Abstract
Notoamide E, a short-lived secondary metabolite, has been proposed as a biosynthetic intermediate to several advanced metabolites isolated from Aspergillus versicolor. In order to verify the role of this indole alkaloid along the biosynthetic pathway, synthetic doubly 13C-labeled notoamide E was fed to Aspergillus versicolor. Analysis of the metabolites showed significant incorporation of notoamide E into the natural products notoamides C and D.
Keywords: Aspergillus versicolor, biosynthesis, prenylated indole alkaloid
Introduction
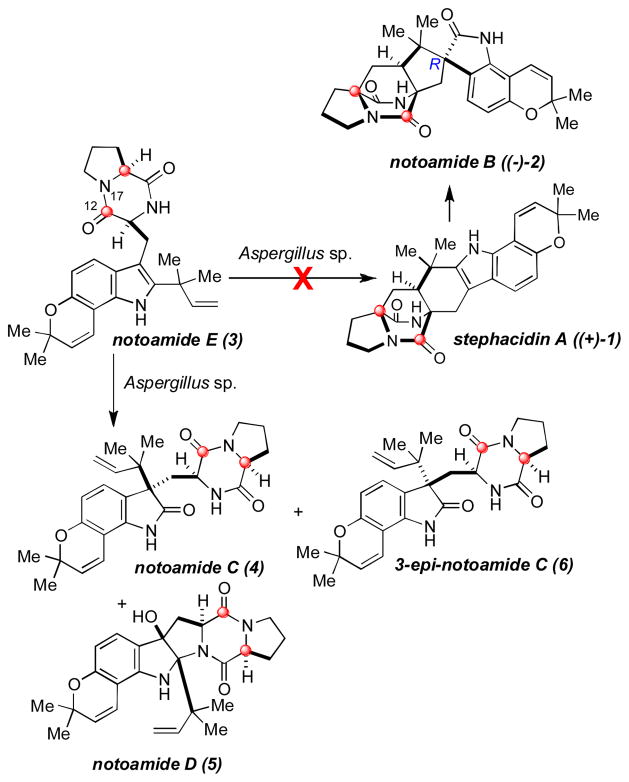
Prenylated indole alkaloids containing either a diketopiperazine ring or a core bicyclo[2.2.2]diazaoctane ring system constitute a unique and well-known family of natural products. These secondary metabolites, specifically the brevianamides,1 paraherquamides,2 stephacidins,3 and notoamides,4,5 are produced by various genera of fungi, mainly Aspergillus and Penicillium spp., and exhibit a wide range of biological activity. Biosynthetically, these structurally complex molecules are derived from tryptophan, a cyclic amino acid such as proline, β-methyl-proline, or pipecolic acid, and one or two isoprene residues. These structural features have made this family of natural products attractive from not only a synthetic perspective,6 but also a biosynthetic standpoint.7 Recently, Tsukamoto and coworkers isolated several secondary metabolites from a marine-derived Aspergillus sp. that hinted at a possible biosynthetic pathway leading from deoxybrevianamide E to (+)-stephacidin A (1), and eventually to (−)-notoamide B (2). Among these metabolites was a new alkaloid, notoamide E (3), which is a short-lived natural metabolite. Notoamide E was found to appear in the culture on day five and completely consumed by day six. It was postulated that the rapid disappearance of 3 was due to the conversion of this metabolite into other downstream metabolites, such as 1, 2, 4, and 5, among others (Figure 1).5,6
Figure 1.
Result of marine Aspergillus sp. feeding experiment with 13C-labeled 3.
We immediately directed our attention to determining the exact role 3 played in the biosynthetic pathway of these unique secondary metabolites. Initially, we were expecting to see the conversion of 3 into (+)-1, (−)-2 and other notoamides.
In order to address these questions, notoamide E was synthesized in doubly 13C-labeled form and fed to the marine Aspergillus sp. Unexpectedly, formation of labeled or unlabeled (+)-1 and (−)-2, as well as all other bridged bicyclo[2.2.2]diazaoctane-containing compounds, were not observed or detected although they are typically the major metabolites produced by this fungus. Furthermore, notoamide C (4) and notoamide D (5) were isolated and found to contain 13C-enrichment at the expected C-12 and C-17 positions. Tsukamoto also reported the isolation of 3-epi-notoamide C (6), a diastereomer to 4 that is not produced by the culture under normal growth conditions. While 6 has not been isolated in the normal medium of either Aspergillus sp. or Aspergillus versicolor, Tsukamoto observed that the yield of 6 (1.26 mg) was nearly four times that of 4 (0.33 mg) in the feeding experiment of notoamide E with Aspergillus sp.5 Three new structurally unique minor metabolites displaying 13C-enrichment were also isolated. These new alkaloids, notoamides E2, E3, and E4,5 are not present in the normal culture of Aspergillus sp. when cultivated on the standard nutrient-rich medium. The lack of formation of (+)-1 and (−)-2, as well as the production of new metabolites, suggest that the presence of excess 3 in the growth medium alters the metabolite profile of this marine-derived Aspergillus sp., and may interrupt the biosynthesis of the major metabolites.5
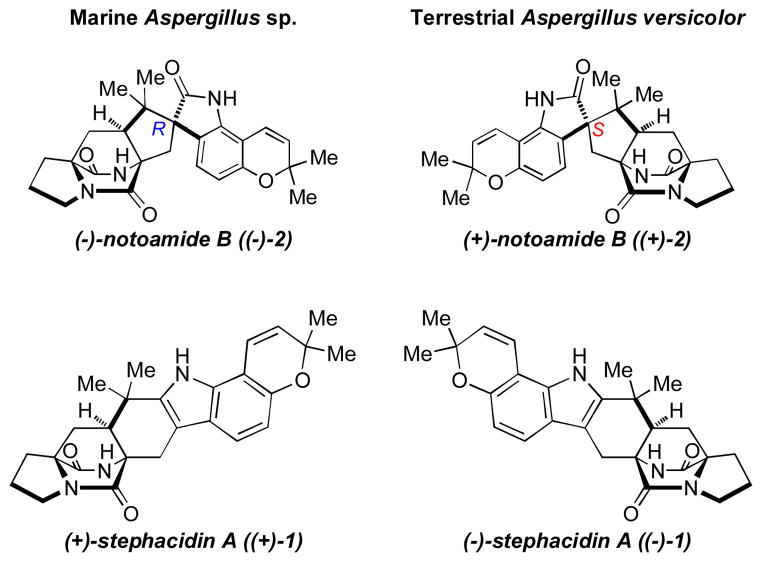
In separate work, Gloer and coworkers isolated (−)-stephacidin A (1), (+)-notoamide B (2) and (+)-versicolamide B from a terrestrial fungus, Aspergillus versicolor.4c These samples possess the opposite absolute configuration to those metabolites previously isolated from both Aspergillus ochraceus WC76466 and the marine-derived Aspergillus sp., and they also represent the isolation of the first set of antipodal stereoisomers within the paraherquamide-stephacidin family (Figure 2).3,4 The isolation of these enantiomeric natural metabolites from an Aspergillus fungus has sparked further interest into elucidating the biosynthetic pathway for the formation of the bicyclo[2.2.2]diazaoctane ring system, which has been strongly implicated as being responsible for the enantio-diverging event.
Figure 2.
Structures of antipodal natural metabolites isolated from marine-derived Aspergillus sp. and terrestrial-derived Aspergillus versicolor.
With the discovery of these antipodal metabolites from different Aspergillus species, questions arose as to whether these unique natural products are biosynthesized along a similar, if not identical, biosynthetic pathway. Although 3 has not yet been isolated from the natural Aspergillus versicolor culture, we reasoned that if the biosynthetic pathways were substantially the same in both species, then Aspergillus versicolor would also incorporate labeled 3 into 4, 5, and 6. Furthermore, based on Tsukamoto’s results,4d we also expected to see the lack of incorporation of 3 into any compounds containing the bridged bicyclo[2.2.2]diazaoctane core, such as (−)-1 and (+)-2.
Materials and Methods
A culture of Aspergillus versicolor NRRL 35600 was obtained from the Department of Agriculture in Peoria, IL. This culture was transferred to malt extract agar slants and allowed to incubate for 14 days. Potato Dextrose Broth was prepared by dissolving 48 g of the medium and 6 g tryptose in 2L of doubly distilled H2O (DDH2O). The solution was heated to aid in dissolving the medium, which was then transferred to fernbach flasks (4 × 500 mL) and autoclaved. Spores of A. versicolor were added to the broth from the agar slants. The fernbach flasks were covered and gently placed in the incubator for 14 days. Doubly 13C-labeled notoamide E (42 mg, 0.096 mmol), the synthesis of which we have recently reported,5,6 was dissolved in 1 mL acetone with 0.2 mL TWEEN 80. The solvent was dried and the residue was dissolved in 300 mL of a sterile trace element solution (35 mM NaNO3, 5.7 mM K2HPO4, 4.2 mM MgSO4·7H2O, 1.3 mM KCl, 36 mM FeSO4·7H2O, 25 mM MnSO4·H2O, 7 mM ZnSO4·7H2O, and 1.5 mM CuCl2·2H2O). The fungal broth was decanted and the fungal cells were washed with 100 mL sterile DDH2O. The precursor/trace element solution (150 mL) was added to each flask using a syringe and needle. The fungal cells were incubated at 25°C for 14 days and each flask was swirled daily to ensure even distribution of the labeled compound. The trace element solution was decanted, and the fungus was pureed in a blender with 1:1 MeOH-CHCl3. The puree was transferred to a 2 L Erlenmeyer flask, diluted to 1.2 L with 1:1 MeOH-CHCl3, and placed in the shaker for 24 hours. Celite (30 g) was added to the flask and allowed to shake for an extra 10 minutes. The suspension was filtered through Whatman #2 paper and the filtrate was stored at 4°C. The mycelia “cake” was diluted with 600 mL 1:1 MeOH-CHCl3 and placed on the shaker for an additional 48 hours. The suspension was filtered through Whatman #2 paper, and the combined filtrates were concentrated under vacuum. The residue was dissolved in 250 mL H2O and extracted with EtOAc (3 × 300 mL). The organic layer was concentrated and partitioned between MeCN and hexanes. The layers were separated, and the MeCN layer was concentrated under vacuum.4c,5,8 The crude material was purified via silica gel flash column chromatography (1% MeOH in DCM – 3% MeOH in DCM) to afford three fractions that were each analyzed by 13C NMR spectroscopy. Enrichment of 13C was found in the latter two fractions, which were further purified via preparative thin layer chromatography (1000 mm, 3% MeOH in DCM x5) to yield 13C-labeled notoamide C (8.1 mg) and notoamide D (11.2 mg).
Results and Discussion
Significant 13C incorporation was observed by 13C NMR spectroscopy at C-12 and C-17 of notoamides C and D. From analysis of the electrospray mass spectra, incorporation of intact doubly 13C-labeled 3 into 4 was determined to be 6.2%, while incorporation into 5 was 6.0%.9,10 Interestingly, upon closer inspection, trace amounts of doubly 13C-labeled 3-epi-notoamide C (6), unlabeled (−)-1, and unlabeled (+)-2 were also detected by LC-MS (Table 1). In contrast to the metabolite profile observed by Tsukamoto and co-workers, only trace amounts of 6 were detected and therefore we were unable to reliably calculate the percentage of intact incorporation. As mentioned above, Tsukamoto observed a complete lack of production of any compounds containing the bicyclo[2.2.2]diazaoctane core, and while (−)-1 and (+)-2 are usually the major metabolites in the normal medium of Aspergillus versicolor, we did isolate trace amounts of these unlabeled compounds (Table 1).4c,5
Table 1.
Intact incorporation of double 13C-labeled Notoamide E (3) into isolated metabolites.
| Metabolite | % specific incorporation | Notes |
|---|---|---|
| notoamide C | 6.2% | 8.1 mg |
| 3-epi-notoamide C | NDa | trace isolated |
| notoamide D | 6.0% | 11.2 mg |
| stephacidin A | 0% | trace isolated |
| notoamide B | 0% | trace isolated |
Not enough material to adequately calculate the % incorporation
In summary, we have shown that the proposed precursor, notoamide E, incorporates into three minor secondary metabolites, 4, 5, and 6 in Aspergillus versicolor. We observed that excess 3 in cultures of Aspergillus versicolor resulted in the production of only trace amounts of the metabolites, (−)-stephacidin A and (+)-notoamide B. The results observed from the feeding experiments of double 13C-labeled 3 with both the Tsukamoto marine-derived Aspergillus sp. and Aspergillus versicolor pose some interesting questions about the biosynthetic pathway of the major metabolites produced by these organisms. During the feeding experiment of 3 with Aspergillus versicolor, we observed that 6 was only produced in trace amounts, unlike the incorporation study with Aspergillus sp. where the yield of 6 was more than 4. This could be a distinguishing difference between the two Aspergillus species. We have also observed an inversion in the amounts of the major and minor metabolites that are normally produced by Aspergillus versicolor when notoamide E was provided to the culture medium. When grown on normal media, notoamides C and D are produced in trace amounts by Aspergillus versicolor. In contrast, when labeled notoamide E was added to the culture media, these substances were isolated as the major metabolites. These results suggests that the addition of excess 3 does not abrogate the oxidative transformations of notoamide E into notoamides C, D and epi-notoamide C. On the other hand, the suppression of formation of stephacidin A and notoamide B observed in the presence of added notoamide E, suggests that these compounds inhibit or divert the enzymatic machinery responsible for the production of the bicyclo[2.2.2]diazaoctane-containing metabolites, especially stephacidin A and notoamide B. Furthermore, results from both sets of feeding studies establish that notoamide E is not a biosynthetic substrate for the putative oxidase that is postulated to mediate the intramolecular Diels-Alder cycloaddition, as evidenced by the lack of incorporation of 3 into any bicyclo[2.2.2]diazaoctane-containing natural products. Notoamide E is, on the other hand, firmly established as a biosynthetic precursor to notoamides C and D. These findings therefore suggest the presence of a branch point in the biosynthetic pathway, just prior to the formation of notoamide E. The details of this putative inhibitory process are currently under investigation as well as efforts to identify the key branch point that diverts to the biosynthetic precursor to stephacidin A, and metabolites derived from stephacidin A (such as notoamide B, stephacidin B, among others) which currently remains enigmatic.
Acknowledgments
We are grateful to the National Institutes of Health (CA70375 to RMW) as well as a grant from the Naito Foundation (to S.T.) for financial support.
Footnotes
Supporting Information Available. Calculations of percent incorporations reported in this study.
References and Notes
- 1.(a) Birch AJ, Wright JJ. Tetrahedron. 1970;26:2329. doi: 10.1016/s0040-4020(01)92812-1. [DOI] [PubMed] [Google Scholar]; (b) Birch AJ, Wright JJS. J Chem Soc, Chem Commun. 1969:644–645. [Google Scholar]; (c) Birch AJ, Russell RA. Tetrahedron. 1972;28:2999. [Google Scholar]
- 2.(a) Yamazaki M, Okuyama E, Kobayashi M, Inoue H. Tetrahedron Lett. 1981;22:135–136. [Google Scholar]; (b) Ondeyka JG, Goegelman RT, Schaeffer JM, Kelemen L, Zitano L. J Antibiot. 1990;43:1375–1379. doi: 10.7164/antibiotics.43.1375. [DOI] [PubMed] [Google Scholar]; (c) Liesch JM, Wichmann CF. J Antibiot. 1990;43:1380–1386. doi: 10.7164/antibiotics.43.1380. [DOI] [PubMed] [Google Scholar]; (d) Banks RM, Blanchflower SE, Everett JR, Manger BR, Reading C. J Antibiot. 1997;50:840–846. doi: 10.7164/antibiotics.50.840. [DOI] [PubMed] [Google Scholar]
- 3.Qian-Cutrone J, Huang S, Shu Y-Z, Vyas D, Fairchild C, Menendez A, Krampitz K, Dalterio R, Klohr SE, Gao Q. J Am Chem Soc. 2002;124:14556–14557. doi: 10.1021/ja028538n. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew Chem Int Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]; (b) Tsukamoto S, Kato H, Samizo M, Nojiri Y, Onuki H, Hirota H, Ohta T. J Nat Prod. 2008;71:2064–2067. doi: 10.1021/np800471y. [DOI] [PubMed] [Google Scholar]; (c) Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Angew Chem Int Ed. 2008;47:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tsukamoto S, Kawabata T, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. Org Lett. 2009;11:1297–1300. doi: 10.1021/ol900071c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Tsukamoto S, Umaoka H, Yoshikawa K, Ikeda T, Hirota H. J Nat Prod. 2010;73:1438–1440. doi: 10.1021/np1002498. [DOI] [PubMed] [Google Scholar]
- 5.Tsukamoto S, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. J Am Chem Soc. 2009;131:3834–3835. doi: 10.1021/ja810029b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Grubbs AW, Artman GD, III, Tsukamoto S, Williams RM. Angew Chem, Int Ed. 2007;46:2257–2261. doi: 10.1002/anie.200604377. [DOI] [PubMed] [Google Scholar]; (b) Greshock TJ, Grubbs AW, Tsukamoto S, Williams RM. Angew Chem, Int Ed. 2007;46:2262–2265. doi: 10.1002/anie.200604378. [DOI] [PubMed] [Google Scholar]
- 7.(a) Williams RM. Chem Pharm Bull. 2002;50:711–740. doi: 10.1248/cpb.50.711. [DOI] [PubMed] [Google Scholar]; (b) Williams RM, Cox RJ. Acc Chem Res. 2003;36:127–139. doi: 10.1021/ar020229e. [DOI] [PubMed] [Google Scholar]; (c) Stocking EM, Sanz-Cervera JF, Williams RM. Angew Chem. 2001;113:1336–1338. doi: 10.1002/1521-3773(20010401)40:7<1296::aid-anie1296>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2001;40:1296–1298. doi: 10.1002/1521-3773(20010401)40:7<1296::aid-anie1296>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.(a) Stocking EM, Williams RM, Sanz-Cervera JF. J Am Chem Soc. 2000;122:9089–9098. [Google Scholar]; (b) Stocking EM, Sanz-Cervera JF, Unkefer CJ, Williams RM. Tetrahedron. 2001;57:5303–5302. [Google Scholar]
- 9.(a) Stocking EM, Sanz-Cervera JF, Williams RM. Angew Chem Int Ed. 2001;40:1296–1298. doi: 10.1002/1521-3773(20010401)40:7<1296::aid-anie1296>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]; (b) Ding Y, Greshock TJ, Miller KA, Sherman DH, Williams RM. Org Lett. 2008;10:4863–4866. doi: 10.1021/ol8019633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calculated according to the method outlined in: Lambert JB, Shurvell HB, Lightner DA, Cooks RG. Organic Structural Spectroscopy. Prentice Hall; Upper Saddle River, NJ: 1998. pp. 447–448.