Abstract
Two reverse-prenylated indole alkaloids, deoxybrevianamide E and 6-hydroxydeoxybrevianamide E, are proposed as biosynthetic precursors for advanced metabolites isolated from the marine-derived Aspergillus sp. In order to uncover the role of the alkaloids in the biosynthetic pathway, the feeding experiments of the [13C]2-[15N]-labeled deoxybrevianamide E and 6-hydroxydeoxybrevianamide E were performed to afford the metabolites, which were produced by oxidation and successive pinacol-type rearrangement of the isoprenyl units.
Keywords: Aspergillus sp, biosynthesis, prenylated indole alkaloids
Introduction
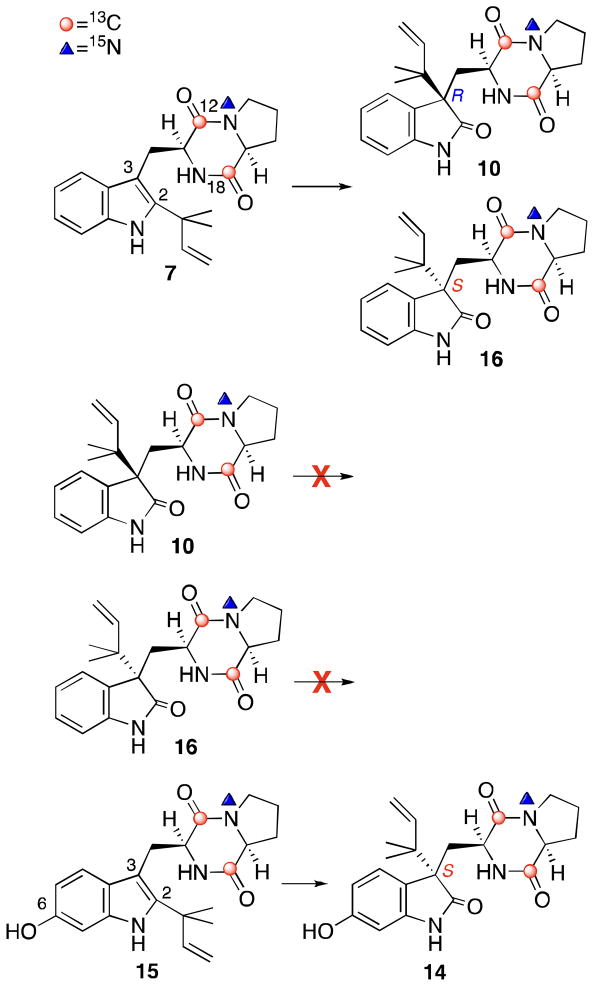
The brevianamides,1 paraherquamides,2 stephacidins,3 malbrancheamides,4 marcfortines,5 asperparalines,6 and notoamides7 are secondary metabolites produced by various genera of fungi, including Aspergillus and Penicillium species and exhibit a wide range of biological activities. These prenylated indole alkaloids are comprised of three different building blocks, tryptophan, a cyclic amino acid, and one or two isoprene units, and show a range of interesting structural features, such as a dioxopiperazine or oxopiperazine within a bicyclo[2.2.2]diazaoctane ring system. Among the families of prenylated indole alkaloids, the notoamides are secondary metabolites produced by the marine-derived Aspergillus sp., which was isolated from the mussle, Mytilus edulis galloprovincialis, collected off the Noto Peninsula in the Sea of Japan. At the beginning of our chemical investigation, four new prenylated indole alkaloids, notoamides A–D (1–4), along with the known alkaloids, sclerotiamide (5), stephacidin A (6), and deoxybrevianamide E (7), were isolated from the fungal culture (Figure 1).7a Based on the structural relationship between these metabolites, we speculated that a possible biosynthetic pathway existed from 7 to 1–6.8 In this biogenesis, we postulated that notoamide E (8) would serve as a key intermediate to other alkaloids, although 8 had not yet been detected in the culture. Closer examination of the fungal metabolites revealed that 8 appears in the culture on day five and is completely consumed by day six.7e This result strongly suggested that the rapid disappearance of 8 was due to the conversion of this metabolite into other downstream metabolites, such as 1–6. Through a feeding experiment of doubly 13C-labeled 8 with the marine-derived Aspergillus sp., we found significant 13C incorporation of 8 into 3 and 4, as well as 3-epi-notoamide C (9) (Figure 1) and three structurally unique metabolites, notoamides E2, E3, and E4.7e Interestingly, 9 and notoamides E2, E3, and E4 were not present in the normal culture of Aspergillus sp. when cultivated on the standard nutrient-rich medium. Surprisingly, the yield of 9 was nearly four times that of 3 in the feeding experiment. This result strongly suggested that the excess amount of the precursor, [13C]2-8, altered the metabolite profile of Aspergillus sp. and resulted in the conversion of 8 into the new alkaloids, 9 and notoamides E2, E3, and E4. Unexpectedly, formation of any labeled bridged bicyclo[2.2.2]diazaoctane-containing alkaloids, such as 1, 2, 5, and 6, was completely halted, although they are typically the major metabolites produced by this fungus. From this result, we proposed an alternative pathway for the formation of the bicyclo[2.2.2]diazaoctane-containing alkaloids, in which the formation of the core bridging bicycle is formed via an intramolecular Diels-Alder (IMDA) reaction, which precedes the construction of the pyranoindole moiety. In this pathway, 7 is first converted to 10 by an R-selective indole oxidase. Oxidation and tautomerization of 10 would yield azadiene intermediate 11, followed by the IMDA reaction to afford the bicyclo[2.2.2]diazaoctane-containing alkaloid 12. Subsequent oxidation and prenylation would afford 2 through its precursor 13 (Figure 2). In order to verify this hypothesis, we decided to perform a feeding experiment of [13C]2-[15N]-7 with the marine-derived Aspergillus sp. Additionally, the isolation of a minor alkaloid, notoamide J (14),7b from the marine-derived Aspergillus sp. strongly attracted our interest toward determining the role of this alkaloid in the biosynthetic pathway of the notoamides. Since 14 contains a hydroxy group at the C-6 position of the indole moiety, 14 is expected to be a precursor of the pyranoindole alkaloids (Figure 3). However, the absolute configuration at the C-3 position of 14 is different from that of the major metabolite 3. Therefore, to determine the biosynthetic role of 14, we performed the feeding experiment of its presumed precursor, [13C]2-[15N]-6-hydroxydeoxybrevianamide E (15).
Figure 1.
Result of the feeding experiment of doubly 13C-labeled 8 with the marine-derived fungus Aspergillus sp.
Figure 2.
Possible biogenesis of the bicyclo[2.2.2]diazaoctane-containing alkaloid 2 from 7.
Figure 3.
Result of the feeding experiment of [13C]2-[15N]-7, 10, 16, and 15.
Results and Discussion
[13C]2-[15N]-Deoxybrevianamide E (7) was synthesized9 and fed to the marine-derived Aspergillus sp. Triply labeled 7 (60 mg, 170μmol) was dissolved in a sterile trace element solution and provided to the fungal culture for 14 days.10 Purification of the fungal metabolites afforded a mixture of [13C]2-[15N]-labeled 10 and 16 (0.34 mg, 0.93μmol) in the ratio of 1:1 (Figure 3). Non-labeled 10 and 16 were previously synthesized11 and the 1H NMR spectra of synthetic non-labeled 10 and 16 and the labeled isolated metabolites matched. Significant 13C incorporation was observed by 13C NMR spectroscopy at C-12 and C-18 of [13C]2-[15N]-labeled 10 (δ173.5 and 166.5) and [13C]2-[15N]-labeled 16 (δ169.8 and 165.4). From analysis of the ESI mass spectrum, incorporation of intact labeled 7 into a mixture of [13C]2-[15N]-labeled 10 and 16 was determined to be 9.7 and 11%, respectively.12 Unexpectedly, no labeled bridged bicyclo[2.2.2]diazaoctane-containing alkaloids such as 12 were obtained. To further examine the downstream metabolites of 10 and 16, synthetic [13C]2-[15N]-labeled 10 and 1613 were fed individually to the Aspergillus sp. culture in the same way described for 7. However, no labeled metabolites were obtained from the putative precursor incorporation studies and the precursors were recovered, which indicated that 10 and 16 were dead-end (shunt) metabolites in the biosynthesis of the notoamides (Figure 3).
[13C]2-[15N]-6-Hydroxydeoxybrevianamide E (15, 57.5 mg, 157μmol) was synthesized14 and fed to the same Aspergillus sp.15 Analysis of the metabolites showed incorporation of triply labeled-15 only into [13C]2-[15N]-notoamide J (14) (0.16 mg, 0.41μmol). 13C-Enrichment was detected at δ165.4 and 169.9, which correspond to the respective C-12 and C-18 positions of 14. Examination of the ESI mass spectrum showed 12.3% incorporation of intact triply labeled 15 into 14. The configuration at the C-3 position was determined to be S on the basis of the CD spectrum, which matched natural 14. The absence of other advanced labeled metabolites in this feeding experiment suggests that 14 is a shunt metabolite in the notoamide biosynthesis.
In conclusion, we performed precursor incorporation experiments of several synthetic [13C]2-[15N]-labeled putative biosynthetic substrates to clarify the biosynthesis of the notoamides in a marine-derived Aspergillus sp. Although we expected to obtain the bridged bicyclo[2.2.2]diazaoctane-containing alkaloids such as 12 or 2 (Figure 2), the feeding experiments of the labeled-7, 10, 16, or 15 did not afford any labeled alkaloids containing the core bridging bicycle (Figure 3). [13C]2-[15N]-Deoxybrevianamide E (7) incorporated into labeled 10 and 16 in a 1:1 ratio. On the other hand, [13C]2-[15N]-6-hydroxydeoxybrevianamide E (15) incorporated into labeled notoamide J (14). Among the prenylated indole alkaloids isolated from the marine-derived Aspergillus sp. that contain the dioxopiperazine ring, notoamides C (3), M,7d and Q7f display the 3R-configuration, while only 14 contains the 3S-configuration. Since 3 is a major alkaloid isolated from the fungal culture, the results of the present feeding experiments of the labeled-7 and 15 were unexpected. The difference of the metabolism between 7 and 15 would be due to the presence of the hydroxy group at the indole ring in 15, and the hydroxy group may play an important role in the pinacol-type rearrangement of the reverse-prenyl group from C-2 to C-3. Surprisingly, three antipodal metabolites containing the bridged bicyclo[2.2.2]diazaoctane ring, (+)-notoamide B ((+)-2), (−)-stephacidin A ((−)-6), and (+)-versicolamide B ((+)-17) were isolated from the closely related terrestrial-derived Aspergillus versicolor (Figure 4), and (+)-2 and (+)-17 possess 3S configuration, which is opposite to those of (−)-2 and (−)-17 isolated from the marine-derived Aspergillus sp.7c It suggests that the terrestrial-derived Aspergillus versicolor contains the metabolic system in which the biosynthesis of the prenylated indole alkaloids proceeds in a parallel yet enantiomeric series of alkaloids relative to the marine-derived Aspergillus sp.16 We recently found that the precursor incorporation experiment of labeled 15 in the terrestrial-derived Aspergillus versicolor afforded 14 with the 3S configuration,14 which is the same result obtained from the marine-derived Aspergillus sp. reported in this paper. Efforts to further elucidate the biosynthetic pathway of the notoamides is currently underway in our laboratory.
Figure 4.
Structures of three pairs of antipodal alkaloids isolated from the marine-derived Aspergillus sp. and the terrestrial-derived Aspergillus versicolor.
Supplementary Material
Acknowledgments
This work was financially supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 23108518 to ST), and by a grant from the Nagase Science and Technology Foundation (to ST), and by the National Institutes of Health (CA70375 to RMW).
Footnotes
Supplementary data (experimental details for the synthesis of isotopically labeled [13C]2-[15N]-deoxybrevianamide E (7) and labeled substrates 10 and 16) associated with this article can be found, in the online version, at doi: 10.1016/j.tetlet.2011.xx.xxx.
References and notes
- 1.(a) Birch AJ, Wright JJ. Tetrahedron. 1970;26:2329. doi: 10.1016/s0040-4020(01)92812-1. [DOI] [PubMed] [Google Scholar]; (b) Birch AJ, Wright JJS. J Chem Soc, Chem Commun. 1969:644–645. [Google Scholar]; (c) Birch AJ, Russell RA. Tetrahedron. 1972;28:2999. [Google Scholar]
- 2.(a) Yamazaki M, Okuyama E, Kobayashi M, Inoue H. Tetrahedron Lett. 1981;22:135–136. [Google Scholar]; (b) Ondeyka JG, Goegelman RT, Schaeffer JM, Kelemen L, Zitano L. J Antibiot. 1990;43:1375–1379. doi: 10.7164/antibiotics.43.1375. [DOI] [PubMed] [Google Scholar]; (c) Liesch JM, Wichmann CF. J Antibiot. 1990;43:1380–1386. doi: 10.7164/antibiotics.43.1380. [DOI] [PubMed] [Google Scholar]; (d) Banks RM, Blanchflower SE, Everett JR, Manger BR, Reading C. J Antibiot. 1997;50:840–846. doi: 10.7164/antibiotics.50.840. [DOI] [PubMed] [Google Scholar]
- 3.Qian-Cutrong J, Huang S, Shu Y-Z, Vyas D, Fairchild C, Menendez A, Krampitz K, Dalterio R, Klohr SE, Gao Q. J Am Chem Soc. 2002;124:14556–14557. doi: 10.1021/ja028538n. [DOI] [PubMed] [Google Scholar]
- 4.(a) Martinez-Luis S, Rodriguez R, Acevedo L, Gonzalez MC, Lira-Rocha A, Mata R. Tetrahedron. 2006;62:1817–1822. [Google Scholar]; (b) Figueroa M, Del Carmen González M, Mata R. Nat Prod Res. 2008;22:709–714. doi: 10.1080/14786410802012361. [DOI] [PubMed] [Google Scholar]
- 5.(a) Polonsky J, Merrien MA, Prange T, Pascard C. J Chem Soc, Chem Commun. 1980:601–602. [Google Scholar]; (b) Prange T, Billion M-A, Vuilhorgne M, Pascard C, Polonsky J. Tetrahedron Lett. 1981;22:1977–1980. [Google Scholar]
- 6.(a) Hayashi H, Nishimoto Y, Nozaki H. Tetrahedron Lett. 1997;38:5655–5658. [Google Scholar]; (b) Banks RM, Blanchflower SE, Everett JR, Manger BR, Reading C. J Antibiot. 1997;50:840–846. doi: 10.7164/antibiotics.50.840. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew Chem Int Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]; (b) Tsukamoto S, Kato H, Samizo M, Nojiri Y, Onuki H, Hirota H, Ohta T. J Nat Prod. 2008;71:2064–2067. doi: 10.1021/np800471y. [DOI] [PubMed] [Google Scholar]; (c) Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Angew Chem Int Ed. 2008;47:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tsukamoto S, Kawabata T, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. Org Lett. 2009;11:1297–1300. doi: 10.1021/ol900071c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Tsukamoto S, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. J Am Chem Soc. 2009;131:3834–3835. doi: 10.1021/ja810029b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Tsukamoto S, Umaoka H, Yoshikawa K, Ikeda T, Hirota H. J Nat Prod. 2010;73:1438–1440. doi: 10.1021/np1002498. [DOI] [PubMed] [Google Scholar]
- 8.(a) Grubbs AW, Artman GD, III, Tsukamoto S, Williams RM. Angew Chem, Int Ed. 2007;46:2257–2261. doi: 10.1002/anie.200604377. [DOI] [PubMed] [Google Scholar]; (b) Greshock TJ, Grubbs AW, Tsukamoto S, Williams RM. Angew Chem, Int Ed. 2007;46:2262–2265. doi: 10.1002/anie.200604378. [DOI] [PubMed] [Google Scholar]
- 9.For the preparation of [13C]2-[15N]-Deoxybrevianamide E (7) see supporting information.
- 10.Culture of Aspergillus sp. was maintained on agar plates (20 g malt extract, 5 g peptone and 20 g agar per liter of 50% seawater) in an incubator at 25°C. The spores from the agar plates was cultured in two 500 mL flasks containing a medium (100 mL × 8) composed of 50% seawater with 2.0% malt extract and 0.5% peptone at 27°C for 7 days. The mycelial cells were filtered by a cheese cross and rinsed with 100 mL of sterile water. [13C]2-[15N]-Deoxybrevianamide E (7) (60 mg, 170μmol) was dissolved in acetone (1 mL) containing Tween 80 (0.2 mL), and the sample was dried up. The precursor/Tween 80 residue was added to three 500 mL flasks containing the sterile trace element solution (150 mL × 12) composed of 35 mM NaNO3, 5.7 mM K2HPO4, 4.2 mM MgSO4, 1.3 mM KCl, 36 μM FeSO4·7H2O, 25 μM MnSO4·H2O, 7 μM ZnSO4·7H2O and 1.5 μM CuCl2·2H2O, and the mixtures were sonicated for 15 minutes to aid in micelle formation. The fungal cells were added to the media and incubated at 27°C for 14 days, and the flasks were swirled daily to ensure even distribution of the precursor. The culture broth was extracted with n-BuOH, and the fungal cells were extracted with MeOH. The both solutions were combined and evaporated. The extract was partitioned between n-hexane and 90% MeOH-H2O. The latter fraction was evaporated, and the residue was purified by ODS column chromatography with 30, 70, and 90% MeOH-H2O and MeOH. The fractions eluted with 70 and 90% MeOH-H2O were combined and purified by ODS HPLC with 65% MeOH-H2O to afford a mixture of [13C]2-[15N]-10 and 16 (0.34 mg, 0.93 μmol). 10: 1H NMR (acetone-d6) δ: 7.27 (d, J = 7.8 Hz, 1H), 7.11 (dt, J = 7.8, 1.4 Hz, 1H), 6.76 (dt, J = 7.8, 1.4 Hz, 1H), 6.75 (d, J = 7.8 Hz, 1H), 6.48 (s, 1H), 6.33 (dd, J = 17.4, 10.6 Hz, 1H), 4.94 (dd, J = 17.4, 1.4 Hz, 1H), 4.88 (dd, J = 10.6, 1.4 Hz, 1H), 4.04 (m, 1H), 3.76 (m, 1H), 3.47 (m, 1H), 3.37 (m, 1H), 2.78 (dd, J = 13.3, 5.0 Hz, 1H), 2.54 (dd, J = 13.3, 7.3 Hz, 1H), 2.20 (m, 1H), 1.90-1.80 (m, 2H), 1.27 (s, 3H), 1.25 (s, 3H). 13C NMR (acetone-d6) δ: 173.5 and 166.5 (d). ESIMS m/z 371 [M + H]+. 16: 1H NMR (acetone-d6) δ: 7.16 (d, J = 6.9 Hz, 1H), 7.00 (dt, J = 7.8, 1.4 Hz, 1H), 6.65 (dt, J = 7.3, 0.9 Hz, 1H), 6.61 (d, J = 7.8 Hz, 1H), 6.53 (s, 1H), 6.51 (dd, J = 17.4, 10.6 Hz, 1H), 4.94 (dd, J = 17.4, 1.8 Hz, 1H), 5.01 (dd, J = 10.6, 1.8 Hz, 1H), 4.39 (dt, J = 8.3, 2.3 Hz, 1H), 4.16 (dt, J = 7.8, 2.8 Hz, 1H), 3.40 (ttt, J = 13.3, 6.0, 2.8 Hz, 1H), 3.16 (ttt, J = 12.4, 7.8, 4.6 Hz, 1H), 2.96 (dt, J = 11.4, 7.3 Hz, 1H), 2.62 (ttt, J = 13.3, 7.0, 5.5 Hz, 1H), 2.01 (m, 1H), 1.95 (m, 1H), 1.72 (m, 1H), 1.60 (m, 1H), 1.39 (s, 3H), 1.35 (s, 3H). 13C NMR (acetone-d6) δ: 169.8 and 165.4 (d). ESIMS m/z 371 [M + H]+.
- 11.For the preparation of 10 and 16 see supporting information.
- 12.The percentage of 13C-enrichment in 10 and 16 from [13C]2-[15N]-7 was calculated according to the method outlined in: Lambert JB, Shurvell HB, Lightner DA, Cooks RG. Organic Structural Spectroscopy. Prentice Hall: Upper Saddle River, NJ; 1998. pp. 447–448.These calculations are based on the comparison of the mass spectrum of the labeled material to the mass spectrum of the unlabeled material.
- 13.For the preparation of [13C]2-[15N]-10 and 16 see supporting information.
- 14.Finefield JM, Sherman DH, Tsukamoto S, Williams RM. J Org Chem. 2011;76:5954–5958. doi: 10.1021/jo200218a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The feeding experiment of [13C]2-[15N]-6-hydroxydeoxybrevianamide E (15) (57.5 mg, 149μmol) with Aspergillus sp. was performed in the same way described for [13C]2-[15N]-deoxybrevianamide E (7)10 to afford [13C]2-[15N]-notoamide J (14). CD(MeOH) [θ]219 −6997, [θ]231 0, [θ]239 +3778, [θ]253 0, [θ]267 −1396, [θ]325 0. 1H NMR (acetone-d6) δ 9.59 (br s, 1H), 7.00 (d, J = 8.2 Hz, 1H), 6.49 (d, J = 8.2, 1.8 Hz, 1H), 6.47 (d, J = 1.8 Hz, 1H), 6.28 (br s, 1H), 6.11 (dd, J = 17.4, 11.0 Hz, 1H), 5.08 (dd, J = 11.0, 1.3 Hz, 1H), 5.01 (dd, J = 17.4, 1.3 Hz, 1H), 3.98 (m, 1H), 3.32 – 3.44 (m, 2H), 3.26 (m, 1H), 3.10 (m, 1H), 1.77 – 1.94 (m, 3H), 1.10 (s, 3H), 1.05 (s, 3H). 13C NMR (acetone-d6) δ: 169.9 and 165.4 (d); ESIMS m/z 385 [M - H]−.
- 16.Miller KA, Tsukamoto S, Williams RM. Nat Chem. 2009;1:63–68. doi: 10.1038/nchem.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.