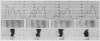Abstract
Serum samples were obtained from 21 normal human fetuses after therapeutic abortion for psychiatric indications. Fetal crown-rump length ranged from 5.2 to 22.5 cm, corresponding to the gestational age of 65-168 days.
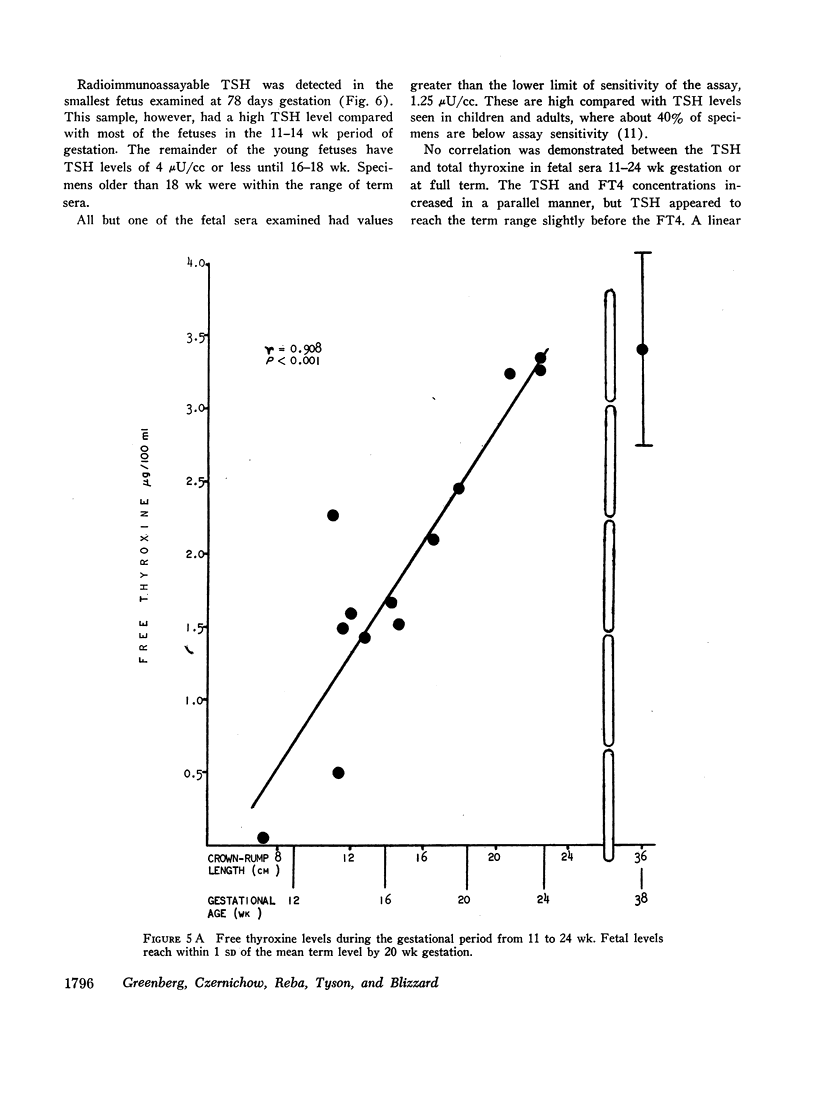
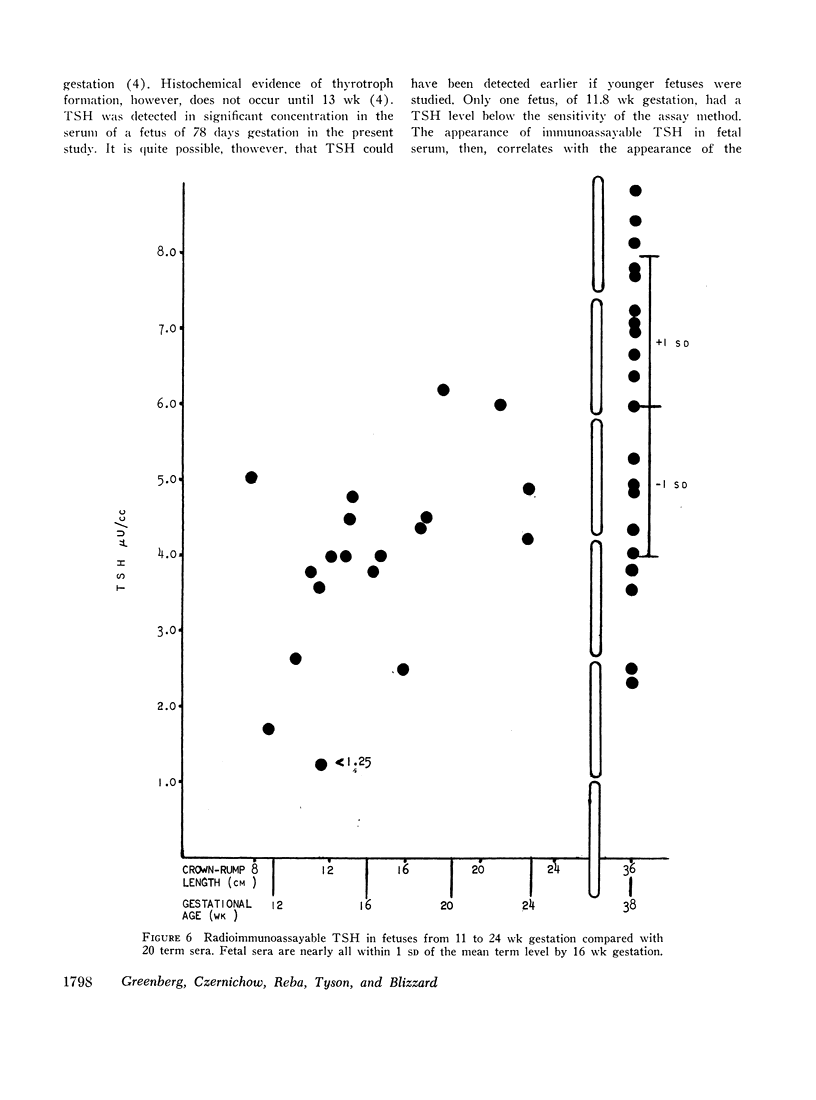
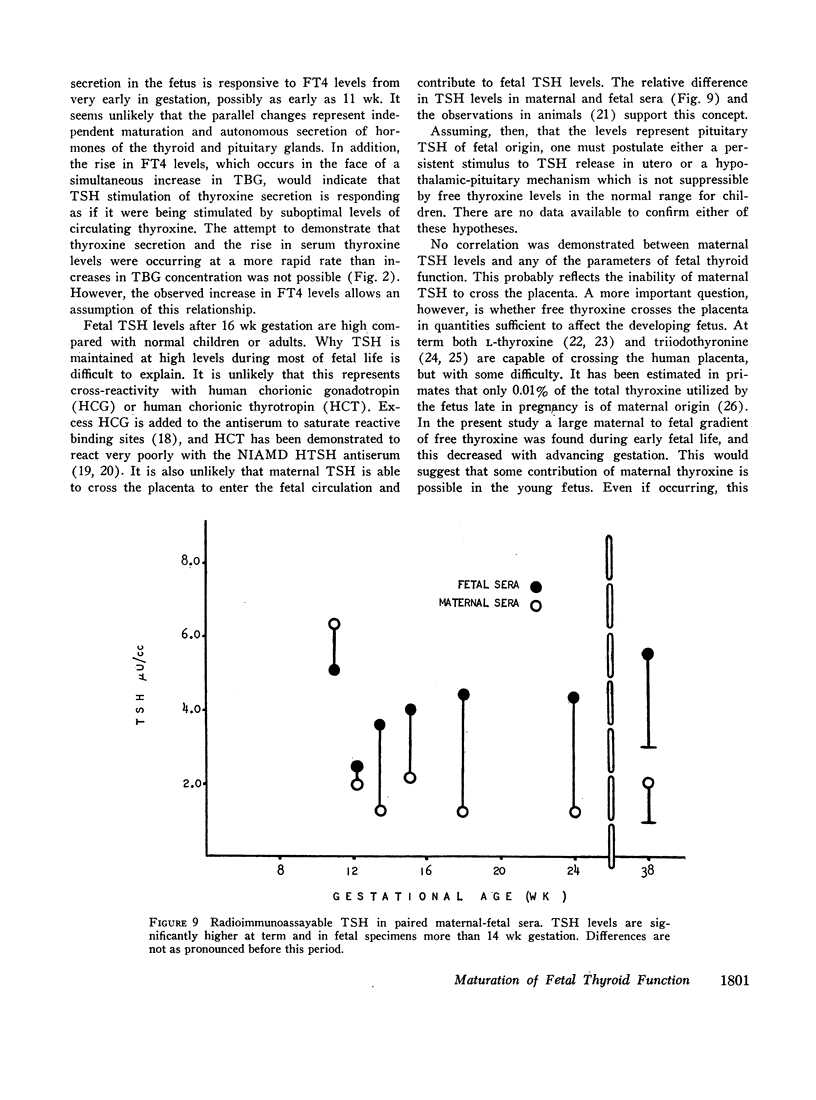
Serum thyroxine, assayed by a modification of the Murphy-Pattee method, was identified in the second smallest fetus examined at 78 days gestation. Thereafter it increased rapidly, maintaining a significant linear correlation with crown-rump length until term (r = 0.800, P < 0.001). Free thyroxine (FT4) also increased in a linear relation to gestational age (r = 0.908, P < 0.001), but reached term levels by 18-20 wk. Radioimmunoassayable thyroid-stimulating hormone (TSH) was detected at 78 days gestation. Levels increased rapidly with advancing gestation, so that by 16 wk almost all were within the range of term infants. After 16 wk gestation, levels were usually greater than 4.0 μU/cc, higher than that seen in normal children.
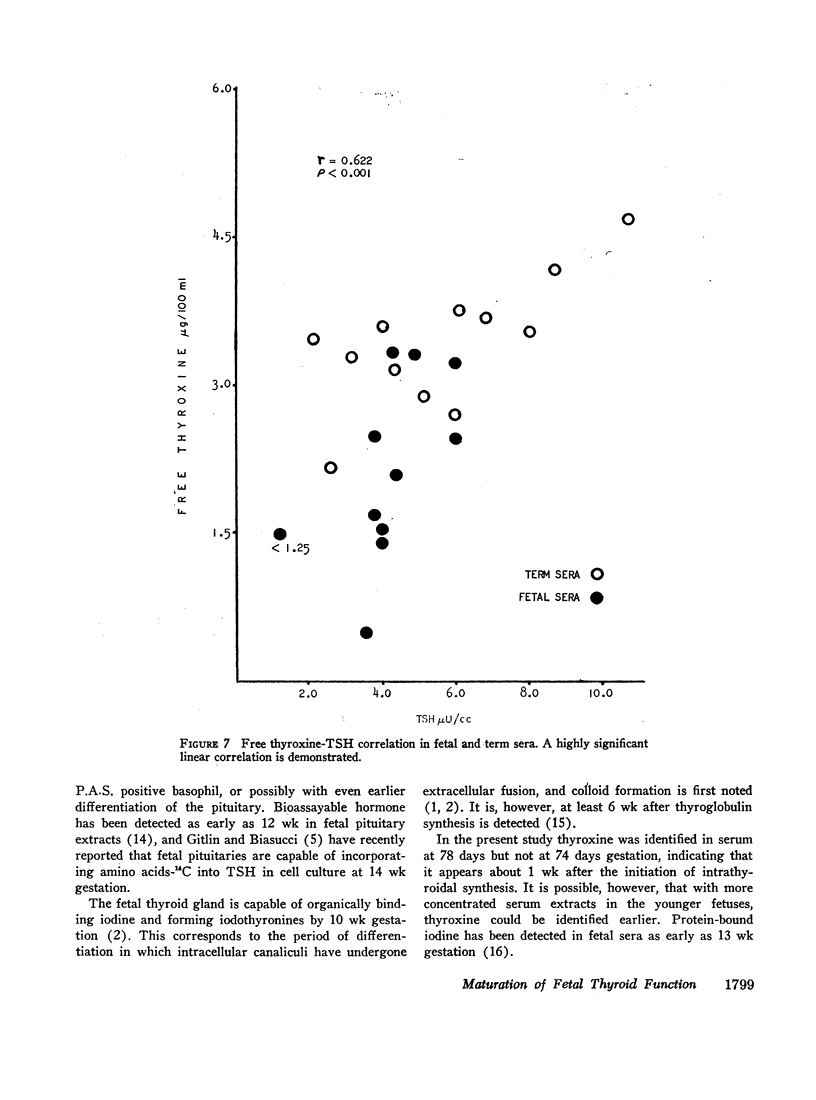
No correlation was demonstrated between the serum TSH levels and total thyroxine. TSH and FT4, however, increased in a parallel manner with a significant positive correlation. This suggested that fetal TSH secretion was responsive to FT4 levels from very early in gestation, possibly as early as 11 wk.
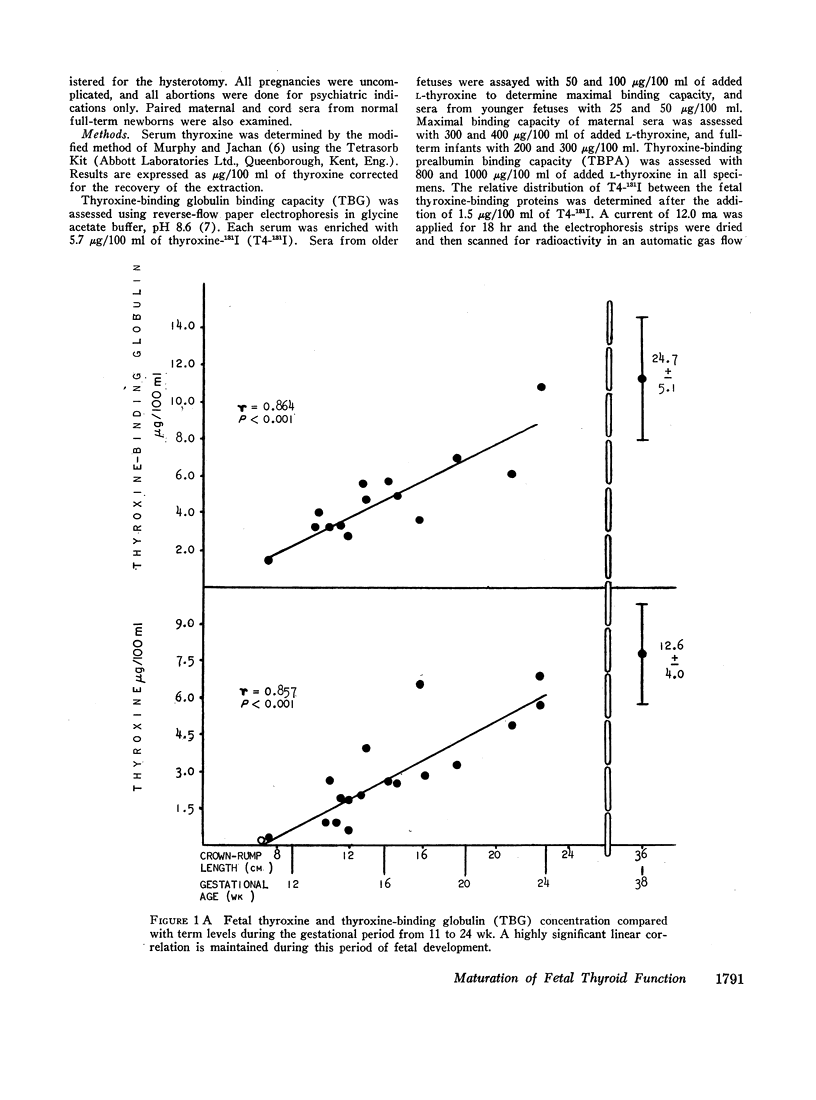
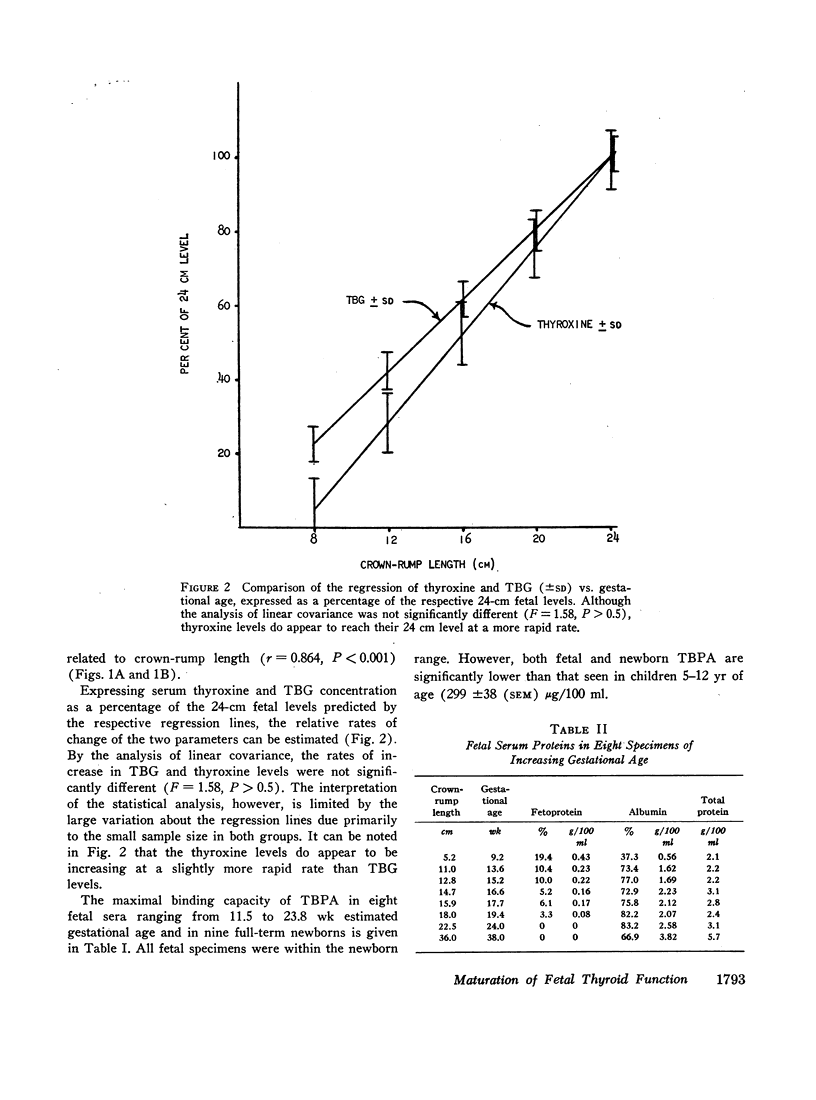
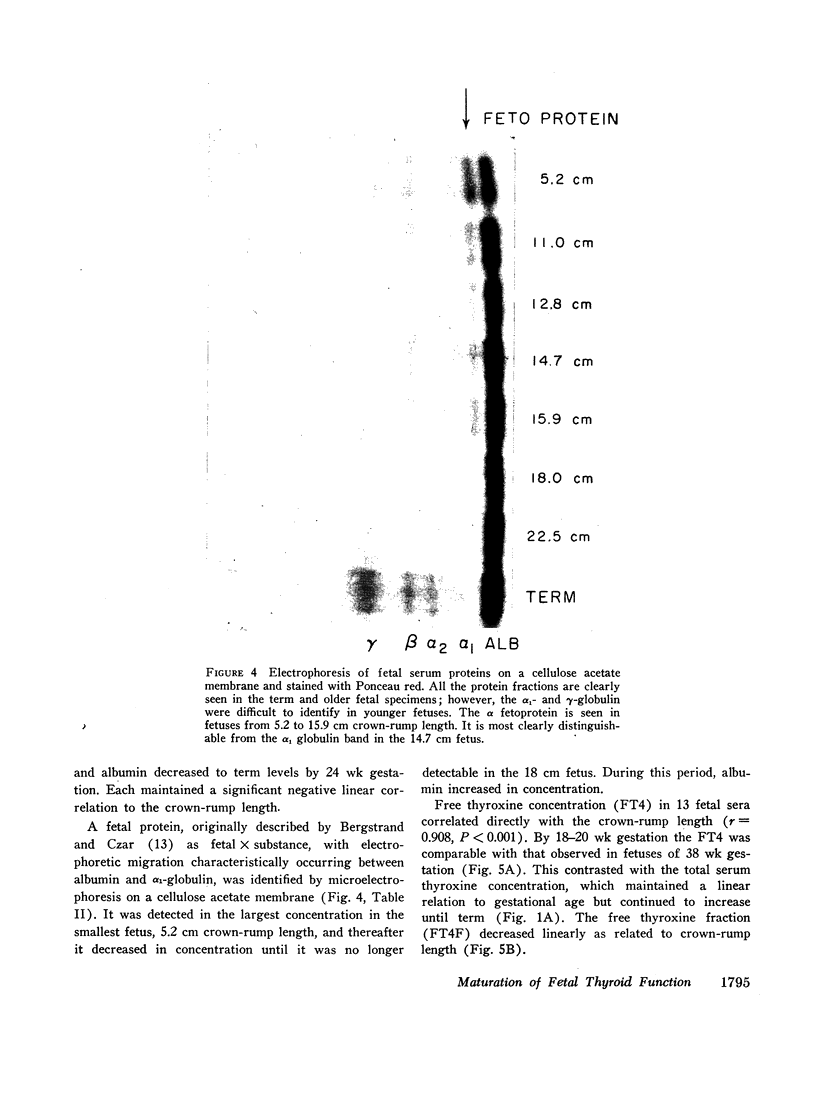
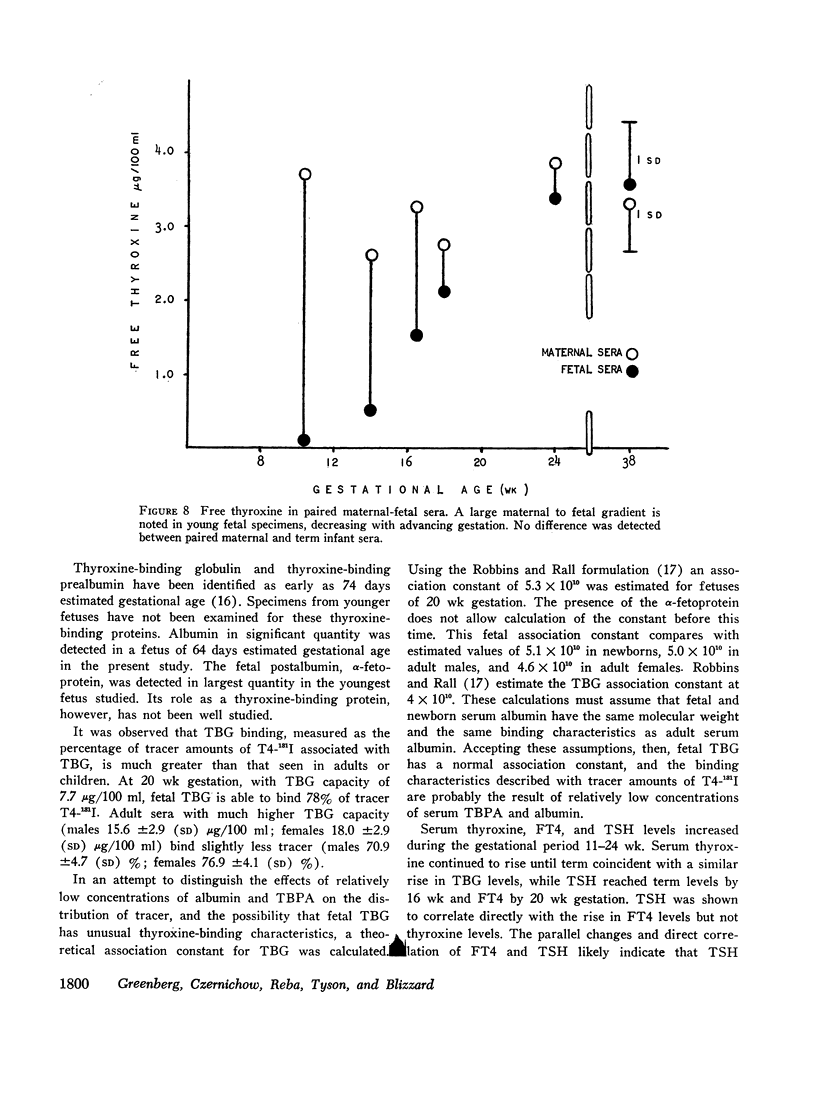
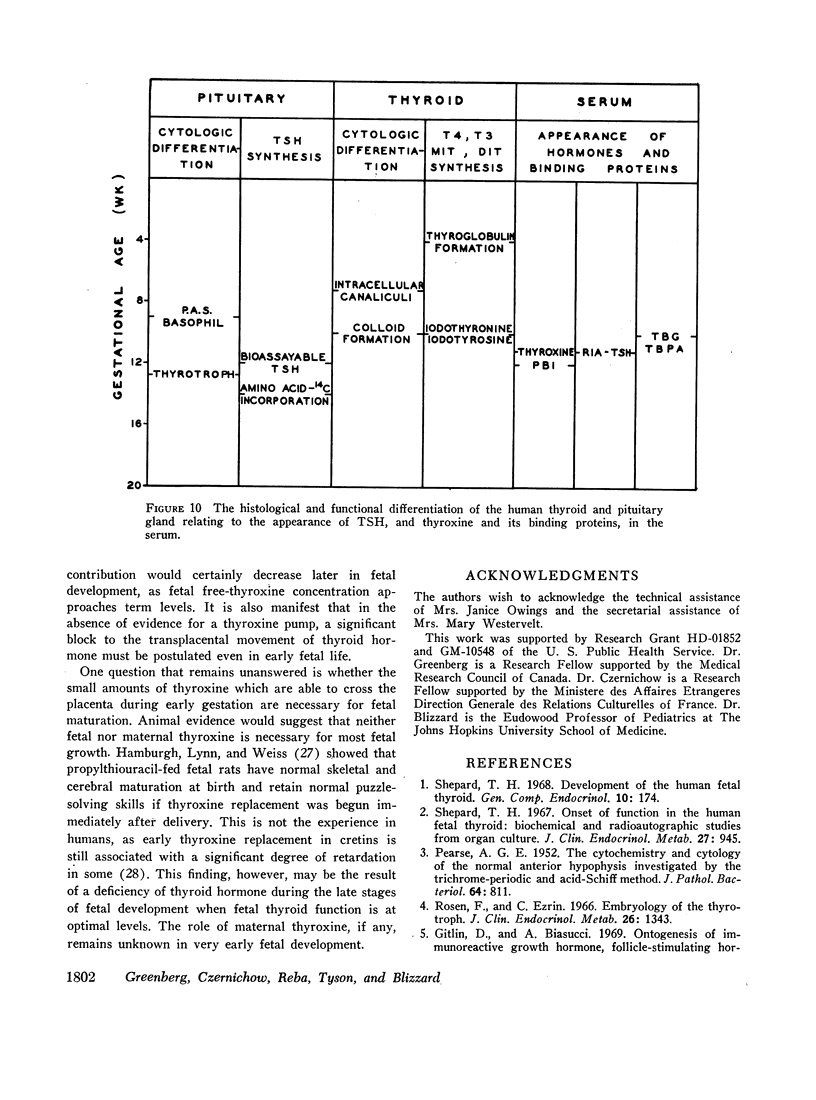
Thyroxine-binding globulin (TBG) was detected in a fetus of 78 days gestation (1.4 μg/100 ml). Levels increased rapidly, paralleling the rise in serum thyroxine and maintaining a linear correlation with crownrump length (r = 0.864, P < 0.001). Thyroxine-binding prealbumin binding capacity (TBPA) in fetuses 14-24 wk gestation was comparable with that seen at term.
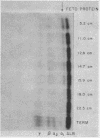
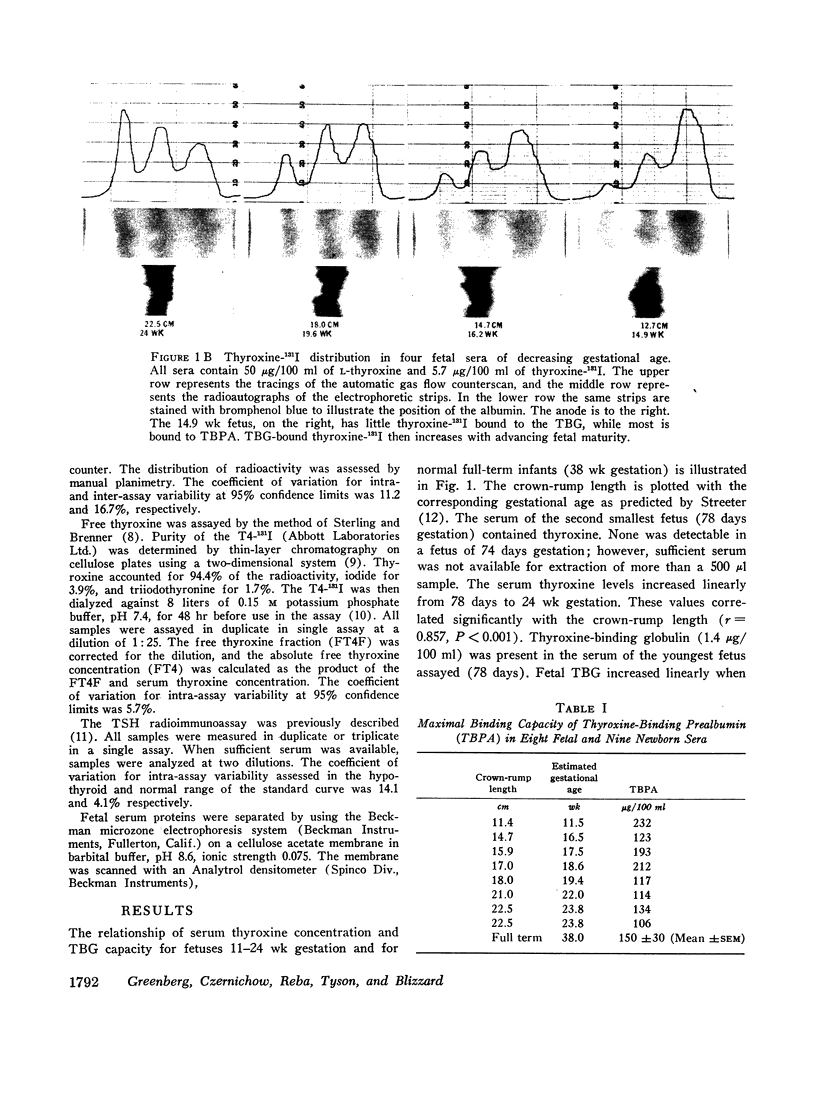
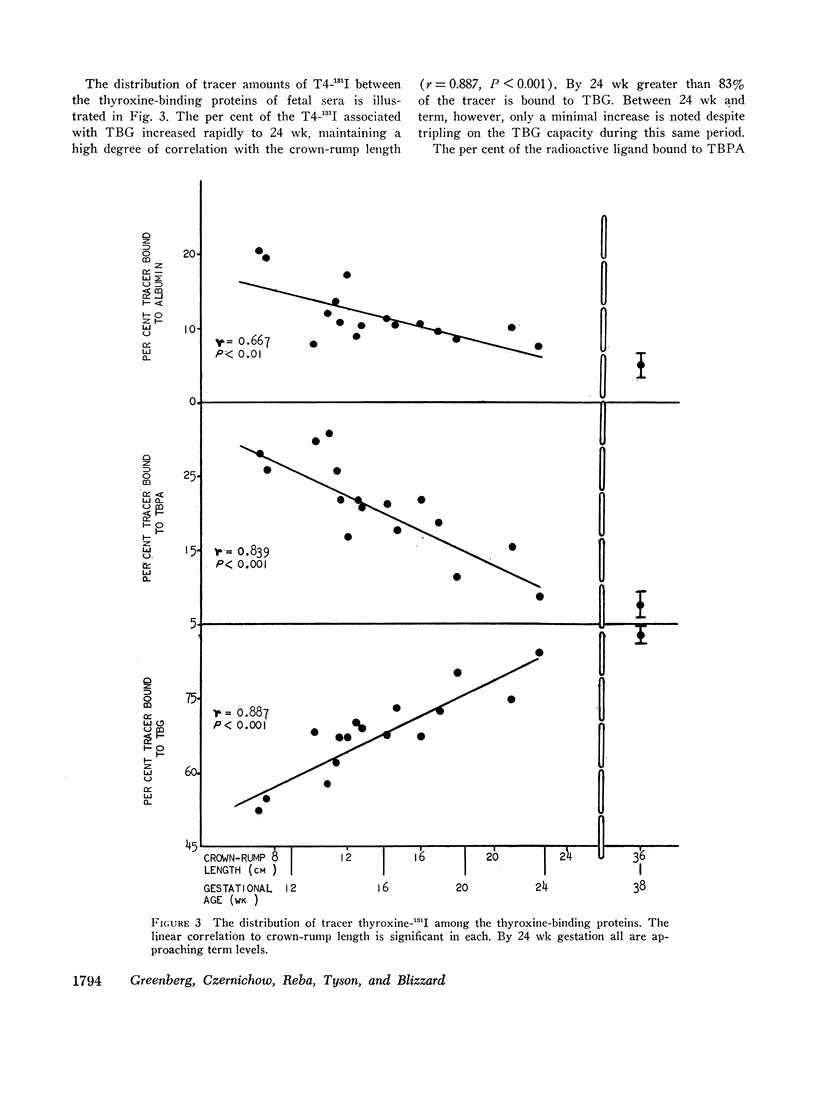
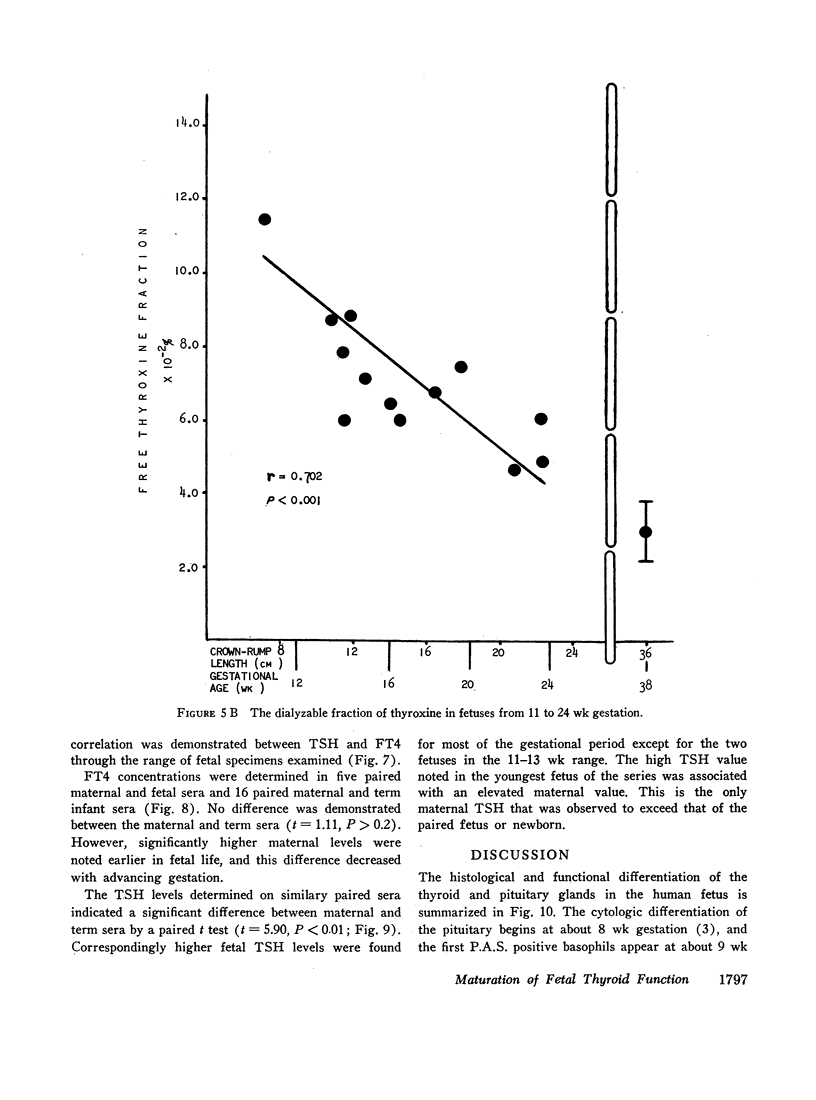
When examining the distribution of tracer amounts of thyroxine-131I (T4-131I) between the thyroxine-binding proteins, it was found that a major fraction was bound to TBPA and albumin during the early part of gestation. This decreased linearly with maturation of the fetus as the fraction bound to TBG increased. By 20 wk gestation fetal TBG was able to bind 78% of tracer despite a TBG capacity of only 7.7 μg/100 ml. This appeared to be the result of relatively low concentrations of TBPA and albumin during this period of gestation. The theoretical association constant calculated for fetal and newborn TBG was found to be similar to that estimated for normal adult males and females.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREOLI M., ROBBINS J. Serum proteins and thyroxineprotein interaction in early human fetuses. J Clin Invest. 1962 May;41:1070–1077. doi: 10.1172/JCI104557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGSTRAND C. G., CZAR B. Paper electrophoretic study of human fetal serum proteins with demonstration of a new protein fraction. Scand J Clin Lab Invest. 1957;9(3):277–286. doi: 10.3109/00365515709079971. [DOI] [PubMed] [Google Scholar]
- Dussault J., Row V. V., Lickrish G., Volpé R. Studies of serum triiodothyronine concentration in maternal and cord blood: transfer of triiodothyronine across the human placenta. J Clin Endocrinol Metab. 1969 Apr;29(4):595–603. doi: 10.1210/jcem-29-4-595. [DOI] [PubMed] [Google Scholar]
- FISHER D. A., LEHMAN H., LACKEY C. PLACENTAL TRANSPORT OF THYROXINE. J Clin Endocrinol Metab. 1964 May;24:393–400. doi: 10.1210/jcem-24-5-393. [DOI] [PubMed] [Google Scholar]
- Favino A., Emrich D., zur Mühlen A von Separation and quantitative determination of 131-I-triiodothyronine and 131-I-thyroxine in human plasma by thin layer chromatography. Acta Endocrinol (Copenh) 1967 Feb;54(2):362–374. doi: 10.1530/acta.0.0540362. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Odell W. D., Hobel C. J., Garza R. Thyroid function in the term fetus. Pediatrics. 1969 Oct;44(4):526–535. [PubMed] [Google Scholar]
- GRUMBACH M. M., WERNER S. C. Transfer of thyroid hormone across the human placenta at term. J Clin Endocrinol Metab. 1956 Oct;16(10):1392–1395. doi: 10.1210/jcem-16-10-1392. [DOI] [PubMed] [Google Scholar]
- Gitlin D., Biasucci A. Ontogenesis of immunoreactive thyroglobulin in the human conceptus. J Clin Endocrinol Metab. 1969 Jun;29(6):849–853. doi: 10.1210/jcem-29-6-849. [DOI] [PubMed] [Google Scholar]
- Greenberg A. H., Czernichow P., Hung W., Shelley W., Winship T., Blizzard R. M. Juvenile chronic lymphocytic thyroiditis: clinical, laboratory and histological correlations. J Clin Endocrinol Metab. 1970 Mar;30(3):293–301. doi: 10.1210/jcem-30-3-293. [DOI] [PubMed] [Google Scholar]
- HAMBURGH M., LYNN E., WEISS E. P. ANALYSIS OF THE INFLUENCE OF THYROID HORMONE ON PRENATAL AND POSTNATAL MATURATION OF THE RAT. Anat Rec. 1964 Oct;150:147–161. doi: 10.1002/ar.1091500206. [DOI] [PubMed] [Google Scholar]
- Hennen G., Pierce J. G., Freychet P. Human chorionic thyrotropin: further characterization and study of its secretion during pregnancy. J Clin Endocrinol Metab. 1969 Apr;29(4):581–594. doi: 10.1210/jcem-29-4-581. [DOI] [PubMed] [Google Scholar]
- Inada M., Sterling K. Thyroxine transport in thyrotoxicosis and hypothyroidism. J Clin Invest. 1967 Sep;46(9):1442–1450. doi: 10.1172/JCI105636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARSE A. G. E. The cytochemistry and cytology of the normal anterior hypophysis investigated by the trichrome-periodic acid-Schiff method. J Pathol Bacteriol. 1952 Oct;64(4):811–826. doi: 10.1002/path.1700640414. [DOI] [PubMed] [Google Scholar]
- PETERSON R. R., YOUNG W. C. The problem of placental permeability for thyrotrophin, propylthiouracil and thyroxine in the guinea pig. Endocrinology. 1952 Feb;50(2):218–225. doi: 10.1210/endo-50-2-218. [DOI] [PubMed] [Google Scholar]
- Raiti S., Holzman G. B., Scott R. L., Blizzard R. M. Evidence for the placental transfer of tri-iodothyronine in human beings. N Engl J Med. 1967 Aug 31;277(9):456–459. doi: 10.1056/NEJM196708312770903. [DOI] [PubMed] [Google Scholar]
- Rosen F., Ezrin C. Embryology of the thyrotroph. J Clin Endocrinol Metab. 1966 Dec;26(12):1343–1345. doi: 10.1210/jcem-26-12-1343. [DOI] [PubMed] [Google Scholar]
- SMITH D. W., BLIZZARD R. M., WILKINS L. The mental prognosis in hypothyroidism of infancy and childhood; a review of 128 cases. Pediatrics. 1957 Jun;19(6):1011–1022. [PubMed] [Google Scholar]
- Schussler G. C., Plager J. E. Effect of preliminary purification of 131-I-thyroxine on the determination of free thyroxine in serum. J Clin Endocrinol Metab. 1967 Feb;27(2):242–250. doi: 10.1210/jcem-27-2-242. [DOI] [PubMed] [Google Scholar]
- Shepard T. H. Development of the human fetal thyroid. Gen Comp Endocrinol. 1968 Apr;10(2):174–181. doi: 10.1016/0016-6480(68)90024-5. [DOI] [PubMed] [Google Scholar]
- Shepard T. H. Onset of function in the human fetal thyroid: biochemical and radioautographic studies from organ culture. J Clin Endocrinol Metab. 1967 Jul;27(7):945–958. doi: 10.1210/jcem-27-7-945. [DOI] [PubMed] [Google Scholar]
- Sterling K., Brenner M. A. Free thyroxine in human serum: simplified measurement with the aid of magnesium precipitation. J Clin Invest. 1966 Jan;45(1):153–163. doi: 10.1172/JCI105320. [DOI] [PMC free article] [PubMed] [Google Scholar]