Abstract
Background
Video-assisted anal fistula treatment (VAAFT) is a novel minimally invasive and sphincter-saving technique for treating complex fistulas. The aim of this report is to describe the procedural steps and preliminary results of VAAFT.
Methods
Karl Storz Video Equipment is used. Key steps are visualization of the fistula tract using the fistuloscope, correct localization of the internal fistula opening under direct vision, endoscopic treatment of the fistula and closure of the internal opening using a stapler or cutaneous-mucosal flap. Diagnostic fistuloscopy under irrigation is followed by an operative phase of fulguration of the fistula tract, closure of the internal opening and suture reinforcement with cyanoacrylate.
Results
From May 2006 to May 2011, we operated on 136 patients using VAAFT. Ninety-eight patients were followed up for a minimum of 6 months. No major complications occurred. In most cases, both short-term and long-term postoperative pain was acceptable. Primary healing was achieved in 72 patients (73.5%) within 2–3 months of the operation. Sixty-two patients were followed up for more than 1 year. The percentage of the patients healed after 1 year was 87.1%.
Conclusions
The main feature of the VAAFT technique is that the procedure is performed entirely under direct endoluminal vision. With this approach, the internal opening can be found in 82.6% of cases. Moreover, fistuloscopy helps to identify any possible secondary tracts or chronic abscesses. The VAAFT technique is sphincter-saving, and the surgical wounds are extremely small. Our preliminary results are very promising.
Keywords: Anal fistula, Fistuloscopy, Video-assisted anal fistula treatment, Complex anal fistula, Sphincter- saving
Introduction
The patient’s first visit and correct initial surgical treatment play a fundamental role in fistula healing. The use of the metallic probe during the first visit or at the beginning of any operative procedure, along with the accurate identification of the internal opening and the location of possible chronic abscesses or secondary tracks are universally considered the keys to successful anal fistula treatment [1]. There are reports in the literature of recurrence rates after surgery of 20% even in simple fistulas largely because of a failure to identify these secondary tracks and the site of the internal opening [2]. Traditional techniques including fistulectomy and the use of a cutting seton have been associated with an incontinence rate approaching 12% in simple fistulas and more in complicated cases and patients who underwent reoperation [3]. Recently, Phillips et al. reported that the lay open technique is still a good option for the management of such complex anal fistulas. Ninety-six per cent of their patients healed and the incidence rates of incontinence for flatus, soft stools and hard stools were 30, 2 and 4%, respectively, with only 8% of patients obliged to use a pad [4]. Over the last few years, many novel attempts have been made to treat complex anal fistulas with minimally invasive techniques including ligation of the intersphincteric fistula tract (the LIFT procedure), anal fistula plugs and the utilization of commercially available fibrin glues [5–7]. This paper describes a new technique, video-assisted anal fistula treatment (VAAFT) developed by the first author (P.M.) in 2006. The main features of this technique include the ability to view the fistula from the inside so that it can be eradicated under direct vision using a fistuloscope. The goals of the VAAFT procedure are accurate identification of the internal opening and the secondary tracts or abscess cavities with formal closure of the internal opening.
Materials and methods
Surgical technique
Video-assisted anal fistula treatment is performed with a kit which includes a fistuloscope (Fig. 1), manufactured by Karl Storz GmbH (Tuttlingen, Germany), an obturator, a unipolar electrode, an endobrush and 0.5 ml of synthetic cyanoacrylate (Glubran 2®—GEM, Viareggio, Italy). The fistuloscope has an 8° angled eyepiece and is equipped with an optical channel and also a working and irrigation channel. Its diameter is 3.3 × 4.7 mm, and its operative length is 18 cm. A removable handle allows easier manoeuvring. The fistuloscope has two taps one of which is connected to a 5,000 ml bag of glycine–mannitol 1% solution, depending on the position of the fistula. Spinal anaesthesia is required. The patient is placed in the lithotomy position. Video-assisted anal fistula treatment has two phases, a diagnostic one and an operative one.
Fig. 1.

The Meinero fistuloscope
Diagnostic phase
The purpose of this phase is to correctly locate the internal fistula opening and possible secondary tracts or abscess cavities. We insert the fistuloscope through the external opening with the glycine–mannitol solution already running. The obturator is visible at the lower edge of the screen to ensure the correct orientation of the fistuloscope. The fistula pathway will appear clearly on the screen (Fig. 2). Sometimes the external opening is surrounded by very tough scar tissue, so we often have to remove this in order to allow easy entry of the fistuloscope. We put the fistuloscope against it and wait for the glycine–mannitol solution to open the fistula tract. Then, we simply advance the fistuloscope along the pathway using slow movements, left/right and up/down. These manoeuvres allow the fistula to accommodate the fistuloscope, and the fistula is straightened. Spinal anaesthesia facilitates this process. Optimal vision of the lumen of the fistula is ensured by the continuous jet of irrigation solution reaching as far as the internal opening, which is the end of the fistula tract (Fig. 3). At this point, the assistant inserts a retractor and the lights in the operating theatre are dimmed so that it is easy to see the fistuloscope light in the rectum. The fistuloscope usually exits through the internal opening. Sometimes the internal opening is very narrow and the location of the orifice can only be identified by observing the fistuloscope light behind the rectal mucosa. The surgeon places two or three sutures at two opposite points of the margin of the internal opening in order to isolate it but not to close it for the time being (Fig. 4).
Fig. 2.
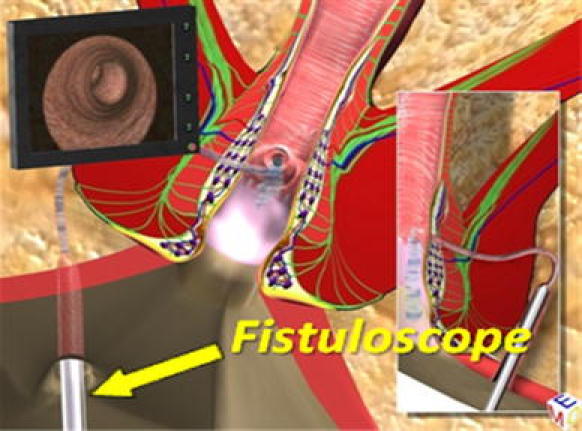
Fistuloscopy with glycine–mannitol 1% solution irrigation
Fig. 3.

Location of internal fistula opening
Fig. 4.
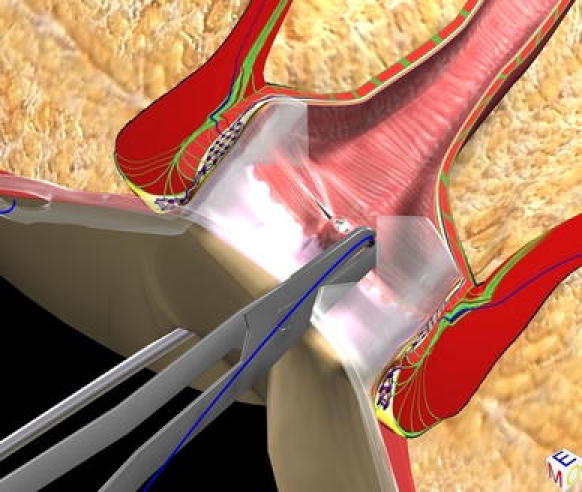
Isolation of internal fistula opening with stitches
Operative phase
The goals in this phase are destruction of the fistula from the inside, cleansing of the fistula tract and finally closure of the internal opening. First, we remove the obturator and replace it with the electrode, which destroys the fistula tract under continuous direct vision (Fig. 5). We proceed centimetre by centimetre from the external opening to the internal opening, cauterizing all fragments of the whitish material adhering to the fistula wall and taking care not to overlook any abscess cavities or any possible fistula tract. Continuing under direct vision, necrotic material is removed with an endo-brush, or when the fistula is straight, with a Volkmann spoon. The continuous jet of the irrigation solution also ensures that all waste material is eliminated into the rectum through the internal opening, which has been isolated by stitches, but not yet closed. At this point, the surgeon returns to the rectum. The assistant maintains tension on the threads in order to lift up the internal fistula opening so that it has the shape of a volcano. A stapler is then inserted at the base of the volcano completing the mechanical cutting and suturing. This procedure can be performed by a semicircular stapler or a linear stapler (roticulator), depending upon the position of the internal opening. The final result is simply a scar in the area where the internal opening was previously located (Fig. 6). When the tissue of the internal opening is thick and tough, the use of the stapler can be difficult and we prefer to close it by fashioning a cutaneous or mucosal flap.
Fig. 5.
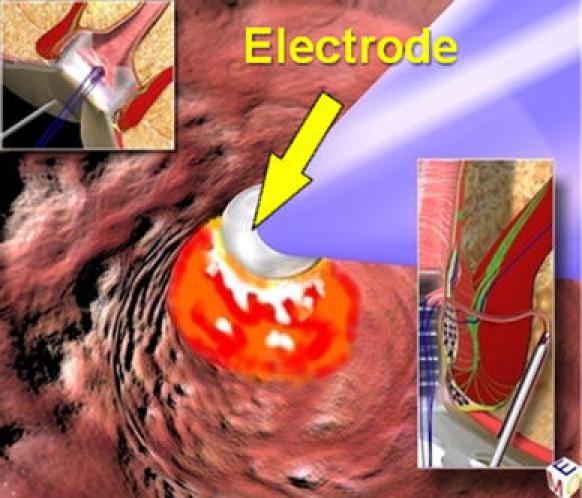
Fistula destruction from the inside by the electrode
Fig. 6.
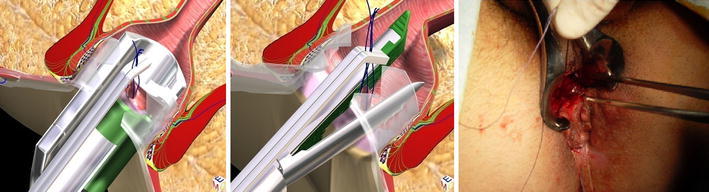
Closure of internal fistula opening with a stapler (semicircular or linear suture line) or a flap
In any event, the internal fistula opening must be closed. At the end of the operation, we apply 0.5 ml of synthetic cyanoacrylate, through a tiny catheter, immediately behind the staple/suture line in order to reinforce the suture itself and ensure that the opening is completely closed. This catheter can be accurately positioned behind the staple/suture line by using the operative channel of the fistuloscope or, if the fistula is straight, directly, without the fistuloscope. The cyanoacrylate must not be placed in the fistula tract because the fistula pathway must be open to allow secretions to drain out in the postoperative period.
From May 2006 to May 2011, 136 patients with a complex anal fistula were managed with this technique. Any fistula that could not be adequately treated by simple fistulotomy was considered “complex” [8]. Our series consisted of 71 males and 27 females, with a median age of 42 years (range 21–77 years). Fistula anatomy was described according to Parks’ classification [9]. Exclusion criteria included Crohn’s disease and cases of simple fistulas. Preoperative assessment included blood tests, virtual or traditional colonoscopy and a chest X-ray where appropriate. Approval was obtained from the Ethics Committee of our Institution and all patients provided informed consent. Thirty-five patients did not require any additional diagnostic investigations, and preoperative assessment of the fistula anatomy was based on clinical grounds alone. Twenty patients underwent magnetic resonance imaging (MRI) or endoanal ultrasonography at our institution, while 81 underwent fistula imaging (MRI, ultrasonography or CT) prior to referral and did not require further testing. Ninety-four patients had already undergone prior surgery for complex anal fistula, 69 of them more than five times. Seven patients had a diverting colostomy. Follow-up was conducted at 2, 6 and 12 months after VAAFT and subsequently, once per year. Twenty-two patients were contacted by phone interview after the first year of follow-up. Ninety-eight out of these 136 patients were followed up for a minimum of 6 months with a median duration of follow-up of 13 months (range 6–60 months).
Results
Seventy-four patients (75.5%) had a high trans-sphincteric fistula (with more than 1 cm of external sphincter involved), 9 patients (9.2%) an extrasphincteric fistula (in 7 cases the result of previous treatments and in 2 cases post-traumatic), 6 patients (6.2%) had a supra-sphincteric fistula and 9 patients (9.2%) had a horseshoe fistula. In 91 cases (92.8%), the fistula pathway was single, whereas in 7 cases (7.2%) it was double. In 16 cases (16.3%), the internal fistula opening was located in the anal canal, in 73 cases (74.5%) at the level of the dentate line and in 9 cases (9.2%) in the rectum. In 81 patients (82.6%), the internal opening of the fistula was located in less than 5 min. In the other 17 (17.3%), it was found by viewing the fistuloscope light in the rectum. The operative time was progressively reduced (from 2 h to 30 min) following improvement in the learning curve. No major complications occurred and no infection or bleeding was observed; however, there were 2 cases of postoperative urinary retention. In one case, scrotal oedema was observed caused by the infiltration of the irrigation solution after rupture of the fistula wall. No cases of allergy to the synthetic cyanoacrylate were reported, and all patients were discharged on the day of surgery. Most patients reported that postoperative pain was acceptable both in the early and in the later postoperative period.
Pain control was based on the visual analogue scale (VAS) score with a mean value of 4.5 (on a scale of 1–10) during the first 48 h. None of the patients reported pain after the first postoperative week. Twenty-one patients (21.4%) did not require analgesics, whereas 49 patients (50%) needed Ketorolac trimetamine on postoperative day 1, 20 (20.4%) required Ketorolac trimetamine for 3 to 4 days and only 8 (8.2%) needed Ketorolac trimetamine for a week. Regarding the last group, in 5 out of 8 patients, the suture was placed in the rectum, where there was no clear correlation between suture level and the development of pain. Primary healing was achieved in 72 patients (73.5%) within 2 to 3 months after surgery. In 26 patients (26.5%), no wound healing was observed. Nineteen of the 26 underwent reoperation with VAAFT, and the other 5 underwent cyanoacrylate reinjection. Nine of the 19 patients reoperated upon with VAAFT healed, whereas 6 have had a recurrence and the other 4 are still under observation. The 5 patients who underwent cyanoacrylate reinjection have all had recurrence. They will be reoperated on once more with VAAFT. Sixty-two patients were followed up for at least 12 months, where 54 (87.1%) have primarily healed their fistula. We did not formally evaluate anal continence in our patients with a validated score before and after surgery. Our aim was only to determine whether the operation might have worsened patients’ continence, and this was evaluated by simply asking the patients about continence problems. All patients denied worsening of faecal continence postoperatively. Among those who had an active job, the longest time off work was 3 days.
Discussion
Current surgical techniques for treating anal fistulas are based on three main principles: identification of the tract and the internal opening, excision of the fistula tract and preservation of anal sphincter function. Fistulotomy/fistulectomy is the gold standard in the treatment of anal fistulas with only minor involvement of the sphincters. Complex fistulas are very challenging for the surgeon because of the high incidence of bowel control impairment after these traditional surgical approaches. The rationale of the VAAFT technique is based on the concept of both detection and perfect closure of the internal fistula opening, in addition to the destruction of the pathway and cleaning which will allow complete and definitive healing. There is great variation in both technical difficulty and efficacy among other sphincter-preserving options for complex cryptoglandular fistulas. Mucosal advancement flaps are technically challenging and are associated with recurrence rates that vary from 2 to 54% [10–14]. These failure rates may result from some mobilization of structures or a tendency for the flap to retract or dehisce. Moreover, advancement flaps are often associated with postoperative incontinence, and the incidence of this complication has been reported to approach 35% in some series [14]. Fibrin glue injection is a technically easy, low-risk technique but results have been disappointing, showing success rates as low as 16% long term [15–20]. Similarly, the use of the anal fistula plug is a simple, sphincter-sparing technique, but very expensive [21] and with reported success rates ranging between 29% and 87% [22–26].
The latest conservative technique reported in literature is the ligation of intersphincteric fistula tract (LIFT) procedure. This approach consists of ligation of the tract in the intersphincteric space, curettage of the tract and closure of the external anal sphincter defect with sutures. This technique, like VAAFT, is based on the principle of a secure closure of the tract near the internal opening and makes possible healing rates ranging from 57 to 94.4% [5, 27–29]. Critical points of this technique are that ligation in the intersphincteric space may be technically demanding for high fistula tracts and for tracts ascending into the intersphincteric plane and crossing the external anal sphincter at a higher level than that of the internal sphincter. Moreover, the exposure of the intersphincteric plane can damage the blood supply to the internal anal sphincter and breach the anal mucosa, leading to a high risk of failure [30]. In any event, the procedure leaves more or less extensive perianal skin wounds, which is not the case with VAAFT. Athanasiadis et al. [31] using a technique of excision of the internal opening, of the intersphincteric tract and of the entire tract up to the external anal sphincter, with a triple suture line designed to close the internal and the external sphincter has reported a technique more invasive and excisional than VAAFT and other surgeons adopting a similar procedure obtained a 59% healing rate [32]. Sir Alan Parks originally proposed a treatment for high anal fistulas consisting of excision of the internal opening with a portion of internal anal sphincter and the cryptoglandular tissue with enlargement of the external opening and curettage of the tract [8]. This approach is based on the principle of “curing” the tract and avoiding division of the external anal sphincter.
Recently, another novel technique for treating complex fistulas, fistula laser closure (FiLaC™), has been proposed [33]. The authors combine conventional closure of the internal opening using a flap with laser obliteration of the fistula tract. In a group of 17 patients with a median follow-up of 9 months, they report an 82.3% healing rate. This approach, like VAAFT, is aimed at destroying the tract and preserving the sphincters, but it is a procedure performed blindly, without advantages in identifying the internal opening, secondary tracts and abscess cavities. The rationale of the VAAFT technique is based on the same principles as other procedures for closing the internal opening and obliterating the track, where the real innovation is the precise identification of the fistula anatomy and of the internal opening by fistuloscopy and fulguration of the tract walls under direct vision. This approach allows the identification and treatment of all the secondary tracts, and the abscess cavities connected to the main pathway. We believe that the adoption of fistuloscopy together with a good technique for closing the internal opening (with a stapler or manually) and reinforcing the closure of the opening from the inner side of the staple/suture line is the most effective way of achieving a high healing rate for complex anal fistulas with preservation of the anal sphincters. In the group with more than a 1-year follow-up, 87.1% of the patients healed. Our data appear promising, confirming the results of Ortiz et al. who reported very low recurrence rates after 1 year using a similar operative principle [34]. As regards costs, there is the initial cost of the fistuloscope and kit, but they are reusable. The cost increases if a stapler is used, but not if a flap is fashioned. The adoption of expensive technology and devices does increase the cost of the procedure, but on the other hand, the short hospital stay (same day discharge), the short recovery time following the operation and the short absence from work result in relative cost effectiveness of the VAAFT procedure.
Conclusions
The VAAFT technique is a minimally invasive and safe technique. The advantages of this technique are evident: it is performed as day surgery, there are no surgical wounds on the buttocks or in the perianal region, and there is complete certainty regarding the location of the internal fistula opening (a key point in all surgical treatment of fistulas). This is coupled with evidence of complete destruction of the fistula from the inside without damage to the anal sphincters. Moreover, the patient does not have postoperative problems with faecal incontinence. VAAFT appears cost effective, requiring a shorter and less expensive preoperative work-up than traditional techniques. The kit is reusable and although the expensive technology involved increases the initial costs of VAAFT (the total cost of the kit ranges between 5,000 and 9,000 EUR), secondary costs are cut by same day discharge, a short recovery period and an early return to work. Our experience is encouraging and needs a longer follow-up of larger series to be validated.
Acknowledgments
This study was supported by Karl Storz GmbH & Co in the form of grants and equipment. The senior author, Piercarlo Meinero, has a patent license agreement with Karl Storz GmbH & Co concerning the fistuloscope kit.
Conflict of interest
The authors have declared that no conflict of interest exists.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s10151-011-0802-5
References
- 1.Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39:723–729. doi: 10.1007/BF02054434. [DOI] [PubMed] [Google Scholar]
- 2.Sangwan YP, Rosen L, Riether RD, Stasik JJ, Sheets JA, Khubchandani IT. Is simple fistula-in-ano simple? Dis Colon Rectum. 1994;37:885–889. doi: 10.1007/BF02052593. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie RD, Sackier JM, Hodde JP. Incontinence rates after cutting seton treatment for anal fistula. Colorectal Dis. 2009;11:564–571. doi: 10.1111/j.1463-1318.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 4.Atkin GK, Martins J, Tozer P, Ranchod P, Phillips RKS. For many high anal fistulas, lay open is still a good option. Tech Coloproctol. 2011;15:143–150. doi: 10.1007/s10151-011-0676-6. [DOI] [PubMed] [Google Scholar]
- 5.Rojanasakul A. LIFT procedure: a simplified technique for fistula-in-ano. Tech Coloproctol. 2009;13:237–240. doi: 10.1007/s10151-009-0522-2. [DOI] [PubMed] [Google Scholar]
- 6.Lupinacci RM, Vallet C, Parc Y, Chafai N, Tiret E. Treatment of fistula-in-ano with the Surgisis(®) AFP(™) anal fistula plug. Gastroenterol Clin Biol. 2010;34:549–553. doi: 10.1016/j.gcb.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Cirocchi R, Santoro A, Trastulli S, et al. Meta-analysis of fibrin glue versus surgery for treatment of fistula-in-ano. Ann Ital Chir. 2010;81:349–356. [PubMed] [Google Scholar]
- 8.Parks AG, Stitz RW. The treatment of high fistula-in-ano. Dis Colon Rectum. 1976;19:487–499. doi: 10.1007/BF02590942. [DOI] [PubMed] [Google Scholar]
- 9.Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 10.Aguilar PS, Plasencia G, Hardy TG, Jr, Hartmann RF, Stewart WR. Mucosal advancement in the treatment of anal fistula. Dis Colon Rectum. 1985;28:496–498. doi: 10.1007/BF02554093. [DOI] [PubMed] [Google Scholar]
- 11.Ozuner G, Hull TL, Cartmill J, Fazio VW. Long-term analysis of the use of transanal rectal advancement flaps for complicated anorectal/vaginal fistulas. Dis Colon Rectum. 1996;39:10–14. doi: 10.1007/BF02048261. [DOI] [PubMed] [Google Scholar]
- 12.Schouten WR, Zimmermann DD, Briel JW. Transanal advancement flap repair of transsphincteric fistulas. Dis Colon Rectum. 1999;42:1419–1423. doi: 10.1007/BF02235039. [DOI] [PubMed] [Google Scholar]
- 13.Mizrahi N, Wexner SD, Zmora O et al (2002) Endorectal advancement flap: are there predictors of failure? Dis Colon Rectum 45:1616–1621 [DOI] [PubMed]
- 14.Sonoda T, Hull T, Piedmonte MR, Fazio VW. Outcomes of primary repair of anorectal and rectovaginal fistulas using the endorectal advancement flap. Dis Colon Rectum. 2002;45:1622–1628. doi: 10.1007/s10350-004-7249-y. [DOI] [PubMed] [Google Scholar]
- 15.Sentovich SM. Fibrin glue for all anal fistulas. J Gastrointest Surg. 2001;5:158–161. doi: 10.1016/S1091-255X(01)80028-7. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan GN, Bartram CI, Phillips RKS, et al. Efficacy of fibrin sealant in the management of complex anal fistula: a prospective trial. Dis Colon Rectum. 2003;46:1167–1174. doi: 10.1007/s10350-004-6708-9. [DOI] [PubMed] [Google Scholar]
- 17.Sentovich SM. Fibrin glue for anal fistulas: long-term results. Dis Colon Rectum. 2003;46:498–502. doi: 10.1007/s10350-004-6589-y. [DOI] [PubMed] [Google Scholar]
- 18.Gisbertz SS, Sosef MN, Festen S, et al. Treatment of fistulas in ano with fibrin glue. Dig Surg. 2005;22:91–94. doi: 10.1159/000085299. [DOI] [PubMed] [Google Scholar]
- 19.Ellis CN, Clark S. Fibrin glue as an adjunct to flap repair of anal fistulas: a randomized, controlled study. Dis Colon Rectum. 2006;49:1736–1740. doi: 10.1007/s10350-006-0718-8. [DOI] [PubMed] [Google Scholar]
- 20.Williams JG, Farrands PA, Williams AB, et al. The treatment of anal fistula: ACPGBI position statement. Colorectal Dis. 2007;9:18–50. doi: 10.1111/j.1463-1318.2007.01372.x. [DOI] [PubMed] [Google Scholar]
- 21.Adamina M, Hoch JS, Burnstein MJ. To plug or not to plug: a cost-effectiveness analysis for complex anal fistula. Surgery. 2010;147:72–78. doi: 10.1016/j.surg.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Johnson EK, Gaw JU, Armstrong DN. Efficacy of anal fistula plug vs. fibrin glue in closure of anorectal fistulas. Dis Colon Rectum. 2006;49:371–376. doi: 10.1007/s10350-005-0288-1. [DOI] [PubMed] [Google Scholar]
- 23.Christoforidis D, Etzioni DA, Goldberg SM, et al. Treatment of complex anal fistulas with the collagen fistula plug. Dis Colon Rectum. 2008;51:1482–1487. doi: 10.1007/s10350-008-9374-5. [DOI] [PubMed] [Google Scholar]
- 24.Lawes DA, Efron JE, Abbas M, et al. Early experience with the bioabsorbable anal fistula plug. World J Surg. 2008;32:1157–1159. doi: 10.1007/s00268-008-9504-1. [DOI] [PubMed] [Google Scholar]
- 25.Malik AI, Nelson RL. Surgical management of anal fistulae: a systematic review. Colorectal Dis. 2008;10:420–430. doi: 10.1111/j.1463-1318.2008.01483.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang JY, Garcia-Aguilar J, Sternberg JA, Abel ME, Varma MG. Treatment of transsphincteric anal fistulas: are fistula plugs an acceptable alternative? Dis Colon Rectum. 2009;52:692–697. doi: 10.1007/DCR.0b013e31819d473f. [DOI] [PubMed] [Google Scholar]
- 27.Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K. Total anal sphincter saving technique for fistula-in-ano: the ligation of intersphinteric fistula tract. J Med Asso Thai. 2007;90:581–586. [PubMed] [Google Scholar]
- 28.Shanwani A, Nor AM, Amri N. Ligation of the intersphincteric fistula tract (LIFT): a sphincter-saving technique for fistula-in-ano. Dis Colon Rectum. 2010;53:39–42. doi: 10.1007/DCR.0b013e3181c160c4. [DOI] [PubMed] [Google Scholar]
- 29.Bleier JI, Moloo H, Goldberg SM. Ligation of the intersphincteric fistula tract: an effective new technique for complex fistulas. Dis Colon Rectum. 2010;53:43–46. doi: 10.1007/DCR.0b013e3181bb869f. [DOI] [PubMed] [Google Scholar]
- 30.Lunniss PJ. LIFT procedure: a simplified technique for fistula-in-ano. Tech Coloproctol. 2009;13:241–242. doi: 10.1007/s10151-009-0523-1. [DOI] [PubMed] [Google Scholar]
- 31.Athanasiadis S, Helmes C, Yazigi R, Kohler A. The direct closure of the internal fistula opening without advancement flap for transsphicteric fistulas-in-ano. Dis Colon Rectum. 2004;47:1174–1180. doi: 10.1007/s10350-004-0551-x. [DOI] [PubMed] [Google Scholar]
- 32.Thomson WH, Fowler AL. Direct appositional (no flap) closure of deep anal fistula. Colorectal Dis. 2004;6:32–36. doi: 10.1111/j.1463-1318.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 33.Wilhelm A. New technique for anal fistula repair using a novel radial emitting laser probe (FILAC™) Tech Coloproctol. 2011;15:239. doi: 10.1007/s10151-011-0726-0. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz H, Marzo M, De Miguel M, et al. Length of follow-up after fistulotomy and fistulectomy associated with endorectal advancement flap repair for fistula in ano. Br J Surg. 2008;95:484–487. doi: 10.1002/bjs.6023. [DOI] [PubMed] [Google Scholar]


