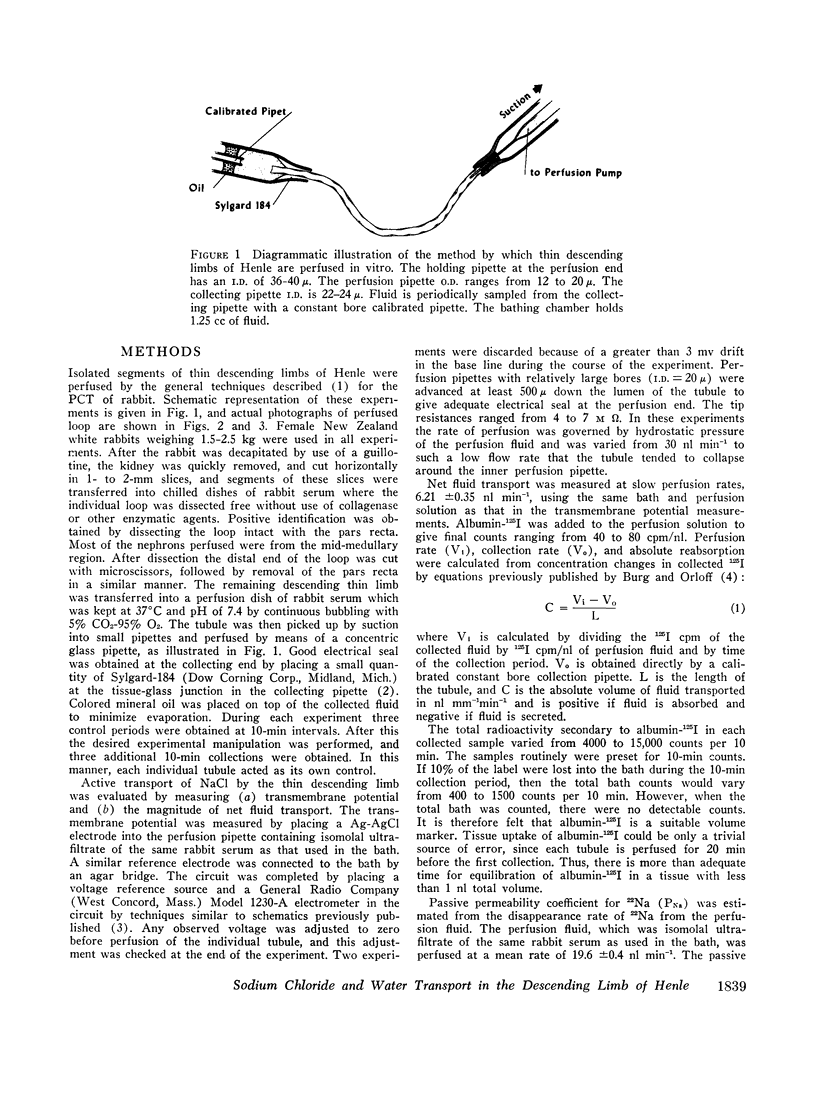
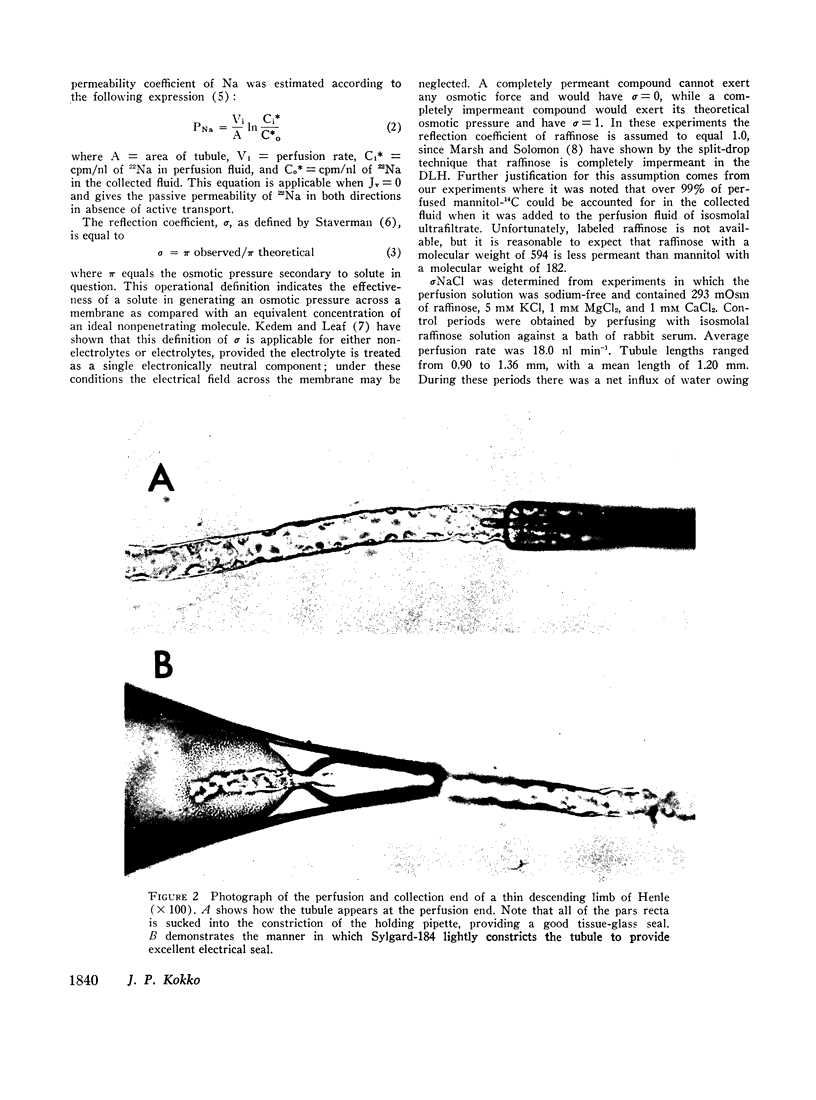
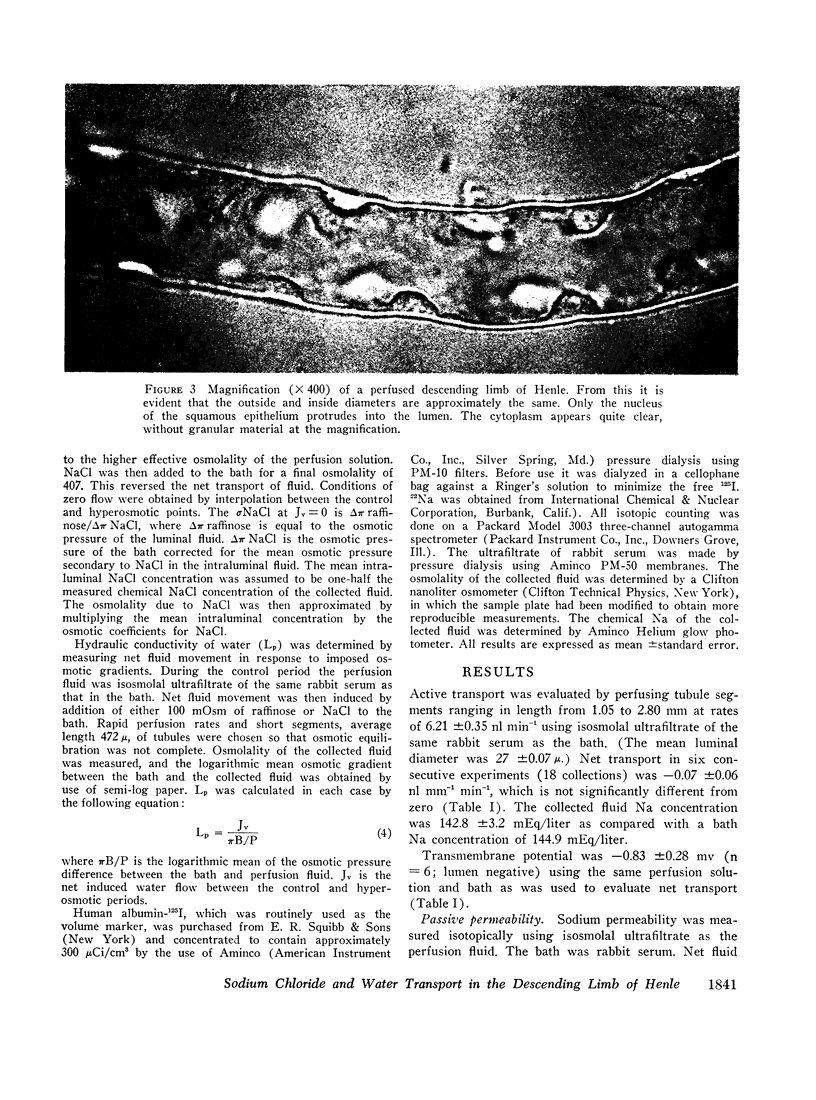
Abstract
The unique membrane characteristics of the thin descending limb of Henle (DLH) play an integral part in the operation of the countercurrent system. We examined these properties in vitro by perfusing isolated thin descending limbs of rabbits. Active transport of NaCl was ruled out by failure to demonstrate either net transport or transmembrane potential difference when perfusing with isosmolal ultrafiltrate of the same rabbit serum as the bath. Transmembrane potential was zero, and net fluid transport was -0.07 ±0.06 nl mm-1 min-1, which also is not significantly different from zero. Passive permeability coefficient for Na(PNa) was determined from the disappearance rate of 22Na from isosmolal perfusion solution. PNa was surprisingly low, 1.61 ±0.27 × 10-5 cm sec-1, a figure which is significantly less than PNa in the proximal convoluted tubule (PCT). Reflection coefficient for NaCl (σNaCl) was measured by perfusing the tubule with Na-free raffinose solution in a bath of rabbit serum to which sufficient NaCl was added to obtain conditions of zero net fluid movement. The measured σNaCl of 0.96 ±0.01 is significantly greater than σNaCl in the PCT. Water permeability to osmotic gradients (Lp) was determined by perfusing with ultrafiltrate of rabbit serum in a bath made hyperosmotic by addition of either 100 mOsm raffinose or NaCl. Lp with raffinose was 1.71 ±0.15 × 10-4 ml cm-2 sec-1 atm-1 and with NaCl 1.62 ±0.05 × 10-4 ml cm-2 sec-1 atm-1, indicating much greater water permeability than in the PCT. In each case the measured increase in osmolality of the collected fluid was primarily due to water efflux without significant influx of solute.
The finding of low permeability to sodium and high permeability to water is consonant with the hypotheses that high interstitial concentration of Na in the medulla generates an effective osmotic pressure which results in concentration of the fluid as it courses through the DLH primarily by abstraction of water without significant net entry of NaCl.
Full text
PDF
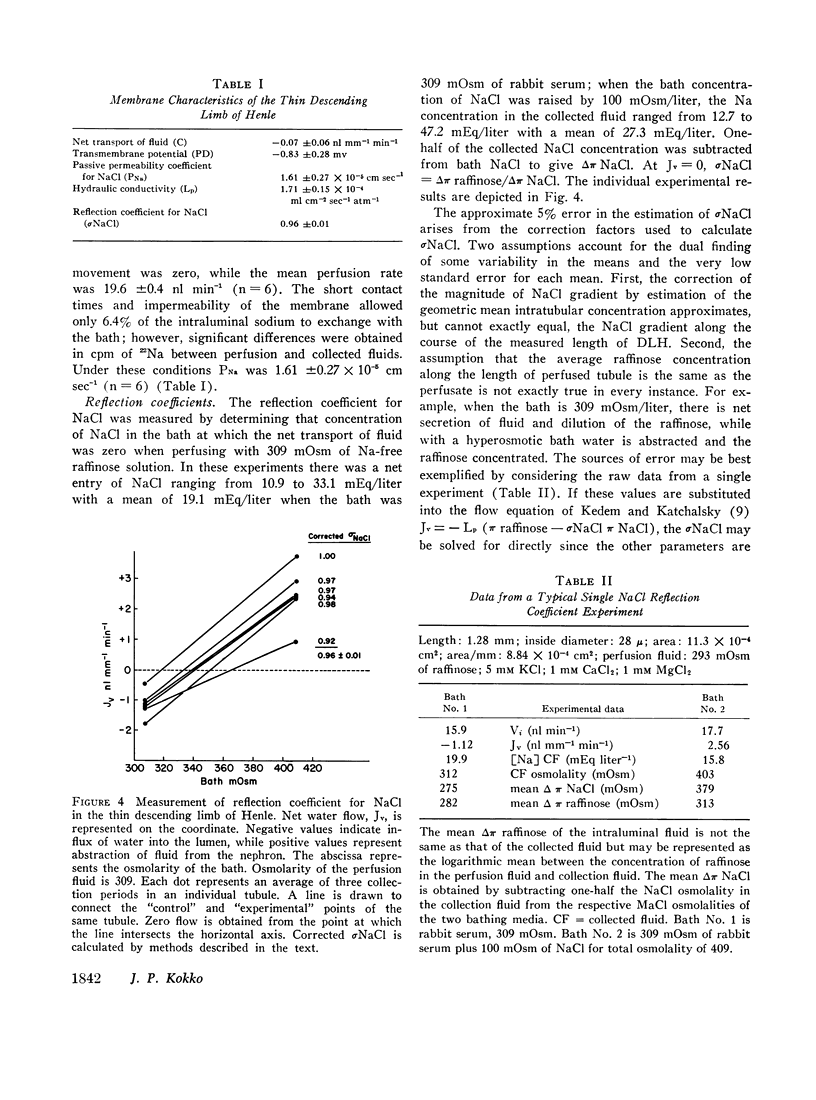




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson H., Costas R., Garcia-Palmieri M. R., Feliberti M., Aixalá R., Blanton J. H., Colón A. A. Coronary heart disease risk factors: a comparison of two puerto rican populations. Am J Public Health Nations Health. 1966 Jul;56(7):1057–1060. doi: 10.2105/ajph.56.7.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B., Issaacson L., Grantham J., Orloff J. Electrical properties of isolated perfused rabbit renal tubules. Am J Physiol. 1968 Oct;215(4):788–794. doi: 10.1152/ajplegacy.1968.215.4.788. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Orloff J. Control of fluid absorption in the renal proximal tubule. J Clin Invest. 1968 Sep;47(9):2016–2024. doi: 10.1172/JCI105888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., LASSITER W. E., MYLLE M., ULLRICH KJ SCHMIDT-NIELSEN B., O'DELL R., PEHLING G. Micropuncture study of composition of loop of Henle fluid in desert rodents. Am J Physiol. 1963 Apr;204:532–535. doi: 10.1152/ajplegacy.1963.204.4.532. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., MYLLE M. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am J Physiol. 1959 Apr;196(4):927–936. doi: 10.1152/ajplegacy.1959.196.4.927. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958 Feb;27(2):229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- Kedem O., Leaf A. The relation between salt and ionic transport coefficients. J Gen Physiol. 1966 Mar;49(4):655–662. doi: 10.1085/jgp.49.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASSITER W. E., GOTTSCHALK C. W., MYLLE M. Micropuncture study of net transtubular movement of water and urea in nondiuretic mammalian kidney. Am J Physiol. 1961 Jun;200:1139–1147. doi: 10.1152/ajplegacy.1961.200.6.1139. [DOI] [PubMed] [Google Scholar]
- MARSH D. J., SOLOMON S. ANALYSIS OF ELECTROLYTE MOVEMENT IN THIN HENLE'S LOOPS OF HAMSTER PAPILLA. Am J Physiol. 1965 Jun;208:1119–1128. doi: 10.1152/ajplegacy.1965.208.6.1119. [DOI] [PubMed] [Google Scholar]
- Marsh D. J. Solute and water flows in thin limbs of Henle's loop in the hamster kidney. Am J Physiol. 1970 Mar;218(3):824–831. doi: 10.1152/ajplegacy.1970.218.3.824. [DOI] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. Permeability of the loop of Henle, vasa recta, and collecting duct to water, urea, and sodium. Am J Physiol. 1968 Jul;215(1):108–115. doi: 10.1152/ajplegacy.1968.215.1.108. [DOI] [PubMed] [Google Scholar]
- ULLRICH K. J., DRENCKHAHN F. O., JARAUSCH K. H. Untersuchungen zum Problem der Harnkonzentrierung und -verdünnung; uber das osmotische Verhalten von Nierenzellen und die begieitende Elektrolytanhäufung im Nierengewebe bei verschiedenen Diuresezuständen. Pflugers Arch. 1955;261(1):62–77. doi: 10.1007/BF00363541. [DOI] [PubMed] [Google Scholar]
- ULLRICH K. J., RUMRICH G., FUCHS G. WASSERPERMEABILITAET UND TRANDTUBULAERER WASSERFLUSS CORTICALER NEPHRONABSCHNITTE BEI VERSCHIEDENEN DIURESEZUSTAENDEN. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jul 1;280:99–119. [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- WIRZ H., HARGITAY B., KUHN W. Lokalisation des Konzentrierungsprozesses in der Niere durch direkte Kryoskopie. Helv Physiol Pharmacol Acta. 1951 Jun;9(2):196–207. [PubMed] [Google Scholar]
- de Rouffignac C., Morel F. Micropuncture study of water, electrolytes, and urea movements along the loops of henle in psammomys. J Clin Invest. 1969 Mar;48(3):474–486. doi: 10.1172/JCI106005. [DOI] [PMC free article] [PubMed] [Google Scholar]