Abstract
OBJECTIVES:
Previous studies have reported that osteoporosis due to estrogen deficiency influences fracture healing. Transforming growth factor (TGF-β) has been found to be involved in fracture healing via the regulation of the differentiation and activation of osteoblasts and osteoclasts. The current study aimed to determine the effects of estrogen on the expression of TGF-β1 during fracture healing in ovariectomized rats.
METHODS:
Thirty female Sprague-Dawley rats weighing 200–250 g were assigned to: (i) a sham-operated group that was given a normal saline; (ii) an ovariectomized control group that was given a normal saline; or (iii) an ovariectomized + estrogen (100 µg/kg/day) group that was treated with conjugated equine estrogen. The right femur of all rats was fractured, and a Kirschner wire was inserted six weeks post-ovariectomy. Treatment with estrogen was given for another six weeks post-fracture. At the end of the study, blood samples were taken, and the right femur was harvested and subjected to biomechanical strength testing.
RESULTS:
The percentage change in the plasma TGF-β1 level before treatment was significantly lower in the ovariectomized control and estrogen groups when compared with the sham group (p<0.001). After six weeks of treatment, the percentage change in the plasma TGF-β1 level in the estrogen group was significantly higher compared with the level in the ovariectomized control group (p = 0.001). The mean ultimate force was significantly increased in the ovariectomized rats treated with estrogen when compared with the ovariectomized control group (p = 0.02).
CONCLUSION:
These data suggest that treatment with conjugated equine estrogen enhanced the strength of the healed bone in estrogen-deficient rats by most likely inducing the expression of TGF-β1.
Keywords: Estrogen, Biomechanics, Fracture, Osteoporosis, Ovariectomy, Repair, TGF-β1
INTRODUCTION
Osteoporotic fracture is of great clinical importance as it carries a high risk of morbidity and mortality.1 Osteoporosis is a metabolic bone disease that results in decreased bone mass and an increased risk of fracture.2 Postmenopausal osteoporosis is considered the most frequent cause of pathological fracture. Albright (1947) was the first to report the primary role of estrogen deficiency in the pathogenesis of postmenopausal osteoporosis.3 Bone is the only tissue in the body that can regenerate without leaving a scar after injury.4 Various local growth factors including bone morphogenetic proteins (BMPs), insulin-like growth factor (I-LGF), transforming growth factor-beta (TGF-β), platelet derived growth factor (PDGF), and fibroblast growth factor (FGF), which are all secreted by osteoblasts, and blood cells have been reported to have a role in maintaining bone metabolism and improving fracture healing by regulating the differentiation of bone cells.5
The TGF-β superfamily is a group of cytokines that includes TGF-β, activins, BMPs, and GDFs that regulate the growth and differentiation of osteoblasts.6 TGF-β is an extracellular protein that is produced mainly by osteoblasts and platelets.7 There are three isoforms of TGF-β found in mammals: TGF-β1, TGF-β2, and TGF-β3. These cytokines are involved in the regulation of cell proliferation and differentiation and in the suppression of the immune system.8 Serum TGF-β has been correlated with the platelet count as most TGF-β is produced by platelets. Therefore, the TGF-β level is commonly measured in the plasma.8 Several studies have investigated the role of TGF-β in promoting bone healing. It has been reported that the topical application of estrogen improved the incisional wound healing associated with increased expression of TGF-β1 in ovariectomized rats when compared with untreated ovariectomized rats.9 Furthermore, it has been found that TGF-β is expressed at high levels in healing fracture calluses.10 Cigarette smoking has been reported to reduce the level of TGF-β1 after surgery, which may delay healing.11 Another study reported that decreased expression of TGF-β1 by osteoblasts may have negative effects on fracture healing in ovariectomized rats.12 Zinc supplementation has been reported to induce the expression of TGF-β in vitro, and therefore, TGF-β1 is essential for the healing process.13 Altogether, these studies suggest that TGF-β may have a role in enhancing bone fracture healing.
Bone loss following post-menopausal estrogen loss can be reversed by estrogen replacement therapy. Hormonal replacement therapy has been used since in the 1930s to manage post-menopausal symptoms.14 It has also been shown to protect against the development of osteoporosis, coronary heart disease, and colorectal carcinoma.15 It has been shown that estrogen replacement therapy (ERT) prevents bone resorption, resulting in the maintenance of skeletal mass and a reduced risk of fracture.16 Despite the beneficial effects of ERT in reducing the risk of fracture, it has been reported that the long-term, unopposed use of estrogen therapy after menopause can increase the risk of endometrial carcinoma and that this risk is reduced when estrogen is combined with progesterone.15,17 However, there have been few reports examining the level of TGF-β1 in the blood during estrogen replacement therapy. The current study aimed to determine the effects of the administration of conjugated equine estrogen on the level of TGF-β1 in the plasma during fracture healing in ovariectomized rats.
MATERIALS AND METHODS
Animals
Thirty female Sprague-Dawley rats weighing 200–250 g were purchased from the Laboratory Animal Resource Unit, Universiti Kebangsaan Malaysia (UKM). The rats were housed individually in clean cages at room temperature with a normal 12-hour light-dark cycle and free access to water and food. The rats were acclimatized for two weeks before they were randomly assigned to the sham-operated (SX, n = 10) or ovariectomized (n = 20) groups. Following a previously described protocol, the rats in the SX group underwent a sham operation, whereas the rats in the ovariectomized group underwent a bilateral ovariectomy at the beginning of the study.18 This study was approved by the Animal Ethics Committee of Universiti Kebangsaan Malaysia (UKM).
Experimental Protocol
Osteoporosis was allowed to develop in the animals for six weeks after the ovariectomy. A pilot study was carried out prior to the main study to confirm the induction of osteoporosis based on the bone-structural histomorphometry.19 Following a previously described protocol, the right femur of all rats underwent a closed fracture at mid-diaphysis six weeks post-ovariectomy.20 Xylazil and ketamine were given in combination as anesthetic agents (Troy Laboratories, Australia) (1:1) at a dose of 0.1 ml/100 g I.M. to all of the rats prior to the fracture procedure. To approach the anterior intercondylar notch, a transverse incision was made on the right knee, and the patella was displaced laterally. A Kirschner wire (K-wire, 1.0 mm) (Jorgensen Lab, USA) was inserted into the anterior intercondylar notch toward the femoral medullary canal as a fixator. Following internal fixation with the K-wire, a guillotine-like blade device weighing 0.5 kg was dropped from 30 cm of height to fall on the middle of the right femur to generate standardized closed fracture with minimal soft tissues trauma.
X-ray images were immediately obtained post-fracture to confirm both the intramedullary placement of the K-wire and the fracture. To prevent infection, 5% Enrofloxacin was given at a dose of 10 mg/kg I.M. (Bayer, Thailand) daily for 10 days with the daily dressing. On the first day post-fracture, the sham (SX, n = 10) group was given a normal saline vehicle, while the ovariectomized rats were further subdivided into two groups: (i) the ovariectomized control (VC, n = 10) group was given a normal saline vehicle; (ii) the ovariectomized + estrogen (CEE, n = 10) group was treated with conjugated equine estrogen (conjugated estrogen, Premarin-Wyeth, Canada) at a dose of 100 µg/kg/day. The estrogen dose used in this study was adopted from earlier studies.20 Animals were weighed every three days to adjust the given dose. Treatment was given daily for six weeks via oral gavage immediately after fracture of the right femur. At the end of the experiment, blood samples were taken from the retro-orbital sinus of the rats just before euthanasia. After euthanasia, the right femur was dissected, and the K-wires were removed. All bone samples were kept at -70°C until use for biomechanical testing. In addition, the uterus was removed from each rat and weighed.
Biochemical Analysis (TGF-β1)
Blood samples were collected three times: at the beginning of the study (before ovariectomy), before the closed fracture (before treatment), and just before sacrifice. All blood samples were taken from the retro-orbital sinus of the rats while they were under anesthesia with diethyl ether. All of the samples were kept in an ice box to prevent clotting. The blood was centrifuged at 4°C and 4,000 rpm for 10 minutes to obtain plasma. Plasma samples were placed in Eppendorf tubes and stored at -70°C. The plasma TGF-β1 level was measured using an enzyme-linked immunosorbent assay (ELISA), as recommended by the manufacturer (TGF-β1 EIA Kit; Assay designs, USA). An ELISA plate reader (Versamax, Sunnyvale, USA) was calibrated against a blank substrate, and the microplate was read at 450 nm. The average net OD was calculated for each standard and sample by subtracting the average blank OD from the average OD for each standard and sample. All sample values that were obtained using the TiterZyme TGF-β1 kit were converted to the NIBSC/WHO TGF-β1 Standard using the following equation:
NIBSC/WHO 87514 value (pg/ml) = Obtained human TGF-β1 value (pg/ml)×0.915
Biomechanical strength testing
Following a previously described protocol, the frozen bone samples were thawed slowly at room temperature overnight.21 On the following day, the bone samples were then analyzed using an Instron Microtester 5848 (Instron, USA) to measure the ultimate force. A load was applied to the middle of the femur diaphysis corresponding to the fracture callus area until the bone was fractured. The ultimate force was then calculated using the Bluhill software package.
Statistical Analysis
Statistical analyses were carried out using SPSS Version 17. The baseline TGF-β1 data were not homogenous, and the homogeneity of the variance (Levene's test) was significant (p<0.05). Because the assumption of the homogeneity of variance was violated, the percentage difference of the TGF-β1 levels was compared instead of the TGF-β1 levels alone. Variables were analyzed using a 2-way mixed repeated measure ANOVA with a post-hoc Tukey's test. The results are presented as the mean±SEM. The level of significance was set at p<0.05.
RESULTS
Uterine weight
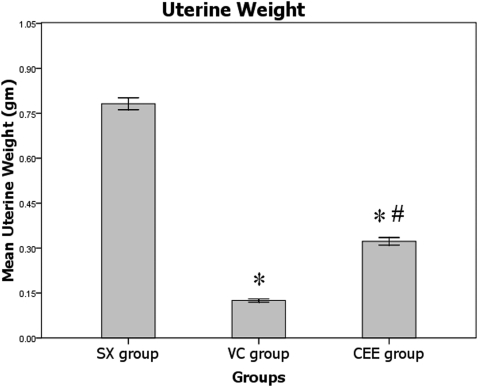
Following six weeks of treatment, marked uterine atrophy was observed in the VC group when compared with the SX group (p<0.05). The uterine weight of the rats treated with ERT was unchanged when compared with the SX group. Finally, the uterine weight in the CEE group was considerably increased following treatment with ERT when compared with the VC group (p<0.05; Figure 1).
Figure 1.
Mean uterine weights after six weeks of treatment. SX, sham-operated group given normal saline; VC, ovariectomized control group given normal saline; CEE, ovariectomized group treated with conjugated equine estrogen at a dose of 100 µg/kg/day; n = 10. * Significant difference compared with the SX group (p<0.05). # Significant difference compared with the VC group (p<0.05). The values are expressed as the mean±SEM.
Biochemical Analysis (TGF-β1)
Biochemical testing was carried out to determine the plasma level of TGF-β1. Blood samples were collected from the rats at baseline (time zero), before treatment and after treatment. The percentage change in the TGF-β1 level before and after treatment with CEE were calculated as follows:
Percentage change before treatment = [TGF-β1 before treatment – baseline] / baseline.
Percentage change after treatment = [TGF-β1 after treatment – baseline] / baseline.
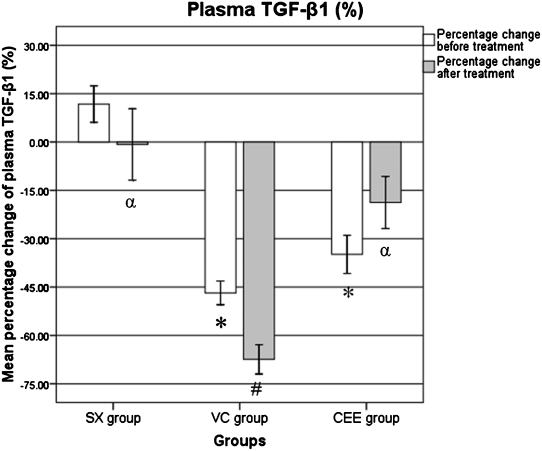
Before treatment, the percentage change in the TGF-β1 level was significantly lower in the CEE and VC groups compared with the SX group (p<0.001). In the VC group, the percentage change in the TGF-β1 level after treatment was significantly lower when compared with the percentage change before treatment (p<0.045). The percentage change in the TGF-β1 level after treatment was significantly higher in the CEE group when compared with the VC group (p = 0.001), and it was identical in the CCE and SX groups (p>0.05; Figure 2).
Figure 2.
The percentage change differences in the plasma TGF-β1 level. SX, sham-operated group given normal saline for six weeks; VC, ovariectomized control group given normal saline for six weeks; CEE, ovariectomized group treated with conjugated equine estrogen (100 µg/kg/day) for six weeks; n = 10. * Significant difference when compared with the sham group (p<0.001). # Significant difference when compared with the BT-BL within the same group (p = 0.045). α Significant difference when compared with the VC group (p = 0.001).
The percentage change in the plasma levels of TGF-β1 is summarized in Table 1.
Table 1.
The percentage change in the plasma TGF-β1 level.
| Group | BT-BL (%) | AT-BL (%) |
| SX | 11.7±5.64 | -0.75±11.08 α |
| VC | -46.7±3.68* | -67.4±4.53*,# |
| CEE | -34.8±5.92* | -18.7±8.08 α |
BT-BL, percentage change before treatment; AT-BL, percentage change after treatment. SX, sham-operated (vehicle normal saline, six weeks); VC, ovariectomized control (normal saline, six weeks); CEE, ovariectomized + estrogen (conjugated equine estrogen 100 µg/kg/day, six weeks); n = 10.
Significant difference when compared with the BT-BL within the same group (p = 0.045).
Significant difference when compared with the sham group (p<0.001).
Significant difference when compared with the AT-BL of the VC group (p = 0.001).
Biomechanical strength testing
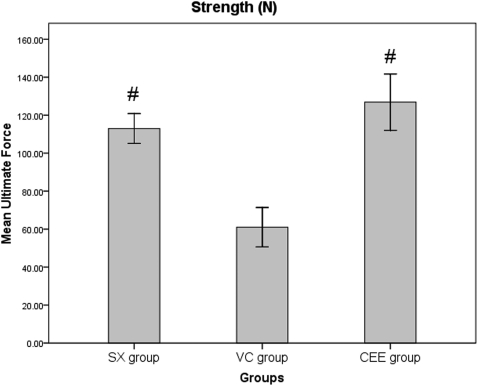
Biomechanical analyses were performed to evaluate the strength of the healed femur following six weeks of treatment. The mean ultimate force (N) of the CEE group was significantly greater than that of the VC group (p = 0.02), and there was no significant difference between the CEE and SX groups (p>0.05) (Figure 3).
Figure 3.
The mean ultimate force (strength) of the fractured right femur after 6 weeks of treatment. SX, sham-operated group treated with normal saline for six weeks; VC, ovariectomized control group treated with normal saline for six weeks; CEE, ovariectomized + estrogen group treated with 100 µg/kg/day of conjugated equine estrogen for six weeks; n = 10. # p<0.05 compared with the VC group. The values are expressed as the mean±SEM.
DISCUSSION
The uterine weight was significantly decreased in the VC when compared with the SX group. Additionally, ovariectomy resulted in marked uterine atrophy in the ovariectomized rats when compared with the SX group, indicating the success of the ovariectomy procedure. The same pattern has been reported in a previous study performed by Fazliana et al., who concluded that the ovariectomy-induced estrogen deficiency resulted in marked uterine atrophy in rats.22 The uterine weight in the CEE group was significantly greater than that of the VC group. Although the ovariectomized rats treated with ERT had a significantly increased uterine weight, the increased uterine weight in the CEE group was still lower than that of SX group. Zhang et al. also demonstrated that the uterine weight is markedly increased in ovariectomized rats treated with estrogen when compared with non-estrogen-treated, ovariectomized rats.23 The treatment with CEE was able to increase the uterine weight of ovariectomized rats, suggesting that the dose of the estrogen therapy used in the current study was adequate.
Several studies have shown that ERT prevents bone resorption, resulting in the maintenance of skeletal mass and a reduction in the risk of fracture.24 The relationship between estrogen and the TGF-β is complex and not completely understood. It has been shown that treatment with estrogen prevents excessive bone loss via the TGF-β-mediated promotion of osteoclast apoptosis.25 Furthermore, Atti et al. reported that TGF-β has a role in the regulation of bone homeostasis as it regulates osteoblast and osteoclast activity.26 Previous studies have found that the local application of TGF-β around the fracture site enhanced fracture healing.27,28
The plasma TGF-β1 level prior to treatment was significantly decreased in the VC and CEE groups when compared with the SX group. Six weeks after ovariectomy, all of the ovariectomized groups showed a significant decrease in the plasma TGF-β1 level when compared with the SX group. Estrogen deficiency resulted in a significant decrease in the expression of TGF-β1 in ovariectomized rats. A similar decreased expression of TGF-β1 in ovariectomized rats (when compared with un- ovariectomized controls) has been reported in other studies.29 This pattern suggests that estrogen deficiency suppresses the expression of TGF-β1, which may interfere with fracture healing.
Following treatment, the percentage change in the TGF-β1 level was significantly lower in the VC group compared with the SX group at the same time point. It was also significantly lower than the mean level before treatment within the same group. Estrogen deficiency has been shown to induce the production of various cytokines and decrease the expression of TGF-β1, thereby inducing osteoclastogenesis.30 Previous studies have demonstrated that estrogen loss results in decreased expression of TGF-β in osteoblasts in ovariectomized rats when compared with the control group.12,29,31 Furthermore, it has been reported that the concentration of TGF-β1 in the blood is lower in patients with delayed fracture healing.32 Therefore, the level of TGF-β1 in the blood may be a marker for fracture healing.
The percentage change in the TGF-β1 level after treatment was significantly higher in the CEE group when compared with the VC group. Therefore, rats treated with estrogen had a higher concentration of TGF-β1 in the blood when compared with the VC group. Treatment with estrogen likely improved osteoporotic fracture healing in ovariectomized rats by inducing the expression of TGF-β1 in osteoblasts.33 Liu et al. found that the TGF-β level in the sham and ovariectomized + ERT groups was greater than the level in the ovariectomized control group.29 In addition, Hughes et al. found that estrogen prevented bone loss through the TGF-β1-mediated induction of osteoclast apoptosis.25 Altogether, this suggests that TGF-β1 may play a role in fracture healing by preventing osteoporotic changes and inducing callus formation.
The TGF-β1 level in the CEE group after six weeks of treatment was not significantly different than the level before treatment. It was also not significantly different when compared with the SX group at the same time point. The TGF-β1 level was increased six weeks post-ovariectomy, and it was nearly identical to the normal concentration of TGF-β1 in the SX group. This suggests that the administration of estrogen enhanced fracture healing in ovariectomized rats by increasing TGF-β1 expression.
The restoration of the biomechanical strength of fractured bones in patients is of great clinical importance.34 Therefore, the biomechanical strength of the fractured femurs was measured to evaluate fracture healing in estrogen-deficient rats. Ultimate force is the most widely used biomechanical parameter for measuring bone strength, and it represents the ultimate compressive force applied to a bone until a fracture occurs.35 There was a significant decrease in the mean ultimate force in ovariectomized rats treated with normal saline when compared with the SX group. Estrogen loss following ovariectomy resulted in a delay in fracture healing, which led to the incomplete restoration of the biomechanical strength of the fractured femurs. Previous studies have found that the biomechanical strength of fractured femurs in non-treated ovariectomized rats was not restored when compared with normal control rats.21,36,37
There was a significant increase in the ultimate force in the CEE group when compared with the VC group. However, the ultimate force was identical in the CEE and SX groups. Treatment with estrogen enhanced fracture healing by restoring the biomechanical strength of healed femur. This is in line with Cao et al., who reported that the strength of the fractured bone in ovariectomized rats treated with estradiol was identical to that in normal control animals.38 Estrogen replacement therapy has been shown to be beneficial in preventing bone loss and decreasing fracture risk.16 Estrogen likely prevents bone loss or excessive resorption of bone by suppressing the differentiation and activity of osteoclasts.30 These data suggest that the restoration of the biomechanical strength of the healed femurs following treatment with estrogen may be attributed to the increased expression of TGF-β1.
The current study demonstrated that estrogen deficiency results in a significant decrease in the expression of TGF-β1 in the blood six weeks post-ovariectomy. Treatment with estrogen induced the expression of TGF-β1 in the blood of estrogen-deficient rats. Therefore, estrogen treatment may have contributed to the restoration of bone strength in the healed bone by increasing the expression of TGF-β1 in the blood. Further studies are required to understand the role of TGF-β in the regulation and differentiation of bone cells.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Universiti Kebangsaan, Malaysia for providing the financial support to conduct the study (UKM-FF-314-2009).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Satomi E, Sitta Mdo C, Machado AN, Garcez Leme LE. Identification and treatment of osteoporosis among elderly patients with hip fractures. Clinics. 2009;64:1201–4. doi: 10.1590/S1807-59322009001200010. 10.1590/S1807-59322009001200010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanik B, Ayrim A, Ozol D, Koktener A, Gokmen D. Influence of obesity on bone mineral density in postmenopausal asthma patients undergoing treatment with inhaled corticosteroids. Clinics. 2009;64:313–8. doi: 10.1590/S1807-59322009000400008. 10.1590/S1807-59322009000400008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albright F. Osteoporosis. Ann Intern Med. 1947;27:861–82. doi: 10.7326/0003-4819-27-6-861. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Gil IFT, Gracia MAA, del Canto Pingarrn M, Jerez LB. Physiological bases of bone regeneration I. Histology and physiology of bone tissue. Med Oral. 2006;11:E47–51. [PubMed] [Google Scholar]
- 5.Kalfas IH. Principles of bone healing. Neurosurg Focus. 2001;10:E1. doi: 10.3171/foc.2001.10.4.2. 10.3171/foc.2001.10.4.2 [DOI] [PubMed] [Google Scholar]
- 6.Centrella M, Horowitz MC, Wozney JM, McCarthy TL. Transforming Growth Factor-{beta} Gene Family Members and Bone. Endocr Rev. 1994;15:27–39. doi: 10.1210/edrv-15-1-27. [DOI] [PubMed] [Google Scholar]
- 7. Roberts AB, Sporn MB.Peptide growth factors and their receptors I. Transforming growth factor-β In:Roberts A B, Sporn M B, editor. Peptide growth factors and their receptors. Berlin: Springer-Verlag 1990419–72. [Google Scholar]
- 8.Kropf J, Schurek JO, Wollner A, Gressner AM. Immunological measurement of transforming growth factor-beta 1 (TGF-beta1) in blood; assay development and comparison. Clin Chem. 1997;43:1965–74. [PubMed] [Google Scholar]
- 9.Ashcroft GS, Dodsworth J, van Boxtel E, Tarnuzzer RW, Horan MA, Schultz GS, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF- 1 levels. Nature Med. 1997;3:1209–15. doi: 10.1038/nm1197-1209. 10.1038/nm1197-1209 [DOI] [PubMed] [Google Scholar]
- 10.Joyce ME, Jingushi S, Bolander ME. Transforming growth factor-beta in the regulation of fracture repair. Orthop Clin North Am. 1990;21:199–209. [PubMed] [Google Scholar]
- 11.Moghaddam A, Weiss S, Wölfl CG, Schmeckenbecher K, Wentzensen A, Grützner PA, et al. Cigarette smoking decreases TGF-[beta]1 serum concentrations after long bone fracture. Injury. 2010;41:1020–5. doi: 10.1016/j.injury.2010.03.014. 10.1016/j.injury.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 12.Xu SW, Yu R, Zhao GF, Wang JW. Early period of fracture healing in ovariectomized rats. Chin J Traumatol. 2003;6:160–6. [PubMed] [Google Scholar]
- 13.Igarashi A, Yamaguchi M. Increase in bone growth factors with healing rat fractures: the enhancing effect of zinc. Int J Mol Med. 2001;8:433–8. doi: 10.3892/ijmm.8.4.433. [DOI] [PubMed] [Google Scholar]
- 14.Cuzick J. Hormone replacement therapy and the risk of breast cancer. Eur J Cancer. 2008;44:2344–9. doi: 10.1016/j.ejca.2008.07.041. 10.1016/j.ejca.2008.07.041 [DOI] [PubMed] [Google Scholar]
- 15.Vecchia CL, Brinton LA, McTiernan A. Menopause, hormone replacement therapy and cancer. Maturitas. 2001;39:97–115. doi: 10.1016/s0378-5122(01)00213-4. 10.1016/S0378-5122(01)00213-4 [DOI] [PubMed] [Google Scholar]
- 16.Ribot C, Trémollières F. Hormone replacement therapy in the management of postmenopausal osteoporosis and prevention of fracture risk. Presse Med. 2006;35:1557–63. doi: 10.1016/s0755-4982(06)74851-5. 10.1016/S0755-4982(06)74851-5 [DOI] [PubMed] [Google Scholar]
- 17.Garrett A, Quinn MA. Hormonal therapies and gynaecological cancers. Best Pract Res Clin Obstet Gynaecol. 2008;22:407–21. doi: 10.1016/j.bpobgyn.2007.08.003. 10.1016/j.bpobgyn.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 18.Adam SK, Das S, Othman F, Jaarin K. Fresh soy oil protects against vascular changes in an estrogen-deficient rat model: an electron microscopy study. Clinics. 2009;64:1113–9. doi: 10.1590/S1807-59322009001100012. 10.1590/S1807-59322009001100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estai MA, Suhaimi F, Soelaiman IN, Shuid AN, Das S. Bone histomorphometric study of young rats following oestrogen deficiency. AJB. 2011;10:12064–70. [Google Scholar]
- 20.Estai MA, Suhaimi FH, Das S, Fadzilah FM, Alhabshi SMI, Shuid AN, Soelaiman IN. Piper sarmentosum enhances fracture healing in ovariectomized osteoporotic rats: a radiological study. Clinics. 2011;66:865–72. doi: 10.1590/S1807-59322011000500025. 10.1590/S1807-59322011000500025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shuid AN, Mohamad S, Mohamed N, Fadzilah FM, Mokhtar SA, Abdullah S, et al. Effects of calcium supplements on fracture healing in a rat osteoporotic model. J Orthop Res. 2010;28:1651–6. doi: 10.1002/jor.21180. 10.1002/jor.21180 [DOI] [PubMed] [Google Scholar]
- 22.Fazliana M, Wan Nazaimoon WM, Gu HF, Östenson CG. Labisia pumila extract regulates body weight and adipokines in ovariectomized rats. Maturitas. 2009;62:91–7. doi: 10.1016/j.maturitas.2008.10.004. 10.1016/j.maturitas.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Liu ZG, Li C, Hu SJ, Liu L, Wang JP, et al. Du-Zhong (Eucommia ulmoides Oliv.) cortex extract prevent OVX-induced osteoporosis in rats. Bone. 2009;45:553–9. doi: 10.1016/j.bone.2008.08.127. 10.1016/j.bone.2008.08.127 [DOI] [PubMed] [Google Scholar]
- 24.Compston J. How to manage osteoporosis after the menopause. Best Pract Res Clin Rheumatol. 2005;19:1007–19. doi: 10.1016/j.berh.2005.06.010. 10.1016/j.berh.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 25.Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2:1132–6. doi: 10.1038/nm1096-1132. 10.1038/nm1096-1132 [DOI] [PubMed] [Google Scholar]
- 26.Atti E, Gomez S, Wahl SM, Mendelsohn R, Paschalis E, Boskey AL. Effects of transforming growth factor-[beta] deficiency on bone development: A Fourier Transform-Infrared imaging analysis* 1. Bone. 2002;31:675–84. doi: 10.1016/s8756-3282(02)00905-5. 10.1016/S8756-3282(02)00905-5 [DOI] [PubMed] [Google Scholar]
- 27.Wildemann B, Bamdad P, Holmer C, Haas NP, Raschke M, Schmidmaier G. Local delivery of growth factors from coated titanium plates increases osteotomy healing in rats. Bone. 2004;34:862–8. doi: 10.1016/j.bone.2004.01.015. 10.1016/j.bone.2004.01.015 [DOI] [PubMed] [Google Scholar]
- 28.De Ranieri A, Virdi AS, Kuroda S, Shott S, Leven RM, Hallab NJ, et al. Local application of rhTGF- 2 enhances peri-implant bone volume and bone-implant contact in a rat model. Bone. 2005;37:55–62. doi: 10.1016/j.bone.2005.03.011. 10.1016/j.bone.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Xu K, Qiao L. Effects of estrogen on the expression of TGF-B in early fracture healing of ovariectomized rats. Bone. 2008;43:S54–S55. 10.1016/j.bone.2008.08.048 [Google Scholar]
- 30.Chen FP, Wang KC, Huang JD. Effect of Estrogen on the Activity and Growth of Human Osteoclasts In Vitro. Taiwan J Obstet Gynecol. 2009;48:350–5. doi: 10.1016/S1028-4559(09)60323-5. 10.1016/S1028-4559(09)60323-5 [DOI] [PubMed] [Google Scholar]
- 31.Ikeda T, Shigeno C, Kasai R, Kohno H, Ohta S, Okumura H, et al. Ovariectomy decreases the mRNA levels of transforming growth factor-beta 1 and increases the mRNA levels of osteocalcin in rat bone in vivo. Biochem Biophys Res Commun. 1993;194:1228–33. doi: 10.1006/bbrc.1993.1954. 10.1006/bbrc.1993.1954 [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann G, Henle P, Küsswetter M, Moghaddam A, Wentzensen A, Richter W, et al. TGF-[beta]1 as a marker of delayed fracture healing. Bone. 2005;36:779–85. doi: 10.1016/j.bone.2005.02.011. 10.1016/j.bone.2005.02.011 [DOI] [PubMed] [Google Scholar]
- 33.Oursler MJO, Cortese C, Keeting P, Anderson MA, Bonde SK, Riggs BL, et al. Modulation of Transforming Growth Factor-{beta} Production in Normal Human Osteoblast-Like Cells by 17 {beta}-Estradiol and Parathyroid Hormone. Endocrinology. 1991;129:3313–20. doi: 10.1210/endo-129-6-3313. 10.1210/endo-129-6-3313 [DOI] [PubMed] [Google Scholar]
- 34.Fu L, Tang T, Miao Y, Hao Y, Dai K. Effect of 1, 25-dihydroxy vitamin D3 on fracture healing and bone remodeling in ovariectomized rat femora. Bone. 2009;44:893–8. doi: 10.1016/j.bone.2009.01.378. 10.1016/j.bone.2009.01.378 [DOI] [PubMed] [Google Scholar]
- 35.Comelekoglu U, Bagis S, Yalin S, Ogenler O, Yildiz A, Sahin NO, et al. Biomechanical evaluation in osteoporosis: ovariectomized rat model. Clin Rheumatol. 2007;26:380–4. doi: 10.1007/s10067-006-0367-2. 10.1007/s10067-006-0367-2 [DOI] [PubMed] [Google Scholar]
- 36.Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, et al. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28:80–8. doi: 10.1016/s8756-3282(00)00414-2. 10.1016/S8756-3282(00)00414-2 [DOI] [PubMed] [Google Scholar]
- 37.Kubo T, Shiga T, Hashimoto J, Yoshioka M, Honjo H, Urabe M, et al. Osteoporosis influences the late period of fracture healing in a rat model prepared by ovariectomy and low calcium diet. J Steroid Biochem Mol Biol. 1999;68:197–202. doi: 10.1016/s0960-0760(99)00032-1. 10.1016/S0960-0760(99)00032-1 [DOI] [PubMed] [Google Scholar]
- 38.Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, et al. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002;17:2237–46. doi: 10.1359/jbmr.2002.17.12.2237. 10.1359/jbmr.2002.17.12.2237 [DOI] [PubMed] [Google Scholar]