Abstract
In this article, the authors present evidence regarding a dissociative subtype of PTSD, with clinical and neurobiological features that can be distinguished from nondissociative PTSD. The dissociative subtype is characterized by overmodulation of affect, while the more common undermodulated type involves the predominance of reexperiencing and hyperarousal symptoms. This article focuses on the neural manifestations of the dissociative subtype in PTSD and compares it to those underlying the reexperiencing/hyperaroused subtype. A model that includes these two types of emotion dysregulation in PTSD is described. In this model, reexperiencing/hyperarousal reactivity is viewed as a form of emotion dysregulation that involves emotional undermodulation, mediated by failure of prefrontal inhibition of limbic regions. In contrast, the dissociative subtype of PTSD is described as a form of emotion dysregulation that involves emotional overmodulation mediated by midline prefrontal inhibition of the same limbic regions. Both types of modulation are involved in a dynamic interplay and lead to alternating symptom profiles in PTSD. These findings have important implications for treatment of PTSD, including the need to assess patients with PTSD for dissociative symptoms and to incorporate the treatment of dissociative symptoms into stage-oriented trauma treatment.
In this article we present evidence that there is a specific dissociative subtype of posttraumatic stress disorder (PTSD), with neurobiological features that distinguish it from nondissociative PTSD. Dissociation is a common feature of PTSD (1–3). It involves disruptions in and fragmentation of the usually integrated functions of consciousness, memory, identity, body awareness, and perception of the self and the environment (4). Although there are many ways to conceptualize dissociation, a useful heuristic is that dissociation involves detachment from the overwhelming emotional content of the experience during and in the immediate aftermath of trauma. Alterations in memory encoding and storage ensue, leading to fragmentation and compartmentalization of memory and impairments in memory retrieval (5, 6). Chronic psychological, sexual, and physical trauma as well as emotional neglect, including parental psychological unavailability, have been etiologically related to dissociation (7–10). It has been hypothesized that such jarring experiences elicit dissociation, promoting a discontinuity of conscious experience and memory (11).
Even though dissociative symptoms are often observed following exposure to chronic psychological trauma, acute traumatic events can also lead to dissociative experiences, often referred to as peritraumatic dissociation. For example, a number of studies of individuals experiencing danger or life threat have shown specific peritraumatic dissociative changes, including alterations in time sense, perception, attentional focus, and awareness of pain among others (12, 13). In addition, depersonalization has been described in a significant percentage of individuals facing acute life threat (reviewed in 9). Information related to the traumatic experiences is often differently encoded in these altered states, resulting in decreased access to information about the trauma once the person returns to his or her baseline state. This may give a subjective sense of “compartmentalization” of the trauma and lead to cognitive fragmentation or emotional detachment from the experience. The cost of this detachment may be avoidance of necessary cognitive and affective processing of trauma in its aftermath (5, 6, 14). Acute dissociative responses to psychological trauma have been found to predict the development of chronic PTSD (15, 16). Moreover, a chronic pattern of dissociation in response to reminders of the original trauma and minor stressors develops in persons who experience acute dissociative responses to psychological trauma (17).
This review will focus on the neurobiological and clinical features of chronic dissociation (defined as detachment states, depersonalization, derealization, and subjective distance from emotional experience for the purpose of this review) in PTSD. Specifically, we will present evidence in favor of a dissociative subtype of PTSD, with clinical and neurobiological features that can be distinguished from nondissociative PTSD. The dissociative subtype is characterized by frequent overmodulation of affect, while the more common undermodulated type involves the predominance of the more commonly studied reexperiencing and hyperarousal symptoms. These findings have important implications for treatment of PTSD, including the need to assess patients with PTSD for dissociative symptoms and to incorporate the treatment of dissociative symptoms into stage-oriented trauma treatment.
Failed Versus Excessive Corticolimbic Inhibition: A Model of Emotional Under- and Overmodulation in PTSD
Neurobiological Studies
Bremner (18) has hypothesized that there may be two subtypes of acute trauma response that represent unique pathways to chronic stress-related psychopathology: one is primarily dissociative and the other predominantly intrusive and hyperaroused. Data from neuroimaging studies have shown that two subtypes of response can persist in persons with chronic PTSD and are associated with distinct patterns of neural activation upon exposure to reminders of traumatic events (19–21). It should be noted that these response patterns are not completely distinct and that individual patients with PTSD may show both response patterns either simultaneously or at different time points. However, PTSD patients with prolonged traumatic experiences such as chronic childhood abuse or combat trauma often show a clinical syndrome that is characterized by chronic symptoms of dissociation (1–3, 15) as opposed to patients who have suffered from more acute traumatic experiences (see clinical section below).
There have been investigations of the neuronal circuitry underlying reexperiencing/hyperaroused versus dissociative responses in chronic PTSD that have used functional MRI (fMRI) and script-driven imagery. In this research paradigm, patients construct a narrative of their traumatic experience that is later read to them while they are in the scanner. Patients are instructed to recall the traumatic memory as vividly as possible during “trauma scripts” and immediately afterward, while the MRI scanner measures oxygen utilization in different brain regions. Results have shown that psychobiological responses to recalling traumatic experiences can differ significantly among patients with chronic PTSD (22, 23). Approximately 70% of patients had the subjective experience of reliving their traumatic experience and showed an increase in heart rate while recalling the traumatic memory (19). The other 30% of PTSD subjects had a dissociative response. The latter predominantly involved subjective states of depersonalization and derealization with no significant concomitant increase in heart rate (19). The neural correlates of reexperiencing states and dissociative states, respectively, in PTSD show opposite patterns of brain activation in brain regions that are implicated in arousal modulation and emotion regulation. In particular these differential patterns are found in the medial prefrontal cortex, the anterior cingulate cortex, and the limbic system.
Emotional undermodulation: failure of corticolimbic inhibition
Patients with reexperiencing/hyperaroused PTSD exhibit abnormally low activation in medial anterior brain regions that are implicated in arousal modulation and emotion regulation more generally, including the ventromedial prefrontal cortex (19) and the rostral anterior cingulate cortex (24). Consistent with impaired cortical modulation, increased activation of the limbic system, especially the amygdala (a brain structure that has been shown to play a key role in fear conditioning), has often been observed in PTSD patients after exposure to traumatic reminders as well as to masked fearful faces (24, 25). We conceptualize this group of patients as experiencing emotional undermodulation in response to traumatic memories. This type of response includes a subjective reliving experience of the traumatic events, such as a flashback. Reexperiencing/hyperarousal reactivity can be viewed as a form of emotion dysregulation that involves emotional undermodulation, mediated by failure of prefrontal inhibition of limbic regions.
Further support for this model is found in studies that take a dimensional approach (involving different symptom severities and associated neural activation patterns within each response subtype) to individual differences in reexperiencing symptoms in response to trauma reminders. This method examines correlations between severity of state reexperiencing to trauma scripts and brain activity in regions associated with awareness and regulation of arousal and emotions (21). These studies have shown that severity of state reexperiencing was positively correlated with activation in the right anterior insula, a brain region that is involved in the neural representation of somatic aspects of emotional states and interoception of feeling states. State reexperiencing was negatively correlated with activation of the rostral portion of the anterior cingulate cortex, a brain region involved in arousal and emotion regulation. These findings are consistent with the phenomenology and clinical presentations of PTSD patients who exhibit pathological emotional undermodulation during reexperiencing states, including a variety of negative emotional states and associated bodily experiences.
Emotional overmodulation: excessive corticolimbic inhibition
In contrast to the reexperiencing/hyperaroused subtype, patients with dissociative PTSD exhibit abnormally high activation in brain regions involved in arousal modulation and emotional regulation, including the dorsal anterior cingulate cortex and the medial prefrontal cortex. The dissociative PTSD patients can be conceptualized as experiencing emotional overmodulation in response to exposure to traumatic memories. This can include subjective disengagement from the emotional content of the traumatic memory through depersonalization or derealization responses, mediated by midline prefrontal inhibition of the limbic regions.
Dimensionally, dissociative response to trauma reminders is negatively correlated with right anterior insula activation and positively correlated with activation in the medial prefrontal cortex and dorsal anterior cingulate cortex (21). In motor vehicle accident-related PTSD, the medial prefrontal cortex cluster that was positively correlated with state dissociative symptoms was negatively correlated with amygdala activity during script-driven imagery (26). This finding provides support for hypothesized hyperinhibition of limbic regions by medial prefrontal areas in states of pathological overmodulation, i.e., during dissociative states in response to trauma-related emotions.
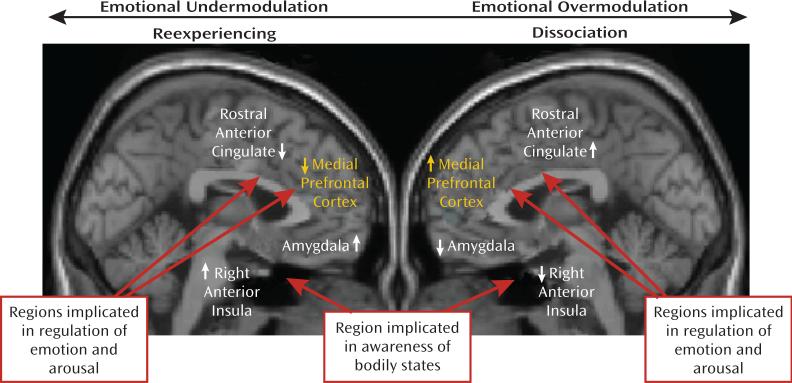
A recent study by Felmingham et al. (27) gives further support to the notion of distinct dissociative and nondissociative reactions in PTSD, based on the corticolimbic inhibition model. Using fMRI, these investigators examined the impact of dissociation on fear processing in two groups of PTSD patients, one with high and the other with low dissociation scores. Felmingham et al. (27) compared brain activation during the processing of consciously and nonconsciously perceived fear stimuli. Patients with dissociative PTSD showed enhanced activation in the ventral prefrontal cortex during conscious fear processing, relative to patients with nondissociative PTSD. The authors suggest that these data support the theory that dissociation is a regulatory strategy invoked to cope with extreme arousal in PTSD through hyperinhibition of limbic regions, with this strategy most active during conscious processing of threat. A model of the two subtypes of PTSD is presented in Figure 1.
FIGURE 1. A Model of Emotional Under- and Overmodulation in PTSDa.
a In this model, reexperiencing/hyperarousal reactivity to traumatic reminders is viewed as a form of emotion dysregulation that involves emotional undermodulation, mediated by failure of prefrontal inhibition of limbic regions. In contrast, the dissociative reactions to traumatic reminders are described as a form of emotion dysregulation that involves emotional overmodulation, mediated by midline prefrontal inhibition of the same limbic regions. Figure adapted from Hopper et al. (21). Copyright © 2007 International Society for Traumatic Stress Studies. Reprinted with permission.
Additional evidence for hyperinhibition of the limbic system, including the amygdala, during dissociative states stems from the pain neurobiology literature. In a study examining healthy subjects, Roeder et al. (28) found decreased amygdala activity in response to painful stimulation during hypnosis-induced states of depersonalization. These findings are also consistent with amygdala deactivation in response to thermal pain stimuli in patients with PTSD as well as with borderline personality disorder patients who generally show high levels of dissociation (29–31). A recent study revealed a similar pattern of increased mid-cingulate and insula activation in patients with borderline personality disorder and comorbid PTSD in conjunction with reduced pain sensitivity during script-induced dissociative states (32).
Experimental studies examining emotional memory suppression in healthy subjects provide additional support for the model that dissociative PTSD involves hyperinhibition of limbic regions with memory suppression associated with increased frontal and decreased hippocampal activity (33). Here, a complex neural network appeared to be involved actively in top-down memory suppression, including dorsolateral and ventrolateral prefrontal cortex (Brodmann's area [BA] 45/46); anterior cingulate cortex (BA 32); the contiguous presupplementary motor area (BA 6); a lateral premotor area in the rostral portion of the dorsal premotor cortex (BA 6/9); and the intraparietal sulcus (BA 7) (also in bilateral BA 47/BA 13 and right putamen). In addition, memory suppression was significantly associated with bilateral hippocampal inhibition. The authors conclude that their study provides a possible neurobiological model for suppression of unwanted memories, consistent with the occurrence of dissociative amnesia (33).
The aforementioned findings, supporting the corticolimbic inhibition model of dissociative PTSD, are consistent with the phenomenology and clinical presentation of patients with dissociative PTSD. Many of these patients require therapeutic approaches that help them to overcome pathological overmodulation of traumatic memories, associated emotions, and bodily experiences. The corticolimbic inhibition model postulates that once a threshold of anxiety is reached, the medial prefrontal cortex inhibits emotional processing in limbic structures (the amygdala), which in turn leads to a dampening of sympathetic output and reduced emotional experiencing (34). Several studies in PTSD patients show that the prefrontal cortex has inhibitory influences on the emotional limbic system. These include PET studies showing a negative correlation between blood flow in the left ventromedial prefrontal cortex and the amygdala during emotional tasks (26), and negative correlations between medial prefrontal cortex and the amygdala during fear conditioning (25). Therefore, the low activation of medial prefrontal regions described in the reexperiencing/hyperaroused PTSD subgroup is consistent with failed inhibition of limbic reactivity. This is associated with emotional undermodulation. In contrast, in the dissociative subgroup, increased activation of medial prefrontal structures is consistent with the notion of hyperinhibition of those same limbic regions. This results in states of pathological emotional overmodulation in response to trauma-related emotions. “Successful” emotional overmodulation appears to involve transient psychological disengagement from trauma-related information. This is marked by alterations in perception and consciousness, as found in depersonalization and derealization states and in dissociative amnesia. Figure 1 summarizes these findings in each subtype of PTSD by contrasting brain regions involved in the reexperiencing/hyperarousal (undermodulation) and dissociative (overmodulation) types of emotional dysregulation to trauma-related stimuli, respectively.
Indeed, this model resembles the signs and symptoms of response to a stressful life event that were originally described by Lindemann (35) and Horowitz (36) in their classic works on stress response syndromes. Horowitz suggested that the responses to stressful life events were expressed in two predominant states. The first was an intrusive state characterized by intrusive feelings and compulsive action. The other was a state of denial marked by dissociative symptoms such as emotional numbing and constriction of ideation. The core problem in PTSD, from his point of view, is under- or overmodulation of affective response to traumatic memories—the emotion modulation system cannot adequately regulate effects of extreme traumatic input.
Evidence of Corticolimbic Inhibition from Dissociative Disorders
Studies of depersonalization disorder provide further support for the corticolimbic model of dissociation. Hollander et al. (37) reported a case study of a patient with primary depersonalization disorder, studied using brain electrical activity mapping. They found left frontal overactivation, indicated by increased anteriorized alpha activity. They demonstrated with SPECT that this patient showed impaired perfusion in the left caudate and increased activity in posterior frontal areas. A subsequent investigation of a group of depersonalization disorder patients examined event-related fMRI to neutral, mild, and intensely happy and sad facial expressions, with simultaneous measurements of skin conductance levels (38). Relative to healthy comparison subjects, depersonalization disorder patients showed a decrease in subcortical limbic activity to increasingly intense happy and sad facial expressions. Moreover, for both happy and sad facial expressions, depersonalization disorder patients, but not healthy subjects, exhibited negative correlations between skin conductance measures and activation in the bilateral dorsal prefrontal cortices. In summary, these studies support the hypothesis that depersonalization disorder subjects exhibit increased prefrontal activity or decreased limbic activity. In turn, this inhibition may result in the hypoemotionality frequently reported in these patients.
Clinical Studies
As described above, PTSD involves both under- and overmodulation of affect and individual patients with PTSD may show both response patterns either simultaneously or at different timepoints. However, PTSD patients with prolonged traumatic experiences such as chronic childhood abuse or combat trauma often exhibit a clinical syndrome that is characterized by chronic and frequent symptoms of dissociation (1–3, 15, 39–41) as opposed to patients who have suffered from more acute or single blow forms of trauma.
Terr (39) proposed two types of traumatic experiences. Type I trauma refers to traumatic conditions that result from single traumatic experiences and include predominantly full and vivid detailed memories, cognitive reappraisals, and misperceptions. In contrast, type II trauma was proposed to be associated with longstanding or repeated exposure to extreme stressors and includes dissociation, denial and numbing, states of self-hypnosis, and rage. Further support for these findings comes from a study by van der Kolk et al. (40) who, as part of a DSM-IV field trial, examined a sample of 395 traumatized treatment-seeking subjects and 125 nontreatment-seeking subjects who had been exposed to traumatic experiences. Results showed that participants who had suffered early onset interpersonal abuse (age ≤14 years) had significantly higher percentages of endorsements of dissociative symptoms than participants with late-onset interpersonal abuse and disaster survivors. Moreover, subjects with dissociative symptoms continued to suffer from dissociation even after they no longer met criteria for PTSD, thus suggesting severe, chronic dissociative symptoms in response to early onset interpersonal violence.
More recently, Ginzburg et al. (42) used signal detection analyses to identify high and low dissociation PTSD subgroups in a sample of 122 women who were seeking treatment for childhood sexual abuse. Specifically, three PTSD symptoms, including hypervigilance, sense of foreshortened future, and sleep difficulties, discriminated between the high and low dissociation subgroups. While these prominent intrusive and hyperarousal symptoms in the dissociative subgroup may seem paradoxical, the dissociation may reflect a compensatory response to higher distress, perhaps mediated by alterations in thalamic activation (43). In addition, Zucker et al. (44) examined differences in dissociative symptoms in 155 subjects with PTSD versus subjects with PTSD who also met criteria for disorders of extreme stress not otherwise specified. The disorders of extreme stress not otherwise specified criteria were developed to characterize a population of traumatized individuals who suffer a “complex” form of PTSD-related multiple episodes of trauma over several developmental epochs, with symptoms of dissociation, emotion dysregulation, somatization, altered relationships and attachments, and alterations in systems of meaning (45). Subjects with both PTSD and disorders of extreme stress not otherwise specified exhibited chronic symptoms of dissociation as evidenced by higher scores on the Dissociative Experiences Scale than participants who were suffering from PTSD only. Moreover, studies in children have suggested that there may be two subtypes of acute trauma response that represent unique pathways to chronic stress-related psychopathology: one is predominantly intrusive and hyperaroused and the other dissociative (46, 47). These studies therefore support the idea that trauma experienced earlier in life creates a significant dissociative component to later PTSD (18). Furthermore, a history of early life trauma is a predictor of the later development of PTSD in response to traumatic stressors (1, 2).
Studies in combat-related PTSD also point to a dissociative subtype of PTSD. Taxometric analyses in a sample of 316 Vietnam veterans consistently revealed a taxon/subtype (subgroup identified by scores that are discontinuous with a dimensional distribution) of highly dissociative individuals. The dissociative subtype of PTSD in this group was also associated with more severe PTSD (3). Furthermore, Putnam et al. (41) examined whether mean dissociation scores as measured by the Dissociative Experiences Scale resulted from uniform distributions of scores in a group of 166 predominantly combat-related PTSD patients. Results showed that the diagnostic group's mean Dissociative Experiences Scale scores were a function of the proportion of subjects within the group who were high dissociators, thus suggesting the existence of a distinct dissociative subtype. An earlier study of Vietnam veterans has also reported that chronic dissociative symptoms are an important element of long-term psychological responses to combat trauma, which is often repetitive in nature (15).
Treatment and Research Implications
Exposure-based treatments for PTSD (48) have the strongest empirical support and involve repeated imaginal and in vivo exposure to trauma-related stimuli. In order for exposure-based treatments to be successful, patients must be able to fully emotionally engage with the trauma-related information. Typically, patients are asked to recall details of their traumatic experience while describing them in the present tense. Exposures are designed to overcome avoidance of such stimuli by providing a safe context in which patients can fully engage with both trauma-related and “corrective” (safety) information. In this way, exposure treatment is designed to overcome and reduce avoidance symptoms, enhance affect management, and facilitate cognitive restructuring of trauma-related memories. In turn, this should bring about the reduction of reexperiencing and hyperarousal symptoms, and, ultimately, elimination of the disorder itself (49).
However, exposure treatments should be used with caution in patients with significant emotional overmodulation, such as dissociative and numbing symptoms. Foa and colleagues (49) have suggested that such symptoms can prevent emotional engagement with trauma-related information and thereby reduce treatment effectiveness (50). In fact, a recent study (51) suggests that levels of dissociation are an important negative predictor of psychotherapy outcome in patients with borderline personality disorder, a disorder that has often been associated with childhood abuse (52). Also, dissociative symptoms block emotional learning in a classical conditioning paradigm (53). Specifically, it has been shown that patients with borderline personality disorder and high levels of dissociation did not show differences in skin conductance and arousal between conditioned stimuli that were paired with an aversive sound and unpaired conditioned stimuli during acquisition and early extinction, while borderline patients with low levels of dissociation and healthy subjects did. These results suggest that emotional, amygdala-based learning processes appear to be inhibited by state dissociation through alteration of acquisition and extinction processes. Therefore, it is crucial, before commencing exposure-based treatments, to assess levels of dissociative psychopathology and provide interventions to reduce dissociative symptomatic responses to trauma-related stimuli. Failure to do so can lead to an actual increase in PTSD and related symptoms, including dissociation, emotion dysregulation, and an increase in the patient's overall distress and functional impairment.
In recognition of such dissociative complexity of symptoms of patients with chronic PTSD related to childhood abuse, Cloitre and colleagues (54) developed an integrative and empirically supported “phase-based” intervention for long-term, child-abuse-related PTSD. Their model accounts for the significant dissociative symptoms frequently associated with repeated childhood maltreatment. Their approach delivers a stage-oriented model that uses skills training in emotion regulation. Before engaging in exposure-based therapy, Cloitre et al. (54) provide data to support the idea that patients must develop mood regulation and grounding skills, identify and modify disordered attachment schemas learned in childhood, and work on competence in social interactions. Future treatment outcome research will need to focus on complex childhood abuse-related PTSD and other types of PTSD that have shown to have considerable dissociative symptoms (e.g., combat-related PTSD [3, 15]). This will help recognize and further develop interventions that are most effective in managing dissociative symptoms and allow them to be optimally timed in a phase-oriented treatment model.
In terms of research, the results described in this review suggest that careful attention must be paid to the differential responses of under- and overmodulation of affect often observed in PTSD. Grouping all PTSD patients, regardless of their different symptom patterns, in the same diagnostic category will hinder our understanding of posttraumatic psychopathology (19, 55). Classification of different PTSD subtypes will enable a more careful analysis of differential responses to psychological trauma and eventually lead to a more sophisticated understanding of the neurobiology and treatment of PTSD.
Future neurobiological research will have to closely examine the relationship between the medial prefrontal and amygdala circuitry during states of under- and overmodulation of affect and brain activation of other brain regions previously proposed as implicated in dissociative PTSD, including the thalamus, superior colliculus, and periaqueductal gray as well as brain stem structures (7, 19). The latter will allow a more detailed understanding of the association between higher and lower brain structures during reexperiencing and dissociative states in this disorder.
Conclusion
Increasingly, both neurobiological and clinical research have shown that PTSD has two subtypes: reexperiencing/hyperaroused and dissociative. These different trauma response subtypes can be viewed as different extremes of emotion dysregulation. The first involves undermodulation and the second overmodulation to trauma-related emotional and somatosensory information. Each response type appears to have distinct CNS correlates, and each response type has been correlated with specific neural activity in brain regions responsible for arousal modulation and emotion regulation, including the medial prefrontal cortex and the anterior cingulate cortex. Findings of lower activation of medial prefrontal regions in the reexperiencing/hyperaroused subtype is consistent with failed inhibition of limbic reactivity and is associated with hyperaroused emotional undermodulation. In contrast, in the dissociative subtype, increased activation of medial prefrontal structures is consistent with hyperinhibition of those same limbic regions in states of pathological emotional overmodulation. These findings have important implications for assessment and treatment of PTSD, including the need to assess patients with PTSD for dissociative symptoms and to incorporate the treatment of dissociative symptoms into a stage-oriented trauma treatment model. They also suggest that grouping all PTSD patients for the purposes of research, regardless of their different symptom patterns, in the same diagnostic category may hamper our understanding of posttrauma psychopathology. Functional neuroimaging may be useful in treatment selection and monitoring of outcome of these different posttraumatic responses.
Acknowledgments
Dr. Schmahl reports receiving a research grant from Lundbeck Research, Inc., and a speakers honorarium from AstraZeneca GmbH.
Footnotes
The remaining authors report no financial relationships with commercial interests.
References
- 1.van der Kolk BA, Pelcovitz D, Roth S, Mandel FS, McFarlane A, Herman JL. Dissociation, somatization, and affect dysregulation: the complexity of adaptation to trauma. Am J Psychiatry. 1996;153(July festschrift suppl):83–93. doi: 10.1176/ajp.153.7.83. [DOI] [PubMed] [Google Scholar]
- 2.Stovall-McClough KC, Cloitre M. Unresolved attachment, PTSD, and dissociation in women with childhood abuse histories. J Consult Clin Psychol. 2006;74:219–228. doi: 10.1037/0022-006X.74.2.219. [DOI] [PubMed] [Google Scholar]
- 3.Waelde LC, Silvern L, Fairbank JA. A taxometric investigation of dissociation in Vietnam veterans. J Trauma Stress. 2005;18:359–369. doi: 10.1002/jts.20034. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 5.Spiegel D. Trauma, dissociation, and memory. In: Yehuda R, McFarlane A, editors. Psychobiology of Posttraumatic Stress Disorder. The New York Academy of Sciences; New York: 1997. pp. 225–237. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel D, Cardena E. Disintegrated experience: the dissociative disorders revisited. J Abnorm Psychol. 1991;100:366–378. doi: 10.1037//0021-843x.100.3.366. [DOI] [PubMed] [Google Scholar]
- 7.Vermetten E, Dorahy MJ, Spiegel D, editors. Traumatic Dissociation Neurobiology and Treatment. American Psychiatric Publishing; Washington, DC: 2007. [Google Scholar]
- 8.Nijenhuis ER, Vanderlinder J, Spinhoven P. Animal defense reactions as a model for trauma-induced dissociative reactions. J Trauma Stress. 1998;11:243–260. doi: 10.1023/A:1024447003022. [DOI] [PubMed] [Google Scholar]
- 9.Loewenstein RJ, Putnam FW. The dissociative disorders. In: Sadock BJ, Sadock VA, editors. Comprehensive Textbook of Psychiatry VIII. 8th ed Vol. 1. Lippincott Williams & Wilkins; Baltimore: 2004. pp. 1844–1901. [Google Scholar]
- 10.Dutra L, Bureau J-F, Holmes B, Lyubchik A, Lyons-Ruth K. Quality of early care and childhood trauma: a prospective study of developmental pathways to dissociation. J Nerv Ment Dis. 2009;197:383–390. doi: 10.1097/NMD.0b013e3181a653b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Kolk BA, van der Hart O, Marmar CR. Dissociation and information processing in posttraumatic stress disorder. In: van der Kolk BA, McFarlane AV, Weisaeth L, editors. Traumatic Stress: The Effects of Overwhelming Experience on Mind, Body, and Society. Guilford; New York: 1996. pp. 303–327. [Google Scholar]
- 12.Birmes P, Carreras D, Ducasse JL, Charlet JP, Warner BA, Lauque D, Schmitt L. Peritraumatic dissociation, acute stress, and early posttraumatic stress disorder in victims of general crime. Can J Psychiatry. 2001;46:649–651. doi: 10.1177/070674370104600711. [DOI] [PubMed] [Google Scholar]
- 13.Morgan CA, III, Hazlett G, Wang S, Richardson EG, Jr, Schnurr P, Southwick SM. Symptoms of dissociation in humans experiencing acute, uncontrollable stress: a prospective investigation. Am J Psychiatry. 2001;158:1239–1247. doi: 10.1176/appi.ajp.158.8.1239. [DOI] [PubMed] [Google Scholar]
- 14.Marmar CR, Weiss DS, Metzler TJ, Delucchi KL, Best SR, Wentworth KA. Longitudinal course and predictors of continuing distress following critical incident exposure in emergency services personnel. J Nerv Ment Dis. 1999;187:15–22. doi: 10.1097/00005053-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Bremner JD, Southwick S, Brett E, Fontana A, Rosenheck R, Charney DS. Dissociation and posttraumatic stress disorder in Vietnam combat veterans. Am J Psychiatry. 1992;149:328–332. doi: 10.1176/ajp.149.3.328. [DOI] [PubMed] [Google Scholar]
- 16.Marmar CR, Weiss DS, Schlenger WE, Fairbank JA, Jordan BK, Kulka RA, Hough RL. Peritraumatic dissociation and posttraumatic stress in male Vietnam theater veterans. Am J Psychiatry. 1994;151:902–907. doi: 10.1176/ajp.151.6.902. [DOI] [PubMed] [Google Scholar]
- 17.Butler LD, Duran REF, Jasiukaitis P, Koopman C, Spiegel D. Hypnotizability and traumatic experience: a diathesis-stress model of dissociative symptomatology. Am J Psychiatry. 1996;153(July festschrift suppl):42–63. doi: 10.1176/ajp.153.8.A42. [DOI] [PubMed] [Google Scholar]
- 18.Bremner JD. Acute and chronic responses to psychological trauma: where do we go from here? (editorial). Am J Psychiatry. 1999;156:349–351. doi: 10.1176/ajp.156.3.349. [DOI] [PubMed] [Google Scholar]
- 19.Lanius RA, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiatr Res. 2006;40:709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Frewen PA, Lanius RA. Toward a psychobiology of posttraumatic self-dysregulation: reexperiencing, hyperarousal, dissociation, and emotional numbing. Ann N Y Acad Sci. 2006;1071:110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- 21.Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- 22.Keane TM. Posttraumatic stress disorder: future directions in science and practice. J Rehabil Res Dev. 2008;45:vii–vix. [PubMed] [Google Scholar]
- 23.Orr SP, Roth WT. Psychophysiological assessment: clinical applications for PTSD. J Affect Disord. 2000;61:225–240. doi: 10.1016/s0165-0327(00)00340-2. [DOI] [PubMed] [Google Scholar]
- 24.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- 26.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 27.Felmingham K, Kemp AH, Williams L, Falconer E, Olivieri G, Peduto A, Bryant R. Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychol Med. 2008;38:1771–1780. doi: 10.1017/S0033291708002742. [DOI] [PubMed] [Google Scholar]
- 28.Roeder CH, Michal M, Overbeck G, van de Ven VG, Linden DEJ. Pain response in depersonalization: a functional imaging study using hypnosis in healthy subjects. Psychother Psycho-som. 2007;76:115–121. doi: 10.1159/000097970. [DOI] [PubMed] [Google Scholar]
- 29.Schmahl C, Bohus M, Esposito F, Treede R-D, Di Salle F, Greffrath W, Ludaescher P, Jochims A, Lieb K, Scheffler K, Hennig J, Seifritz E. Neural correlates of antinociception in borderline personality disorder. Arch Gen Psychiatry. 2006;63:659–667. doi: 10.1001/archpsyc.63.6.659. [DOI] [PubMed] [Google Scholar]
- 30.Geuze E, Westenberg HGM, Jochims A, De Kloet CS, Bohus M, Vermetten E, Schmahl C. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76–85. doi: 10.1001/archpsyc.64.1.76. [DOI] [PubMed] [Google Scholar]
- 31.Kraus A, Esposito F, Seifritz E, Di Salle F, Ruf M, Valerius G, Bohus M, Schmahl C. Amygdala deactivation as a neural correlate of pain processing in patients with borderline personality disorder and co-occurrent posttraumatic stress disorder. Biol Psychiatry. 2009;65:819–822. doi: 10.1016/j.biopsych.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 32.Ludaescher P, Valerius G, Stiglmayr C, Muchnik J, Lanius R, Schmahl C. Pain sensitivity and neural processing during dissociative states in patients with borderline personality disorder with and without comorbid PTSD – a pilot study. J Psych Neurosci. doi: 10.1503/jpn.090022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JDE. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- 34.Sierra M, Berrios GE. Depersonalization: neurobiological perspectives. Biol Psychiatry. 1998;44:898–908. doi: 10.1016/s0006-3223(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 35.Lindemann E. Symptomatology and management of acute grief (1944). Am J Psychiatry. 1994;151(June suppl):155–160. doi: 10.1176/ajp.151.6.155. [DOI] [PubMed] [Google Scholar]
- 36.Horowitz MJ. Stress-response syndromes: a review of posttraumatic and adjustment disorders. Psychiatr Serv. 1986;37:241–249. doi: 10.1176/ps.37.3.241. [DOI] [PubMed] [Google Scholar]
- 37.Hollander E, Carrasco JL, Mullen LS, Trungold S, DeCaria CM, Towey J. Left hemispheric activation in depersonalization disorder: a case report. Biol Psychiatry. 1992;31:1157–1162. doi: 10.1016/0006-3223(92)90161-r. [DOI] [PubMed] [Google Scholar]
- 38.Lemche E, Surguladze SA, Giampietro VP, Anilkumar A, Brammer MJ, Sierra M, Chitnis X, Williams SC, Gasston D, Joraschky P, David AS, Phillips ML. Limbic and prefrontal responses to facial emotion expressions in depersonalization. Neuroreport. 2007;18:473–477. doi: 10.1097/WNR.0b013e328057deb3. [DOI] [PubMed] [Google Scholar]
- 39.Terr LC. Childhood traumas: an outline and overview. Am J Psychiatry. 1991;148:10–20. doi: 10.1176/ajp.148.1.10. [DOI] [PubMed] [Google Scholar]
- 40.van der Kolk BA, Pelcovitz D, Roth S, Mandel FS, McFarlane A, Herman JL. Dissociation, somatization, and affect dysregulation: the complexity of adaptation to trauma. Am J Psychiatry. 1996;153(July festschrift suppl):83–93. doi: 10.1176/ajp.153.7.83. [DOI] [PubMed] [Google Scholar]
- 41.Putnam FW, Carlson EB, Ross CA, Anderson G, Clark P, Torem M, Bowman ES, Coons P, Chu JA, Dill DL, Loewenstein RJ, Braun BG. Patterns of dissociation in clinical and nonclinical samples. J Nerv Ment Dis. 1996;184:673–679. doi: 10.1097/00005053-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Ginzburg K, Koopman C, Butler LD, Palesh O, Kraemer HC, Classen CC, Spiegel D. Evidence for a dissociative subtype of post-traumatic stress disorder among help-seeking childhood sexual abuse survivors. J Trauma Dissociation. 2006;7:7–27. doi: 10.1300/J229v07n02_02. [DOI] [PubMed] [Google Scholar]
- 43.Krystal JH, Bennett A, Bremner JD, Southwick SM, Charney DS. Toward a cognitive neuroscience of dissociation and altered memory functions in post-traumatic stress disorder. In: Friedman MJ, Charney DS, Deutch AY, editors. Neurobiological and Clinical Consequences of Stress: From Normal Adaptation to PTSD. Lippincott-Raven; Philadelphia: 1995. pp. 239–269. [Google Scholar]
- 44.Zucker M, Spinazzola J, Blaustein M, van der Kolk BA. Dissociative symptomatology in posttraumatic stress disorder and disorders of extreme stress. J Trauma Dissociation. 2006;7:19–31. doi: 10.1300/J229v07n01_03. [DOI] [PubMed] [Google Scholar]
- 45.Herman JL. Sequelae of prolonged and repeated trauma: evidence for a complex posttraumatic syndrome (DESNOS) In: Davidson JRT, Foa EB, editors. Posttraumatic Stress Disorder: DSM-IV and Beyond. American Psychiatric Press; Washington, DC: 1993. pp. 213–228. [Google Scholar]
- 46.Perry BD, Pollard RA, Blakley TL, Baker WL, Vigilante D. Childhood trauma, the neurobiology of adaptation and use-dependent development of the brain: how states become traits. Infant Ment Health J. 1995;16:271–291. [Google Scholar]
- 47.Kaplow JB, Dodge KA, Amaya-Jackson L, Saxe GN. Pathways to PTSD, part II: sexually abused children. Am J Psychiatry. 2005;162:1305–1310. doi: 10.1176/appi.ajp.162.7.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Institute of Medicine . Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. The National Academies; Washington, DC: 2007. [Google Scholar]
- 49.Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychol Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 50.Jaycox LH, Foa EB, Morral AR. Influence of emotional engagement and habituation on exposure therapy for PTSD. J Consult Clin Psychol. 1998;66:185–192. doi: 10.1037//0022-006x.66.1.185. [DOI] [PubMed] [Google Scholar]
- 51.Kleindienst N, Limberger MF, Ebner-Priemer UW, Mauchnik J, Dyer A, Berger M, Schmahl C, Bohus M. Dissociation predicts poor response to dialectical behavioral therapy in female patients with borderline personality disorder. J Pers Disord. doi: 10.1521/pedi.2011.25.4.432. in press. [DOI] [PubMed] [Google Scholar]
- 52.Ball JS, Links PS. Borderline personality disorder and childhood trauma: evidence for a causal relationship. Curr Psychiatry Rep. 2009;11:63–68. doi: 10.1007/s11920-009-0010-4. [DOI] [PubMed] [Google Scholar]
- 53.Ebner-Priemer UW, Mauchnik J, Kleindienst N, Schmahl C, Peper M, Rosenthal MZ, Flor H, Bohus M. Emotional learning during dissociative states in borderline personality disorder. J Psychiatry Neurosci. 2009;34:214–222. [PMC free article] [PubMed] [Google Scholar]
- 54.Cloitre M, Koenen KC, Cohen LR, Han H. Skills training in affective and interpersonal regulation followed by exposure: a phase-based treatment for PTSD related to childhood abuse. J Consult Clin Psychol. 2002;70:1067–1074. doi: 10.1037//0022-006x.70.5.1067. [DOI] [PubMed] [Google Scholar]
- 55.Sar V, Ross C. Dissociative disorders as a confounding factor in psychiatric research. Psychiatr Clin North Am. 2006;29:129–144. ix. doi: 10.1016/j.psc.2005.10.008. [DOI] [PubMed] [Google Scholar]