogenetic abnormalities detected by conventional cytogenetic analysis in 94 myeloma patients.
| Cytogenetic changes | No. of patients (%) | p-value* | ||
| All patients(n = 94) | Non-carrier patients(n = 80) | Carrier patients(n = 14) | ||
| Normal | 65 (69.1) | 60 (75.0) | 5 (35.7) | 0.003 |
| Abnormal | 29 (30.9) | 20 (25.0) | 9 (64.3) | |
| Hypodiploidy | 15 (16.0) | 12 (15.0) | 3 (21.4) | |
| Del(13) | 14 (14.9) | 11 (13.8) | 3 (21.4) | |
| 11q abnormalities | 5 (5.3) | 4 (5.0) | 1 (7.1) | |
By χ2 test.
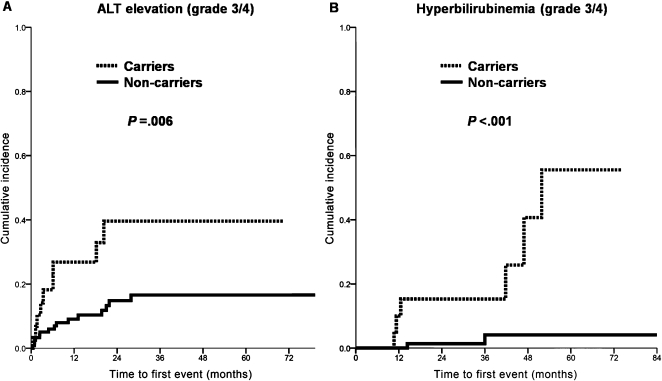
A higher rate of post-chemotherapy liver injury has been reported in patients with hematological malignancies and HBV infections.6 To investigate the incidence of hepatic adverse events in carrier patients with multiple myeloma, alterations in the total bilirubin levels were recorded throughout the treatment course. Among the 17 patients who were HBV carriers, antiviral prophylaxis, either with lamivudine (n = 11) or entecavir (n = 1), was prescribed for 12 (70.6%). No prophylaxis was administered to HCV carriers. Table 3 shows the incidence of hepatic adverse events during the entire treatment course. The carrier group had a significantly higher incidence of grade 3-4 ALT elevation (30.0% vs. 12.0%, p = 0.014). Grade 3-4 events for AST elevation were also more common in the carrier group (20.0% vs. 8.8%, p = 0.078). Hyperbilirubinemia with all grades of adverse events or with grade 3-4 adverse events was more frequently encountered in the carrier group (rates of grade 3-4 events, 20.0% vs. 1.6%, p<0.001). Curves for the three-year cumulative incidence of hepatic adverse events were plotted. The grade 3-4 ALT elevations were observed in 39.6% of carriers and 16.6% of non-carriers (p = 0.006, Figure 1A). The incidence of hyperbilirubinemia also differed significantly between the carrier and non-carrier groups (15.3% vs. 4.1%, p<0.001, Figure 1B). The incidence of hyperbilirubinemia continued to increase in the carrier group beyond three years, in contrast to the plateau observed in the non-carrier group. One patient died of acute hepatitis attributable to HBV reactivation. Despite lamivudine prophylaxis, the patient developed severe jaundice, became seropositive for the hepatitis B envelope antigen, and showed a marked increase in the level of HBV DNA of up to 9.6×108 copies/mL in the eleventh month after treatment. Otherwise, no HCV reactivation was observed in this cohort during the study period.
Table 3.
Incidence of hepatic adverse events among 155 patients with multiple myeloma.
| Non-carriers, no. (%)(n = 125) | No. of carrier patients (%) | p-value* | |||
| All(n = 30) | HBV carriers†(n = 17) | HCV carriers†(n = 14) | |||
| ALT elevation | |||||
| All grades | 82 (65.6%) | 17 (56.7%) | 9 (52.9%) | 9 (64.3%) | 0.360 |
| Grade 3-4 | 15 (12.0%) | 9 (30.0%) | 5 (29.4%) | 4 (28.6%) | 0.014 |
| AST elevation | |||||
| All grades | 64 (51.2%) | 17 (56.7%) | 10 (58.8%) | 8 (57.1%) | 0.590 |
| Grade 3-4 | 11 (8.8%) | 6 (20.0%) | 4 (23.5%) | 2 (14.3%) | 0.078 |
| Hyperbilirubinemia | |||||
| All grades | 29 (23.2%) | 13 (43.3%) | 7 (41.2%) | 6 (42.9%) | 0.026 |
| Grade 3-4 | 2 (1.6%) | 6 (20.0%) | 4 (23.5%) | 2 (14.3%) | <0.001 |
Fisher's exact test or χ2 test as appropriate; carrier patients compared with patients who were not carriers.
One patient was a carrier of both HBV and HCV.
ALT, alanine transaminase; AST, aspartate transaminase.
Figure 1.
Cumulative incidences of (a) grade 3 alanine transaminase elevation and (b) grade 3 hyperbilirubinemia.
The estimated probability of OS among carrier patients was less than that among non-carrier patients (median OS 16.0 vs. 42.4 months, hazard ratio [HR] 1.8, 95% CI, 1.07-3.0, p = 0.026). Regarding myeloma-related deaths, the median disease-specific survivals for carrier and non-carrier patients were 16.0 and 45.5 months, respectively. Eight patients died of causes not related to myeloma (two in the carrier group and six in the non-carrier group). Five of these deaths were attributed to cardiovascular complications, and one patient died of HBV reactivation with liver failure, as described above. Other factors influenced OS in the univariate analysis. These factors included age, ISS stage, the presence of cytogenetic abnormalities, the platelet count, and the levels of β-2-microglobulin (β2m), albumin, serum calcium, and serum creatinine (Table 4). In the multivariate analysis, chronic hepatitis virus infection was not an independent factor related to OS. However, being more than 65 years old (HR 2.858, 95% CI, 1.412-5.785, p = 0.004), the presence of cytogenetic abnormalities (HR 3.036, 95% CI, 1.584-5.817, p = 0.001), and a serum creatinine level greater than 2 mg/dL (HR 3.050, 95% CI 1.541-6.035, p = 0.001) remained statistically significant prognostic factors.
Table 4.
Univariate and multivariate analyses for overall survival in patients with multiple myeloma.
| Univariate | Multivariate* | |||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Viral hepatitis (carriers vs. non-carriers) | 1.801 | 1.072 – 3.023 | 0.026 | - | - | - |
| Age (≥65 vs. <65) | 2.735 | 1.727 – 4.333 | <0.001 | 2.858 | 1.412 – 5.785 | 0.004 |
| Sex (male vs. female) | 0.908 | 0.593 – 1.393 | 0.659 | - | - | - |
| Plasmacytoma (presence vs. absence) | 0.979 | 0.566 – 1.694 | 0.941 | - | - | - |
| ISS stage | ||||||
| 2 vs. 1 | 3.101 | 1.509 – 2.618 | 0.002 | |||
| 3 vs. 1 | 4.631 | 2.506 – 8.556 | <0.001 | - | - | - |
| Cytogenetics (abnormal vs. normal) | 2.123 | 1.202 – 3.749 | 0.009 | 3.036 | 1.584 – 5.817 | 0.001 |
| Bone lesions (≥2 vs. 0-1) | 1.252 | 0.800 – 1.958 | 0.325 | - | - | - |
| β2m (≥3.5 vs. <3.5 mg/L) | 5.756 | 2.665 – 12.430 | <0.001 | 3.767 | 0.860 – 16.504 | 0.079 |
| Albumin (<3.5 vs. ≥3.5 g/dL) | 1.727 | 1.172 – 2.546 | 0.006 | - | - | - |
| Hemoglobin (<10 vs. ≥10 g/dL) | 2.137 | 1.404 – 3.254 | <0.001 | - | - | - |
| Platelet count (< 100×109 vs. ≥100×109/L) | 1.529 | 0.963 – 2.427 | 0.072 | - | - | - |
| Serum calcium (≥12 vs. <12 mg/dL) | 2.986 | 1.748 – 5.101 | <0.001 | - | - | - |
| Serum creatinine (≥2.0 vs. <2.0 mg/dL) | 2.519 | 1.709 – 3.713 | <0.001 | 3.050 | 1.541 – 6.035 | 0.001 |
| Serum ALT (abnormal vs. normal) | 1.256 | 0.674 – 2.341 | 0.473 | - | - | - |
Variables significant at p<0.1 in the univariate model were entered in the Cox regression multivariate model using conditional backward analysis.
HR, hazard ratio; CI, confidence interval; ALT, alanine transaminase; β2m, beta-2-microglobulin; ISS, International Staging System.
DISCUSSION
The results of the current study reveal that the impact of chronic HBV and HCV infections is not only clinically significant for patients with lymphoma, a population known to be vulnerable to viral hepatitis, but also for patients with multiple myeloma. Here, we showed that the prevalences of chronic HBV and HCV infections in myeloma patients were 11.0% and 9.0%, respectively. Carrier status was associated with the presence of cytogenetic abnormalities, and carriers experienced more hepatic adverse events during the entire treatment course. Moreover, patients who were hepatitis virus carriers had a worse OS. To the best of our knowledge, this is the first study to investigate the clinical significance of chronic hepatitis virus infection in patients with multiple myeloma.
Many studies have investigated the prevalence of hepatitis virus infections in patients with hematological malignancies. However, only a few studies have included significant numbers of multiple myeloma patients. Table 5 provides a summary of these studies. Most studies regarding the prevalence of HCV infection have been conducted in HCV-endemic areas, such as Southern Europe and East Asia, and these studies have suggested that HCV infection has a pathogenic role in lymphoproliferative disorders, including multiple myeloma.6, However, some reports have revealed similar incidences of HCV infection in myeloma patients and in controls.22-27 In addition, the results from several population-based studies that evaluated the incidence of myeloma among HCV carriers have produced contradictory results.28-31 Few studies have explored the association between HBV and multiple myeloma. Using a similar serologic method adopted by previous studies for detection, our study provided results from an endemic area and revealed a higher incidence of HCV infection in individuals with multiple myeloma. Nevertheless, larger epidemiologic studies and further experiments elucidating the molecular mechanisms connecting hepatitis viruses and the pathogenesis of myeloma are warranted.
Table 5.
Summary of studies (with ≥50 enrolled patients) exploring the prevalence of chronic hepatitis virus infection in multiple myeloma patients.
| AuthorYear | Country | No. of MM patients | No. (%) of HBV carriers | % of HBV among controls | No. (%) of HCV carriers | % of HCV among controls | Major findings |
| 1995 Cavanna20 | Italy | 90 | - | - | 15 (16.7) | 1.7 | Higher prevalence of HCV infection in patients with LPDs |
| 1996 Silvestri17 | Italy | 78 | - | - | 3 (3.8) | 3.2 | Prevalence and relative risk of being infected by HCV is higher in B-NHL but not MM patients |
| 1996 Musto21 | Italy | 90 | - | - | 10 (11.1) | 5.4 | Higher prevalence of HCV infection in patients with non-cryoglobulinemic B-LPD including MM |
| 1997 De Rosa18 | Italy | 56 | - | - | 9 (16.1) | 1.9 | Higher prevalence of HCV infection in patients with B-LPD including MM |
| 2004 Bianco22 | Italy | 107 | - | 5 (4.7) | 5.6 | HCV infection may be associated with some lymphoid and myeloid malignancies but not MM and HD | |
| 2004 De Sanjose23 | Spain | 74 | - | - | 2 (2.7) | 3.8 | Excess risk associated with HCV in some lymphoma subtypes (not including MM), but not statistically significant |
| 2005 Takai6 | Japan | 124 | 4 (3.2) | 1.2 | 8 (6.5) | 2.6 | Liver injury in HBV carriers was more severe than that in HCV carriers and non-carriers in the whole study population (acute leukemia, NHL, and MM) |
| 2006 Takeshita19 | Japan | 81 | - | - | 4 (4.9) | 2.5 | Higher prevalence of HCV infection in B-NHL including MM |
| 2007 Veneri24 | Italy | 139 | - | - | 1 (0.7) | 5.4 | No increase of HCV prevalence in patients with MM |
| 2008 Anderson25 | U.S. | 9,995 | 31 (0.3) | 0.2 | 20 (0.2) | 0.2 | HCV is associated with elevated risk of NHL and AML but not MM* |
| 2008 Okan26 | Japan | 67 | 2 (3.0) | 4.9 | 1 (1.5) | 1.1 | No significant difference in the combined prevalence of HBV and HCV infections in patients with LPD |
| 2011 Franceschi27 | European Union | 238 | 6 (2.5) | 0.6 | 1 (0.4) | 0.6 | HBV is associated with the risk of MM, HD, and NHL |
| Current study | Taiwan | 155 | 17 (11.0) | 17.3 | 14 (8.4) | 4.4 | Hepatitis virus carriage was associated with the presence of cytogenetic abnormalities, more hepatic adverse events and worse survival |
AML, acute myeloid leukemia; HBV, hepatitis B virus; HCV, hepatitis C virus; HD, Hodgkin's disease; LPD, lymphoproliferative disorders; MM, multiple myeloma; NHL, non-Hodgkin's lymphoma.
Diagnosis of HBV and HCV infection was based on the International Classification of Diseases codes for Medicare.
A unique feature of the carrier patients in our study was their greater incidence of cytogenetic abnormalities, which was a strong prognostic factor for a worse OS. As early as the 1970s, chromosome abnormalities were observed in the lymphocytes of patients with chronic hepatitis.32 Subsequent studies showed that oxidative DNA damage occurs in circulating leukocytes as an early event in chronic HCV infection.33 Furthermore, Cardin et al. demonstrated that the level of 8-hydroxydeoxyguanosine, an indicator of oxidative DNA damage, in circulating leukocytes correlated well with the severity of HCV-related liver disease in a population-based study.34 Another study also illustrated that the levels of DNA damage in lymphocytes correlated independently with the severity of both chronic hepatitis B and chronic hepatitis C.35 Recently, the genotoxic effects of HBV and HCV were demonstrated in peripheral blood lymphocytes via the occurrence of DNA fragmentation.36 According to the findings above, chronic viral hepatitis might contribute to the susceptibility of plasma cells, to the terminal differentiation of lymphocytes, to genomic instability and to subsequent cytogenetic abnormalities. However, the associations and the molecular mechanisms underlying these results require further exploration and confirmation. In addition to cytogenetic changes, we also found that myeloma patients who were chronic viral hepatitis carriers experienced more and earlier hepatic adverse events during treatment. This result is consistent with the finding of Takai et al. that the incidence of post-chemotherapy liver injury is higher in HBV carriers among patients with hematological malignancies.6 Moreover, the increase in hepatic adverse events during treatment could not be explained by the difference in the therapies received (as shown in Table 1). Taken together, the features of this patient subgroup suggest that patients with chronic viral hepatitis are possibly a distinct subgroup of multiple myeloma patients. Determination of carrier status, especially in an endemic area, and close follow-up of liver dysfunction should be mandatory for these patients.
Due to the retrospective nature of this study, molecular-based cytogenetic technique data, such as those from fluorescence in situ hybridization (FISH), were not available. However, conventional cytogenetic abnormalities retain their prognostic value in some circumstances. As reported, multiple myeloma cells are difficult to karyotype because of their low proliferative rates. Therefore, an abnormal karyotype is indicative of higher proliferation and a poor prognosis.37 Many studies have suggested the prognostic value of hypodiploidy or chromosome 13 deletion detected by conventional cytogenetic analysis in myeloma patients.14-16 In our study, the presence of complex karyotypes and a significant portion of patients with the two above-mentioned abnormalities might explain the adverse outcomes for patients with abnormal cytogenetic changes. Further studies incorporating FISH analysis are necessary to confirm the association between hepatitis carriage and cytogenetic abnormalities.
To prevent HBV reactivation during chemotherapy, lamivudine is recommended as prophylaxis in lymphoma patients positive for HBsAg and in patients with past HBV infections.38-39 This strategy should perhaps also be applied to myeloma patients, although the basis for this recommendation is less clear. In this study, most of the anti-viral prophylaxis for HBV-infected patients (11/12) was given in the context of secondary prophylaxis. This fact might be attributed to the limited reimbursement for prophylactic agents in previous years. Therefore, the impact of anti-viral prophylaxis on the development of hepatic adverse events might not have been observed. Future studies highlighting the role of anti-viral prophylaxis in myeloma patients with HBV infections are warranted.
In conclusion, our study determined the prevalence of HBV and HCV infection in myeloma patients in an endemic area and proposed that these patients might comprise a distinct subgroup with more hepatic adverse events and worse survival rates. Serologic tests at diagnosis are necessary to identify these patients, and regular monitoring for liver dysfunction should be mandatory. Anti-viral prophylaxis should play a role in the treatment of HBV-infected patients, although the efficacy of such prophylaxis requires validation in subsequent studies. In addition, the association between cytogenetic abnormalities in multiple myeloma patients and hepatitis virus infection warrants further study to clarify the mechanism behind the relationship.
ACKNOWLEDGMENTS
This study was supported by grants from Taipei Veterans General Hospital (V97B1-011 and V99A-153) and the Taiwan Clinical Oncology Research Foundation.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Katzel JA, Hari P, Vesole DH. Multiple myeloma: charging toward a bright future. CA Cancer J Clin. 2007;57:301–18. doi: 10.3322/CA.57.5.301. 10.3322/CA.57.5.301 [DOI] [PubMed] [Google Scholar]
- 2.Mark T, Niesvizky R, Coleman M. Novel agents in myeloma: an exciting saga. Cancer. 2009;115:236–42. doi: 10.1002/cncr.24040. 10.1002/cncr.24040 [DOI] [PubMed] [Google Scholar]
- 3.Vento S, Cainelli F, Longhi MS. Reactivation of replication of hepatitis B and C viruses after immunosuppressive therapy: an unresolved issue. Lancet Oncol. 2002;3:333–40. doi: 10.1016/s1470-2045(02)00773-8. 10.1016/S1470-2045(02)00773-8 [DOI] [PubMed] [Google Scholar]
- 4.Lam KC, Lai CL, Trepo C, Wu PC. Deleterious effect of prednisolone in HBsAg-positive chronic active hepatitis. N Engl J Med. 1981;304:380–6. doi: 10.1056/NEJM198102123040702. 10.1056/NEJM198102123040702 [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Hsiung CA, Su IJ, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37:1320–8. doi: 10.1053/jhep.2003.50220. 10.1053/jhep.2003.50220 [DOI] [PubMed] [Google Scholar]
- 6.Takai S, Tsurumi H, Ando K, et al. Prevalence of hepatitis B and C virus infection in haematological malignancies and liver injury following chemotherapy. Eur J Haematol. 2005;74:158–65. doi: 10.1111/j.1600-0609.2004.00376.x. 10.1111/j.1600-0609.2004.00376.x [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Xu RH, Luo HY, Zhang DS, Jiang WQ, Huang HQ, et al. Clinical and prognostic analysis of hepatitis B virus infection in diffuse large B-cell lymphoma. BMC Cancer. 2008;8:115. doi: 10.1186/1471-2407-8-115. 10.1186/1471-2407-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuckerman E, Zuckerman T, Douer D, Qian D, Levine AM. Liver dysfunction in patients infected with hepatitis C virus undergoing chemotherapy for hematologic malignancies. Cancer. 1998;83:1224–30. 10.1002/(SICI)1097-0142(19980915)83:6<1224::AID-CNCR23>3.0.CO;2-6 [PubMed] [Google Scholar]
- 9.Ramos CA, Saliba RM, de Pádua L, Khorshid O, Shpall EJ, Giralt S, et al. Impact of hepatitis C virus seropositivity on survival after allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Haematologica. 2009;94:249–57. doi: 10.3324/haematol.13756. 10.3324/haematol.13756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CH, Yang PM, Huang GT, Lee HS, Sung JL, Sheu JC. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J Formos Med Assoc. 2007;106:148–55. doi: 10.1016/S0929-6646(09)60231-X. 10.1016/S0929-6646(09)60231-X [DOI] [PubMed] [Google Scholar]
- 11.Chen MH, Hsiao LT, Chiou TJ, Liu JH, Gau JP, Teng HW, et al. High prevalence of occult hepatitis B virus infection in patients with B cell non-Hodgkin's lymphoma. Ann Hematol. 2008;87:475–80. doi: 10.1007/s00277-008-0469-9. 10.1007/s00277-008-0469-9 [DOI] [PubMed] [Google Scholar]
- 12.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57. 10.1046/j.1365-2141.2003.04355.x [PubMed] [Google Scholar]
- 13.Anderson KC, Alsina M, Bensinger W, Biermann JS, Chanan-Khan A, Cohen AD, et al. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw. 2009;7:908–42. doi: 10.6004/jnccn.2009.0061. [DOI] [PubMed] [Google Scholar]
- 14.Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84:1095–110. doi: 10.4065/mcp.2009.0603. 10.4065/mcp.2009.0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tricot G, Barlogie B, Jagannath S, Bracy D, Mattox S, Vesole DH, et al. Poor prognosis in multiple myeloma is associated only with partial or complete deletions of chromosome 13 or abnormalities involving 11q and not with other karyotype abnormalities. Blood. 1995;86:4250–6. [PubMed] [Google Scholar]
- 16.Smadja NV, Bastard C, Brigaudeau C, Leroux D, Fruchart C. Hypodiploidy is a major prognostic factor in multiple myeloma. Blood. 2001;98:2229–38. doi: 10.1182/blood.v98.7.2229. 10.1182/blood.V98.7.2229 [DOI] [PubMed] [Google Scholar]
- 17.vestri F, Pipan C, Barillari G, Zaja F, Fanin R, Infanti L, et al. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87:4296–301. [PubMed] [Google Scholar]
- 18.De Rosa G, Gobbo ML, De Renzo A, Notaro R, Garofalo S, Grimaldi M, et al. High prevalence of hepatitis C virus infection in patients with B-cell lymphoproliferative disorders in Italy. Am J Hematol. 1997;55:77–82. doi: 10.1002/(sici)1096-8652(199706)55:2<77::aid-ajh5>3.0.co;2-#. 10.1002/(SICI)1096-8652(199706)55:2<77::AID-AJH5>3.0.CO;2-# [DOI] [PubMed] [Google Scholar]
- 19.Takeshita M, Sakai H, Okamura S, Higaki K, Oshiro Y, Uike N, et al. Prevalence of hepatitis C virus infection in cases of B-cell lymphoma in Japan. Histopathology. 2006;48:189–98. doi: 10.1111/j.1365-2559.2005.02311.x. 10.1111/j.1365-2559.2005.02311.x [DOI] [PubMed] [Google Scholar]
- 20.Cavanna L, Sbolli G, Tanzi E, Romanò L, Civardi G, Buscarini E, et al. High prevalence of antibodies to hepatitis C virus in patients with lymphoproliferative disorders. Haematologica. 1995;80:486–7. [PubMed] [Google Scholar]
- 21.Musto P, Dell'Olio M, Carotenuto M, Mangia A, Andriulli A. Hepatitis C virus infection: a new bridge between hematologists and gastroenterologists. Blood. 1996;88:752–4. [PubMed] [Google Scholar]
- 22.Bianco E, Marcucci F, Mele A, Musto P, Cotichini R, Sanpaolo MG, Iannitto E, et al. Prevalence of hepatitis C virus infection in lymphoproliferative diseases other than B-cell non-Hodgkin's lymphoma, and in myeloproliferative diseases: an Italian Multi-Center case-control study. Haematologica. 2004;89:70–6. [PubMed] [Google Scholar]
- 23.de Sanjose S, Nieters A, Goedert JJ, Domingo-Domenech E, Fernandez de Sevilla A, Bosch R, et al. Role of hepatitis C virus infection in malignant lymphoma in Spain. Int J Cancer. 2004;111:81–5. doi: 10.1002/ijc.11727. 10.1002/ijc.11727 [DOI] [PubMed] [Google Scholar]
- 24.Veneri D, Franchini M, Zanotti R, Frattini F, Randon F, Rinaldi M, et al. Prevalence of hepatitis C virus infection among patients with lymphoproliferative disorders: a single center survey. Am J Hematol. 2007;82:1031. doi: 10.1002/ajh.20965. 10.1002/ajh.20965 [DOI] [PubMed] [Google Scholar]
- 25.Anderson LA, Pfeiffer R, Warren JL, Landgren O, Gadalla S, Berndt SI, et al. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev. 2008;17:3069–75. doi: 10.1158/1055-9965.EPI-08-0408. 10.1158/1055-9965.EPI-08-0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okan V, Yilmaz M, Bayram A, Kis C, Cifci S, Buyukhatipoglu H, et al. Prevalence of hepatitis B and C viruses in patients with lymphoproliferative disorders. Int J Hematol. 2008;88:403–8. doi: 10.1007/s12185-008-0175-3. 10.1007/s12185-008-0175-3 [DOI] [PubMed] [Google Scholar]
- 27.Franceschi S, Lise M, Trépo C, Berthillon P, Chuang SC, Nieters A, et al. Infection with hepatitis B and C viruses and risk of lymphoid malignancies in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Epidemiol Biomarkers Prev. 2011;20:208–14. doi: 10.1158/1055-9965.EPI-10-0889. 10.1158/1055-9965.EPI-10-0889 [DOI] [PubMed] [Google Scholar]
- 28.Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15:2078–85. doi: 10.1158/1055-9965.EPI-06-0308. 10.1158/1055-9965.EPI-06-0308 [DOI] [PubMed] [Google Scholar]
- 29.Duberg AS, Nordström M, Törner A, Reichard O, Strauss R, Janzon R, et al. Non-Hodgkin's lymphoma and other nonhepatic malignancies in Swedish patients with hepatitis C virus infection. Hepatology. 2005;41:652–9. doi: 10.1002/hep.20608. 10.1002/hep.20608 [DOI] [PubMed] [Google Scholar]
- 30.Rabkin CS, Tess BH, Christianson RE, Wright WE, Waters DJ, Alter HJ, et al. Prospective study of hepatitis C viral infection as a risk factor for subsequent B-cell neoplasia. Blood. 2002;99:4240–2. doi: 10.1182/blood-2002-01-0226. 10.1182/blood-2002-01-0226 [DOI] [PubMed] [Google Scholar]
- 31.ano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–7. doi: 10.1001/jama.297.18.2010. 10.1001/jama.297.18.2010 [DOI] [PubMed] [Google Scholar]
- 32.Stefanescu DT, Moanga M, Teodorescu M, Brucher J. Chromosome abnormalities in chronic active hepatitis. J Clin Pathol. 1972;25:705–7. doi: 10.1136/jcp.25.8.705. 10.1136/jcp.25.8.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farinati F, Cardin R, Degan P, De Maria N, Floyd RA, Van Thiel DH, et al. Oxidative DNA damage in circulating leukocytes occurs as an early event in chronic HCV infection. Free Radic Biol Med. 1999;27:1284–91. doi: 10.1016/s0891-5849(99)00161-6. 10.1016/S0891-5849(99)00161-6 [DOI] [PubMed] [Google Scholar]
- 34.Cardin R, Saccoccio G, Masutti F, Bellentani S, Farinati F, Tiribelli C. DNA oxidative damage in leukocytes correlates with the severity of HCV-related liver disease: validation in an open population study. J Hepatol. 2001;34:587–92. doi: 10.1016/s0168-8278(00)00098-2. 10.1016/S0168-8278(00)00098-2 [DOI] [PubMed] [Google Scholar]
- 35.Bolukbas C, Bolukbas FF, Kocyigit A, Aslan M, Selek S, Bitiren M, et al. Relationship between levels of DNA damage in lymphocytes and histopathological severity of chronic hepatitis C and various clinical forms of hepatitis B. . J Gastroenterol Hepatol. 2006;21:610–6. doi: 10.1111/j.1440-1746.2005.04069.x. 10.1111/j.1440-1746.2005.04069.x [DOI] [PubMed] [Google Scholar]
- 36.Grossi S, Sumberaz A, Gosmar M, Mattioli F, Testino G, Martelli A. DNA damage in peripheral blood lymphocytes of patients with cirrhosis related to alcohol abuse or to hepatitis B and C viruses. Eur J Gastroenterol Hepatol. 2008;20:22–5. doi: 10.1097/MEG.0b013e3282f163fe. 10.1097/MEG.0b013e3282f163fe [DOI] [PubMed] [Google Scholar]
- 37.Rajkumar SV, Fonseca R, Dewald GW, Therneau TM, Lacy MQ, Kyle RA, et al. Cytogenetic abnormalities correlate with the plasma cell labeling index and extent of bone marrow involvement in myeloma. Cancer Genet Cytogenet. 1999;113:73–7. doi: 10.1016/s0165-4608(99)00009-6. 10.1016/S0165-4608(99)00009-6 [DOI] [PubMed] [Google Scholar]
- 38.Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: a randomized trial. Hepatology. 2008;47:844–53. doi: 10.1002/hep.22106. 10.1002/hep.22106 [DOI] [PubMed] [Google Scholar]
- 39.Koo YX, Tan DS, Tan IB, Tao M, Chow WC, Lim ST. Hepatitis B virus reactivation and role of antiviral prophylaxis in lymphoma patients with past hepatitis B virus infection who are receiving chemoimmunotherapy. Cancer. 2010;116:115–21. doi: 10.1002/cncr.24742. [DOI] [PubMed] [Google Scholar]