Abstract
The velvet protein, VeA, is involved in the regulation of diverse cellular processes. In this study, we explored functions of FgVeA in the wheat head blight pathogen, Fusarium graminearum,using a gene replacement strategy. The FgVEA deletion mutant exhibited a reduction in aerial hyphae formation, hydrophobicity, and deoxynivalenol (DON) biosynthesis. Deletion of FgVEA gene led to an increase in conidial production, but a delay in conidial germination. Pathogencity assays showed that the mutant was impaired in virulence on flowering wheat head. Sensitivity tests to various stresses exhibited that the FgVEA deletion mutant showed increased resistance to osmotic stress and cell wall-damaging agents, but increased sensitivity to iprodione and fludioxonil fungicides. Ultrastructural and histochemical analyses revealed that conidia of FgVeA deletion mutant contained an unusually high number of large lipid droplets, which is in agreement with the observation that the mutant accumulated a higher basal level of glycerol than the wild-type progenitor. Serial analysis of gene expression (SAGE) in the FgVEA mutant confirmed that FgVeA was involved in various cellular processes. Additionally, six proteins interacting with FgVeA were identified by yeast two hybrid assays in current study. These results indicate that FgVeA plays a critical role in a variety of cellular processes in F. graminearum.
Introduction
Fusarium graminearum Schwabe [teleomorph Gibberella zeae (Schwein.) Petch], a homothallic ascomycete, is the major causal agent of Fusarium head blight (FHB), which is a devastating disease of cereal crops worldwide [1]. While yield loss caused by the disease is a major concern, the mycotoxins, such as deoxynivalenol (DON) and its derivatives, produced by the fungus in infected grains pose a serious threat to human and animal health [2]. Despite the high economic impact of FHB, efficient strategies for the management of FHB are not available yet, which could be explained in part by our limited information for F. graminearum biology. Therefore, a better understanding of regulation mechanisms of fungal development, virulence, and DON biosynthesis in F. graminearum will be essential to facilitate the development of efficient control strategies against FHB.
The velvet protein encoded by VEA gene has been shown to be involved in the regulation of diverse cellular processes, including control of asexual and sexual development as well as secondary metabolisms in several fungal species [3], [4]. The VeA was first characterized in Aspergillus nidulans as an inhibitor of light-dependent conidiation in 1960 [5], and was later shown to be a negative regulator of asexual development [6]. A VEA deletion mutant of A. nidulans failed to form fruiting bodies, and the opposite effect was observed when the gene was over-expressed, which confirmed that VeA is a positive regulator of sexual development and simultaneously a negative regulator of asexual development [7]. It is interesting that in A. parasiticus, genetically related to A. nidulans, deletion of VEA resulted to a reduction of conidiation [8]. These results indicate that the role of VeA in sexual development varies significantly among different fungal species.
In last few years, effects of VeA on secondary metabolism have been well investigated in Aspergillus spp. In A. nidulans, VeA is necessary for expression of the transcription factor AflR, which activates the mycotoxin sterigmatocystin biosynthesis gene cluster [9]. Similarly, in A. parasiticus and A. flavus, VeA is essential for the expression of two transcription factors AflR and AflJ, which are necessary for activation of aflatoxin biosynthesis genes. Consequently, the mycotoxin aflatoxin biosynthesis is completely blocked in VEA deletion mutants of these fungi [10], [11]. In addition to its multiple functions in secondary metabolism and fungal development, recent evidence showed that VeA negatively regulated catabolism of branched chain amino acid and ethanol metabolism at the transcriptional level [12].
VeA proteins are conserved throughout the fungal kingdom [13]. Recently, functions of VeA have been investigated in several other filamentous fungi including Acremonium chrysogenum [14], Fusarium verticillioides [13], [15], Mycosphaerella graminicola [16] and Penicillium chrysogenum [17]. In these fungal species, VEA deletion mutants present some new phenotypic characteristics, which were not described in Aspergillus spp. For example, deletion of VEA gene (FvVE1) in F. verticillioides suppressed aerial hyphal growth and reduced colony surface hydrophobicity on solid media. In addition, deletion of FvVE1 markedly increased the ratio of macroconidia to microconidia [13]. The VEA deletion mutants of M. graminicola were hypersensitive to shaking [16]. In F. fujikuroi, FfVel1 (a homolog of VeA of Aspergillus spp.) can act as a positive regulator for biosyntheses of gibberellins, fumonisins and fusarin CA, simultaneously as a negative regulator for another secondary metabolite bikaverin [18]. Furthermore, FfVel1 deletion mutants failed to infect rice seedlings [18]. In contrast, pathogenicity was not altered in the VEA deletion mutant of M. graminicola [16]. These studies indicate that functions of VeA in different fungal species may vary significantly.
The purpose of this study was to investigate functions of FgVEA gene encoding a VeA-homologous protein in F. graminearum. Although the role of VeA in controlling synthesis of secondary metabolites is a common feature, notably, in current study, we observed that the FgVEA deletion mutants of F. graminearum presented some new phenotypic characteristics, which were not previously described in other fungi.
Results
Sequence analysis of FgVEA
The FgVEA (F. graminearum genome accession number FGSG_11955.3) was originally identified through homology searches of the F. graminearum genome sequence by using BLASTP algorithm with the FvVe1 of F. verticillioides [13] as query. In F. graminearum genome database, FGSG_11955.3 missed a 225-bp fragment. After sequenced the full length genomic DNA and cDNA sequence for FgVEA, we found that the gene including one intron is 1,656-bp in length, and encodes a 532-amino-acid protein. The predicted amino acid sequence of FgVeA shares 79%, 78%, 52%, and 47% identities to FfVel1 of F. fujikuroi, FvVe1 of F. verticillioides, VeA of A. nidulans, and PcVelA of P. chrysogenum, respectively. Alignment of predicted amino acid sequences showed that N-terminal regions of VeA from different fungi including F. graminearum are highly conserved (Fig. S1B). Further in silico analyses demonstrated that FgVeA has a putative pat4 nuclear localization signal (NLS) from amino acids 474 to 477.
Deletion of FgVEA in F. graminearum
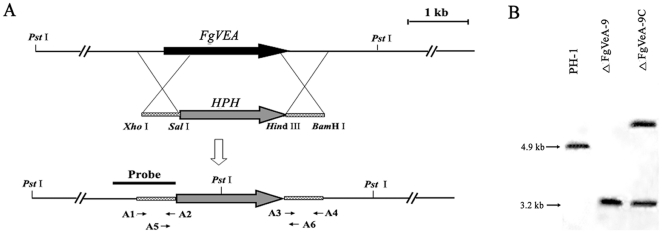
To investigate functions of the velvet protein FgVeA in F. graminearum, we generated gene deletion mutants using a homology recombination strategy (Fig. 1A). Among thirteen hygromycin-resistant transformants, eight FgVEA deletion mutants were identified by PCR analysis with the primer pair A5 + A6 (Table S1). The primer pair amplified 1,759- and 1,537-bp fragments from FgVEA deletion mutants and the wild-type progenitor PH-1, respectively. When probed with a 1,162-bp DNA fragment of FgVEA, the deletion mutant ΔFgVeA-9 had an anticipated 3,213-bp band, but lacked a 4,912-bp band which was present in the progenitor (Fig. 1B). The Southern hybridization pattern confirmed that the ΔFgVeA-9 is a null mutant resulting from a single homologous recombination event at the FgVEA locus. The complemented strain ΔFgVeA-9C contains a single copy of wild-type FgVEA, which was inserted into genome of the FgVEA deletion mutant ΔFgVeA-9 (Fig. 1B).
Figure 1. Schematic representation of the FgVEA deletion strategy. (A).
FgVEA and hygromycin resistance cassette (HPH) are denoted by large black and gray arrows, respectively. Annealing sites of PCR primers are indicated with arrows (see supplementary Table 1 for the primer sequences). (B) A 1,162-bp fragment of FgVEA was used as a probe in Southern blot hybridization analysis. Genomic DNA preparations of the wild-type PH-1, the FgVEA deletion mutant ΔFgVeA-9, and the complement strain ΔFgVeA-9C were digested with Pst I.
Effects of FgVeA on hyphal growth and pigment formation in F. graminearum
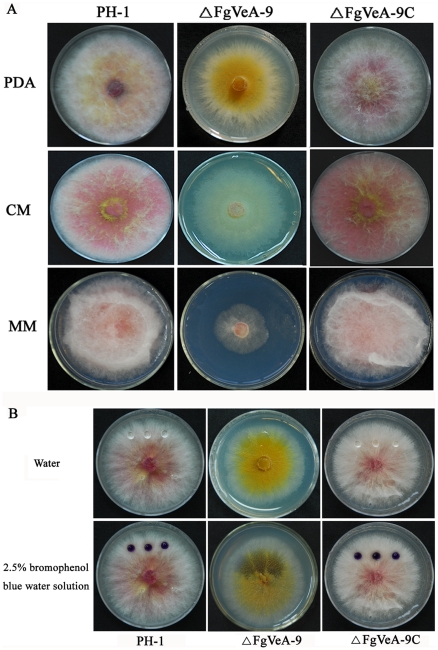
The deletion of FgVEA dramatically affected colony morphology of F. graminearum on solid media. The mycelial growth rate of ΔFgVeA-9 was significantly slower than that of wild type progenitor PH-1 and the complemented strain ΔFgVeA-9C on MM medium (Fig. 2A). In addition, the ΔFgVeA-9 exhibited reduced aerial hyphal growth on solid media PDA, CM and MM (Fig. 2A), although scanning electron microscopy examination showed that the hyphae of ΔFgVeA-9 were not significantly different from those of wild type progenitor (data not shown). The phenotypic defects of ΔFgVeA-9 mutant on solid media were restored by genetic complementation with the wild-type FgVEA in the complemented strain ΔFgVeA-9C (Fig. 2A).
Figure 2. Impact of FgVEA on colony morphology and pigment formation.
(A) The wild-type strain PH-1, FgVEA deletion mutant ΔFgVeA-9, and complemented strain ΔFgVeA-9C were grown on solid media, PDA, CM, MM for 4 days at 25°C. (B) On each of the fungal colonies, 20 µl of water or 2.5% bromophenol blue solution was pipetted on the colony surface and photographed 10 min later. Spherical water droplets formed on colonies of PH-1 and ΔFgVeA-9C, whereas the droplet dispersed immediately on colonies of ΔFgVeA-9.
The hydrophobic property on the cell surface is a distinguishable feature of aerial hyphae and contributes to hyphal formation in many fungal species [19], [20]. Deletion of FgVEA led to inhibition of aerial hyphae gorwth, which suggested a reduction of hydrophobicity on cell surface of the mutant. To confirm this deduction, 20 µl of water was placed on the colony surface of each strain grown on the solid medium PDA. As shown in Fig. 2B, the water formed spherical droplets on the colony of wild-type progenitor without extending or being absorbed for at least 30 min. In contrast, the water was absorbed into the mycelia of ΔFgVeA-9 within 10 sec. The absorption difference was easily visualized when 2.5% bromophenol blue water solution was placed on the colony surface (Fig. 2B). These results indicate that FgVeA is important for colony surface properties in F. graminearum.
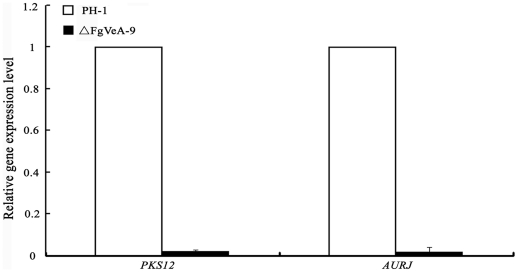
On CM medium, it was clear that ΔFgVeA-9 revealed a significant reduction in red pigment formation (Fig. 2A). To further confirm this observation, we assayed the expression of PKS12 and AURJ genes encoding a type I polyketide synthase and O-methyltransferase, respectively, which are necessary for red pigment biosynthesis [21]. Quantitative real-time PCR (qRT-PCR) analyses showed that expression levels of both PKS12 and AURJ in ΔFgVeA-9 were decreased by 98% as compared to those in wild type progenitor PH-1 (Fig. 3). These results indicate that FgVeA was involved in the regulation of pigment biosynthesis in F. graminearum.
Figure 3. Relative expression levels of PKS12 and AURJ in the FgVEA deletion mutant ΔFgVeA-9.
RNA samples were extracted from mycelia of each strain after grown in potato dextrose broth for 2 days. The relative expression of PKS12 and AURJ in ΔFgVeA-9 is the relative amount of cDNA of each gene in the wild-type strain. Line bars in each column denote standard errors of three experiments.
Effects of FgVeA on conidial differentiation and germination
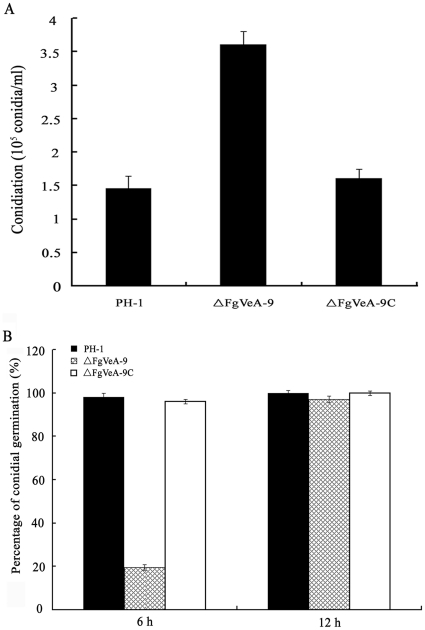
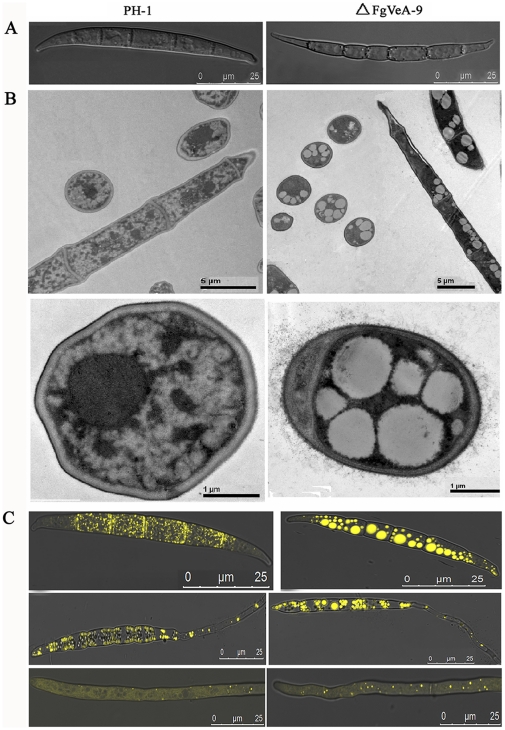
The VeA had been found to regulate asexual development in several other fungal species [4]. Consequently, we examined the conidiation, conidial germination, and cellar structure for the FgVEA deletion mutant ΔFgVeA-9. In MBL medium, ΔFgVeA-9 produced significantly more conidia than the wild type progenitor or the complemented strain (Fig. 4A). When cultured the conidia in 2% glucose, only approximately 20% conidia of ΔFgVeA-9 were able to germinate within 6 hr of incubation, but almost all conidia of wild type progenitor or the complemented strain germinated under the same condition. When incubation time was extended to 12 h, all the conidia of ΔFgVeA-9 were able to germinate (Fig. 4B), and form normal unbranched germ tubes, indicating that deletion of FgVEA led to a delay in conidial germination. Scanning electron microscopic examination showed that conidia of ΔFgVeA-9 were slightly slender than those of wild type PH-1 (Fig. 5A). In order to characterize the conidia of ΔFgVeA-9 in detail, we examined conidial structure using transmission electron microscopy. As shown in Fig. 5B, a few large lipid droplets were observed in ΔFgVeA-9 conidia, but not in those of wild type strain. The lipid droplets were further verified by histochemical staining with Nile Red. The large discrete lipid droplets were highlighted in the ungerminated and germinating conidia of ΔFgVeA-9, but not in the wild-type strain (Fig. 5C). The large discrete lipid droplets were degraded in the hyphae of ΔFgVeA-9 (Fig. 5C). In the following serial analysis of gene expression (SAGE) experiment, we also paid attention on the expression of the genes involved in fatty acid biosynthesis and metabolism. As shown in Table S2, among 41 genes, 25 and 1 genes involved in fatty acid metabolism were up- and down-regulated, respectively, more than five folds. These results strongly indicate that FgVeA was involved in the regulation of lipid metabolism in F. graminearum.
Figure 4. Impact of FgVEA on conidiation and conidial germination of F. graminearum. (A).
Conidia were quantified after incubation of the wild-type strain PH-1, FgVEA deletion mutant ΔFgVeA-9, and complemented strain ΔFgVeA-9C in 10 ml mung bean liquid medium for 4 days in a shaker. (B) Percentages of germinated conidia of PH-1, ΔFgVeA-9, and ΔFgVeA-9C after incubated in 2% glucose for 6 h or 12 h. Bars denote standard errors from three repeated experiments.
Figure 5. Ultrastructural and histochemical analyses of lipid droplets within conidia and hyphae of the mutant ΔFgVeA-9.
(A) Differential interference contrast (DIC) images of conidia were captured with an electronic microscope. (B) Lipid drops within conidia of the wild type PH-1 and the ΔFgVeA-9 mutant examined with a transmission electronic microscope. (C) Lipid drops in conidia (top), germinating conidia (middle), and hyphae (bottom) were stained with Nile Red and examined under a microscope with episcopic fluorescence.
Sensitivity of FgVEA deletion mutant to osmotic stresses and fungicides
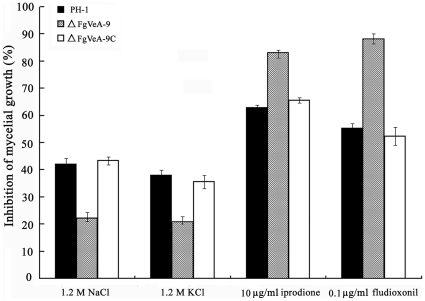
In F. verticillioides, the FvVE1 deletion mutants exhibited a dramatic increase in production of aerial hyphae and radial growth on solid media amended with osmotic stabilizers NaCl, KCl, sorbitol, and sucrose [13]. Thus, we examined sensitivity of ΔFgVeA-9 to osmotic stresses mediated by these osmotic stabilizers. As shown in Fig. 6, ΔFgVeA-9 exhibited significantly increased resistance to ionic osmotic stress mediated by 1.2 M NaCl and 1.2 M KCl. Previous studies have shown that phenylpyrrole and dicarboximide fungicides activate the high osmolarity glycerol (HOG) pathway [22], [23], and the fungicide-resistant mutants of F. graminearum revealed increased sensitivity to osmotic stabilizers, while the fungicide-sensitive strain was tolerant to osmotic stress [24]. Therefore, we were interested in determining the sensitivity of ΔFgVeA-9 to the phenylpyrrole fungicide fludioxonil and the dicarboximide fungicide iprodione. As expected, ΔFgVeA-9 showed increased sensitivity to the fludioxonil and iprodione (Fig. 6).
Figure 6. Sensitivity of the PH-1, ΔFgVeA-9 and ΔFgVeA-9C to osmotic stresses and fungicides.
Osmotic stresses were mediated by addition of 1.2 M NaCl or 1.2 M KCl in potato dextrose agar (PDA) medium. The fungicides iprodione and fludioxonil were added into PDA at 10 µg/ml and 0.1 µg/ml, respectively. Bars denote standard errors from three repeated experiments.
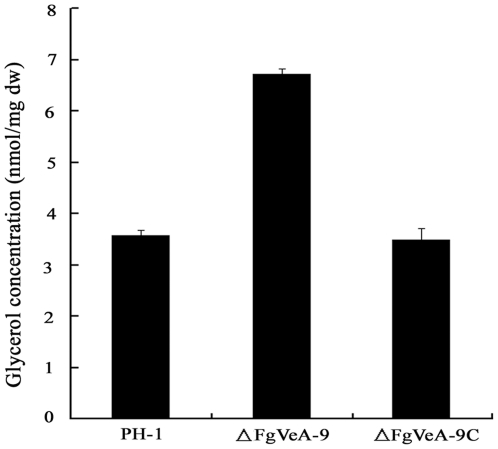
It has been reported that osmotic stress can induce glycerol accumulation in fungi via the HOG-like pathway [25]. We therefore analyzed glycerol accumulation in mycelia of the mutant ΔFgVeA-9. As shown in Fig. 7, the level of glycerol concentration in ΔFgVeA-9 was significantly higher than that in the wild-type PH-1 and the complemented strain ΔFgVeA-9C, which could partially explain the reason why ΔFgVeA-9 exhibited increased resistance to osmotic stresses.
Figure 7. Effects of FgVeA on the glycerol biosynthesis.
Intracellular glycerol concentration (nmol/mg dried mycelia) in mycelia of the wild-type strain PH-1, FgVEA deletion mutant ΔFgVeA-9, and complemented strain ΔFgVeA-9C were analyzed after incubation in PDB for 2 days. Bars denote standard errors from three repeated experiments.
Resistance of the FgVEA deletion mutant to cell wall damaging agents
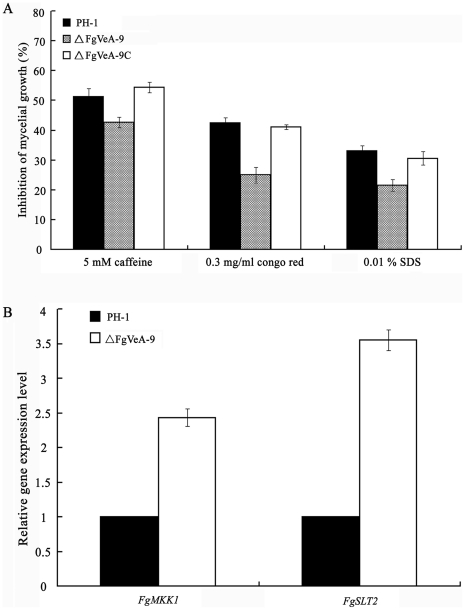
The deletion of FgVEA led to an increase in resistance to osmotic stabilizers, which suggests that FgVeA might be involved in the regulation of cell member and/or cell wall integrity. To address this, we determined sensitivity of ΔFgVeA-9 to cell member damaging agent SDS, and to cell wall damaging agents: congo red and caffeine. Compared to the wild type progenitor and the complemented strain, ΔFgVeA-9 displayed increased resistance to these compounds (Fig. 8A). To further confirm the involvement of FgVeA in the regulation of cell wall integrity (CWI) pathway, we determined the expressions of FgMKK1 (FGSG_07295) and FgSLT2 (FGSG_10313), which are homologous to the S. cerevisiae CWI core element genes, Mkk1 and Slt2, respectively. As shown in Fig. 8B, expression levels of FgMKK1 and FgSLT2 in ΔFgVeA-9 were 2.43 and 3.55-folds, respectively, higher than those in the wild-type strain. These results further supported that FgVeA was associated with the CWI pathway.
Figure 8. Effects of FgVeA on cell wall integrity of F. graminearum.
(A) Sensitivity of the wild type PH-1, FgVEA deletion mutant ΔFgVeA-9, and complemented strain ΔFgVeA-9C to cell wall damaging agents. (B) Relative expression levels of FgSLT1 and FgMKK1 in PH-1 and mutant ΔFgVeA-9. The relative expression of FgSLT1 and FgMKK1 in ΔFgVeA-9 is the relative amount of cDNA of each gene in the wild-type strain. Line bars in each column of each figure denote standard errors of three repeated experiments.
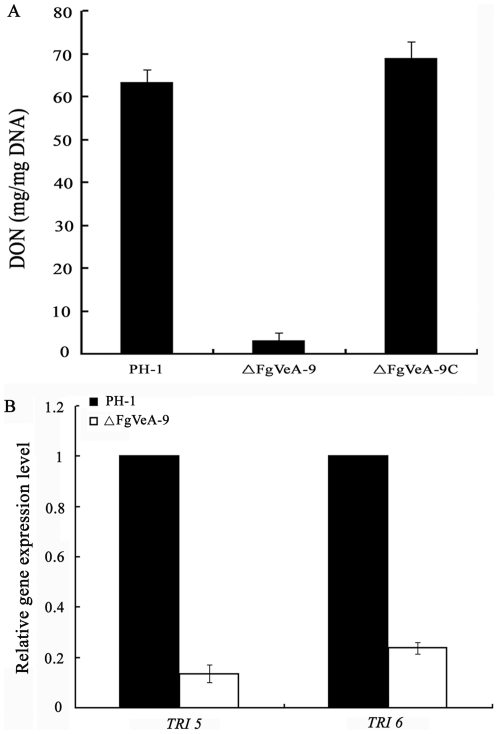
Role of FgVeA in the regulation of deoxynivalenol (DON) biosynthesis
Previous studies have shown that VeA proteins were involved in the regulation of secondary metabolism in several fungi [4]. F. graminearum produces various secondary metabolites including the mycotoxin DON. Therefore, we analyzed DON biosynthesis in ΔFgVeA-9. When cultured on wheat kernels for 20 days, the amount of DON produced by the wild-type strain were 21 times higher than that produced by ΔFgVeA-9 (Fig. 9A). Complementation of the gene restored the ability of the fungus to produce DON production. To further confirm this finding, we assayed the expression of TRI5 and TRI6 by quantitative real-time PCR (qRT-PCR) using RNA samples isolated from mycelia grown in GYEP medium. The expression levels of TRI5 and TRI6 in the mutant ΔFgVeA-9 was decreased by 87% and 76%, respectively, as compared to those in wild type progenitor (Fig. 9B). These results indicate that FgVeA was necessary for the regulation of DON biosynthesis in F. graminearum.
Figure 9. Effects of FgVeA on the biosynthesis of DON. (A).
Amount of DON (per mg fungal DNA) produced by FgVEA deletion mutant ΔFgVeA-9, and complemented strain ΔFgVeA-9C was detected in infected wheat kernels after 20 days of incubation. Line bars in each column denote standard errors of three repeated experiments. (B) Relative expression levels of TIR5 and TRI6 in PH-1 and ΔFgVeA-9. The relative expression of TIR5 or TRI6 in ΔFgVeA-9 is the relative amount of cDNA of each gene in the wild-type progenitor. Line bars in each column denote standard errors of three repeated experiments.
FgVeA is essential for virulence of F. graminearum
DON has been identified as a virulence factor in F. graminearum [26]–[28]. Since the FgVEA deletion mutants were impaired in DON biosynthesis, we further analyzed the virulence of FgVEA deletion mutant by point inoculating conidial suspension on flowering wheat head. Fifteen days after inoculation, ΔFgVeA-9 caused infection only in the inoculated spikelet, but not in nearby spikelets (Fig 10). Under the same conditions, however, scab symptoms developed in more than 90% spikelets when wheat heads were point-inoculated with the wild-type PH-1, or the FgVEA complemented strain FgVeA-9C (Fig. 10).
Figure 10. Virulence of the wild type PH-1, FgVEA deletion mutant ΔFgVeA-9, complemented strain ΔFgVeA-9C on flowering wheat heads.
Wheat heads were point-inoculated with conidial suspension of each strain, and infected wheat heads were examined 15 days after inoculation.
Serial analysis of gene expression (SAGE) reveals that FgVeA is associated with various metabolism pathways
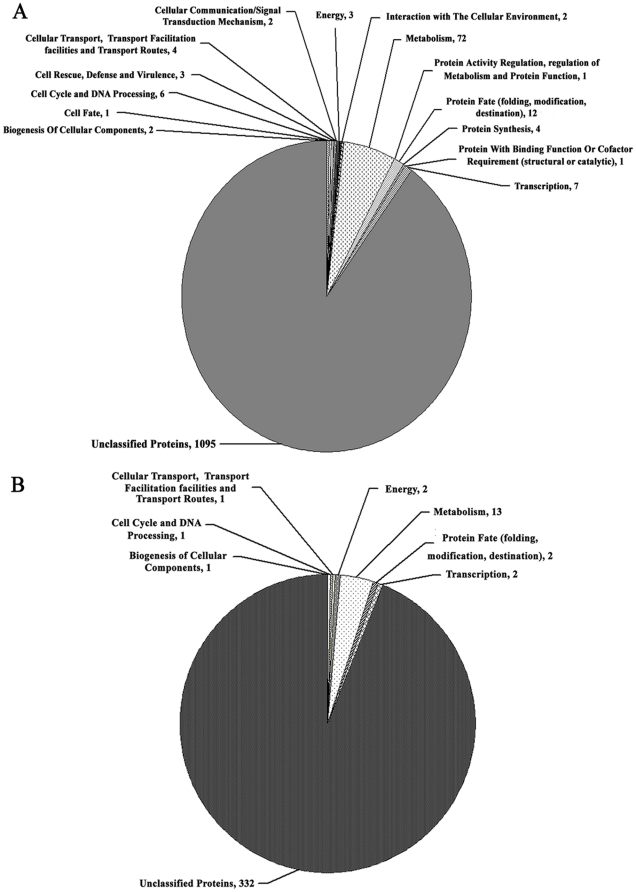
To further elucidate the function of FgVeA as well as identify genes that it may impact, we conducted SAGE assays for ΔFgVeA-9 and the wild-type progenitor PH-1. After removal of low quality (<3) tags, a total of 125,406 and 131,640 distinct tags were obtained for PH-1 and ΔFgVeA-9, respectively. Among these distinct tags, 70.02% and 75.5% can be uniquely mapped to the reference sequences for PH-1 and ΔFgVeA-9, respectively. For SAGE data, the analysis is usually limited to a predefined tag showing at least 5-fold difference in abundance at a P value ≤0.05 [29]. With this criterion, we identified 1,215 genes up-regulated (>5-folds) and 354 genes down-regulated (<0.2-folds) in ΔFgVeA-9 compared to PH-1. To obtain better understanding of the overall gene expression profile, the up- and down-regulated genes were mapped in the chromosomes, and they seemed to be distributed evenly in the chromosomes (Fig. S2). Using the FunCat program (http://mips.helmholtz-muenchen.de/proj/funcatDB/search_main_frame.html), the up- and down-regulated genes were further grouped into several functional categories, and most genes were classed as “unknown function”. Among the 120 classified genes, which were up-regulated in ΔFgVeA-9, 72 (60%) were grouped into the functional category of metabolism (Fig. 11A). Among the 22 classified down-regulated genes, again, 13 (59%) were associated with various metabolisms (Fig. 11B). In addition, expressions of 26 genes associated peroxisome biogenesis and SNARE interactions in vesicular transport pathway were changed dramatically in the FgVEA mutant (Figs. S3A and B). These results further support that FgVeA is involved in various cellular processes.
Figure 11. Pie chart grouping the genes up- and down-regulated in expression in ΔFgVeA compared with PH-1. (A).
A total of 1,215 genes were up-regulated more than 5 folds in the mutant ΔFgVeA-9 compared with wild-type PH-1. (B) A total of 354 genes were down-regulated more than 5 folds in the mutant ΔFgVeA-9 compared with wild-type PH-1. The expressions of genes were detected by the serial analysis of gene expression method.
FgVeA interact with several proteins containing methyltransferase domain
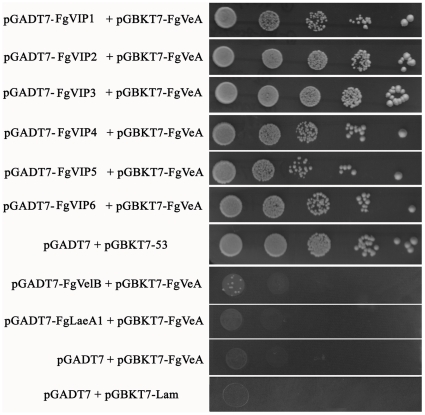
Recent work in A. nidulans indicated that the positive regulation of secondary metabolites is most likely achieved through the physical interactions of VeA with the velvet-like protein VelB and the putative methyltransferase LaeA in nucleus [30]. Yeast two-hybrid analysis confirmed the VeA-VelB and VeA-LaeA interactions, where VelB and LaeA do not interact in A. nidulans, suggesting that VeA acts as a bridge between VelB and LaeA [30]. Genome-wide search for the homolog of VelB and LaeA in F. graminearum showed that the fungus contains a VelB homolog (here named FgVelB, FGSG_01362), and a LaeA homolog designed FgLaeA1 (FGSG_00657). Deletion of FgLaeA1 led to a reduction in red pigment formation on PDA medium (Fig. S4). Surprisingly, yeast two-hybrid assay showed that FgVeA did not interact with FgVelB or FgLaeA1 (Fig. 12). Using yeast two-hybrid approach, we did identify six FgVeA interacting proteins (designed FgVIPs), that are homologous to FgLaeA1 (Fig. 12). All these six FgVIP proteins [FgVIP1 (FGSG_07660), FgVIP2 (FGSG_03525), FgVIP3 (FGSG_05685), FgVIP4 (FGSG_03567), FgVIP5 (FGSG_08741), and FgVIP6 (FGSG_03011)] contain a conserved methyltransferase domain. It was surprised that except for a slight change in pigment formation, the deletion mutants of these six genes were indistinguishable from the wild type progenitor on PDA plates (Fig. S4), and all mutants were pathogenic on wheat head (data not show). These results strongly indicate that the velvet complex in F. graminearum was quite different from that in A. nidulans. An important challenge at this time is to elucidate the dynamics and functions of this large protein complex in F. graminearum.
Figure 12. Yeast two-hybrid analysis of the interaction between FgVeA, FgVelB and FgLaeA1 or six FgVeA interacting proteins (named FgVIP1-6).
The pair of plasmids pGBKT7-53 and pGADT7 was served as a positive control. The pairs of plasmids pGBKT7-Lam and pGADT7, pGADT7 and pGBKT7-FgVeA were used as negative controls. Growth of the transformed yeast was assayed on the medium containing 5 mM 3-aminotriazole (3-AT), but lacking His, Leu and Trp. Columns in each panel represent serial decimal dilution.
Discussion
Recent studies in several filamentous fungi have shown that the VeA protein plays an important role in fungal growth, colony morphology, development, and secondary metabolism. However, certain variations in the role have been observed in different fungi even within the genus of Aspergillus [4]. For example, complementation of the A. nidulans VEA deletion mutant with the velvet gene from F. verticillioides could not rescue the wild-type phenotypes of A. nidulans, indicating species-specific functions of VeA in different fungi [4]. Bioinformatic analyses have demonstrated that fungal VeA proteins consist of conserved N-terminal and variable C-terminal regions, which may be responsible for conserved and species-specific functions of VeA, respectively. Consistent with conserved N-terminal as well as variable C-terminal functions of the velvet proteins, in this study, we found some of the phenotypes in FgVEA mutant were similar to those reported in other fungi, while others were novel and unique to F. graminearum.
The first study of VeA ortholog in other fungal species beyond the genus Aspergillus was conducted by Li et al. for the plant pathogen F. verticillioides [13]. In F. verticillioides, conidia of FvVE1 deletion mutant exhibited three unnormal types of germination: hyperbranched hyphae, microcycle conidiation and yeast-like growth [13]. Different from F. verticillioides, in current study, we found that deletion of F. graminearum FgVEA led to a delay in conidial germination, but conidia of FgVEA deletion mutant germinated to form normal unbranched germ tubes. In F. verticillioides, deletion of FvVE1 caused a notable activation of conidiation and increased the ratio of macroconida to microconidia [13]. In this study, we also observed that the FgVEA deletion mutant of F. graminearum produced significant more conidia than wild type progenitor. In contrast, deletion of FfVel1 in F. fujikuroi led to a significant reduction in conidiation [18]. The opposite effect of VeA on conidiation had also been found in Aspergillus species. In A. nidulans and A. flavus, deletion of VEA resulted in an increase in asexual development [6], [31]. However, conidiation was reduced when VEA was deleted in A. parasiticus, a species genetically related to A. flavus [8]. These results strongly indicated that the roles of VeA in conidiation and conidial germination vary significantly in different fungal species.
A previous study of F. verticillioides showed that deletion of the FvVE1 gene resulted in decreased hydrophobicity of the colony surface and impairment in aerial hyphae formation [13]. Similarly, we observed a significant reduction of aerial hyphal growth and hydrophobicity in FgVEA mutant compared to the wild-type progenitor PH-1. The reduced hydrophobicity on cell surface in FgVEA deletion mutant suggested an alteration in cell wall composition. We therefore tested sensitivity of the FgVEA deletion mutant to cell wall damaging agents, congo red and caffeine. As expectedly, the FgVEA deletion mutant showed resistant to cellulose-binging chemical congo red and to caffeine, which is in agreement with the overexpression of 1,3-beta-glucan synthase gene, FgGLS2 (FGSG_07946) in the mutant (Table S2). These results indicated that FgVeA had an important role in maintaining normal cell wall composition and integrity.
Secondary metabolism had been reported to be positively regulated by velvet proteins in several fungi. F. graminearum, a pathogen of important cereal crops, produces various secondary metabolites including the trichothecene mycotoxin, DON [32]. In this study, we observed that FgVeA positively regulated expression of DON biosynthesis genes and DON production. Similar to DON production, the FvVE1 deletion mutant of F. verticillioides produced significantly less gibberellin than the wild type strain did [18]. The dramatic down regulation of secondary metabolites in Fusarium spp. was consistent with the observation from Aspergillus spp., where VeA had been found to activate production of mycotoxin sterigmatosystin in A. nidulans [33], aflatoxin and cyclopiazonic acid in A. flavus [31], [34] as well as aflatoxin in A. parasiticus [8].
A previous study showed that the FvVE1 deletion mutant of F. verticillioides produced more red pigment (polyketide bikaverin) than the wild type strain. In contrast, the FvVE1 overexpression mutant showed significantly less coloration. Consistently with these observations, expressions of bikaverin cluster genes were significantly up-regulated in the FvVEl deletion mutant and down-regulated in the overexpression mutant. F. graminearum also produced a red pigment, the polyketide aurofusarin [35]. In contrast to the bikaverin biosynthesis in F. verticillioides, in current study, we found that the FgVEA deletion mutant produced dramatically less red pigment on solid media. Furthermore, expressions of seven aurofusarin biosynthesis genes were down-regulated significantly in the mutant (Table S2). These results indicate that the role of VeA in the regulation of pigment biosynthesis is species-dependent.
In response to osmotic pressure, fungi usually accumulated glycerol within their cells via HOG pathway to increase the internal turgor pressure [36]. In current study, we found that the FgVEA deletion showed increased tolerance to osmotic stress mediated by NaCl and KCl. This finding was in agreement with the observation that the mutant accumulated a higher level of glycerol than the wild-type progenitor. These results suggest that FgVeA was associated with the HOG pathway in F. graminearum.
In this study, phenotypic characterization of the FgVEA deletion mutant showed that FgVeA was essential for virulence of F. graminearum. The impairment in virulence of FgVEA deletion mutant appeared to be due to defects in multiple regulatory functions. First, the deletion of FgVEA led to a delay in conidial germination and retardation in mycelium growth. Second, the FgVEA mutant produced a dramatically low level of DON, which played a significant role in spread of the fungus within a spike [37]. In addition, it had been demonstrated that the hydrophobic property on the cell surface and normal fungal cell wall were important for viability, fungal morphology, and virulence [19], [38], the reduced hydrophobicity of cell surface in FgVEA deletion mutant may partially lead to impairment in virulence of the mutant on wheat head. The involvement of VeA protein in virulence had also been reported in F. fujikuroi [18], but not in M. graminicola. The FfVel1 mutant of F. fujikuroi was nonpathogenic on rice seedlings; but in M. graminicola, the VEA deletion mutant remained high virulence on wheat leaves [16]. These results indicated that the roles of VeA in pathogenicity vary significantly in different fungi.
In this study, we observed that conidia of ΔFgVeA contained more lipid droplets than the wild-type progenitor. SAGE data also showed that an increased number of genes associated with fatty acid biosynthesis and metabolism were up-regulated significantly in the mutant (Table S2). To our knowledge, it was the first report that VeA was involved in lipid metabolism in fungi. Interestingly, previous analysis in our laboratory revealed that the deletion of a type 2C protein phosphatase gene FgPTC3 also led to accumulation of large lipid droplets in F. graminearum conidia [39]. Furthermore, several phenotypes of the FgVEA deletion mutant, including reduction in aerial hyphal formation and DON biosynthesis, resistance to cell wall damaging agents, and impairment in pathogenicity, were similar to those of the FgPTC3 deletion mutant. These results indicated that FgPtc3 and FgVeA had some common functions in regulation of various cellular processes. This inference was further supported by SAGE analyses of gene expression profiling in FgPTC3 and FgVEA mutants. Among 1215 up-regulated genes in the FgVEA deletion mutant, 721 genes (59.3%) were also up-regulated in the FgPTC3 deletion mutant. Again 354 down-regulated genes in the FgVEA deletion mutant, 218 genes (61.6%) were also down-regulated in the FgPTC3 deletion mutant (Fig. S5). The significant overlap in genes regulated by FgVeA and FgPtc3 strongly indicated that both components were involved in some common signaling pathways in F. graminearum. Thus, it will be interesting to investigate relationships between these two components and their downstream network in F. graminearum, which would be helpful in understanding biology of F. graminearum.
Materials and Methods
Strains and culture conditions
F. graminearum wild-type strain PH-1 was used as a parental strain for transformation experiments. The wild-type strain and transformants generated in this study were grown on potato dextrose agar (PDA), minimal medium (MM) or complete medium (CM) for mycelial growth tests, and in mung bean liquid (MBL) medium [40] for sporulation analysis.
Sequence analysis of FgVEA
The FgVEA were originally identified through homology searches of the F. graminearum genome sequence (available at http://www.broadinstitute.org/annotation/genome/fusarium_group/MultiHome.html) by using BLAST with the FvVE1 from F. verticillioides [13] as query. To verify the existence and the size of intron in FgVEA, RNA was extracted from mycelia of the wild-type strain PH-1 using the TaKaRa RNAiso Reagent (TaKaRa Biotech. Co., Dalian, China) and used for reverse transcription with a RevertAid H Minus First Strand cDNA Synthesis kit (Fermentas Life Sciences, Burlington, Canada) according to the manufacturer's instructions. Reverse transcription PCR amplification of FgVEA cDNAs were performed using the primer pair Va-F1 + Va-R1 (Table S1). PCR amplifications were performed with the following parameters: initial denaturation at 95°C for 3 min, followed by 35 cycles of denaturation at 94°C for 40 s, annealing at 54°C for 40 s, extension at 72°C for 2 min, and final extension at 72°C for 10 min. The resultant PCR products were purified, cloned and sequenced.
Construction of vector for the deletion of FgVEA
The FgVEA gene deletion vector pCA-FgVeA-Del was constructed by inserting two flanking sequences of FgVEA gene into two sides of the HPH (hygromycin resistance) gene in the pBS-HPH1 vector [41]. The upstream flanking sequence fragment of FgVEA was amplified from PH-1 genomic DNA using the primer pair A1 + A2 (Table S1). The 616-bp fragment was inserted into Xho I-Sal I sites of the pBS-HPH1 vector to generate the plasmid pBS-FgVeA-up. Subsequently, a 554-bp downstream flanking sequence fragment of FgVEA was amplified from the PH-1 genomic DNA using the primer pair A3 + A4 and was inserted into the Hind III-BamH I site of pBS- FgVeA-up vector to generate the plasmid pBS- FgVeA-UD. Finally, the 2,673-bp fragment containing FgVeA-upstream-HPH-FgVeA-downstream cassette (Fig. 1A) was obtained by digestion of plasmid pBS-FgVeA-UD with Xho I and BamH I, and ligated into the Xho I-BamH I site in pCAMBIA 1300 (CAMBIA, Canberra, Australia). The resultant FgVEA deletion vector pCA-FgVeA-Del was transformed into Agrobacterium tumefaciens strain C58C1 by electroporation, the A. tumefaciens-mediated fungal transformation was performed as described previously [42].
Complementation of the FgVEA gene deletion mutant
The FgVEA deletion mutant (ΔFgVeA-9) was complemented with a full-length FgVEA gene, to confirm that the phenotype changes in FgVEA deletion mutant were due to the deletion of the gene. The FgVEA complement plasmid pCA-FgVeA-C was constructed using the backbone of pCAMBIA1300. First, a Xho I-Kpn I NEO cassette containing a trpC promoter was amplified from plasmid pBS-RP-Red-A8-NEO [43] with primers neo-F + neo-R (Table S1), and cloned into the Xho I-Kpn I site of pCAMBIA1300 to create plasmid pCA-neo. Then, a full-length FgVEA gene including 2,129-bp promoter region and 854-bp terminator region was amplified from genomic DNA of the wild-type strain PH-1 using the primer pair Va-com-F + Va-com-R (Table S1), and subsequently cloned into Pst I and Hind III sites of pCA-neo to generate the complement plasmid pCA-FgVeA-C. Before plasmid pCA-FgVeA-C was transformed into A. tumefaciens strain C58C1, FgVEA in this plasmid was sequenced to ensure flawlessness of the sequence. Transformation of ΔFgVeA-9 with full-length FgVEA gene was conducted as described above except that geneticin was used as a selection agent.
Mycelial growth and conidiation assays
Mycelial growth tests under different conditions were performed on PDA or MM plates supplemented with the following products: NaCl, KCl, iprodione, fludioxonil, caffeine, congo red, SDS at concentrations indicated in figure legends. Each plate was inoculated with a 5-mm mycelial plug taken from the edge of a 3-day-old colony. There were three replicate plates for each treatment. Plates were incubated at 25°C for 4 days in the dark, and then colony diameter in each plate was measured and the original mycelial plug diameter (5 mm) subtracted from each measurement. The percentage of the mycelial radial growth inhibition (RGI) was calculated using the formula RGI = ((C–N)/(C–5))*100, where, C is colony diameter of the control, and N is that of a treatment. Each experiment was repeated three times.
For conidiation assays, ten mycelial plugs (5-mm in diameter) of each strain taken from the periphery of a 3-day-old colony were inoculated in a 50-ml flask containing 10 ml of MBL medium. The flasks were incubated at 25°C for 4 days in a shaker (180 rpm). For each strain, the number of conidia in the broth was determined using a hemacytometer. The experiment was repeated three times.
Microscopic examination of hyphal and conidial morphology
Hyphal and conidial morphology of the strains were examined with the Leica TCS SP5 imaging system. For transmission electron microscopy, conidia were fixed with 2.5% glutaraldehyde for 24 h at 4°C, then samples were washed with 0.1 M phosphate buffered saline (PBS) for three times, fixed in 1% osmic acid for 3 h at room temperature. After the fixed tissues were rinsed 3 times (15 min each) with 0.1 M PBS, and dehydrated in graded ethanol solutions, the samples were embedded in Lowicryl K4M resin (Electron Microscopy Sciences, Fort Washington, PA, USA). Ultrathin sections (70 nm) were cut from the embedded tissue blocks and mounted onto nickel grids before observation. The sections were examined under an electron microscope JEM-1200EX (JEOL, Japan).
Histochemical analysis of lipid droplets
Lipid droplets in the conidia were visualized by staining with a Nile Red staining solution [44], [45] consisting of 20 mg/mL polyvinylpyrrolidone and 2.5 µg/mL Nile Red Oxazone (9-diethylamino-5H-benzo[α] phenoxazine-5-one, Sigma) in 50 mM Tris-maleate buffer (pH 7.5). Briefly, after incubation in MBL medium for 3 d, conidia of each strain were harvested and mounted in the Nile Red staining solution. Within a few seconds, lipid droplets began to fluoresce when viewed under a microscope with episcopic fluorescence attachment.
Determination of intracellular glycerol accumulation
Each strain was grown in potato dextrose broth (PDB) for 2 days at 25°C in a shaker. Mycelia of each strain were harvested and ground in liquid nitrogen. Then, mycelial powder (100 mg) was transferred to a 2-ml microcentrifuge tube containing 0.1 ml glycerol extraction buffer (Shanghai Chaoyan Biotechnology Co.). After mixing by a vortex shaker (HaLiDa, Jiangsu, China) three times for 30 s each, the tubes were centrifuged at 5000 g for 20 min. The resulting supernatant was transferred to a new tube, and 10 µl of each supernatant was mixed with 190 µl detection buffer of a glycerol assay kit (Shanghai Chaoyan Biotechnology Co.). After the mixture was incubated at 37°C for 15 min, the glycerol concentration was determined by a spectrophotometer (SPECTRAmax Plus) at 550 nm. The experiment was repeated three times.
Yeast two hybrid analysis
To construct plasmids for yeast two hybrid screen analysis, the coding sequence of the full length FgVEA, FgVelB, FgLaeA1, FgVIP1, FgVIP2, FgVIP3, FgVIP4, FgVIP5 or FgVIP6 was amplified from the cDNA of PH-1. The FgVelB, FgVIP1 and FgVIP4 fragments were inserted into the Nde I-BamH I sites of the yeast GAL4 binding domain vector pGBKT7 and GAL4 activation domain vector pGADT7 (Clontech, Mountain View, CA, USA). The FgVIP3 and FgVIP5 PCR fragments were inserted into the Nde I-EcoR I sites of the yeast GAL4 binding domain vector pGBKT7 and GAL4 activation domain vector pGADT7. The FgLaeA1 and FgVIP2 PCR fragments were inserted into the Sma I-BamH I sites of the yeast GAL4 binding domain vector pGBKT7 and GAL4 activation domain vector pGADT7, respectively. The FgVIP6 fragment was inserted into the EcoR I -BamH I sites of the yeast GAL4 binding domain vector pGBKT7 and GAL4 activation domain vector pGADT7. The yeast two hybrid plasmids AD-FgLaeA1 + BD-FgVeA, AD-FgVelB + BD-FgVeA, AD-FgVIP1 + BD-FgVeA, AD-FgVIP2 + BD-FgVeA, AD-FgVIP3 + BD-FgVeA, AD-FgVIP4 + BD-FgVeA, AD-FgVIP5 + BD-FgVeA, AD-FgVIP6 + BD-FgVeA were co-transformed into the S. cerevisiae reporter strain AH109 according to LiAc/SS-DNA/PEG transformation procedure [46]. The pair of plasmid pGBKT7-53 and pGADT7 was served as a positive control. The pairs of plasmids pGBKT7-Lam and pGADT7, pGADT7 and pGBKT7-FgVeA were used as negative controls. Transformants were grown at 30°C for 72 h on synthetic medium lacking Leu and Trp, and then transferred to the medium lacking His, Leu and Trp and containing 5 mM 3-aminotriazole (3-AT) to identify binding activity. Three independent experiments were performed to confirm yeast two hybrid results.
SAGE analysis of gene expression profiling in ΔFgVeA-9
The wild type progenitor and ΔFgVeA-9 were grown in PDB for 2 days at 25°C in a shaker. Then mycelia of each strain were harvested and used for RNA extraction. The library constructions used for SAGE analysis were obtained from the total RNA of wild-type strain and ΔFgVeA-9 mutant using the kit for preparing samples for digital gene expression-Tag profiling with DpnII (Illumina Inc., California, USA) according to the manufacturer's protocol. The experiment was performed by BGI Co. (Shenzhen, China) using Illumina Cluster Station and Illumina HiSeq (TM) 2000 System. Since tags detected by SAGE with a frequency less than 3 transcripts per million (tpm) may not be reliable [47], only tags with a frequency ≥3 tpm were used in data analysis in this study. The unique tags were then aligned to all the known transcripts of F. graminearum using Novoalign aligner (Novocraft Technologies, Kuala Lumpur, Malaysia). The frequencies of each SAGE tag in the FgVEA deletion mutant ΔFgVeA-9 and wild-type strain PH-1 were compared, and the statistical significance (P value) was calculated according to Audic and Claverie test using the program IDEG6 [48]. The P value is a measure of confidence that the gene is differentially expressed in the two compared samples.
Pathogenicity assays on flowering wheat heads
After incubation in MBL medium for 4 days, conidia of each strain were collected by filtration through three layers of gauze and subsequently re-suspended in sterile distilled water to a concentration of 1×106 conidia/ml. A10-µl aliquot of conidial suspension was injected into a floret in the central section spikelet of single flowering wheat heads of susceptible cultivar Jimai22. There were ten replicates for each strain. After inoculation, the plants were kept at 22±2°C under 95–100% humidity. Fifteen days after inoculation, the infected spikelets in each inoculated wheat head were recorded. The experiment was repeated for four times.
Analysis of DON production and expression level of TRI5 and TRI6
A 30-g aliquot of healthy wheat kernels was sterilized and inoculated with 1 ml spore suspension (106 spores/ml) of the wild-type strain PH-1, complemented strain ΔFgVeA-9C and deletion mutant ΔFgVeA-9. After incubation at 25°C for 20 days, DON was extracted using a previously described protocol [49], and the amount of F. graminearum DNA in each sample was determined using a quantitative real-time PCR method [50]. The DON extracts were purified with PuriToxSR DON column TC-T200 (Trilogy analytical laboratory), and the amount of DON (per mg fungal DNA) in each sample was determined by using a HPLC system Waters 1525. The experiment was repeated three times independently, and data were analyzed using analysis of variance (SAS version 8.0; SAS Institute, Cary, NC).
To determine expression level of TIR5 and TRI6, the mycelia of the wild-type progenitor PH-1, and the ΔFgVeA-9 were inoculated into GYEP medium (5% glucose, 0.1% yeast extract, 0.1% peptone) and cultured for 2 days at 25°C in the dark. Total RNA was extracted from mycelia of each sample, the expression of TRI5 and TRI6 were determined using a quantitative real-time PCR method. The experiment was repeated three times.
Supporting Information
Phylogenetic analysis and alignment of VeA proteins from F. graminearum, F. fujikuroi, F. verticillioides, A. nidulans , and P. chrysogenum . (A) Phylogenetic analysis of amino acid sequences of VeA from F. graminearum, F. fujikuroi, F. verticillioides, A. nidulans and P. chrysogenum. (B) Alignment of amino acid sequences of VeA from F. graminearum with those from F. fujikuroi, F. verticillioides, A. nidulans, and P. chrysogenum.
(TIF)
A total of 1215 up-regulated (red) and 354 down-regulated (green) genes in FgVEA deletion mutant were mapped in chromosomes.
(TIF)
Effects of FgVEA deletion on expression of F. graminearum genes involved in peroxisome biogenesis (A) and SNARE interactions in vesicular transport pathway (B). The up- and down-regulated genes in the FgVEA deletion mutant are indicated in red- and green- boxes, respectively. Numbers nearby boxes represent fold changes of gene expression.
(TIF)
Colony morphology of FgLaeA1, and six FgVIP (FgVeA interacting protein) deletion mutants grown on PDA medium. The wild-type strain PH-1, FgLaeA1 deletion mutant ΔFgLaeA1-4, the FgVIP deletion mutants ΔFgVIP1-2, ΔFgVIP2-7, ΔFgVIP3-8, ΔFgVIP4-4, ΔFgVIP5-6 and ΔFgVIP6-9 were grown on PDA for 4 days at 25°C. The photos were taken from top (A) and bottom (B) of plates, respectively.
(TIF)
The gene expression profiling in FgPTC3 and FgVEA deletion mutants. The 721 genes out of 1,215 up-regulated more than 5 folds in ΔFgVeA-9 were also up-regulated in the FgPTC3 deletion mutant ΔFgPtc3- 8 (left). The 218 genes out of 354 down-regulated more than 5 folds in ΔFgVeA-9 were also down-regulated in the FgPTC3 deletion mutant ΔFgPtc3- 8 (right). A total of 1863 up-regulated and 546 down-regulated genes were detected in the two mutants. The expressions of genes were detected by the serial analysis of gene expression method.
(TIF)
Oligonucleotide primers used in this study.
(DOC)
Expression changes of the genes involved in fatty acid metabolism, cell wall and aurofusarin biosyntheses in F. graminearum FgVEA deletion mutant ΔFgVeA-9 detected by serial analysis of gene expression method.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the earmarked fund for Modern Agro-industry Technology Research System (CARS-3-1-15), National Science Foundation (30971933), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT0943). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Starkey DE, Ward TJ, Aoki T, Gale LR, Kistler HC, et al. Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genet Biol. 2007;44:1191–1204. doi: 10.1016/j.fgb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Sutton JC. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Trans Br Mycol Soc. 1982;70:187–192. [Google Scholar]
- 3.Bayram O, Krappmann S, Seiler S, Vogt N, Braus GH. Neurospora crassa ve-1 affects asexual conidiation. Fungal Genet Biol. 2008;45:127–138. doi: 10.1016/j.fgb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Calvo AM. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet Biol. 2008;45:1053–1061. doi: 10.1016/j.fgb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Käfer E. Origins of translocations in Aspergillus nidulans. . Genetics. 1965;52:217–232. doi: 10.1093/genetics/52.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yager LN. Early developmental events during asexual and sexual sporulation in Aspergillus nidulans. Biotechnology. 1992;23:19–41. [PubMed] [Google Scholar]
- 7.Kim HS, Han KY, Kim KJ, Han DM, Jahng KY, et al. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet Biol. 2002;37:72–80. doi: 10.1016/s1087-1845(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 8.Calvo AM, Bok J, Brooks W, Keller NP. VeA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl Environ Microbiol. 2004;70:4733–4739. doi: 10.1128/AEM.70.8.4733-4739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CZ, Liou GY, Yuan GF. Comparison of the aflR gene sequences of strains in Aspergillus section Flavi. Microbiology. 2006;152:161–170. doi: 10.1099/mic.0.27618-0. [DOI] [PubMed] [Google Scholar]
- 10.Meyers DM, Obrian G, Du WL, Bhatnagar D, Payne GA. Characterization of aflJ, a gene required for conversion of pathway intermediates to aflatoxin. Appl Environ Microbiol. 1998;64:3713–3717. doi: 10.1128/aem.64.10.3713-3717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du W, Obrian GR, Payne GA. Function and regulation of aflJ in the accumulation of aflatoxin early pathway intermediate in Aspergillus flavus. Food Addit Contam. 2007;24:1043–1050. doi: 10.1080/02652030701513826. [DOI] [PubMed] [Google Scholar]
- 12.Roze LV, Chanda A, Laivenieks M, Beaudry RM, Artymovich KA, et al. Volatile profiling reveals intracellular metabolic changes in Aspergillus parasiticus: veA regulates branched chain amino acid and ethanol metabolism. BMC Biochemistry. 2010;11:33. doi: 10.1186/1471-2091-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Myung K, Guse D, Donkin B, Proctor RH, et al. FvVE1 regulates filamentous growth, the ratio of microconidia to macroconidia and cell wall formation in Fusarium verticillioides. Mol Microbiol. 2006;62:1418–1432. doi: 10.1111/j.1365-2958.2006.05447.x. [DOI] [PubMed] [Google Scholar]
- 14.Dreyer J, Eichhorn H, Friedlin E, Kürnsteiner H, Kück U. A homologue of the Aspergillus velvet gene regulates both cephalosporin C biosynthesis and hyphal fragmentation in Acremonium chrysogenum. Appl Environ Microbiol. 2007;73:3412–3422. doi: 10.1128/AEM.00129-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myung K, Li S, Butchko RAE, Busman M, Proctor RH, et al. FvVE1 regulates biosynthesis of the mycotoxins fumonisins and fusarins in Fusarium verticillioides. J Agric Food Chem. 2009;57:5089–5094. doi: 10.1021/jf900783u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi YE, Goodwin SB. MVE1, encoding the velvet gene product homolog in mycosphaerella graminicola, is associated with aerial mycelium formation, melanin biosynthesis, hyphal swelling, and light signaling. Appl Environ Microbiol. 2011;77:942–953. doi: 10.1128/AEM.01830-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoff B, Kamerewerd J, Sigl C, Mitterbauer R, Zadra I, et al. Two components of a velvet-like complex control hyphal morphogenesis, conidiophore development, and penicillin biosynthesis in Penicillium chrysogenum. Eukaryotic Cell. 2010;8:1236–1250. doi: 10.1128/EC.00077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiemann P, Brown DW, Kleigrewe K, Bok JW, Keller NP, et al. FfVel1 and FfLae1, components of a velvet-like complex in Fusarium fujikuroi, affect differentiation, secondary metabolism and virulence. Mol Microbiol. 2010;77:972–994. doi: 10.1111/j.1365-2958.2010.07263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kershaw MJ, Talbot NJ. Hydrophobins and repellents: proteins with fundamental roles in fungal morphogenesis. Fungal Genet Biol. 1998;23:18–33. doi: 10.1006/fgbi.1997.1022. [DOI] [PubMed] [Google Scholar]
- 20.Wösten HA, Richter M, Willey JM. Structural proteins involved in emergence of microbial aerial hyphae. Fungal Genet Biol. 1999;27:153–160. doi: 10.1006/fgbi.1999.1130. [DOI] [PubMed] [Google Scholar]
- 21.Frandsen RJN, Nielsen NJ, Maolanon N, Sørensen JC, Olsson S, et al. The biosynthetic pathway for aurofusarin in Fusarium graminearum reveals a close link between the naphthoquinones and naphthopyrones. Mol Microbiol. 2006;61:1069–1080. doi: 10.1111/j.1365-2958.2006.05295.x. [DOI] [PubMed] [Google Scholar]
- 22.Kojima K, Takano Y, Yoshimi A, Tanaka C, Kikuchi T, et al. Fungicide activity through activation of a fungal signaling pathway. Mol Microbiol. 2004;53:1785–1796. doi: 10.1111/j.1365-2958.2004.04244.x. [DOI] [PubMed] [Google Scholar]
- 23.Yan LY, Yang QQ, Sundin SW, Li HY, Ma ZH. The mitogen-activated protein kinase kinase BOS5 is involved in regulating vegetative differentiation and virulence in Botrytis cinerea. Fungal Genet Biol. 2010;47:753–760. doi: 10.1016/j.fgb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Jiang JH, Yun YZ, Fu J, Shim WB, Ma ZH. Involvement of a putative response regulator FgRrg-1 in osmotic stress response, fungicide resistance and virulence in Fusarium graminearum. . Mol Plant Pathol. 2011;12:425–436. doi: 10.1111/j.1364-3703.2010.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wojda I, Alonso-Monge R, Bebelman JP, Mager WH, Siderius M. Response to high osmotic conditions and elevated temperature in Saccharomyces cerevisiae is controlled by intracellular glycerol and involves coordinate activity of MAP kinase pathways. Microbiology. 2003;149:1193–1204. doi: 10.1099/mic.0.26110-0. [DOI] [PubMed] [Google Scholar]
- 26.Proctor RH, Hohn TM, Mccormick SP. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant-Microbe Interact. 1995;8:593–601. doi: 10.1094/mpmi-8-0593. [DOI] [PubMed] [Google Scholar]
- 27.Desjardins AE, Proctor RH, Bai GH, McCormick SP, Shaner G, et al. Reduced virulence of trichothecene-nonproducing mutants of Gibberella zeae in wheat field tests. Mol Plant-Microbe Interact. 1996;9:775–781. [Google Scholar]
- 28.Seong KY, Pasquali M, Zhou XY, Song J, Hilburn K, et al. Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol Microbiol. 2009;72:354–367. doi: 10.1111/j.1365-2958.2009.06649.x. [DOI] [PubMed] [Google Scholar]
- 29.Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997;7:986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- 30.Bayram O, Krappmann S, Ni M, Bok JW, Helmstaedt K, et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 2008;320:1504–1506. doi: 10.1126/science.1155888. [DOI] [PubMed] [Google Scholar]
- 31.Duran RM, Cary JW, Calvo AM. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl Microbiol Biotechnol. 2007;73:1158–1168. doi: 10.1007/s00253-006-0581-5. [DOI] [PubMed] [Google Scholar]
- 32.Kimura M, Tokai T, Takahashi-Ando N, Ohsato S, Fujimura M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: pathways, genes, and evolution. Biosci Biotechnol Biochem. 2007;71:2105–2123. doi: 10.1271/bbb.70183. [DOI] [PubMed] [Google Scholar]
- 33.Kato N, Brooks W, Calvo AM. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot Cell. 2003;2:1178–1186. doi: 10.1128/EC.2.6.1178-1186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amaike S, Keller NP. Distinct roles for VeA and LaeA in development and pathogenesis of Aspergillus flavus. Eukaryot Cell. 2009;8:1051–1060. doi: 10.1128/EC.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medentsev AG, Akimenko VK. Naphthoquinone metabolites of the fungi. Phytochemistry. 1998;47:935–959. doi: 10.1016/s0031-9422(98)80053-8. [DOI] [PubMed] [Google Scholar]
- 36.Rispail N, Soanes DM, Ant C, Czajkowski R, Grünler A, et al. Comparative genomics of MAP kinase and calcium–calcineurin signaling components in plant and human pathogenic fungi. Fungal Genet Biol. 2009;46:287–298. doi: 10.1016/j.fgb.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Bai GH, Desjardins AE, Plattner RD. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia. 2002;153:91–98. doi: 10.1023/a:1014419323550. [DOI] [PubMed] [Google Scholar]
- 38.Muller C, McIntyre M, Hansen K, Nielsen J. Metabolic engineering of the morphology of Aspergillus oryzae by altering chitin synthesis. Appl Environ Microbiol. 2002;68:1827–1836. doi: 10.1128/AEM.68.4.1827-1836.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang JH, Yun YZ, Yang QQ, Shim WB, Wang ZY, et al. A Type 2C Protein Phosphatase FgPtc3 Is Involved in Cell Wall Integrity, Lipid Metabolism, and Virulence in Fusarium graminearum. Plos One. 2011;6:e25311. doi: 10.1371/journal.pone.0025311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai GH, Shaner G. Variation in Fusarium graminearum and cultivar resistance to wheat scab. Plant Dis. 1996;80:975–979. [Google Scholar]
- 41.Liu XH, Lu JP, Zhang L, Dong B, Min H, et al. Involvement of a Magnaporthe grisea serine/threonine kinase gene, MgATG1, in appressorium turgor and pathogenesis. Eukaryot Cell. 2007;6:997–1005. doi: 10.1128/EC.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullins ED, Chen X, Romaine P, Raina R, Geiser DM, et al. Agrobacterium-mediated transformation of Fusarium oxysporum: An efficient tool for insertional mutagenesis and gene transfer. Phytopathology. 2001;91:173–180. doi: 10.1094/PHYTO.2001.91.2.173. [DOI] [PubMed] [Google Scholar]
- 43.Dong B, Liu XH, Lu JP, Zhang FS, Gao HM, et al. MgAtg9 trafficking in Magnaporthe oryzae. Autophagy. 2009;5:946–953. doi: 10.4161/auto.5.7.9161. [DOI] [PubMed] [Google Scholar]
- 44.Thines E, Weber RWS, Talbot NJ. MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell. 2000;12:1703–1718. doi: 10.1105/tpc.12.9.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber RWS, Wakley GE, Pitt D. Histochemical and ultrastructural characterization of vacuoles and spherosomes as components of the lytic system in hyphae of the fungus Botrytis cinerea. Histochem J. 1999;31:293–301. doi: 10.1023/a:1003713901179. [DOI] [PubMed] [Google Scholar]
- 46.Schiestl RH, Gietz RD. High-efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 47.Jongeneel CV, Iseli C, Stevenson BJ, Riggins GJ, Lal A, et al. Comprehensive sampling of gene expression in human cell lines with massively parallel signature sequencing. Proc Natl Acad Sci USA. 2003;100:4702–4705. doi: 10.1073/pnas.0831040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romualdi C, Bortoluzzi S, D'Alessi F, Danieli GA. IDEG6: a web tool for detection of differentially expressed genes in multiple tag sampling experiments. Physiol Genomics. 2003;12:159–162. doi: 10.1152/physiolgenomics.00096.2002. [DOI] [PubMed] [Google Scholar]
- 49.Mirocha CJ, Kolaczkowski E, Xie WP, Yu H, Jelen H. Analysis of deoxynivalenol and its derivatives (batch and single kernel) using gas chromatography mass spectrometry. J Agric Food Chem. 1998;46:1414–1418. [Google Scholar]
- 50.Yin YN, Liu X, Ma ZH. Simultaneous detection of Fusarium asiaticum and Fusarium graminearum in wheat seeds using a real-time PCR method. Lett in Appl Microbiol. 2009;48:680–686. doi: 10.1111/j.1472-765X.2009.02595.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic analysis and alignment of VeA proteins from F. graminearum, F. fujikuroi, F. verticillioides, A. nidulans , and P. chrysogenum . (A) Phylogenetic analysis of amino acid sequences of VeA from F. graminearum, F. fujikuroi, F. verticillioides, A. nidulans and P. chrysogenum. (B) Alignment of amino acid sequences of VeA from F. graminearum with those from F. fujikuroi, F. verticillioides, A. nidulans, and P. chrysogenum.
(TIF)
A total of 1215 up-regulated (red) and 354 down-regulated (green) genes in FgVEA deletion mutant were mapped in chromosomes.
(TIF)
Effects of FgVEA deletion on expression of F. graminearum genes involved in peroxisome biogenesis (A) and SNARE interactions in vesicular transport pathway (B). The up- and down-regulated genes in the FgVEA deletion mutant are indicated in red- and green- boxes, respectively. Numbers nearby boxes represent fold changes of gene expression.
(TIF)
Colony morphology of FgLaeA1, and six FgVIP (FgVeA interacting protein) deletion mutants grown on PDA medium. The wild-type strain PH-1, FgLaeA1 deletion mutant ΔFgLaeA1-4, the FgVIP deletion mutants ΔFgVIP1-2, ΔFgVIP2-7, ΔFgVIP3-8, ΔFgVIP4-4, ΔFgVIP5-6 and ΔFgVIP6-9 were grown on PDA for 4 days at 25°C. The photos were taken from top (A) and bottom (B) of plates, respectively.
(TIF)
The gene expression profiling in FgPTC3 and FgVEA deletion mutants. The 721 genes out of 1,215 up-regulated more than 5 folds in ΔFgVeA-9 were also up-regulated in the FgPTC3 deletion mutant ΔFgPtc3- 8 (left). The 218 genes out of 354 down-regulated more than 5 folds in ΔFgVeA-9 were also down-regulated in the FgPTC3 deletion mutant ΔFgPtc3- 8 (right). A total of 1863 up-regulated and 546 down-regulated genes were detected in the two mutants. The expressions of genes were detected by the serial analysis of gene expression method.
(TIF)
Oligonucleotide primers used in this study.
(DOC)
Expression changes of the genes involved in fatty acid metabolism, cell wall and aurofusarin biosyntheses in F. graminearum FgVEA deletion mutant ΔFgVeA-9 detected by serial analysis of gene expression method.
(DOC)