Abstract
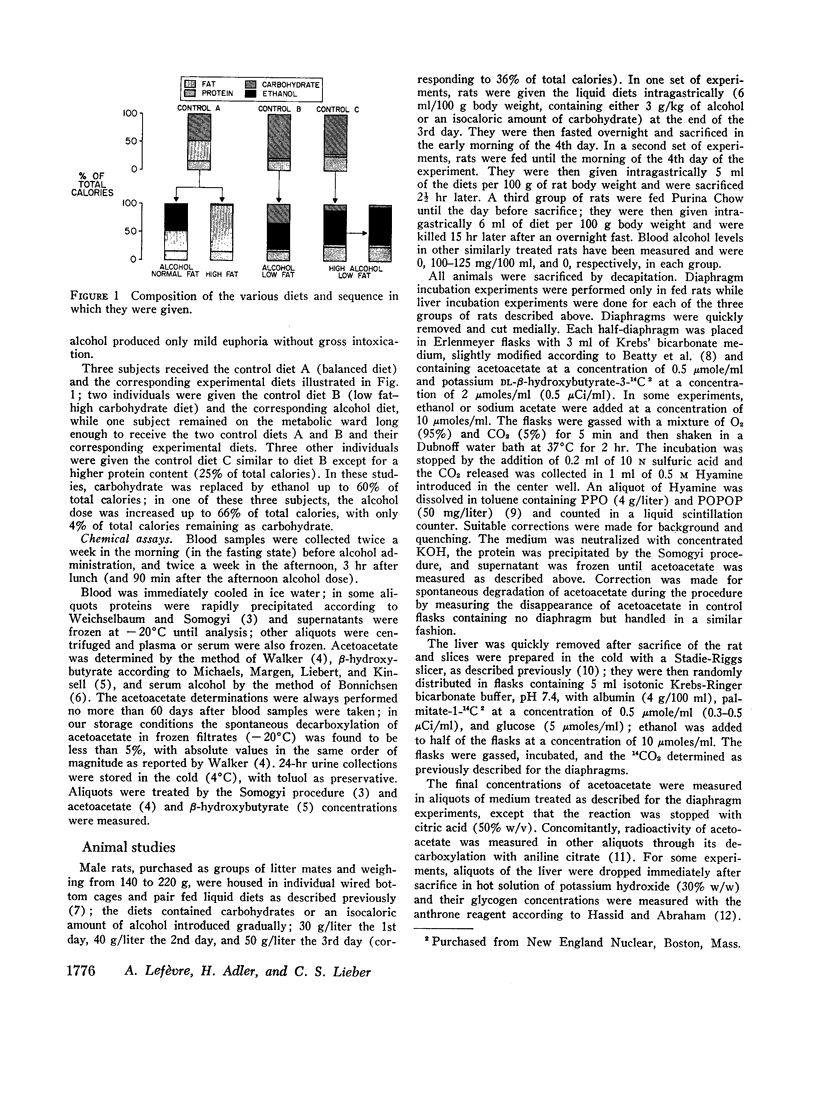
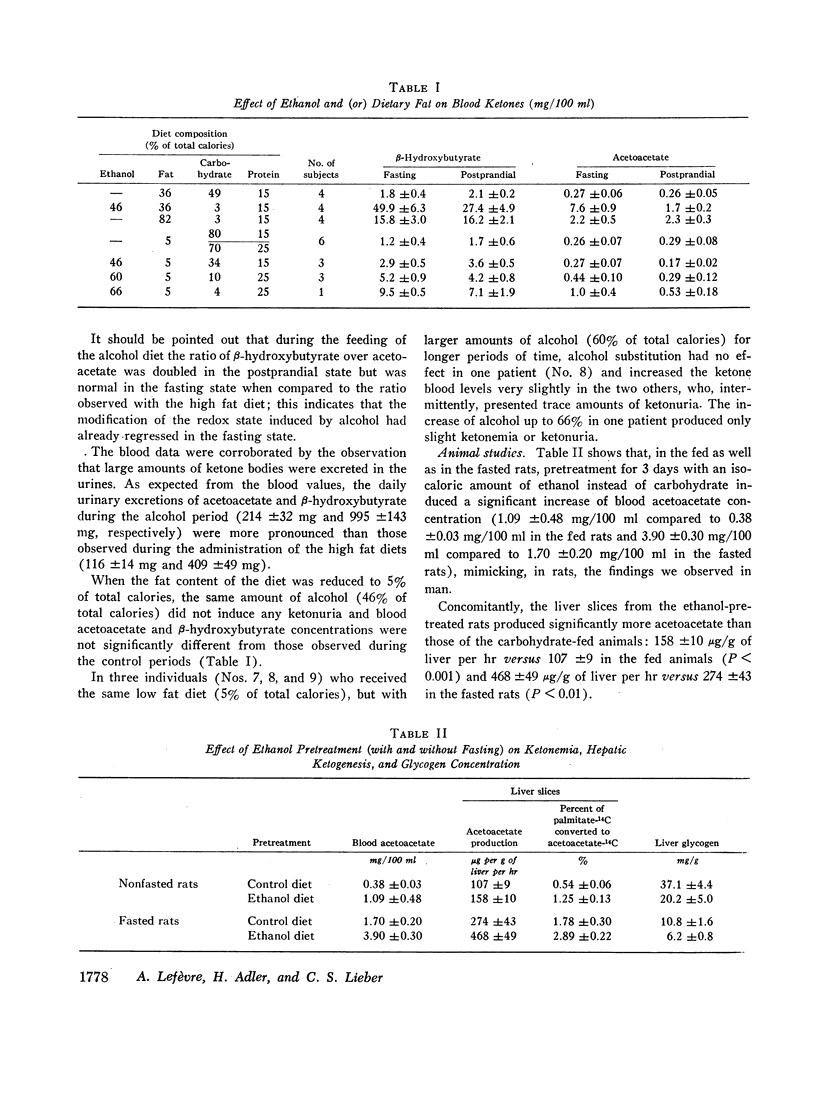
Ketonuria has been observed in alcoholics. To study the mechanism of this effect, healthy, volunteers were given adequate diets (36% of calories as lipid and 15% as protein) for 18 days, with isocaloric replacement of carbohydrate (46% of calories) by either ethanol or additional fat. The latter resulted in a high fat diet, with 82% of calories as lipid. After about 1 wk of alcohol, massive and persistent ketonuria developed. Compared with the control period, there was a 30-fold increase in fasting blood acetoacetate and β-hydroxybutyrate (P < 0.001). With the high fat diet, acetoacetate and β-hydroxybutyrate increased 8- to 10-fold (P < 0.001). In the postprandial state, ethanol also induced hyperketonemia, but less markedly than when ethanol followed an overnight fast. With low fat diets (5% of calories), alcohol (46% of total calories) did not induce ketonuria or hyperketonemia, suggesting that a combination of alcohol and dietary fat is necessary. The addition of alcohol to rat liver slices did not affect ketogenesis. In rats pretreated with alcohol for 3 days, however, ketonemia developed, hepatic glycogen was decreased, and liver slices (incubated with palmitate-14C and glucose) had a significant increase in acetoacetate production, when compared to carbohydrate pretreated controls. Alcohol pretreatment or addition of alcohol in vitro had no effect on acetoacetate utilization by rat diaphragms, and decreased only slightly the conversion of β-hydroxybutyrate-14C to 14CO2. Thus, the hyperketonemia and ketonuria observed after alcohol consumption cannot be attributed to an immediate effect of alcohol, but is the consequence of a delayed change in intermediary metabolism characterized by increased hepatic ketone production from fatty acids, possibly linked to ethanol-induced glycogen depletion and depression of citric acid cycle activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson E. A., Arky R. A. Acute antilipolytic effects of ethyl alcohol and acetate in man. J Lab Clin Med. 1968 Jul;72(1):105–117. [PubMed] [Google Scholar]
- BEATTY C. H., MARCO A., PETERSON R. D., BOCEK R. M., WEST E. S. Acetoacetic acid metabolism by skeletal muscle fibers from control and diabetic rats. J Biol Chem. 1960 Oct;235:2774–2777. [PubMed] [Google Scholar]
- Bremer J. Pathogenesis of ketonemia. Scand J Clin Lab Invest. 1969 Apr;23(2):105–108. doi: 10.3109/00365516909077011. [DOI] [PubMed] [Google Scholar]
- Crouse J. R., Gerson C. D., DeCarli L. M., Lieber C. S. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man. J Lipid Res. 1968 Jul;9(4):509–512. [PubMed] [Google Scholar]
- DeCarli L. M., Lieber C. S. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967 Mar;91(3):331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- FREUND G. THE CALORIE DEFICIENCY HYPOTHESIS OF KETOGENESIS TESTED IN MAN. Metabolism. 1965 Sep;14:985–990. doi: 10.1016/0026-0495(65)90114-9. [DOI] [PubMed] [Google Scholar]
- FROSANDER O. A., RAEIHAE N., SALASPURO M., MAEENPAEAE P. INFLUENCE OF ETHANOL ON THE LIVER METABOLISM OF FED AND STARVED RATS. Biochem J. 1965 Jan;94:259–265. doi: 10.1042/bj0940259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman L., Lieber C. S. Effect of ethanol on plasma glycerol in man. Am J Clin Nutr. 1967 May;20(5):400–403. doi: 10.1093/ajcn/20.5.400. [DOI] [PubMed] [Google Scholar]
- Iseri O. A., Lieber C. S., Gottlieb L. S. The ultrastructure of fatty liver induced by prolonged ethanol ingestion. Am J Pathol. 1966 Apr;48(4):535–555. [PMC free article] [PubMed] [Google Scholar]
- JONES J. A., BLECHER M. ON THE MECHANISM OF BETA-OXIDATION OF LONG CHAIN FATTY ACIDS BY LIVER MITOCHONDRIA FROM NORMAL AND ALLOXAN-DIABETIC RATS. J Biol Chem. 1965 Jan;240:68–70. [PubMed] [Google Scholar]
- Jones D. P., Perman E. S., Lieber C. S. Free fatty acid turnover and triglyceride metabolism after ethanol ingestion in man. J Lab Clin Med. 1965 Nov;66(5):804–813. [PubMed] [Google Scholar]
- KATZ J., CHAIKOFF I. L. Synthesis via the Krebs' cycle in the utilization of acetate by rat liver slices. Biochim Biophys Acta. 1955 Sep;18(1):87–101. doi: 10.1016/0006-3002(55)90012-3. [DOI] [PubMed] [Google Scholar]
- LIEBER C. S., SCHMID R. The effect of ethanol on fatty acid metabolism; stimulation of hepatic fatty acid synthesis in vitro. J Clin Invest. 1961 Feb;40:394–399. doi: 10.1172/JCI104266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDQUIST F., TYGSTRUP N., WINKLER K., MELLEMGAARD K., MUNCK-PETERSEN S. Ethanol metabolism and production of free acetate in the human liver. J Clin Invest. 1962 May;41:955–961. doi: 10.1172/JCI104574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane B. P., Lieber C. S. Ultrastructural alterations in human hepatocytes following ingestion of ethanol with adequate diets. Am J Pathol. 1966 Oct;49(4):593–603. [PMC free article] [PubMed] [Google Scholar]
- Lieber C. S., Lefèvre A., Spritz N., Feinman L., DeCarli L. M. Difference in hepatic metabolism of long- and medium-chain fatty acids: the role of fatty acid chain length in the production of the alcoholic fatty liver. J Clin Invest. 1967 Sep;46(9):1451–1460. doi: 10.1172/JCI105637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C. S. Metabolic effects produced by alcohol in the liver and other tissues. Adv Intern Med. 1968;14:151–199. [PubMed] [Google Scholar]
- Lieber C. S., Rubin E. Alcoholic fatty liver in man on a high protein and low fat diet. Am J Med. 1968 Feb;44(2):200–206. doi: 10.1016/0002-9343(68)90151-4. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., Spritz N., DeCarli L. M. Role of dietary, adipose, and endogenously synthesized fatty acids in the pathogenesis of the alcoholic fatty liver. J Clin Invest. 1966 Jan;45(1):51–62. doi: 10.1172/JCI105323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C. S., Spritz N. Effects of prolonged ethanol intake in man: role of dietary adipose, and endogenously synthesized fatty acids in the pathogenesis of the alcoholic fatty liver. J Clin Invest. 1966 Sep;45(9):1400–1411. doi: 10.1172/JCI105448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYES P. A. A calorie deficiency hypothesis of ketogenesis. Metabolism. 1962 Aug;11:781–799. [PubMed] [Google Scholar]
- MICHAELS G. D., MARGEN S., LIEBERT G., KINSELL L. W. Studies in fat metabolism. I. The colorimetric determination of ketone bodies in biological fluids. J Clin Invest. 1951 Dec;30(122):1483–1485. doi: 10.1172/JCI102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat A. K. Effects of ethanol infusion on the redox state and metabolite levels in rat liver in vivo. Eur J Biochem. 1968 Dec 5;6(4):585–592. doi: 10.1111/j.1432-1033.1968.tb00485.x. [DOI] [PubMed] [Google Scholar]
- SCHLIERF G., GUNNING B., UZAWA H., KINSELL L. W. THE EFFECTS OF CALORICALLY EQUIVALENT AMOUNTS OF ETHANOL AND DRY WINE ON PLASMA LIPIDS, KETONES AND BLOOD SUGAR IN DIABETIC AND NONDIABETIC SUBJECTS. Am J Clin Nutr. 1964 Aug;15:85–89. doi: 10.1093/ajcn/15.2.85. [DOI] [PubMed] [Google Scholar]
- WALKER P. G. A colorimetric method for the estimation of acetoacetate. Biochem J. 1954 Dec;58(4):699–704. doi: 10.1042/bj0580699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Krebs H. A. Activity and intracellular distribution of enzymes of ketone-body metabolism in rat liver. Biochem J. 1968 Jul;108(3):353–361. doi: 10.1042/bj1080353. [DOI] [PMC free article] [PubMed] [Google Scholar]






