Abstract
Posttraumatic stress disorder (PTSD) is associated with long-term changes in neurobiology. Brain areas involved in the stress response include the medial prefrontal cortex, hippocampus, and amygdala. Neurohormonal systems that act on the brain areas to modulate PTSD symptoms and memory include glucocorticoids and norepinephrine. Dysfunction of these brain areas is responsible for the symptoms of PTSD. Brain imaging studies show that PTSD patients have increased amygdala reactivity during fear acquisition. Other studies show smaller hippocampal volume. A failure of medial prefrontal/anterior cingulate activation with re-experiencing of the trauma is hypothesized to represent a neural correlate of the failure of extinction seen in PTSD. The brain has the capacity for plasticity in the aftermath of traumatic stress. Antidepressant treatments and changes in environment can reverse the effects of stress on hippocampal neurogenesis, and humans with PTSD showed increased hippocampal volume with both paroxetine and phenytoin.
Keywords: PET, depression, cortisol, glucocorticoids, stress, PTSD
Introduction
Childhood abuse is a pervasive problem that is often associated with lasting psychopathology. For instance, 16% of women have a history of childhood sexual abuse (rape or fondling) based on nationwide surveys (McCauley et al., 1997). Ten percent of women (13 million) suffer from posttraumatic stress disorder (PTSD) at some time in their lives (Kessler et al., 1995), and PTSD is twice as common in women as in men. Childhood sexual abuse is the most common cause of PTSD in women (Kessler et al., 1995). This paper reviews the long-term effects of childhood abuse on brain and neurobiology, as well as the functional plasticity of the brain in the aftermath of trauma. Findings are reviewed in PTSD and other mental disorders related to early abuse, including borderline personality disorder (BPD) and dissociative identity disorder (DID).
Psychological effects of trauma
Trauma results in a range of mental symptoms, including PTSD, BPD, DID, substance abuse, anxiety, and depression. Most of the research has been done in PTSD patients, however these patients frequently have co-morbid symptoms with these other disorders, which led to the use of the term “trauma-spectrum disorders” (Bremner, 2002). Risk factors for PTSD include prior history of stress, low years of education, prior psychiatric history, young age, and lack of social support (Bremner et al., 1995c). In one study Vietnam combat veterans with a history of childhood abuse had fourfold increased relative risk of PTSD (Bremner et al., 1993b). Childhood abuse was the factor most strongly associated with risk for PTSD, even after controlling for level of combat exposure, months in Vietnam, and participation in atrocities. Twin studies also show that there is a genetic contribution to PTSD risk (Goldberg et al., 1990).
Effects of stress on memory and the hippocampus
Studies in animals show that stress impacts adversely on the brain, especially on the hippocampus. Stress, acting through increased excitatory amino acids, decreased brain-derived neurotrophic factor (BDNF), and/or increased glucocorticoids, is associated with a loss of branching of neurons in the hippocampus and an inhibition of hippocampal neurogenesis (Uno et al., 1989; Sapolsky et al., 1990; Nibuya et al., 1995; Smith et al., 1995;Sapolsky, 1996; Duman et al., 1997). These effects are reversed by a variety of antidepressant treatments (Malberg et al., 2000; Duman et al., 2001; Santarelli et al., 2003; Duman, 2004). In addition, an enriched environment has been shown to promote hippocampal neurogenesis (Kempermann et al., 1997, 1998).
Consistent with the effects of stress on brain structures that mediate memory, including the hippocampus, prefrontal cortex, and amygdala, PTSD is associated with a wide range of memory deficits (Bremner, 2003). Memory can be categorized as declarative (memory for facts or lists, mediated in part by the hippocampus) or non-declarative (memory for things like riding a bike, or conditioned responses) (Schacter, 1996). PTSD patients show deficits in declarative memory, enhanced responses to conditioning, and perseverative errors (possibly related to frontal lobe dysfunction) (Elzinga and Bremner, 2002).
Studies in PTSD showed deficits in hippocampal function as measured with neuropsychological tests of declarative memory function (Bremner et al., 1993a, 1995a, 2004b; Uddo et al., 1993; Yehuda et al., 1995; Vasterling et al., 2002, 2006; Vasterling and Bremner, 2006). One recent study showed a decline in verbal declarative memory function from before to after Iraq deployment, showing that combat exposure resulted in changes in cognitive function (Vasterling et al., 2006). Several studies have also shown smaller hippocampal volume and/or N-acetyl aspartate (NAA, a marker of neuronal integrity) measured with magnetic resonance imaging (MRI) in PTSD (Bremner et al., 1995b, 1997b, 2003c; Stein et al., 1997; Freeman et al., 1998; Schuff et al., 2001; Villarreal et al., 2002; Lindauer et al., 2004; Shin et al., 2004; Kitayama et al., 2005; Vythilingam et al., 2005; Jatzko et al., 2006). Two recent meta-analyses showed that this effect was seen for both left and right hippocampus, and was seen equally in men and women (Kitayama et al., 2005; Smith, 2005; Jatzko et al., 2006). However effects were only seen in adults (including those with early life stress) and not in children (De Bellis et al., 1999, 2001; Carrion et al., 2001). Findings from animal studies in fact show that early life stress may not have an immediate effect on the hippocampus, but may only manifest during the adult phase of development (Brunson et al., 2001).
Bremner has outlined a model of trauma-spectrum disorders (Bremner, 2002). These psychiatric disorders, ranging from depression to BPD, DID and PTSD, are all linked to stress and share (at least in part) common bases in the brain. Studies in these disorders in fact show that exposure to early childhood abuse is associated with smaller hippocampal volume, including depression (Vythilingam et al., 2002), PTSD (Bremner et al., 1997b, 2003c), BPD (Driessen et al., 2000; Schmahl et al., 2003), and DID (Vermetten et al., 2006a). In addition, these disorders are associated with increased cortisol response to symptom provoking stressors for PTSD (Bremner et al., 2003a; Elzinga et al., 2003) and BPD (Elzinga et al., unpublished data, 12/12/06). BPD (Driessen et al., 2000; Schmahl et al., 2003) and DID (Vermetten et al., 2006a) (but not PTSD) are also associated with smaller amygdala volume.
Stress and neurohormonal systems
Alterations in the hypothalamic-pituitary-adrenal (HPA) axis have also been associated with stress-related psychiatric disorders. Corticotropin releasing factor (CRF) plays an important role in the stress response. Chronic stress exposure is associated with increases in CRF in animal studies (Arborelius et al., 1999). Central CRF administration is associated with fear-related behaviors (decreased exploration, increased startle, decreased grooming). Stress-induced lesions of the hippocampus result in a removal of inhibition of CRF release from the hypothalamus. Other findings from animal studies include a blunted adrenocorticotropin hormone (ACTH) response to CRF challenge, increased cortisol in the periphery, and resistance to negative feedback of dexamethasone (Arborelius et al., 1999). Two studies have shown increased concentrations of CRF in PTSD (Bremner et al., 1997a; Baker et al., 1999). Some studies (Yehuda et al., 1991b, 1994, 1996), but not others (Young and Breslau, 2004a, b) found decreased cortisol in 24 h urines or in diurnal salivary samples. Two studies using comprehensive measurement of plasma cortisol at multiple time points found lower cortisol concentrations in the afternoon (Yehuda et al., 1996; Bremner et al., 2007). Women with early childhood sexual abuse and PTSD were found to have lower afternoon cortisol and an increase in cortisol pulsatility compared to controls (Bremner et al., 2007). Other studies found increased lymphocyte glucocorticoid receptors (Yehuda et al., 1991a), super-suppression of cortisol with low-dose (0.5 mg) dexamethasone (Yehuda et al., 1993), blunted ACTH response to CRF, increased cortisol response to stressors (Bremner et al., 2003a) and to traumatic reminders of early trauma (Elzinga et al., 2003). Women with depression and early trauma also had increased cortisol response to public speaking (Heim et al., 2000).
There has been considerable interest in the relationship between stress, aging, and dehydroepiandosterone (DHEA). DHEA declines with aging (Orentreich et al., 1992; Barrett-Connor and Edelstein, 1994; Flynn, 1999; Johnson et al., 2002) and there has been considerable interest in the ability of DHEA supplements to block the normal effects of aging, although there is no convincing data that DHEA has such effects. DHEA also is important in the stress response. Chronic stress increases DHEA and DHEA-S (Fuller et al., 1984). DHEA also has antistress effects, blocking the effects of glucocorticoids on peripheral tissues as well as the hippocampus (Kimonides et al., 1998; Kaminska et al., 2000) and decreasing anxiety (Prasad et al., 1997). Studies of DHEA in patients with stress-related psychiatric disorders are contradictory, while studies in adult depressed patients showed both increases (Heuser et al., 1998) and decreases (Goodyer et al., 1996; Herbert et al., 1996) as well as no change (Michael et al., 2000; Young et al., 2002) in levels. Studies of DHEA in PTSD have been equally contradictory, with one study citing lower concentrations relative to controls (Kanter et al., 2001) while the other showed elevations (Spivak et al., 2000). We recently measured DHEA and DHEA-S at multiple time points over a 24 h period in women with early abuse and PTSD, and found elevations in both DHEA and DHEA-S (Bremner et al., 2007).
We performed a comprehensive assessment of memory, cortisol, DHEA, and the hippocampus in women with sexual abuse before 13, with and without PTSD, and healthy nonabused women. All subjects underwent assessment of hippocampal structure with MRI, assessment of hippocampal function with PET in conjunction with a paragraph encoding declarative memory task, assessment of HPA axis function at baseline and with a stressful challenge, and neuropsychological testing of declarative memory function. Early childhood sexual abuse before the age of 13 was defined as rape or molestation as assessed with the Early Trauma Inventory (Bremner et al., 2000). All subjects were free of psychotropic medication for 4 weeks before study.
Women with a history of early childhood sexual abuse and the diagnosis of PTSD (N = 10) were compared to abused non-PTSD women (N = 12) for hippocampal function using PET. All subjects were scanned during encoding of a paragraph and control task in conjunction with injection of 0–15 water and PET imaging of the brain. MR images were obtained for measurement of hippocampal volume, with an additional group of nonabused normal women (total N = 33). Subjects (N = 56) were also admitted to the GCRC for a 24 h period, for measurement of plasma cortisol, DHEA, and estradiol measured at 15 min intervals for 24 h. Salivary cortisol was measured after reading of a traumatic script related to personalized childhood abuse experiences.
In addition, salivary cortisol was measured before and after a 20 min cognitive challenge (arithmetic, color-word naming, problem solving under time pressure, and negative feedback). Women with abuse and PTSD had smaller hippocampal volume (Bremner et al., 2003b), a failure of hippocampal activation with declarative memory tasks (Bremner et al., 2003b), lower plasma cortisol concentrations in the afternoon (Bremner et al., 2007), increased cortisol pulsatility (Bremner et al., 2007), increased plasma DHEA concentrations (Bremner et al., 2007), increased cortisol response to stress (Bremner et al., 2003a), increased cortisol response to traumatic reminders (Elzinga et al., 2003), and impaired declarative memory measured with neuropsychological testing (Bremner et al., 2004b).
Neurohormonal modulation of memory
Glucocorticoids affect learning and memory. Elevations of glucocorticoids within the physiological range result in reversible deficits in memory function in animals (Oitzl and de Kloet, 1992; Bodnoff et al., 1995) as well as human subjects (Newcomer et al., 1994, 1999; Kirschbaum et al., 1996; Lupien et al., 1997, 1999, 2002; de Quervain et al., 2000; Wolf et al., 2001). Glucocorticoids released during stress, possibly acting through the hippocampus, may explain in part the acutely reversible as well as chronic effects that stress has on declarative memory (Kirschbaum et al., 1996; Porter and Landfield, 1998; de Kloet et al., 1999; Wolf, 2003). Greater deficits are seen in younger subjects in comparison to older subjects, hypothesized to be secondary to age-related decreases in glucocorticoid receptor density (Newcomer et al., 1995). Impairment of working memory by glucocorticoids may require noradrenergic stimulation to have its effect (Elzinga and Roelofs, 2005). We used a protocol of 1 mg of dexamethasone, followed by 2 mg one day later, and found an impairment in declarative memory function (percent retention of a paragraph after a delay) in healthy subjects, but not patients with depression (Bremner et al., 2004d) or PTSD (Bremner et al., 2005c). We hypothesized that this might be due to disease-related decreases in glucocorticoid receptor function. This is consistent with the idea of PTSD as an “accelerated aging” (Bremner and Narayan, 1998) related to common theories of progressive hippocampal atrophy and dysfunction in both processes. We have also shown that endogenous cortisol release stimulated by a cognitive stress challenge in healthy subjects impaired delayed recall of words and a spatial memory task at 24 h (Elzinga et al., 2005).
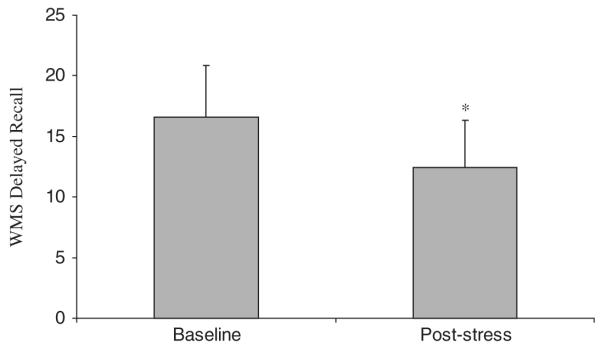
In women (with and without PTSD), with a history of early abuse, memory functioning was also affected after exposure to personalized scripts (Elzinga et al., 2003). For neutral paragraphs encoded after exposure to the trauma scripts there was an impairment in delayed recall relative to paragraphs encoded in a no-stress condition (Fig. 1). Recall 24 h later of an emotional paragraph presented immediately after the trauma scripts was positively correlated with cortisol response to the stressful challenge, meaning that cortisol enhanced consolidation of emotional memories. Another study in male healthy subjects has shown that endogenous cortisol levels in healthy subjects who became upset during a social speech task were positively correlated with enhanced delayed memory recall of pictures, which was especially prominent for recall of unpleasant pictures (Abercrombie et al., 2005). Taken together, these findings are consistent with animal models suggesting that glucocorticoid effects on learning require emotional arousal (Roozendaal, 2000).
Fig. 1.
Effects of a traumatic script on memory recall. There was a significant difference in delayed paragraph recall for paragraphs encoded after exposure to traumatic scripts compared to paragraphs encoded at a pre-stress baseline (t(22) = −3.39, p<0.01). This showed that stress impaired the ability to consolidate declarative memory.
Catecholamines released during stress also modulate the encoding and retrieval of memory (McGaugh, 2000). Administration of epinephrine (which is released from the adrenal) affects memory consolidation with an inverted U-shaped curve. Memory improves up to a point and decreases with high doses (Gold and van Buskirk, 1975; Liang et al., 1986). Lower doses of norepinephrine injected into the amygdala promote memory for an inhibitory avoidance task while higher doses inhibit memory (Liang et al., 1990). In humans, noradrenergic beta-blocker medications blocked the formation of emotional memories (Cahill et al., 1994), while enhanced norepinephrine release was associated with enhanced encoding of emotional memories (Southwick et al., 2002). Vasopressin and oxytocin have been shown to modulate memory formation in both animals (McGaugh, 2000) and human subjects (including those with PTSD) (Pitman et al., 1993).
Fear conditioning and extinction
One of the most classic laboratory paradigms that has been used as a model for PTSD is conditioned fear. In animal models, the pairing of light and shock leads to fear responses to the light alone. With exposure to light alone there is a gradual decrease in fear responding (called “extinction to fear”) (Davis, 1992). Re-exposure to the light-shock at a later time point results in a rapid return of fear responding (Quirk, 2002). Medial prefrontal cortical inhibition of the amygdala (which plays a critical role in fear responses) is felt to represent the neural mechanism of extinction to fear responding (Quirk et al., 2006). This brain area is known to mediate emotion, as represented by the famous case of Phineas Gage (Damasio et al., 1994). Phineas Gage was a 19th century railroad worker who was injured by a spike that entered through his eye socket and lesioned his medial prefrontal cortex (mPFC). Areas involved included the orbitofrontal, anterior cingulate (25/24/32), and mesofrontal cortex (9). Speech and cognition remained intact. He had marked deficits in his ability to judge social contexts, behave appropriately in social contexts, and assess emotional nonverbal signals from others. Based on these findings and others, the mPFC has been judged to play a critical role in the emotion and social function.
This medial prefrontal area also plays an important role in the modulation of the neurohormonal response to stress. This area mediates peripheral cortisol and sympathetic responses to stress (Diorio et al., 1993). Dysfunction of this area could explain altered neurohormonal responses to stress in PTSD patients.
Studies in PTSD have shown dysfunction in the medial prefrontal cortical response to stress and traumatic reminders. We previously found decreased medial prefrontal function in combat veterans with PTSD exposed to combat-related PTSD (Bremner et al., 1999b). In a second study we showed that women with PTSD related to early childhood sexual abuse had a decrease in medial prefrontal function in response to scripts of early childhood sexual abuse (Bremner et al., 1999a). A second study of exposure to emotional word pairs (e.g., rape-mutilate) showed decreased medial prefrontal and hippocampal function in abused women with PTSD (Bremner et al., 2003d). Another study used “Stroop” words (say the color of a color word, e.g., green, which leads to slowing of response time, due to inhibition of response) with an emotional Stroop component (name the color of a word like “rape”). The Stroop paradigm is associated with activation of anterior cingulate. Studies of the emotional Stroop (e.g., say the color of the word rape) has been associated with a slower response time in abuse-related (Foa et al., 1991) or combat-related PTSD (McNally et al., 1990). We studied neural correlates of the emotional Stroop in women with a history of early abuse with and without PTSD. We found that performance of the emotional Stroop was associated with decreased function in the mPFC in the PTSD patients (Bremner et al., 2004c).
We also have assessed neural correlates of conditioned fear in PTSD. Pairing of light and shock leads to increased fear responding and increased startle to light alone (conditioned fear). Conditioned fear and startle response are mediated by the central nucleus of the amygdala. Failure of extinction occurs with lesions of the mPFC (which inhibits the amygdala).
We studied fear conditioning with PET in women with a history of early abuse and PTSD and healthy nonabused women (Bremner et al., 2005b). Subjects were exposed to repeated and intermittent exposure to a blue square on a screen in the absence of shock (habituation), exposure to a blue square with a shock (fear acquisition), and then exposure to the blue square in the absence of shock (extinction). On a separate control day, they received random shocks instead of paired exposures; otherwise the protocol was the same. PTSD subjects experienced increased anxiety with fear acquisition and extinction. PTSD subjects also had increased amygdala blood flow during fear acquisition and decreased medial prefrontal blood flow during extinction. Increased amygdala blood flow during fear acquisition in the PTSD patients was correlated with increased PTSD symptoms, anxiety, and dissociation during fear acquisition. Increased amygdala blood flow during fear acquisition was correlated with decreased medial prefrontal blood flow during fear extinction in all of the subjects. There was a highly significant negative correlation between increased anxiety and decreased medial prefrontal blood flow during extinction in the PTSD patients (r = 0.90; p = 0.006).
Effects of treatment on the brain in PTSD
We have also assessed the effects of the selective serotonin reuptake inhibitor paroxetine on brain and cognition in PTSD. Previous multisite randomized placebo-controlled trials have shown efficacy for paroxetine over placebo in PTSD (Marshall et al., 2001; Tucker et al., 2001). Antidepressants have also been shown to promote neurogenesis in the hippocampus, a brain area involved in learning and memory (Duman et al., 1997). In an open-label study we showed a 5% increase in hippocampal volume after 9 months of treatment with paroxetine, as well as a 30% improvement in verbal declarative memory function measured with neuropsychological testing (Vermetten et al., 2003). Paroxetine treatment was also associated with a decreased cortisol and heart rate response to a stressful task (Vermetten et al., 2006b).
Glutamate, dissociation, and PTSD
Alterations in glutamatergic function has also been implicated in PTSD as well as dissociation (Krystal et al., 1996; Chambers et al., 1999). Symptoms of dissociation are an important part of the psychopathological response to stress. Symptoms of dissociation measured with the Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998) are:
Do things seem to be moving in slow motion?
Do things seem to be unreal to you, as if you are in a dream?
Do you feel as if you are watching the situation as an observer or spectator?
Do you feel disconnected from your own body?
Do you see things as if you were in a tunnel, or looking through a wide-angle photographic lens?
Does this experience seem to take much longer than you would have expected?
Increased dissociative symptoms at the time of trauma predict long-term PTSD (Bremner et al., 1992; Marmar et al., 1994). Although symptoms of dissociation are not part of the DSM criteria for PTSD, they are part of the criteria for acute stress disorder, and symptoms of dissociation are frequently seen in PTSD patients. PTSD patients are observed clinically to have an increased dissociative response to the original trauma, and then have chronic increased susceptibility to dissociative responses to minor stressors and traumatic reminders.
Although the neurobiology of dissociation has been studied less than PTSD, alterations in stress hormones likely play a role in these symptoms. One particular neurotransmitter system that has been hypothesized to play a role in dissociative symptoms is the excitatory amino acid glutamate (Krystal et al., 1994, 1996; Chambers et al., 1999). Glutamate is released during stress (Moghaddam et al., 1997), and high levels of glutamate are associated with toxicity to the hippocampus. Glutamate acts at the N-methyl-D-aspartic acid (NMDA) receptor, and is highly concentrated in the hippocampus. Glutamate is involved in memory at the molecular level. Excessive levels of glutamate can cause cytotoxicity as seen in patients with epilepsy. Stress inhibits glucose utilization, and thereby impairs reuptake of glutamate in glia with associated cytotoxicity.
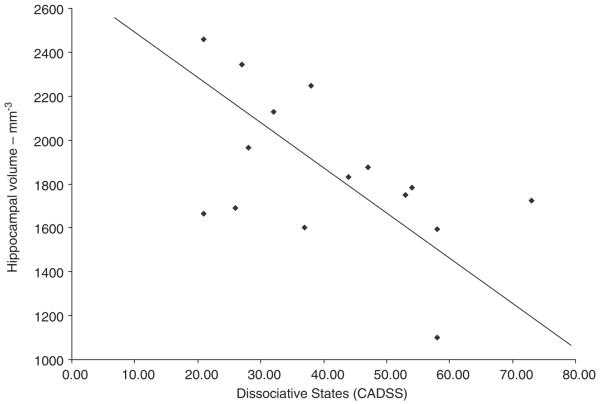
Several lines of evidence support alterations of glutamatergic function in dissociation. The NMDA antagonist, Ketamine, when administered to normal subjects, results in an increased dissociative symptoms as measured with the CADSS (Krystal et al., 1994). In addition, increased dissociative states correlate with smaller volume of the hippocampus (which as noted above has a high concentration of NMDA receptors) in women with early abuse and PTSD (Stein et al., 1997; Bremner et al., 2003b). A correlation between dissociative states as measured with the CADSS and smaller hippocampal volume was seen in women with early abuse and DID (Fig. 2). Phenytoin (dilantin) is an antiepileptic drug that is efficacious in the treatment of epilepsy. Phenytoin modulates glutamatergic function and blocks the effects of stress on the hippocampus in animal studies (Watanabe et al., 1992). We conducted a pilot project in nine PTSD subjects of the effect of phenytoin on symptoms of PTSD and the brain. Phenytoin resulted in a decrease in PTSD symptoms (Bremner et al., 2004a) as well as a 5% increase in right hippocampal and right cerebral volume (Bremner et al., 2005a).
Fig. 2.
Relationship between hippocampal volume measured with MRI and dissociative states measured with the CADSS in women with early abuse and the diagnosis of dissociative identity disorder. There was a significant negative correlation between hippocampal volume and dissociative states (r = −0.54; df = 14; p<0.05), suggesting that increased levels of dissociation were related to smaller hippocampal volume. This correlation was not shown for amygdala volume. In addition there was not an association between level of PTSD symptoms and hippocampal volume in these patients.
Conclusions
We have presented evidence for long-term alterations in brain and neurobiology in PTSD. Brain areas involved in the stress response include the mPFC, hippocampus, and amygdala. Neurohormonal systems that act on the brain areas to modulate PTSD symptoms and memory include glucocorticoids and norepinephrine. Dysfunction of these brain areas is responsible for symptoms of PTSD.
The related symptom area of dissociation is felt to be related to alterations in glutamatergic function; however, more research is needed in this area.
Brain imaging studies show that PTSD patients have increased amygdala reactivity during fear acquisition. Other studies show smaller hippocampal volume. A failure of medial prefrontal/anterior cingulate activation with re-experiencing of the trauma is hypothesized to represent a neural correlate of the failure of extinction seen in PTSD.
The brain has the capacity for plasticity in the aftermath of traumatic stress. Antidepressant treatments and changes in environment can reverse the effects of stress on hippocampal neurogenesis. In humans with PTSD, paroxetine increases hippocampal volume and improves memory function in conjunction with improving PTSD symptoms. Phenytoin, which blocks the effects of stress on the hippocampus in animal studies, also increases hippocampal volume in PTSD patients.
Future studies should use brain imaging and neurobiology to assess plasticity in PTSD. These can include both functional neuroimaging and neuroreceptor imaging to track the course of change during treatment, or to predict which traumatized individuals will develop chronic PTSD. The information from such studies will provide valuable information that will guide the development of new treatments.
Discussion: Chapter 12
OITZL: Did I miss something about the temporal aspect of PTSD? That is, are there differences between individuals who may have had PTSD for a limited duration like 6 months and those with chronic PTSD who have had PTSD for many years?
BREMNER: We’re starting to study this in people that are coming from Iraq. The argument that we make is that people coming from Iraq in the first 6 months to a year after their return have a different type of PTSD than some of the older PTSD veterans from Vietnam. Until now the subjects we have studied have chronic forms of PTSD, such as the PTSD related to abuse in early childhood or Vietnam combat veterans with PTSD. We are looking at adolescents with abuse-related PTSD in which the PTSD is more acute, but that study is not completed. And then we do have a current study that is looking at returning Iraqi veterans trying to get people in the first 6 months after they were discharged from service. We are doing a brain imaging and intervention that involves mindfulness-based stress reduction.
JOËLS: The number of newborn cells in adult brain is extremely low. It is not well known how many newborn cells there are in children, but from the little that is known it is not a lot. Even if these numbers are doubled and you look over a couple of weeks, the increase in hippocampal volume or 5% you see in your studies after paroxetine treatment cannot easily be explained by these newborn cells. So where do you think this increase of volume comes from? Is it maybe a change in blood flow or something else?
BREMNER: It could be that in addition to new neurons developing with paroxetine treatment, there are other contributions to increased volume such as an increase in dendritic branching. It could also be that treatment results in an increase in water content. However, we have preliminary data showing an increase in N-acetyl aspartate (NAA) measured with magnetic resonance spectroscopy (MRS) in the hippocampus of PTSD patients. NAA is a marker of neuronal integrity, so this suggests that paroxetine has effects on neuronal structure.
SECKL: I would like to ask a slightly different type of question and also about your findings with DHEA, which among us as endocrinologists has been a graveyard for many people’s careers. But it is a pretty unique association: Do you think there is a fundamental underlying diathesis in this condition with this altered 17 hydroxylase? What is your thinking about this finding?
BREMNER: Our study showed that DHEA is elevated in women with abuse-related PTSD, and also that there was an elevation in DHEA to cortisol ratio where there was even more of a difference between the patients and the controls. From the standpoint of PTSD pathology, you can argue that it is a paradoxical finding since DHEA supposedly should have antiglucocorticoid-protective effect on the hippocampus. Here we are getting elevated levels, and smaller hippocampal volume and lower cortisol in the same subjects. In terms of the pathophysiology of why it is elevated, I do not know if I have a good explanation. If you look at the depression literature it is a mixed literature. In PTSD there are two studies that have a single sample, one significantly decreased and one significantly increased. Do you want to comment? I am not an endocrinologist.
YEHUDA: You have a lot of data and it is really impressive that you can get so many measures on the same subjects, and that is really what helps you make progress in the field because we tend to make these observations usually in different subjects; to me that is terrific. However, I am wondering I think there is a point in your presentation where you present like this is the theory and here are the data, the data don’t fit the theory, and then we come back then about the theory, e.g., you have one slide about traumatic stress spectrum and all that, and depression and PTSD should be alike and elevated cortisol and cortisol damages the brain. Ok all that’s fine and we know that is in the literature out there. But now you have collected your data, you are one of the few people on the planet that have multiple measures in the same cohort. So you can say, “Hey wait a minute, I have low cortisol at baseline and small hippocampuses, ha!” So what does that do to those slides in the beginning of the talk that summarize the literature and form the basis of your model? How do you feel about extending that?
BREMNER: There’s some pattern that you know, e.g., that we can see correlations between memory performance measured at the time of the 24 h cortisol measure. In normal subjects there is an inverse correlation, so the higher the cortisol the lower the memory function which makes sense in terms of what is known about how administration of cortisol impairs declarative memory function in normal subjects. Because of time I did not show the trial in which we have given dexamethasone to healthy subjects in a 3-day protocol. After 3 days memory function becomes impaired in healthy subjects but not in the PTSD patients. We can see low cortisol and small hippocampal volume in the same subjects. And then we put them through a trauma-specific task that gives them anxiety and cortisol release is increased. So there could be some pattern cortisol release that causes hippocampal damage and memory impairment. But…
YEHUDA: How can it do that when it is low? How does low basal cortisol cause hippocampal damage?
BREMNER: Well low basal cortisol does not cause hippocampal damage. And we do not find correlations between hippocampal volume and cortisol in our patients with PTSD from traumas 20 years ago.
YEHUDA: You did not show cortisol and hippocampal volume correlation?
BREMNER: No we don’t have this correlation; there is not a correlation in PTSD. So you know whether cortisol at one point in time resulted at the time of trauma in a kind of damage that would be a speculation. I do not know if we could ever really look at that. But what it looks like more is that there is a pattern of findings in subjects at baseline that, maybe, points at some central pathological process that leads to lower cortisol and smaller hippocampal volume that may not even be related in terms of the pathogenesis.
YEHUDA: It is at the heart of the matter of when we decide that maybe cortisol does not damage the hippocampus. I mean how much data do you need to look at the literature and say “Ha, this does not apply here.” But it does not sound that there ever is going to be evidence of such a correlation for some of us.
DE KLOET: There is a literature out there, in which it is stated explicitly that high cortisol concentrations are damaging to the hippocampus, but in my opinion that cannot be generalized because there many situations like exercise that also produce high cortisol, but that are paradoxical not damaging at all. Only if levels of cortisol are chronically elevated for prolonged periods of time under conditions of distress one may see deterioration of the immune system, metabolic changes, and impairment of brain function. The question then is: What is special in the pattern of cortisol secretion that it damages the brain?
YEHUDA: The point is that at some time the data that we get should force us to have new models and abandon models that don’t fit the data. Because then we are just trying to make that data fit models that are no good and you can have low cortisol and small hippocampal volume and somehow it still becomes important to say at some time there was no high cortisol, why, maybe, maybe not, maybe that does not fit, I am just wondering at what point we do that.
DE KLOET: I think the jury is out. There is a need for good controlled studies to measure the pulsatile patterns of cortisol to demonstrate what the actual significance of cortisol is, whether it is a predisposing factor or a consequence of the PTSD condition.
Abbreviations
- ACTH
adrenocorticotropin
- BDNF
brain-derived neurotrophic factor
- BPD
borderline personality disorder
- CRF
corticotropin releasing factor
- DHEA
dehydroepiandosterone
- DID
dissociative identity disorder
- mPFC
medial prefrontal cortex
- MRI
magnetic resonance imaging
- NAA
N-acetyl aspartic acid
- NMDA
N-methyl-D-aspartic acid
- PET
positron emission tomography
- PTSD
posttraumatic stress disorder
References
- Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2005;31:187–196. doi: 10.1016/j.psyneuen.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Edelstein SL. A prospective study of dehydroepiandrosterone sulfate and cognitive function in an older population: the Rancho Bernardo study. J. Am. Geriatr. Soc. 1994;42:420–423. doi: 10.1111/j.1532-5415.1994.tb07491.x. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J. Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Does Stress Damage the Brain? Under-standing Trauma-related Disorders from a Mind-Body Perspective. W.W. Norton; New York: 2002. [Google Scholar]
- Bremner JD. Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacol. Bull. 2003;37:6–25. [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam F, Marmar C, Southwick SM, Lubin H, Charney DS. Measurement of dissociative states with the Clinician Administered Dissociative States Scale (CADSS) J. Trauma. Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens M, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatry. 1997a;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Mletzko T, Welter S, Siddiq S, Reed L, Williams C, Heim CM, Nemeroff CB. Treatment of posttraumatic stress disorder with phenytoin: an open label pilot study. J. Clin. Psychiatry. 2004a;65:1559–1564. doi: 10.4088/jcp.v65n1120. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Mletzko T, Welter S, Quinn S, Williams C, Brummer M, Siddiq S, Reed L, Heim CM, Nemeroff CB. Effects of phenytoin on memory, cognition and brain structure in posttraumatic stress disorder: a pilot study. J. Psychopharmacol. 2005a;19:159–165. doi: 10.1177/0269881105048996. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M. The effects of stress on memory and the hippocampus throughout the life cycle: implications for childhood development and aging. Dev. Psychopathol. 1998;10:871–886. doi: 10.1017/s0954579498001916. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatry. 1999a;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall PR, Capelli S, Scott TM, McCarthy G, Charney DS. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995a;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall PR, Scott TM, Bronen RA, Delaney RC, Seibyl JP, Southwick SM, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am. J. Psychiatry. 1995b;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall PR, Vermetten E, Staib L, Bronen RA, Mazure CM, Capelli S, McCarthy G, Innis RB, Charney DS. MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol. Psychiatry. 1997b;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS. Deficits in short-term memory in posttraumatic stress disorder. Am. J. Psychiatry. 1993a;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Brett E, Fontana A, Rosenheck A, Charney DS. Dissociation and posttraumatic stress disorder in Vietnam combat veterans. Am. J. Psychiatry. 1992;149:328–332. doi: 10.1176/ajp.149.3.328. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Charney DS. Etiological factors in the development of posttraumatic stress disorder. In: Mazure CM, editor. Does Stress Cause Psychiatric Illness? Vol. 46. American Psychiatric Press; Washington, DC: 1995c. pp. 149–186. [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. Am. J. Psychiatry. 1993b;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol. Psychiatry. 1999b;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Kelley ME. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 h in women with childhood sexual abuse-related posttraumatic stress disorder. J. Nerv. Ment. Dis. 2007 doi: 10.1097/NMD.0b013e3181594ca0. (in press) [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress. Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Nafzal N, Vythilingam M. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder (PTSD) J. Nerv. Ment. Dis. 2004b;192:643–649. doi: 10.1097/01.nmd.0000142027.52893.c8. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual abuse-related posttraumatic stress disorder. Psychol. Med. 2005b;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol. Psychiatry. 2004c;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Anderson G, Heninger GR, Southwick SM, Charney DS. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003a;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Newcomer JW, Charney DS. Effects of glucocorticoids on declarative memory function in major depression. Biol. Psychiatry. 2004d;55:811–815. doi: 10.1016/j.biopsych.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Newcomer JW, Charney DS. Effects of dexamethasone on declarative memory function in posttraumatic stress disorder (PTSD) Psychiatry Res. 2005c;129:1–10. doi: 10.1016/j.psychres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg P, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder (PTSD) Am. J. Psychiatry. 2003b;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatry. 2003c;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib L, Soufer R, Charney DS. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder (PTSD) related to early childhood sexual abuse. Biol. Psychiatry. 2003d;53:289–299. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol. Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Bremner JD, Moghaddam B, Krystal JH. Glutamate and posttraumatic stress disorder: toward a psychobiology of dissociation. Semin. Clin. Neuropsychiatry. 1999;4:274–281. doi: 10.153/SCNP00400274. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol. Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. A.E. Bennett Research Award: developmental traumatology: Part II. Brain development. Biol. Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch. Gen. Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Duman RS. Depression: a case of neuronal life and death? Biol. Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch. Gen. Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg JE, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J. Pharmacol. Exp. Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- Elzinga BM, Bakker A, Bremner JD. Stress-induced cortisol elevations are associated with impaired delayed, but not immediate recall. Psychiatry Res. 2005;134:211–223. doi: 10.1016/j.psychres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural subst-rates of memory the final common pathway in PTSD? J. Affect. Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K. Cortisol induced impairments of working memory require acute sympathetic activation. Behav. Neurosci. 2005;119:98–103. doi: 10.1037/0735-7044.119.1.98. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CS, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuro-psychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Flynn MA. Dehydroepiandrosterone replacement in aging humans. J. Clin. Endocrinol. Metab. 1999;84:1527–1533. doi: 10.1210/jcem.84.5.5672. [DOI] [PubMed] [Google Scholar]
- Foa EB, Feske U, Murdock TB, Kozak MJ, McCarthy PR. Processing of threat related information in rape victims. J. Abnorm. Psychology. 1991;100:156–162. doi: 10.1037//0021-843x.100.2.156. [DOI] [PubMed] [Google Scholar]
- Freeman TW, Cardwell D, Karson CN, Komoroski RA. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn. Reson. Med. 1998;40:66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- Fuller GB, Hobson WC, Reyes FI, Winter JS, Faiman C. Influence of restraint and ketamine anesthesia on adrenal steroids, progesterone, and gonadotropins in rhesus monkeys. Proc. Soc. Exp. Biol. Med. 1984;175:487–490. doi: 10.3181/00379727-175-41825. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk R. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav. Biol. 1975;13:145–153. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- Goldberg J, True WR, Eisen SA, Henderson WG. A twin study of the effects of the Vietnam War on posttraumatic stress disorder. JAMA. 1990;263:1227–1232. [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altharn PM, Pearson J, Secher SM, Shiers HM. Adrenal steroids during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) presentation. Psychol. Med. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Herbert J, Goodyer IM, Altham PM, EPearson J, Secher SM, Shiers HM. Adrenal secretion and major depression in 8- to 16-year-olds, II: influence of co-morbidity at presentation. Psychol. Med. 1996;26:257–263. doi: 10.1017/s0033291700034656. [DOI] [PubMed] [Google Scholar]
- Heuser I, Deuschle M, Luppa P, Schweiger U, Standhardt H, Weber B. Increased diurnal plasma concentrations of dehydroepiandrosterone in depressed patients. J. Clin. Endocrinol. Metab. 1998;83:3130–3133. doi: 10.1210/jcem.83.9.5081. [DOI] [PubMed] [Google Scholar]
- Jatzko A, Rothenhofer S, Schmitt A, Gaser C, Demirakca T, Weber-Fahr W, Wessa M, Magnotta V, Braus DF. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. J. Affect. Disord. 2006;94:121–126. doi: 10.1016/j.jad.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Bebb RA, Sirrs SM. Uses of DHEA in aging and other disease states. Ageing Res. Rev. 2002;1:29–41. doi: 10.1016/s0047-6374(01)00369-4. [DOI] [PubMed] [Google Scholar]
- Kaminska M, Harris J, Gijsbers K, Dubrovsky B. Dehydroepiandrosterone sulfate (DHEAS) counter-acts decremental effects of corticosterone on dentate gyrus LTP: implications for depression. Brain Res. Bull. 2000;52:229–234. doi: 10.1016/s0361-9230(00)00251-3. [DOI] [PubMed] [Google Scholar]
- Kanter ED, Wilkinson CW, Radant AD, Petrie EC, Dobie DJ, McFall ME, Peskind ER, Raskind MA. Glucocorticoid feedback sensitivity and adrenocortical responsiveness in posttraumatic stress disorder. Biol. Psychiatry. 2001;50:238–245. doi: 10.1016/s0006-3223(01)01158-1. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and-treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J. Affect. Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Bennett A, Bremner JD, Southwick SM, Charney DS. Recent developments in the neurobiology of dissociation: implications for posttraumatic stress disorder. In: Michelson LK, Ray WJ, editors. Handbook of Dissociation: Theoretical, Empirical, and Clinical Perspectives. Plenum Press; New York: 1996. pp. 163–190. [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS. Subanesthetic effects of the non-competitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. Modulating effects of post-training epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Liang KC, McGaugh JL, Yao HY. Involvement of amygdala pathways in the influence of post-training intra-amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Res. 1990;508:225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, den Heeten GJ, Gersons BP. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol. Psychiatry. 2004;56:356–363. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S, Nair NP, Hauger RL, McEwen BS, Meaney MJ. Stress-induced declarative memory impairment in healthy elderly subjects: relationship to cortisol reactivity. J. Clin. Endocrinol. Metab. 1997;82:2070–2075. doi: 10.1210/jcem.82.7.4075. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav. Neurosci. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Briere S, Kin N.M.K. Ng Ying, Meaney MJ, Nair NPV. Acute modulation of aged human memory by pharmacological manipulation of glucocorticoids. J. Clin. Endocrinol. Metab. 2002;87:3798–3807. doi: 10.1210/jcem.87.8.8760. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmar CR, Weiss DS, Schlenger DS, Fairbank JA, Jordan BK, Kulka RA, Hough RL. Peritraumatic dissociation and posttraumatic stress in male Vietnam theater veterans. Am. J. Psychiatry. 1994;151:902–907. doi: 10.1176/ajp.151.6.902. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am. J. Psychiatry. 2001;158:1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EG. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- McGaugh JL. Memory: a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Kaspi RJ, Riemann BC, Zeitlin SB. Selective processing of threat cues in posttraumatic stress disorder. J. Abnorm. Psychol. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol. Psychiatry. 2000;48:989–995. doi: 10.1016/s0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997;17:2127–2912. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J. Neurosci. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Kelly AK, Paras L, Craft S. Age-related differences in glucocorticoid effect on memory in human subjects. Soc. Neurosci. Abstr. 1995;21:161. [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch. Gen. Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electro-convulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav. Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J. Clin. Endocrinol. Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Lasko NB. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1993;48:107–117. doi: 10.1016/0165-1781(93)90035-f. [DOI] [PubMed] [Google Scholar]
- Porter NM, Landfield PW. Stress hormones and brain aging. Nat. Neurosci. 1998;1:3–4. doi: 10.1038/196. [DOI] [PubMed] [Google Scholar]
- Prasad A, Imamura M, Prasad C. Dehydroepiandrosterone decreases behavioral despair in high- but not low-anxiety rats. Physiol. Behav. 1997;62:1053–1057. doi: 10.1016/s0031-9384(97)00239-4. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ-F, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat. Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn. Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol. Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL. Searching for Memory: The Brain, the Mind, and the Past. Basic Books; New York: 1996. [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psych. Res.: Neuroimaging. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol. Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, Lasko N, Segal E, Makris N, Richert K, Levering J, Schacter DL, Alpert NM, Fischman AJ, Pitman RK, Rauch SL. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNA in the hippocampus. J. Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Horner B, Morgan CA, Bremner JD, Davis M, Cahill L, Gold PB, Charney DS. Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. Am. J. Psychiatry. 2002;159:1420–1422. doi: 10.1176/appi.ajp.159.8.1420. [DOI] [PubMed] [Google Scholar]
- Spivak B, Maayan R, Kotler M, Mester R, Gil-Ad I, Shtaif B, Weizman A. Elevated circulatory level of GABA-A antagonistic neurosteroids in patients with combat-related posttraumatic stress disorder. Psychol. Med. 2000;30:1227–1231. doi: 10.1017/s0033291799002731. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled flexible-dosage trial. J. Clin. Psychiatry. 2001;62:860–868. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- Uddo M, Vasterling JJ, Braily K, Sutker PB. Memory and attention in posttraumatic stress disorder. J. Psychopathol. Behav. Assess. 1993;15:43–52. [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J. Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Bremner JD. The impact of the 1991 Gulf War on the mind and brain: findings from neuropsychological and neuroimaging research. Philos. Trans. R Soc. Lond. Biol. Sci. 2006;361:593–604. doi: 10.1098/rstb.2006.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Sutker PH. Attention, learning, and memory performance and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychologia. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Proctor SP, Amoroso P, Kane R, Heeren T, White RF. Neuropsychological outcomes of army personnel following deployment to the Iraq War. JAMA. 2006;296:519–529. doi: 10.1001/jama.296.5.519. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdala volumes in dissociative identity disorder. Am. J. Psychiatry. 2006a;163:1–8. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Schmahl C, De Kloet C, Southwick WM, Charney DS, Bremner JD. Alterations in stress reactivity after long-term treatment with paroxetine in women with posttraumatic stress disorder. Ann. N.Y. Acad. Sci. 2006b;1071:184–202. doi: 10.1196/annals.1364.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, Kodituwakku PW, Hart BL, Escalona R, Brooks WM. Reduced hippocampal volume and total white matter in posttraumatic stress disorder. Biol. Psychiatry. 2002;52:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport CD, Miller AH, Vermetten E, Anderson E, Bronen R, Staib L, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Luckenbaugh DA, Lam T, Morgan CA, III, Lipschitz D, Charney DS, Bremner JD, Southwick SM. Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psychiatry Res. 2005;139:89–99. doi: 10.1016/j.pscychresns.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Watanabe YE, Gould H, Cameron D, Daniels D, McEwen BS. Phenytoin prevents stress and corticosterone induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Wolf OT. HPA axis and memory. Best Pract. Res. Clin. Endocrinol. Metab. 2003;17:287–299. doi: 10.1016/s1521-690x(02)00101-x. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Keefe RS, Harvey PD, Levengood RA, Gerber DK, Geni J, Siever LJ. Learning and memory in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry. 1995;152:137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Lowry MT, Southwick SM, Mason JW, Giller EL. Increased number of glucocorticoid receptors in posttraumatic stress disorder. Am. J. Psychiatry. 1991a;148:499–504. doi: 10.1176/ajp.148.4.499. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner JD, Charney DS, Mason J. Enhanced suppression of cortisol with low dose dexamethasone in posttraumatic stress disorder. Am. J. Psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum EL, Giller EL, Mason JW. Low urinary cortisol in PTSD. J. Nerv. Ment. Dis. 1991b;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Levengood RA, Trestman RL, Siever LJ. Circadian regulation of basal cortisol levels in posttraumatic stress disorder. Ann. N.Y. Acad. Sci. 1994;378:380. doi: 10.1111/j.1749-6632.1994.tb39260.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol. Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Young AH, Gallagher P, Porter RJ. Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. Am. J. Psychiatry. 2002;159:1237–1239. doi: 10.1176/appi.ajp.159.7.1237. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: an epidemiologic community study. Arch. Gen. Psychiatry. 2004a;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder: a community epidemiologic study. Biol. Psychiatry. 2004b;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]