Abstract
Objective
The anterior cingulate cortex (ACC) plays an important role in emotion, and studies in animals have shown changes in ACC structure with early life stress. The purpose of this study was to measure volume of the ACC in PTSD.
Method
Magnetic resonance imaging (MRI) was used to measure ACC volume in 8 subjects with abuse-related PTSD and 13 healthy subjects without PTSD. ACC volume included Brodmann’s area [BA] 24 and 32.
Results
Right ACC volume in PTSD patients was significantly smaller than in non-PTSD subjects.
Conclusion
These results are consistent with smaller ACC volume in PTSD.
Keywords: Posttraumatic stress disorder, Anterior cingulate, Magnetic resonance, Volumetry, Brodmann’s area
1. Introduction
Posttraumatic stress disorder (PTSD) is a highly prevalent disorder affecting 8% of the population by some estimates (Kessler et al., 1995). Understanding the neurobiology of PTSD is important for the development of new treatments for this disorder. The medial prefrontal cortex, a brain area which makes up a part of the frontal lobe, plays an important role in the negative feedback regulation of hypothalamic–pituitary–adrenal (HPA) activity during physiologic and behavioral stress. In recent animal studies, repeated restraint stress or corticosterone injections induced apical dendritic reorganization by reducing total branch number of pyramidal neurons in the anterior cingulate cortex (ACC) (Radley et al., 2005; Cerqueira et al., 2005a,b). Furthermore, neuroimaging studies demonstrated functional and morphologic abnormalities in ACC of the patients with PTSD. Brain functional studies using positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) showed decreased function in ACC (Bremner et al., 2004; Bremner et al., 1999; Yang et al., 2004; Lanius et al., 2003; Shin et al., 2001), and morphometric MRI studies demonstrated smaller volume of the ACC (Rauch et al., 2003; Yamasue et al., 2003) in comparison to non-PTSD subjects. However, volumetric studies of ACC in PTSD using MRI have had conflicting findings; one study using the conventional manual tracing method found significant volume reduction in pregenual ACC but not in dorsal ACC (Brodmann’s area [BA] 24) (Rauch et al., 2003), and the other using voxel-based analysis found a gray-matter volume reduction in dorsal ACC (Yamasue et al., 2003). Additionally, there was no difference in ACC volume between acute PTSD and healthy subjects but was found shape differences in a recent study (Corbo et al., 2005). However, no studies have examined the effects of early childhood abuse and PTSD on ACC volume. The purpose of the current study was to clarify the structural changes of ACC in subjects with abuse-related PTSD.
2. Method
Magnetic resonance imaging was used to measure ACC volume in 8 subjects with abuse-related PTSD (7 women and 1 man, mean=43 years, S.D.=7) and 13 control subjects without current or past history of PTSD or other psychiatry disorder (12 women and 1 man, mean=37, S.D.=8). The 8 subjects with PTSD met DSM-IV criteria for current PTSD, according to the Clinician Administered PTSD Scale (CAPS; mean score=70, S.D.=37). All of the subjects were recruited in New Heaven, Connecticut, USA. Psychiatric disorder was assessed using the Structured Clinical Interview for DSM-IV (SCID). Five of the patients had a past history of major depression, and two had a current panic disorder or the past history of it, and one subject had a past history of alcohol abuse.
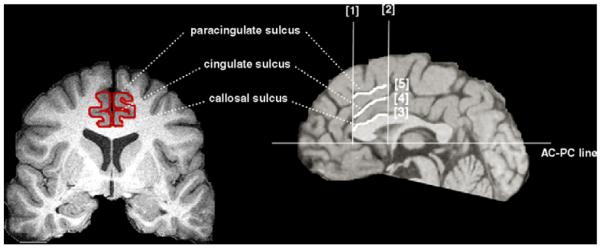
Magnetic resonance images were acquired by using methods described in detail elsewhere (Vermetten et al., 2003). The measurement were performed on all coronal slices which were bounded below (Fig. 1): [1] the most rostral coronal plane containing the genu of the corpus callosum, [2] the most caudal coronal plain containing the clear view of the anterior commissure, [3] the callosal sulcus, [4] the cingulate sulcus, [5] the paracingulate sulcus, referring to the atlas of Talairach and Tournoux (1988), the diagram of Vogt et al. (1995) and the probabilistic map of Paus et al. (1996). The gray and white matter boundaries were distinguished by differential signal intensities in the T1-weighted images.
Fig. 1.
Boundaries for the ACC are illustrated. [1] The most rostral coronal plane containing the genu of the corpus callosum, [2] the most caudal coronal plain containing the anterior comissure, [3] the callosal sulcus, [4] the cingulate sulcus, and [5] the paracingulate sulcus. Within this area of dorsal ACC, volume was measured in the two ways; the one was “Whole ACC” volume which included both Brodmann’s area [BA] 24 and 32 (the area between [3] and [5]), and the other was “Partial ACC” volume which included only BA 24 (between [3] and [4]).
Within the area, we described above (dorsal ACC), volume was measured in the two ways; the one was “Whole ACC” volume which mostly included both BA 24 and 32 (the area between [3] and [5]), and the other was “Partial ACC” volume which mostly included only BA 24 (between [3] and [4]). We have previously reported good inter-rater reliability (ICC=0.67, P <0.05) for measuring ACC (Bremner et al., 2002).
Statistical analysis was performed using one-way analysis of variance (ANOVA), and the threshold was set to P=0.05.
3. Results
As shown in Fig. 2, the absolute volumes of “Whole ACC” volume in patients with PTSD were: Right: 1112.2 ± 183.8; Left: 1116.4 ± 196.5, and in non-PTSD controls: Right: 1376.3 ± 295.4; Left: 1334.6 ± 302.5 (mm3; AVE ± S.D.). The volumes of “Partial ACC” volume in patients with PTSD were: Right: 780.6 ± 150.4; Left: 802.7 ± 226.0, and in non-PTSD controls: Right: 822.1 F170.6; Left: 764.8 ± 119.6 (mm3;AVE±S.D.). According to these results, “Whole ACC” volume on the right side in PTSD patients was significantly smaller (19.2%) than in non-PTSD controls (P <0.05). On the left side, “Whole ACC” volume in PTSD was smaller (16.3%) than in the non-PTSD but was not statistically significant. There was no significance between the volumes of both right and left in “Partial ACC” volumes.
Fig. 2.
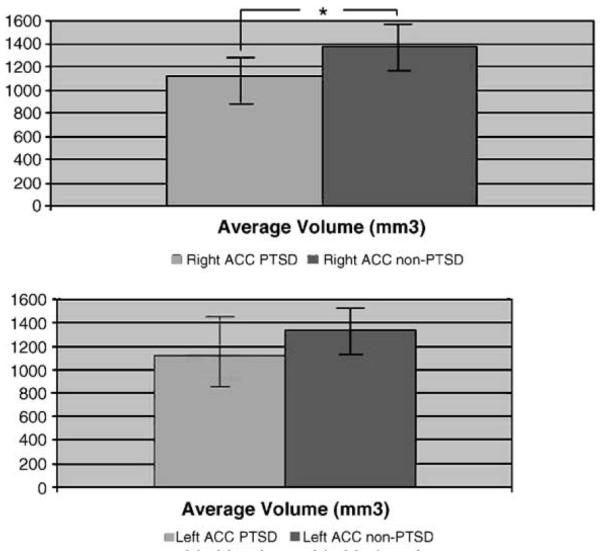
Whole ACC volume (BA 24 and BA 32) in subjects with abuse-related PTSD and non-PTSD. Whole ACC volume was significantly reduced on the right side in PTSD compared with non-PTSD (*P <0.05).
4. Discussion
In the present study, right dorsal ACC volume in PTSD, including both BA 24 and 32, was significantly smaller than in non-PTSD. This area corresponds to the region of decreased function reported for ACC in functional imaging studies (Bremner et al., 2004; Yang et al., 2004; Lanius et al., 2003; Shin et al., 2001). In addition, there was no significant difference in partial ACC volume, including only BA 24 in PTSD in comparison to the non-PTSD controls. This result mostly agrees to a previous report using the conventional manual tracing method, which is similar to ours (Rauch et al., 2003). Furthermore, our findings suggest that volume reduction in BA 32 may be of greater magnitude than in BA 24 in the area of dorsal ACC.
On the other hand, there is considerable variability of pattern and asymmetry in human cingulate and paracingulate sulci (Paus et al., 1996). Future studies are needed to replicate the current findings. In addition, the current study does not address whether the changes are stress-related or are related to a pre-existing difference in brain structure. These questions can be addressed in longitudinal studies.
These results, interpreted in conjunction with other functional and structural imaging studies, suggest that PTSD is associated with altered function and structure of the ACC. Alterations in this brain region may underlie changes in emotion that are a part of PTSD.
References
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biol. Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol. Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Catania C, Sotiropoulos I, Schubert M, Kalisch R, Almeida OF, Auer DP, Sousa N. Corticosteroid status influences the volume of the rat cingulate cortex—a magnetic resonance imaging study. J. Psychiatr. Res. 2005a;39:451–460. doi: 10.1016/j.jpsychires.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J. Neurosci. 2005b;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo V, Clement MH, Armony JL, Pruessner JC, Brunet A. Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biol. Psychiatry. 2005;58:119–124. doi: 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Post-traumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RWJ, Gati JS, Menon RS. Recall of emotional states in posttraumatic stress disorder: An fMRI investigation. Biol. Psychiatry. 2003;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- Paus T, Tomaiuolo F, Otaky N, MacDonald D, Petrides M, Atlas J, Morris R, Evans AC. Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb. Cortex. 1996;6:207–214. doi: 10.1093/cercor/6.2.207. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb. Cortex (Advance Access) 2005 May 18; doi: 10.1093/cercor/bhi104. doi:10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. NeuroReport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol. Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Palnner Stereotaxic Atlas of the Human Brain. Thieme Medical; New York, NY: 1988. [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increase verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry. 2003;5:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Nimchinski EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. PNAS. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci. Lett. 2004;370:13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]