Abstract
Rho kinase (ROCK) proteins are Rho-GTPase activated serine/threonine kinases that function as modulators of actin-myosin cytoskeletal dynamics via regulation of Lin11, Isl-1 & Mec-3 domain (LIM) kinase, myosin light chain (MLC), and MLC phosphatase. A strong correlation between cytoskeletal rearrangements and tumor cell invasion, metastasis, and deregulated microenvironment interaction has been reported in the literature, and the utilization of pharmacological inhibitors of ROCK signaling for the treatment of cancer is actively being pursued by a number of pharmaceutical companies. Indeed, in many preclinical models ROCK inhibitors have shown remarkable efficacy in reducing tumor growth and metastasis. Interestingly, ROCK signaling has been shown to be either pro-apoptotic or pro-survival in a cell type and context dependent manner, though the molecular mechanisms controlling ROCK-mediated cell fate decisions are unknown. This review summarizes the many pleiotropic roles of ROCK signaling in survival and apoptosis, and suggests that controlled modulation of ROCK activity in tumor cells has the potential to significantly affect tumor survival and patient outcome.
Keywords: ROCK, Rho kinase, ROCK signaling, ROCK pathways, review
ROCK PROTEINS
Rho associated protein kinases (ROCK, also known as Rho kinase) belong to a family of serine/threonine kinases modulated by interactions with Rho GTPases to serve as key regulators of actin cytoskeletal dynamics, and therefore control cell migration and motility(1). Specifically, ROCK proteins promote the formation of stress fibers and focal adhesions (Figure 1), but have also been implicated in diverse processes such as cell junction integrity and cell cycle control(2). ROCK activity is responsible for stabilization of actin microfilaments as well as promoting cellular contraction and cell substratum contact. ROCK stimulates actin polymerization via an inhibitory phosphorylation of the actin severing LIM kinase (Figure 2). ROCK promotes cellular contraction and attachment via an activating phosphorylation of myosin light chain (MLC) to increase myosin ATPase activity, and an inhibitory phosphorylation of MLC phosphatase leading to increased activation of MLC (Figure 3). Additionally, numerous other downstream targets of ROCK proteins have been identified including, but not limited to, intermediate filaments, ezrin/radixin/moesin (ERM) family proteins, collapsing response mediator protein 2 (CRMP2), calponin and adducin.
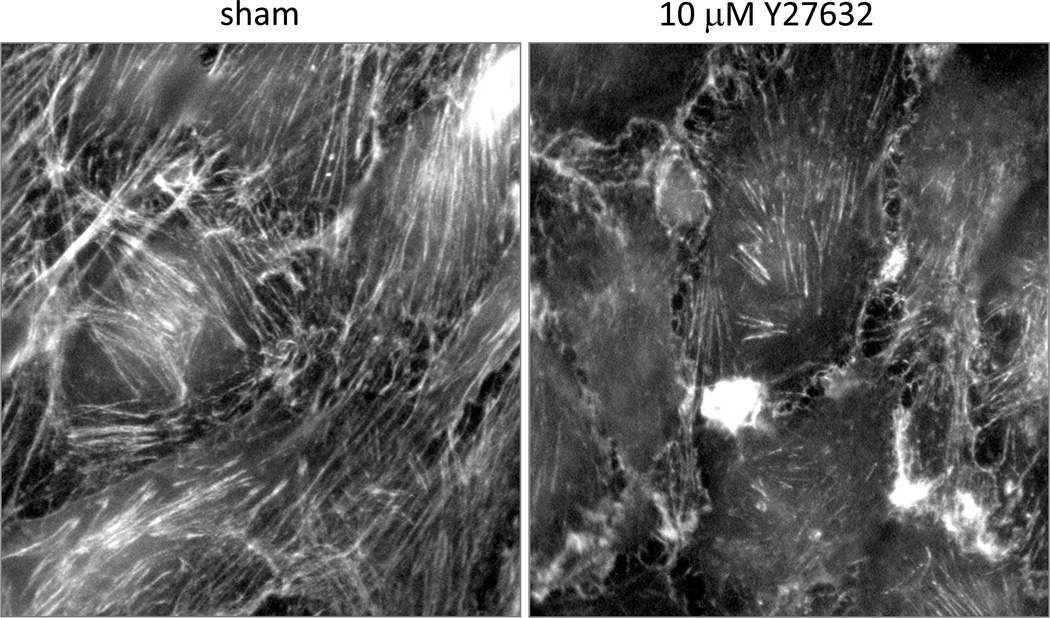
Figure 1. ROCK activity in actin polymerization.
MS1 endothelial cells were sham treated or treated with 10 µM of the ROCK1 and 2 pharmacological inhibitor Y27632. Cells were then stained with FITC-labelled phalloidin which specifically binds to polymerized actin microfilaments. Disruption of total ROCK activity results in a dramatic reduction in the quantity of polymerized actin.
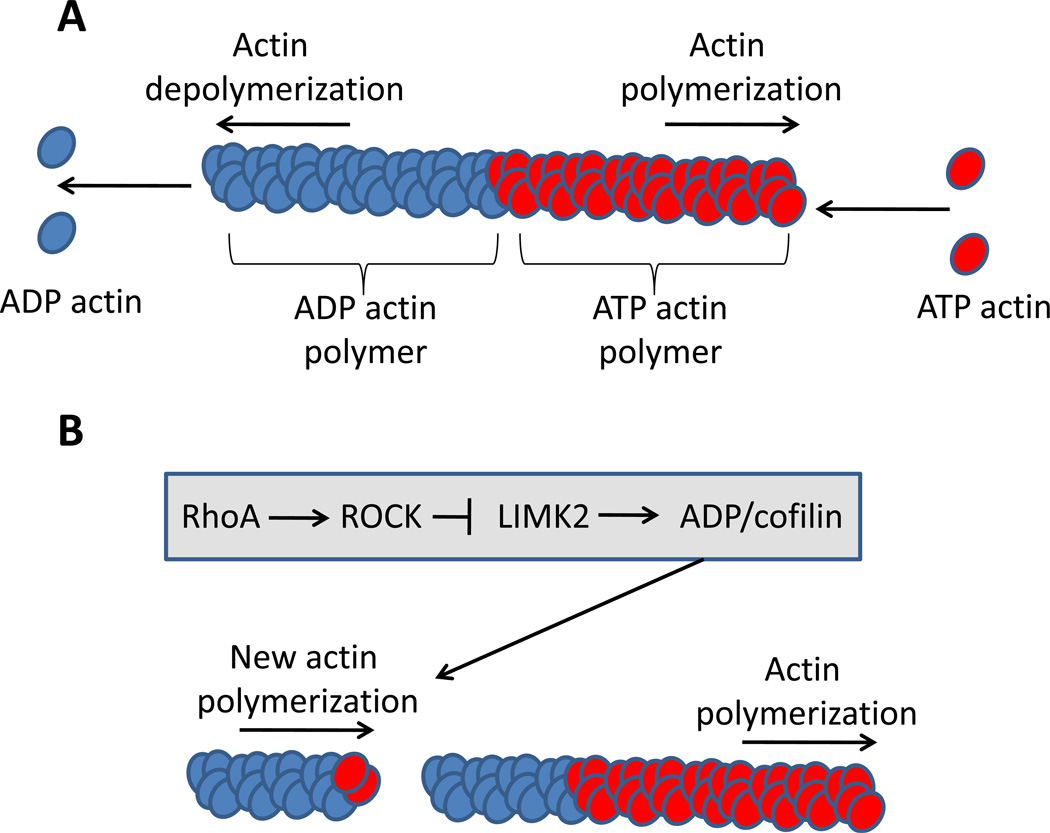
Figure 2. ROCK control of actin polymerization.
(A) Individual subunits of ATP-bound globular actin (G-actin) assemble into long filamentous polymers (F-actin), creating a double helix structure. Hydrolysis of the ATP destabilizes the polymer, causing dissolution of F-actin polymers into G-actin monomers. (B) ROCK stimulates stabilization of actin polymerization via an inhibitory phosphorylation of Lin11, Isl1, Mec3 (LIM) domain kinase (LIMK), which when active promotes ADP/cofilin-mediated actin severing.
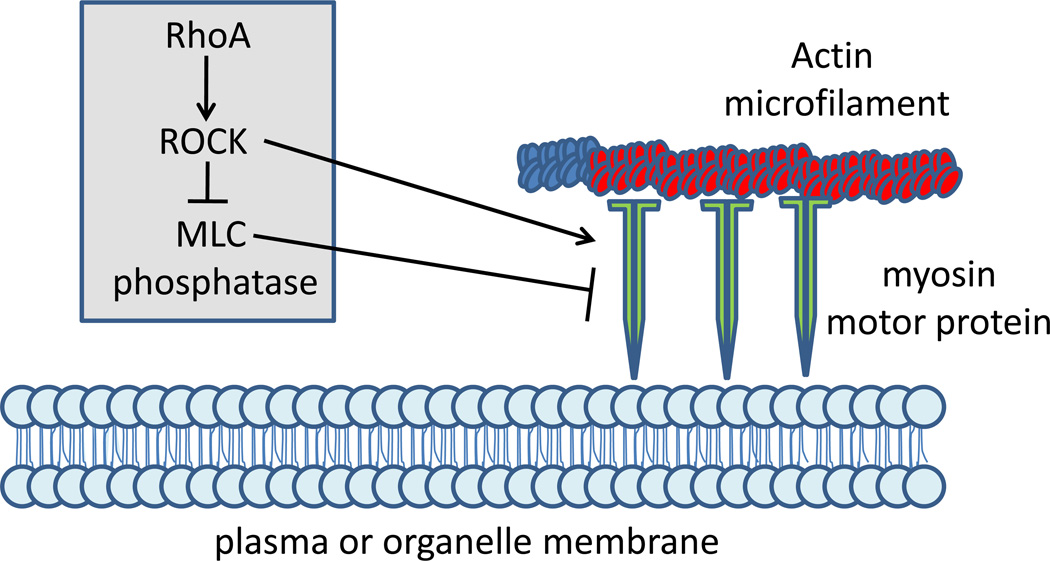
Figure 3. ROCK control of cellular contractility.
Actin filaments in association with myosin motor proteins control cellular movement, cell division and other biological processes across all cell types. ROCK promotes cellular contraction and attachment via an activating phosphorylation of myosin light chain (MLC) to increase myosin ATPase activity, and an inhibitory phosphorylation of MLC phosphatase leading to increased activation of MLC.
Two paralogs of ROCK have been identified in mammals (ROCK1 and ROCK2). These proteins were originally isolated as RhoA-GTP interacting proteins, and share 65% overall identity and 92% identity in their kinase domains(1). ROCK1 and ROCK2 are widely expressed from C. elegans to mammals and demonstrate both overlapping and unique tissue expression patterns and signaling functions within the cell. ROCK1 and ROCK2 knockout mice show distinct phenotypes, suggesting these proteins perform, at least to some degree, non-overlapping roles during development. ROCK1 knockout mice exhibit failure of eyelid and ventral body wall closure resulting in lethality soon after birth(3), while ROCK2 knockout mice exhibit embryonic lethality due to intrauterine growth retardation and placental dysfunction(4). The generation of heterozygote ROCK1 and ROCK2 mice leads to viable, fertile litters with no obvious phenotypic abnormalities, however a detailed examination of ROCK1(+/−) mice revealed reduced neointima formation following carotid artery ligation correlating with decreased vascular smooth muscle cell proliferation and survival, decreased levels of proinflammatory adhesion molecule expression, and decreased leukocyte infiltration(5). Moreover, ROCK1(+/−) mice exhibit increased resistance to perivascular fibrosis, accompanied by decreased expression of tissue growth factor-beta, connective tissue growth factor and type III collagen(6). ROCK2(+/−), but not ROCK1(+/−), mice demonstrate no obvious cardiac phenotype, however they display decreased platelet endothelial cell adhesion molecule staining of endothelial cells in the lung, suggesting that ROCK2 plays a strong role in capillary development(7).
Deregulation of Rho/ROCK signaling has been reported across diverse tumors types. For instance, Rho-signaling proteins are elevated in, and contribute to the metastatic behavior of a variety of tumors(8–12). Several preclinical and clinical studies have utilized inhibitors of Rho/ROCK signaling for anticancer therapeutics in prostate, lung, melanoma, glioblastoma and many other tumor types with remarkable success(13–17). Many of the positive outcomes claimed from targeting Rho/ROCK signaling have been attributed to a reduction in invasion/metastatic potential of the cancer cells; however a wealth of findings have demonstrated that ROCK proteins are key modulators of cell survival and apoptosis, suggesting that cell viability may also be affected by ROCK inhibition.
AN OVERVIEW OF APOPTOSIS
Apoptosis is a controlled form of cell death that involves cell shrinkage, membrane blebbing, cellular disintegration, chromosome condensation, and subsequent removal of the apoptotic fragments by phagocytosis(1). This process is initiated by activation of caspase cystein proteases which cleave a large number of downstream protein targets to induce orderly morphological and biochemical changes within the cell, involving reorganization of actin microfilaments, microtubules, and intermediate filaments. The initial stages of apoptosis involve partial detachment of the cell from the extracellular matrix (ECM) due to caspase-mediated cleavage of focal adhesion kinase (FAK) as well as other structural proteins linking actin to focal adhesions(18). Following focal adhesion disassembly at the cell periphery, extensive cellular retraction occurs due to a loss of stress fibers and reorganization of actin microfilaments to form short fibers that bundle together and increase the tensile strength of the cell. As a consequence of retraction, cells undergoing apoptosis round up and reassemble new focal adhesion complexes ventral to the retracted cell body. Moreover, the formation of dynamic membrane protrusions called blebs is driven by modulation of actin-myosin activity, creating hydrodynamic forces during contraction to induce collapse at points of structural weakness within the cell(19, 20). Occurring concomitant with this process is caspase-8 mediated activation of deoxyriboneuclease, which catalyzes internucleosomal DNA cleavage(21). Finally, apoptotic bodies of varying size and composition are produced in an actin/myosin dependent manner and are phagocytized by nearby cells and scavenging immune cells, to be ultimately degraded by lysosomal enzymes(22).
Apoptosis can be activated in the cell by two major processes: the intrinsic and extrinsic apoptotic pathways. The extrinsic apoptotic pathway responds to secreted death ligands (such as apoptosis stimulating fragment [Fas] ligand, tumor necrosis factor [TNF] alpha and tumor necrosis factor alpha related apoptosis inducing ligand [TRAIL]) that bind specifically to transmembrane death receptors (such as TNF-R, Fas and TRAIL-R) in the target cell, initiating a signal for apoptosis. Death ligand activation of these receptors induces the formation of a death-inducing signaling complex (DISC) composed of the death ligand, a trimeric death receptor and death domain containing adaptor proteins which trigger cleavage of caspases into their active form(23). This process leads to further rounds of caspase cleavage and activation, resulting in cellular apoptosis.
The intrinsic apoptotic pathway is initiated as a p53 induced cascade in response to DNA damage, defective cell cycle progression, or other severe cell stresses. This pathway is regulated by the fine balance of B-cell CLL/lymphoma 2 (Bcl2) family proteins within the cell(24). The Bcl2 proteins are apoptotic regulatory proteins that modulate mitochondrial membrane permeability, with some members being pro apoptotic and others anti-apoptotic. Under normal conditions, the anti-apoptotic Bcl2 proteins (such as Bcl2, Bcl-xl, BclW, bifunctional Bcl2 family protein 1 [Bfl1], myeloid leukemia cell differentiation protein 1 [Mcl1], Bcl2 related protein A1 [A1], and Bcl2 homologue of ovary [Boo]) maintain mitochondrial integrity by counteracting the activation and function of pro-apoptotic Bcl2 family members (such as Bcl2 associated X protein [Bax], Bcl2 homologous antagonist killer [Bak], BclX5, Bcl2 associated death promoter [Bad], BH3 interacting domain death agonist [Bid], Blc2 interacting killer [Bik], and hara-kiri [Hrk]) whose role is to induce mitochondrial damage. When pro-apoptotic Bcl2 proteins are activated, cytochrome-c is released from the mitochondria where it binds with apoptotic protease activating factor 1 (Apaf-1), forming the apoptosome. The activation of initiator caspases by the apoptosome begins a cascade of cleavage events ultimately leading to cellular apoptosis.
ROLE OF ROCK PROTEINS IN APOPTOSIS/SURVIVAL
Both disassembly and excessive crosslinking of the actin microfilament cytoskeletal architecture has been extensively demonstrated to induce apoptosis in numerous cell types(25–28) through modulation of signaling components such as Bcl2 activation(29), death receptor activation(30, 31), caspase activation(32), and p53 signaling(33). Moreover, an intimate association exists between cytoskeletal dynamics, the extracellular microenvironment, cell-to-cell adhesions and cell-to-substratum adhesions, where alterations in any of these components could be detrimental to the survival of the cell(34). ROCK protein signaling reportedly acts in either a pro- or anti-apoptotic fashion depending on cell type, cell context and microenvironment. For instance, ROCK proteins are essential for multiple aspects of both the intrinsic and extrinsic apoptotic processes, including regulation of cytoskeletal-mediated cell contraction and membrane blebbing, nuclear membrane disintegration, modulation of Bcl2-family member and caspase expression/activation and phagocytosis of the fragmented apoptotic bodies (discussed extensively below, Figure 4). In contrast, ROCK signaling exhibited pro-survival roles in a number of experimental studies (Figure 5)(14, 15, 35–40). Though a wealth of data exists to suggest both pro- and anti-survival roles for ROCK proteins, the molecular mechanisms that modulate these pleitropic roles are largely unknown.
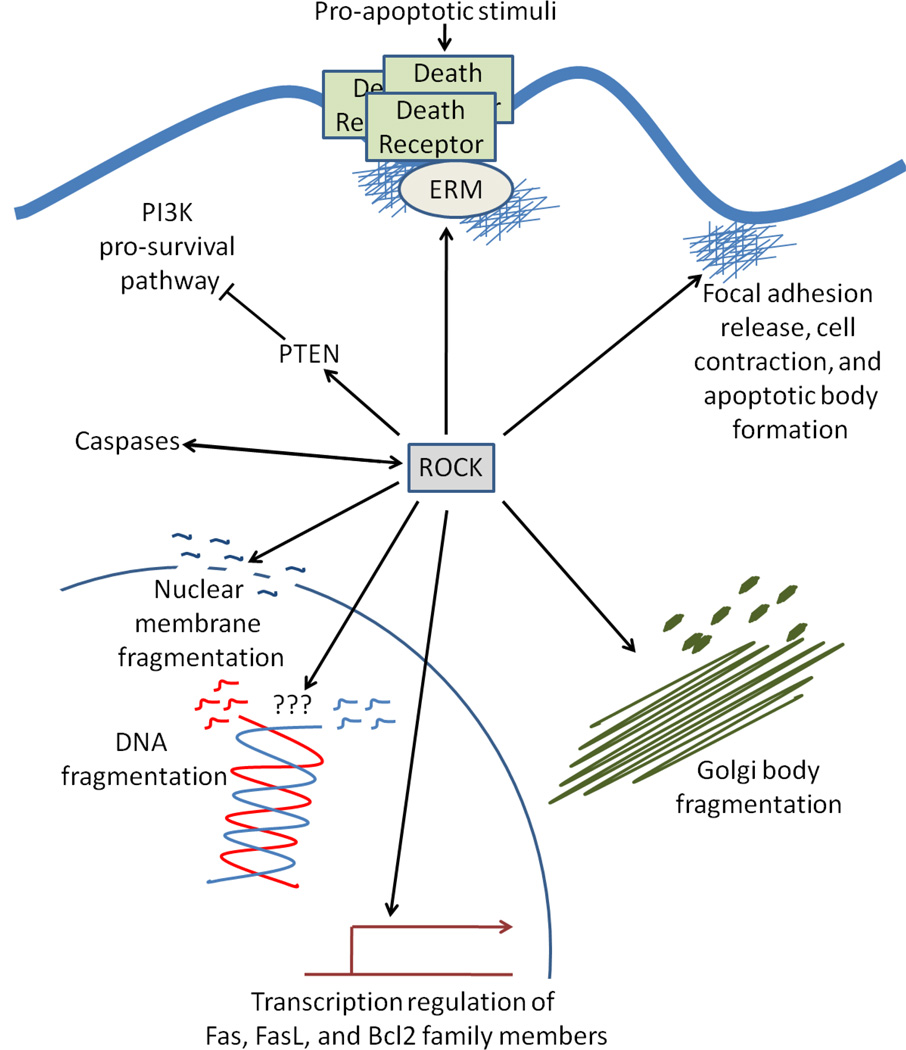
Figure 4. Essential role of ROCK in apoptosis.
ROCK proteins are activated by caspase cleavage and promote the cleavage of procaspases into their active caspase forms. ROCK activity is necessary for multiple aspects of both intrinsic and extrinsic apoptosis including death receptor activation via ezrin, radixin, and moesin (ERM) proteins, apoptotic bleb and body formation, nuclear and organelle fragmentation, and DNA fragmentation. Moreover, ROCK phosphorylates and inhibits phosphatase and tensin homology (PTEN), thus blocking the pro-survival phosphoinositide 3-kinase (PI3K) pathway.
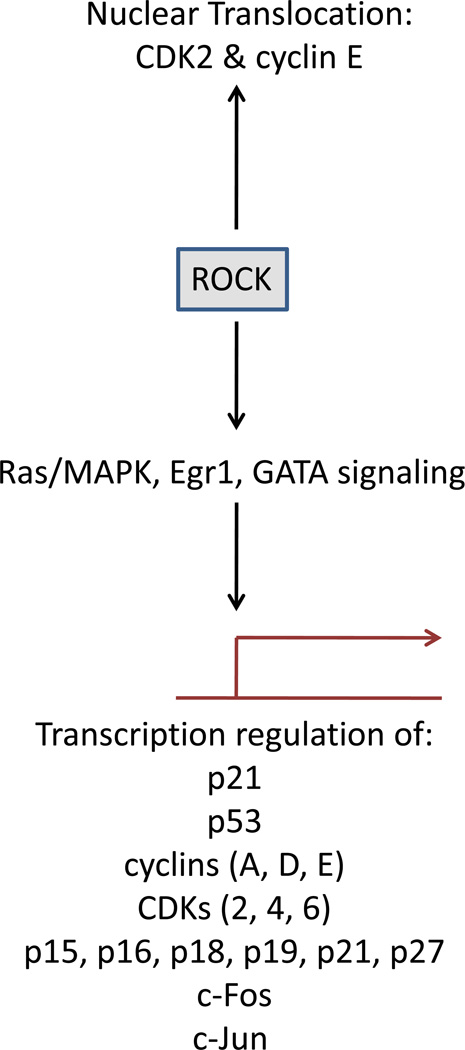
Figure 5. Role of ROCK activity in cell survival.
ROCK activity is necessary for progression from the G1 to S-phase of the cell cycle by controlling the expression of cyclins, cyclin dependent kinases (CDKs), and numerous other cell cycle regulators. Additionally, ROCK activity has been shown to promote CDK2 and cyclin E translocation into the nucleus.
ROCK PROTEIN REGULATION OF APOPTOSIS
Essential roles of ROCK proteins in apoptosis
ROCK proteins are direct targets of caspase activity, whereby caspase 2 and 3 cleavage of ROCK proteins occurs in early apoptosis, thus removing the ROCK autoinhibitory C-terminal domain. This results in constitutive kinase activity of ROCK and its subsequent regulation of actin-myosin cellular contraction(41–47). Of note, ROCK cleavage also occurs early in apoptosis in a caspase-independent manner during endothelial release of microparticles and during invasion of colorectal cancer cells(48, 49). Granzyme-B has been shown to directly cleave ROCK2 in a caspase-independent manner, leading to cytotoxic lymphocyte granule-induced apoptosis(46). Whether via a caspase dependent or independent route, ROCK cleavage is an essential step for apoptosis given that pharmacological inhibition of its kinase activity effectively abrogates apoptosis in a number of cell types.
In blebbing cells, caspase-cleaved ROCK-mediated phosphorylation of MLC is increased, thereby inducing contraction of cortical actin within the cell(50,19, 44, 45). Indeed, transfection of cells with either a truncated (constitutively activated) ROCK1 gene or overexpression of a wild type ROCK2 gene is sufficient to induce MLC-mediated membrane blebbing independently of apoptotic stimuli(44, 51). Studies using cytoskeletal or ROCK inhibitors have identified multiple stages in apoptotic blebbing. For instance, caspase independent blebbing (zeiosis) occurs immediately after cytochrome c release from the mitochondria into the cytoplasm, whereby surface swellings at the active edges of cells form small blebs that dynamically extend and retract(52). This early phase of apoptotic blebbing, which occurs at the point where adherent cells begin to retract away from their neighbors and partially detach from the substratum(53), is critically dependent on ROCK/MLC cytoskeletal signaling(52). Late phase blebbing leads to morphologically distinct blebs that are relatively stable, fewer in number than those seen during early blebbing, exhibit an absence of visible organelles, and contain a distinct layer of endoplasmic reticulum which envelops chromatin. Formation of these late blebs is efficiently blocked with Latrunculin A (an actin microfilament inhibitor), Blebbistatin (an inhibitor of myosin II), or Nocodazole (a microtubule inhibitor); however pharmacological inhibition of ROCK proteins only partially prevents the formation of late blebs(52).
In hypertrophic cardiomyocytes, Rho/ROCK signaling is necessary for apoptotic DNA fragmentation via activation of p53 and Bax(54). Conversely, inhibition of RhoA or ROCK protein signaling in heptatic stellate cells increases DNA fragmentation and condensation of nuclear chromatin(55). These limited data suggest that a more thorough examination is necessary before any consensus can be made regarding the role of ROCK proteins in apoptotic DNA fragmentation. Apoptotic nuclear disintegration, an actin microfilament-dependent and microtubule-independent process, requires ROCK modulation of the actin-myosin contractile force coupled with a ROCK-independent caspase-mediated degradation of nuclear lamin proteins(56). In addition to regulating nuclear disintegration, ROCK signaling is necessary for Golgi organelle fragmentation in apoptotic adrenal medulla pheochromocytoma cells(57). In this model, overexpression of constitutively active ROCK proteins induces Golgi fragmentation even in the absence of apoptotic stimuli. Moreover, ROCK proteins regulate protein traffic to and from cellular organelles during the apoptotic cascade. For instance, when myeloid leukemia cells become apoptotic, activated extracellular signal regulated kinase (ERK) is unable to translocate into the nuclei. Pharmacological inhibition of ROCK signaling is not only capable of rescuing these cells from apoptosis, but successfully restores the nuclear translocation of activated ERK(58). Furthermore, in apoptotic myeloid leukemia and fibroblast cells, caspase-independent ROCK signaling leads to nuclear exclusion of C1/C2 heterogenous nuclear ribonucleoproteins (hnRNPs), which play important roles in the packaging of nascent transcripts, alternative splicing and translational regulation(59). ROCK-mediated control of protein localization is well documented, as modulation of actin polymerization by ROCK has been shown to regulate nuclear localization of serum response factor (SRF) and sex determining region Y-box 9 (Sox9) during non-apoptotic conditions(60, 61). ROCK control of subcellular protein localization could potentially be a commonplace mechanism by which rapidly changing cytoskeletal dynamics during apoptosis alters cellular function.
Apoptotic body formation is driven by actin-myosin contraction initiated by caspase-mediated activating cleavage of ROCK1. In fibroblast and B-lymphoma cells this process is prevented by pharmacological inhibition or small interfering RNA (siRNA) knockdown of ROCK1, but not by inhibition of ROCK2(57, 62). Moreover, ROCK activation is necessary for efficient phagocytosis of fragmented apoptotic bodies, and has been demonstrated to control the expression of N-acetylglucosamine (GlcNAc), a carbohydrate that serves as a major phagocytic marker(57, 63).
ROCK control of extrinsic apoptosis
The extrinsic apoptotic receptor Fas is linked to the actin cytoskeleton via an interaction with ezrin, radixin and moesin (ERM) proteins, whose function is to connect transmembrane proteins to the cytoskeleton(64, 65). The disruption of actin cytoskeleton dynamics or down-regulation of either ezrin or moesin inhibits extrinsic apoptotic signaling by blocking Fas aggregation and redistribution of Fas into lipid rafts, and by preventing association of flavin adenine dinucleotide (Fad) associated protein with death domain (FADD) with its procaspases(31, 64, 66). These data suggest that ligand-mediated activation of the extrinsic apoptotic pathway initiates a cytoskeleton driven clustering of the activated death receptor with its downstream death domain proteins and their associated caspases. This process is dependent on ROCK signaling as pharmacological inhibition or siRNA downregulation of ROCK proteins blocks clustering of FAS proteins to lipid rafts, inhibits ROCK-mediated phosphorylation of ezrin and disrupts procaspase 8 and 10 association with FAS and FADD(64, 66–68). A similar ROCK-driven cytoskeletal regulation has been demonstrated for extrinsic apoptotic induction following ligand driven Fas receptor clustering(69, 70). In addition to modulating death receptor activity, ROCK signaling controls the expression levels of several extrinsic apoptotic regulators. Pharmacological inhibition of ROCK signaling results in a decrease in Fas, FasL and TRAIL expression during androgen induced apoptotic regression of prostate cancer cells and following cisplatin cytotoxicity in neuroblastoma cells(71–73). In contrast, ROCK inhibition reportedly enhances FasL expression in melanoma tumors(35).
ROCK control of intrinsic apoptosis
ROCK proteins perform a key role in cell cycle inhibition and impinge on the p53-driven intrinsic apoptotic cascade at multiple points from initial activation to output. However, the ROCK-mediated regulation of cell cycle and intrinsic apoptotic regulators seems to function in a cell type and context specific manner as conflicting results have been reported throughout the literature. Moreover, unlike that seen for ROCK regulation of the extrinsic apoptotic pathway, few consistent mechanisms have been proposed as to how ROCK proteins control intrinsic apoptotic regulation. ROCK inhibition has been shown to increase phosphorylation of p53 in neuronal cells, suggesting that ROCK signaling promotes murine double minute oncogene 2 (Mdm2)-mediated ubiquitination and degradation of p53(74, 75). In contrast, fasudil treatment following nephropathy leads to decreased p53 expression, suggesting the opposite(76). No direct physical association has been reported in the literature between ROCK and p53, indicating that ROCK mediated regulation of p53 levels is likely modulated through indirect signaling crosstalk. For instance, ROCK activity has been shown to regulate phosphoinositol-3-kinase (PI3K)/Ak transforming (AKT) signaling (a negative regulator of p53 stability) through ROCK-dependent assembly of focal adhesions(77). A large proteomic screen demonstrated that ROCK2 physically associates and is activated by the serine/threonine kinase Polo-like kinase (Plk1)(78, 79), an important regulator of mitotic events such as centrosome maturation, mitotic entry, spindle formation, sister chromatid cohesion, and cytokinesis. This interaction could modulate p53 status given that Plk1 is a strong inhibitor of p53 function through a direct physical interaction between the two proteins(80). Moreover, Plk1 induces an inhibitory phosphorylation on the Sumo E3 ligase topoisomerase I-binding protein (Topors) leading to inhibition of p53 sumyolation and its subsequent ubiquitination and degradation(81). Another possibility that deserves further study involves ROCK/LIMK mediated regulation of tubulin-dynein motor protein transport into the nucleus. p53 has been shown to localize to cellular microtubules, and transport of p53 into the nucleus following DNA damage is tubulin-dynein motor protein depedent(82–84). ROCK activity has repeatedly been demonstrated to control microtubule activity in a number of systems ranging from cell protrusions to tubulin-dynein vesicular trafficking(85–87), but whether p53 nuclear localization is regulated via this ROCK/LIMK/motor protein process has yet to be determined.
In a number of studies, ROCK signaling reportedly controlled Bcl-2 family member gene expression in favor of apoptosis(54, 73, 88–90) and modulates activation of multiple caspases(54, 64, 67, 77, 88, 91). ROCK modulation of Bcl2 expression may occur via the PI3K mediated pathway (discussed below) or through c-jun N-terminal kinase (JNK) activation. The JNK pathway is primarily activated by cytokines or exposure to various environmental stresses and plays an important role in regulating stress-induced apoptosis by triggering cytochrome c release from the mitochondria through modulation of Bcl2 and Bcl-xl activity(92). It has been demonstrated that ROCK1 directly phosphorylates JNK-interacting protein (JIP)-3, a scaffolding protein responsible for recruitment and activation of JNK protein, leading to subsequent triggering of apoptosis(93). This process can be prevented by sequestration of ROCK1 into stress granules, thus blocking ROCK1 interaction with JIP3 and protecting cells from apoptosis(94).
ROCK PROTEIN REGULATION OF CELL SURVIVAL
Control of cell survival by Rock proteins
Inhibition of ROCK promotes survival following balloon surgery and stent implantation of the carotid artery(95, 96), in autologous vein grafts(97), in pulmonary hypertension(98–100), following renal damage(101–106), in vaculogenic erectile dysfunction(107–110) and in diabetic retinal microvasculopathy(111). While it is highly likely that the effects of ROCK inhibition on numerous disease models are multifactorial, few mechanisms have been proposed to explain such observations. The PI3K/Akt pathway plays a central role in promoting cell survival by regulation of the activity and expression of Bcl2 family members, forkhead box 0 (FoxO) transcription factors, and p53 stability(112). PI3K activation is countered by phosphatase and tensin homolog (PTEN), a phosphatase that dephosphorylates proteins and phosphoinositide substrates(113). Activation of ROCK proteins by caspase cleavage or oncogene overexpression induces a direct phosphorylation of PTEN by ROCK, leading to the increased phosphatase activity and enhanced protein stability of PTEN. Activated PTEN then directly counters the pro-survival PI3K/AKT pathway, suggesting that ROCK activation blocks cell survival(41, 114–116). In addition, the PI3K/Akt pathway promotes the nitric oxide-mediated survival of endothelial cells by stimulating the expression of endothelial nitric oxide synthase (eNOS), the enzyme that converts the amino acid arginine to nitric oxide(117). Thus, ROCK-mediated activation of PTEN leads to a subsequent decrease in nitric oxide (NO) production and reduced cell survival of endothelial cells(118, 119), however ROCK’s regulation of NO-driven survival is reportedly PI3K/Akt independent in some cells and involves activation of the PKC pathway(120–122).
ROCK signaling regulates chemotherapy resistance in several tumor cell types, and thus affects overall tumor resilience and survival. For instance, in multiple myeloma cells, ROCK-mediated attachment to the extracellular matrix is an essential component of cell adhesion-mediated drug resistance, a process whereby integrin interactions lead to up-regulation of anti-apoptotic Bcl2 family members and overexpression of multidrug resistant genes(123). Similarly, inhibition of ROCK activity leads to enhancement of cisplatin-induced cytotoxicity in lung carcinoma cells through a focal adhesion kinase-independent mechanism(124). Conversely, following cisplatin injury to a panel of cultured neuroblastoma cells, pharmacological inhibition of ROCK activity resulted in increased cell survival, rapid acquisition of a chemoresistant phenotype and enhanced in vivo tumor survival. The increased chemoresistance in ROCK-inhibited neuroblastoma cells was attributed primarily to enhanced DNA damage repair, with observable alterations in the expression of multidrug resistance genes, p53, p21, Bcl2 family members and death receptors and their ligands(73).
ROCK PROTEIN REGULATION OF PROLIFERATION
Control of cell cycle progression by ROCK proteins
siRNA or pharmacological inhibition of ROCK blocks the G1/S transition in a number of cell types. Indeed, ROCK signaling promotes cell cycle progression into the S phase through a diverse array of downstream targets including upregulation of cyclin A/D1/D3 and cyclin dependent kinase (CDK) 2/4/6, nuclear translocation of CDK2 and cyclin E, and downregulation of the cell cycle inhibitors cyclin dependent kinase 4 inhibitor B (CDKN4B), CDKN2A, CDKN2C, CDKN2D (p21), CDKN1A, and CDKN1B(125–130) ROCK utilizes multiple downstream signaling cascades to modulate proliferation where it activates Ras/MAPK to regulate cyclin D and p21 expression, and, alternatively, LIM Kinase 2 to regulate cyclin A expression(128). Moreover, ROCK increases the expression of the F-box protein s-phase kinase-associated protein 2 (Skp2) which is required for the degradation of the cell cycle inhibitor p27(Kip1)(126, 128). Inhibition of ROCK signaling leads to cell cycle arrest in the G1 phase, decreased JNK, extracellular signal-regulated kinase (ERK), Ephrin-related tyrosine kinase (ELK), early growth response protein 1 (Egr1), and globin transcription factor (GATA) transcription factor activation, decreased c-FBJ murine osteosarcoma viral oncogene homolog (c-fos), jun proto-oncogene (c-jun), FasL, and Bcl2 expression, and increased Bax expression(15, 35, 39, 96, 131–134). Alternatively, a handful of papers suggest ROCK activity is capable of blocking cell cycle progression under certain conditions. For instance, during phorbol ester-induced apoptosis in prostate cancer cells, increased expression of the cell cycle inhibitor p21 is dependent on ROCK-mediated regulation of cytoskeleton dynamics(135). Additionally, pharmacological inhibition of ROCK activity in human Wharton’s jelly stem cells leads to downregulation of the pro-apoptotic Bax gene and the cell cycle regulators p21 and p53, as well as upregulation of the anti-apoptotic Bcl2 gene(89, 90), suggesting ROCK can inhibit cell cycle progression under certain conditions.
In addition to the requirement of early growth factor-mediated progression through the cell cycle, microenvironment-dependent changes in cell shape and cytoskeleton regulation modulate the G1/S transition whereby the major mitogenic-responsive pathways such as Ras, Rho, and PI3K are regulated by integrin mediated cell adhesion to the ECM(136). Disrupted integrin signaling is responsible for the change from anchorage dependent to anchorage independent cell growth in tumor cells, demonstrating a strong linkage between the extracellular microenvironment, cell adhesion, cellular morphology, and cell survival. Fibronectin/integrin interactions have been shown to stimulate cell proliferation in a ROCK-dependent manner by suppression of p21 and stimulation of cyclin D1 mRNA expression levels(130, 137, 138). Moreover, the degree of cell spreading on the ECM is a potent modulator of cell proliferation(139), and density dependent growth control is regulated by cell-to-cell adhesions via cadherin-mediated activation of p21 and p27(140–143) and reduction in the strength and stability of cell-ECM contacts(144). Interestingly, cells that are restricted from spreading, such as fully confluent monolayers, exhibit a shape-dependent failure to increase the expression of cyclin D1, down-regulate p27 and phosphorylate retinoblastoma protein in late G1(145, 146) and low ROCK activity(147). Cell spreading and mechanical stretch (mimicking non-confluent cell density) has been shown to activate RhoA and ROCK in smooth muscle cells, resulting in membrane association of RhoA, leading to ROCK-dependent hyperphosphorylation of Rb and enhanced proliferation(147–149). The loss of cadherin-mediated cell-to-cell contacts as seen in subconfluent cultures leads to the formation of a signaling complex composed of ROCK, novel protein kinase C (nPKC), and sarcoma proto-oncogene (Src) family kinases (SFKs), resulting in protein kinase D-dependent activation of the pro-proliferation nuclear factor kappa-B (NFkappaB) protein(37). These findings suggest that the extracellular microenvironment, particularly the effect of cell density, may affect the outcome of ROCK signaling in the control of cell fate. Therefore, simple differences in cell plating density may explain the numerous conflicting observations regarding the role of ROCK proteins in cell survival.
IMPLICATIONS FOR CANCER THERAPY
Despite the obvious complexity and the ever growing number of publications linking ROCK as well as cytoskeletal regulation in the cellular decision between life and death, no sufficient comprehensive mechanism has been established which comes close to explaining the fundamental intricacies governing the pleiotropic roles of the ROCK proteins in cell survival. Despite this shortcoming, targeting of ROCK signaling in animal models of tumor progression has manifested outstanding results in many cases particularly with regard to tumor cell invasion and metastasis, suggesting that manipulation of this pathway could hold the key to pushing cancer cells just over the edge so that patients might gain an upper hand not afforded by chemotherapy or radiation alone. Perhaps the greatest challenge to researchers and clinicians is the dissection of these conflicting signaling roles, thereby learning which tumor types and what physiological conditions are appropriate for the proper manipulation of ROCK signaling.
ACKNOWLEDGEMENTS
Preparation of this manuscript was supported by a National Heart, Lung, and Blood Institute Award (HL098931) awarded to BAB.
References
- 1.Shi J, Wei L. Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz) 2007;55:61–75. doi: 10.1007/s00005-007-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, Saint-Geniez M, Campaigniac JP, Liao JK, D'Amore PA. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24:3186–3195. doi: 10.1096/fj.09-145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamai T, Arai K, Sumi S, Tsujii T, Honda M, Yamanishi T, Yoshida KI. The rho/rho-kinase pathway is involved in the progression of testicular germ cell tumour. BJU Int. 2002;89:449–453. doi: 10.1046/j.1464-4096.2001.01920.x. [DOI] [PubMed] [Google Scholar]
- 9.Kamai T, Tsujii T, Arai K, Takagi K, Asami H, Ito Y, Oshima H. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin Cancer Res. 2003;9:2632–2641. [PubMed] [Google Scholar]
- 10.Kaneko K, Satoh K, Masamune A, Satoh A, Shimosegawa T. Expression of ROCK-1 in human pancreatic cancer: its down-regulation by morpholino oligo antisense can reduce the migration of pancreatic cancer cells in vitro. Pancreas. 2002;24:251–257. doi: 10.1097/00006676-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kleer CG, van Golen KL, Zhang Y, Wu ZF, Rubin MA, Merajver SD. Characterization of RhoC expression in benign and malignant breast disease: a potential new marker for small breast carcinomas with metastatic ability. Am J Pathol. 2002;160:579–584. doi: 10.1016/S0002-9440(10)64877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Zhao LQ, Xiong MM, Wang XQ, Yang GR, Qiu ZL, Wu M, Liu ZH. Gene expression profiles at different stages of human esophageal squamous cell carcinoma. World J Gastroenterol. 2003;9:9–15. doi: 10.3748/wjg.v9.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somlyo AV, Bradshaw D, Ramos S, Murphy C, Myers CE, Somlyo AP. Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem Biophys Res Commun. 2000;269:652–659. doi: 10.1006/bbrc.2000.2343. [DOI] [PubMed] [Google Scholar]
- 14.Rattan R, Giri S, Singh AK, Singh I. Rho/ROCK pathway as a target of tumor therapy. J Neurosci Res. 2006;83:243–255. doi: 10.1002/jnr.20707. [DOI] [PubMed] [Google Scholar]
- 15.Routhier A, Astuccio M, Lahey D, Monfredo N, Johnson A, Callahan W, Partington A, Fellows K, Ouellette L, Zhidro S, Goodrow C, Smith A, Sullivan K, Simone P, Le L, Vezuli B, Zohni M, West E, Gleason D, Bryan B. Pharmacological inhibition of Rho-kinase signaling with Y-27632 blocks melanoma tumor growth. Oncol Rep. 2010;23:861–867. [PubMed] [Google Scholar]
- 16.Spencer C, Montalvo J, McLaughlin SR, Bryan BA. Small molecule inhibition of cytoskeletal dynamics in melanoma tumors results in altered transcriptional expression patterns of key genes involved in tumor initiation and progression. Cancer Genomics Proteomics. 2011;8:77–85. [PMC free article] [PubMed] [Google Scholar]
- 17.Amine A, Rivera S, Opolon P, Dekkal M, Biard DS, Bouamar H, Louache F, McKay MJ, Bourhis J, Deutsch E, Vozenin-Brotons MC. Novel anti-metastatic action of cidofovir mediated by inhibition of E6/E7, CXCR4 and Rho/ROCK signaling in HPV tumor cells. PLoS One. 2009;4:e5018. doi: 10.1371/journal.pone.0005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levkau B, Herren B, Koyama H, Ross R, Raines EW. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J Exp Med. 1998;187:579–586. doi: 10.1084/jem.187.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002;9:493–504. doi: 10.1038/sj.cdd.4400987. [DOI] [PubMed] [Google Scholar]
- 20.Ndozangue-Touriguine O, Hamelin J, Breard J. Cytoskeleton and apoptosis. Biochem Pharmacol. 2008;76:11–18. doi: 10.1016/j.bcp.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Mukae N, Yokoyama H, Yokokura T, Sakoyama Y, Sakahira H, Nagata S. Identification and developmental expression of inhibitor of caspase-activated DNase (ICAD) in Drosophila melanogaster. J Biol Chem. 2000;275:21402–21408. doi: 10.1074/jbc.M909611199. [DOI] [PubMed] [Google Scholar]
- 22.deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem. 2003;39:105–117. doi: 10.1042/bse0390105. [DOI] [PubMed] [Google Scholar]
- 23.Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect. 2009;11:1050–1062. doi: 10.1016/j.micinf.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Rolland SG, Conradt B. New role of the BCL2 family of proteins in the regulation of mitochondrial dynamics. Curr Opin Cell Biol. 2010;22:852–858. doi: 10.1016/j.ceb.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinoue A, Takigawa T, Miura T, Nishimura Y, Suzuki S, Shiota K. Disruption of actin cytoskeleton and anchorage-dependent cell spreading induces apoptotic death of mouse neural crest cells cultured in vitro. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:130–137. doi: 10.1002/ar.a.20150. [DOI] [PubMed] [Google Scholar]
- 26.Cabado AG, Leira F, Vieytes MR, Vieites JM, Botana LM. Cytoskeletal disruption is the key factor that triggers apoptosis in okadaic acid-treated neuroblastoma cells. Arch Toxicol. 2004;78:74–85. doi: 10.1007/s00204-003-0505-4. [DOI] [PubMed] [Google Scholar]
- 27.Celeste Morley S, Sun GP, Bierer BE. Inhibition of actin polymerization enhances commitment to and execution of apoptosis induced by withdrawal of trophic support. J Cell Biochem. 2003;88:1066–1076. doi: 10.1002/jcb.10449. [DOI] [PubMed] [Google Scholar]
- 28.White SR, Williams P, Wojcik KR, Sun S, Hiemstra PS, Rabe KF, Dorscheid DR. Initiation of apoptosis by actin cytoskeletal derangement in human airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:282–294. doi: 10.1165/ajrcmb.24.3.3995. [DOI] [PubMed] [Google Scholar]
- 29.Martin SS, Vuori K. Regulation of Bcl-2 proteins during anoikis and amorphosis. Biochim Biophys Acta. 2004;1692:145–157. doi: 10.1016/j.bbamcr.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Bijian K, Takano T, Papillon J, Le Berre L, Michaud JL, Kennedy CR, Cybulsky AV. Actin cytoskeleton regulates extracellular matrix-dependent survival signals in glomerular epithelial cells. Am J Physiol Renal Physiol. 2005;289:F1313–F1323. doi: 10.1152/ajprenal.00106.2005. [DOI] [PubMed] [Google Scholar]
- 31.Gajate C, Mollinedo F. Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy. J Biol Chem. 2005;280:11641–11647. doi: 10.1074/jbc.M411781200. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki Y, Tsuruga M, Zhou D, Fujita Y, Shang X, Dang Y, Kawasaki K, Oka S. Cytoskeletal disruption accelerates caspase-3 activation and alters the intracellular membrane reorganization in DNA damage-induced apoptosis. Exp Cell Res. 2000;259:64–78. doi: 10.1006/excr.2000.4970. [DOI] [PubMed] [Google Scholar]
- 33.Rubtsova SN, Kondratov RV, Kopnin PB, Chumakov PM, Kopnin BP, Vasiliev JM. Disruption of actin microfilaments by cytochalasin D leads to activation of p53. FEBS Lett. 1998;430:353–357. doi: 10.1016/s0014-5793(98)00692-9. [DOI] [PubMed] [Google Scholar]
- 34.Mammoto A, Mammoto T, Ingber DE. Rho signaling and mechanical control of vascular development. Curr Opin Hematol. 2008;15:228–234. doi: 10.1097/MOH.0b013e3282fa7445. [DOI] [PubMed] [Google Scholar]
- 35.Sarrabayrouse G, Synaeve C, Leveque K, Favre G, Tilkin-Mariame AF. Statins stimulate in vitro membrane FasL expression and lymphocyte apoptosis through RhoA/ROCK pathway in murine melanoma cells. Neoplasia. 2007;9:1078–1090. doi: 10.1593/neo.07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore M, Marroquin BA, Gugliotta W, Tse R, White SR. Rho kinase inhibition initiates apoptosis in human airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:379–387. doi: 10.1165/rcmb.2003-0019OC. [DOI] [PubMed] [Google Scholar]
- 37.Cowell CF, Yan IK, Eiseler T, Leightner AC, Doppler H, Storz P. Loss of cell-cell contacts induces NF-kappaB via RhoA-mediated activation of protein kinase D1. J Cell Biochem. 2009;106:714–728. doi: 10.1002/jcb.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krijnen PA, Sipkens JA, Molling JW, Rauwerda JA, Stehouwer CD, Muller A, Paulus WJ, van Nieuw Amerongen GP, Hack CE, Verhoeven AJ, van Hinsbergh VW, Niessen HW. Inhibition of Rho-ROCK signaling induces apoptotic and non-apoptotic PS exposure in cardiomyocytes via inhibition of flippase. J Mol Cell Cardiol. 2010;49:781–790. doi: 10.1016/j.yjmcc.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Shibata R, Kai H, Seki Y, Kusaba K, Takemiya K, Koga M, Jalalidin A, Tokuda K, Tahara N, Niiyama H, Nagata T, Kuwahara F, Imaizumi T. Rho-kinase inhibition reduces neointima formation after vascular injury by enhancing Bax expression and apoptosis. J Cardiovasc Pharmacol. 2003;42 Suppl 1:S43–S47. doi: 10.1097/00005344-200312001-00011. [DOI] [PubMed] [Google Scholar]
- 40.Svoboda KK, Moessner P, Field T, Acevedo J. ROCK inhibitor (Y27632) increases apoptosis and disrupts the actin cortical mat in embryonic avian corneal epithelium. Dev Dyn. 2004;229:579–590. doi: 10.1002/dvdy.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, Schwartz RJ. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci U S A. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coudray AM, Louvet C, Kornprobst M, Raymond E, Andre T, Tournigand C, Faivre S, De Gramont A, Larsen AK, Gespach C. Increased anticancer activity of the thymidylate synthase inhibitor BGC9331 combined with the topoisomerase I inhibitor SN-38 in human colorectal and breast cancer cells: induction of apoptosis and ROCK cleavage through caspase-3-dependent and -independent mechanisms. Int J Oncol. 2005;27:553–561. [PubMed] [Google Scholar]
- 43.Ark M, Ozdemir A, Polat B. Ouabain-induced apoptosis and Rho kinase: a novel caspase-2 cleavage site and fragment of Rock-2. Apoptosis. 2010;15:1494–1506. doi: 10.1007/s10495-010-0529-1. [DOI] [PubMed] [Google Scholar]
- 44.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 45.Coleman ML, Marshall CJ. A family outing: small GTPases cyclin' through G1. Nat Cell Biol. 2001;3:E250–E251. doi: 10.1038/ncb1101-e250. [DOI] [PubMed] [Google Scholar]
- 46.Sebbagh M, Hamelin J, Bertoglio J, Solary E, Breard J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med. 2005;201:465–471. doi: 10.1084/jem.20031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueda H, Morishita R, Itoh H, Narumiya S, Mikoshiba K, Kato K, Asano T. Galpha11 induces caspase-mediated proteolytic activation of Rho-associated kinase, ROCK-I, in HeLa cells. J Biol Chem. 2001;276:42527–42533. doi: 10.1074/jbc.M102529200. [DOI] [PubMed] [Google Scholar]
- 48.Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C, Dignat-George F, Anfosso F. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–1876. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- 49.Ehrenschwender M, Siegmund D, Wicovsky A, Kracht M, Dittrich-Breiholz O, Spindler V, Waschke J, Kalthoff H, Trauzold A, Wajant H. Mutant PIK3CA licenses TRAIL and CD95L to induce non-apoptotic caspase-8-mediated ROCK activation. Cell Death Differ. 2010;17:1435–1447. doi: 10.1038/cdd.2010.36. [DOI] [PubMed] [Google Scholar]
- 50.Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998;140:627–636. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song Y, Hoang BQ, Chang DD. ROCK-II-induced membrane blebbing and chromatin condensation require actin cytoskeleton. Exp Cell Res. 2002;278:45–52. doi: 10.1006/excr.2002.5565. [DOI] [PubMed] [Google Scholar]
- 52.Lane JD, Allan VJ, Woodman PG. Active relocation of chromatin and endoplasmic reticulum into blebs in late apoptotic cells. J Cell Sci. 2005;118:4059–4071. doi: 10.1242/jcs.02529. [DOI] [PubMed] [Google Scholar]
- 53.Mills JC, Stone NL, Pittman RN. Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J Cell Biol. 1999;146:703–708. doi: 10.1083/jcb.146.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- 55.Ikeda H, Nagashima K, Yanase M, Tomiya T, Arai M, Inoue Y, Tejima K, Nishikawa T, Omata M, Kimura S, Fujiwara K. Involvement of Rho/Rho kinase pathway in regulation of apoptosis in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G880–G886. doi: 10.1152/ajpgi.00039.2003. [DOI] [PubMed] [Google Scholar]
- 56.Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, Stewart CL, Olson MF. Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol. 2005;168:245–255. doi: 10.1083/jcb.200409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orlando KA, Stone NL, Pittman RN. Rho kinase regulates fragmentation and phagocytosis of apoptotic cells. Exp Cell Res. 2006;312:5–15. doi: 10.1016/j.yexcr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Lai JM, Wu S, Huang DY, Chang ZF. Cytosolic retention of phosphorylated extracellular signal-regulated kinase and a Rho-associated kinase-mediated signal impair expression of p21(Cip1/Waf1) in phorbol 12-myristate-13- acetate-induced apoptotic cells. Mol Cell Biol. 2002;22:7581–7592. doi: 10.1128/MCB.22.21.7581-7592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HH, Chien CL, Liao HK, Chen YJ, Chang ZF. Nuclear efflux of heterogeneous nuclear ribonucleoprotein C1/C2 in apoptotic cells: a novel nuclear export dependent on Rho-associated kinase activation. J Cell Sci. 2004;117:5579–5589. doi: 10.1242/jcs.01482. [DOI] [PubMed] [Google Scholar]
- 60.Liu HW, Halayko AJ, Fernandes DJ, Harmon GS, McCauley JA, Kocieniewski P, McConville J, Fu Y, Forsythe SM, Kogut P, Bellam S, Dowell M, Churchill J, Lesso H, Kassiri K, Mitchell RW, Hershenson MB, Camoretti-Mercado B, Solway J. The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am J Respir Cell Mol Biol. 2003;29:39–47. doi: 10.1165/rcmb.2002-0206OC. [DOI] [PubMed] [Google Scholar]
- 61.Haudenschild DR, Chen J, Pang N, Lotz MK, D'Lima DD. Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis Rheum. 2010;62:191–200. doi: 10.1002/art.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parent N, Sane AT, Droin N, Bertrand R. Procaspase-2S inhibits procaspase-3 processing and activation, preventing ROCK-1-mediated apoptotic blebbing and body formation in human B lymphoma Namalwa cells. Apoptosis. 2005;10:313–322. doi: 10.1007/s10495-005-0805-7. [DOI] [PubMed] [Google Scholar]
- 63.Orlando KA, Pittman RN. Rho kinase regulates phagocytosis, surface expression of GlcNAc, and Golgi fragmentation of apoptotic PC12 cells. Exp Cell Res. 2006;312:3298–3311. doi: 10.1016/j.yexcr.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 64.Hebert M, Potin S, Sebbagh M, Bertoglio J, Breard J, Hamelin J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J Immunol. 2008;181:5963–5973. doi: 10.4049/jimmunol.181.9.5963. [DOI] [PubMed] [Google Scholar]
- 65.Parlato S, Giammarioli AM, Logozzi M, Lozupone F, Matarrese P, Luciani F, Falchi M, Malorni W, Fais S. CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J. 2000;19:5123–5134. doi: 10.1093/emboj/19.19.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rebillard A, Jouan-Lanhouet S, Jouan E, Legembre P, Pizon M, Sergent O, Gilot D, Tekpli X, Lagadic-Gossmann D, Dimanche-Boitrel MT. Cisplatin-induced apoptosis involves a Fas-ROCK-ezrin-dependent actin remodelling in human colon cancer cells. Eur J Cancer. 2010;46:1445–1455. doi: 10.1016/j.ejca.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 67.Lai JM, Hsieh CL, Chang ZF. Caspase activation during phorbol ester-induced apoptosis requires ROCK-dependent myosin-mediated contraction. J Cell Sci. 2003;116:3491–3501. doi: 10.1242/jcs.00660. [DOI] [PubMed] [Google Scholar]
- 68.Connell LE, Helfman DM. Myosin light chain kinase plays a role in the regulation of epithelial cell survival. J Cell Sci. 2006;119:2269–2281. doi: 10.1242/jcs.02926. [DOI] [PubMed] [Google Scholar]
- 69.Soderstrom TS, Nyberg SD, Eriksson JE. CD95 capping is ROCK-dependent and dispensable for apoptosis. J Cell Sci. 2005;118:2211–2223. doi: 10.1242/jcs.02343. [DOI] [PubMed] [Google Scholar]
- 70.Hoogwater FJ, Nijkamp MW, Smakman N, Steller EJ, Emmink BL, Westendorp BF, Raats DA, Sprick MR, Schaefer U, Van Houdt WJ, De Bruijn MT, Schackmann RC, Derksen PW, Medema JP, Walczak H, Borel Rinkes IH, Kranenburg O. Oncogenic K-Ras turns death receptors into metastasis-promoting receptors in human and mouse colorectal cancer cells. Gastroenterology. 2010;138:2357–2367. doi: 10.1053/j.gastro.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 71.Papadopoulou N, Charalampopoulos I, Anagnostopoulou V, Konstantinidis G, Foller M, Gravanis A, Alevizopoulos K, Lang F, Stournaras C. Membrane androgen receptor activation triggers down-regulation of PI-3K/Akt/NF-kappaB activity and induces apoptotic responses via Bad, FasL and caspase-3 in DU145 prostate cancer cells. Mol Cancer. 2008;7:88. doi: 10.1186/1476-4598-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papadopoulou N, Charalampopoulos I, Alevizopoulos K, Gravanis A, Stournaras C. Rho/ROCK/actin signaling regulates membrane androgen receptor induced apoptosis in prostate cancer cells. Exp Cell Res. 2008;314:3162–3174. doi: 10.1016/j.yexcr.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 73.Street CA, Routhier AA, Spencer C, Perkins AL, Masterjohn K, Hackathorn A, Montalvo J, Dennstedt EA, Bryan BA. Pharmacological inhibition of Rho-kinase (ROCK) signaling enhances cisplatin resistance in neuroblastoma cells. Int J Oncol. 2010;37:1297–1305. doi: 10.3892/ijo_00000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin Q, Baudry M, Liao G, Noniyev A, Galeano J, Bi X. A novel function for p53: regulation of growth cone motility through interaction with Rho kinase. J Neurosci. 2009;29:5183–5192. doi: 10.1523/JNEUROSCI.0420-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qin Q, Liao G, Baudry M, Bi X. Cholesterol perturbation in mice results in p53 degradation and axonal pathology through p38 MAPK and Mdm2 activation. PLoS One. 2010;5:e9999. doi: 10.1371/journal.pone.0009999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park JW, Park CH, Kim IJ, Bae EH, Ma SK, Lee JU, Kim SW. Rho kinase inhibition by fasudil attenuates cyclosporine-induced kidney injury. J Pharmacol Exp Ther. 2011;338:271–279. doi: 10.1124/jpet.111.179457. [DOI] [PubMed] [Google Scholar]
- 77.Del Re DP, Miyamoto S, Brown JH. Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. J Biol Chem. 2008;283:35622–35629. doi: 10.1074/jbc.M804036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, Ong SE, Gammeltoft S, Carr SA, Yaffe MB. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J. 2007;26:2262–2273. doi: 10.1038/sj.emboj.7601683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, Wang J, Jiao H, Liao J, Xu X. Cytokinesis and cancer: Polo loves ROCK'n' Rho(A) J Genet Genomics. 2010;37:159–172. doi: 10.1016/S1673-8527(09)60034-5. [DOI] [PubMed] [Google Scholar]
- 80.Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, Fukuzawa M, Nakagawara A. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J Biol Chem. 2004;279:25549–25561. doi: 10.1074/jbc.M314182200. [DOI] [PubMed] [Google Scholar]
- 81.Yang X, Li H, Zhou Z, Wang WH, Deng A, Andrisani O, Liu X. Plk1-mediated phosphorylation of Topors regulates p53 stability. J Biol Chem. 2009;284:18588–18592. doi: 10.1074/jbc.C109.001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T. p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol. 2000;2:709–717. doi: 10.1038/35036335. [DOI] [PubMed] [Google Scholar]
- 83.Trostel SY, Sackett DL, Fojo T. Oligomerization of p53 precedes its association with dynein and nuclear accumulation. Cell Cycle. 2006;5:2253–2259. doi: 10.4161/cc.5.19.3291. [DOI] [PubMed] [Google Scholar]
- 84.Giannakakou P, Nakano M, Nicolaou KC, O'Brate A, Yu J, Blagosklonny MV, Greber UF, Fojo T. Enhanced microtubule-dependent trafficking and p53 nuclear accumulation by suppression of microtubule dynamics. Proc Natl Acad Sci U S A. 2002;99:10855–10860. doi: 10.1073/pnas.132275599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gorovoy M, Niu J, Bernard O, Profirovic J, Minshall R, Neamu R, Voyno-Yasenetskaya T. LIM kinase 1 coordinates microtubule stability and actin polymerization in human endothelial cells. J Biol Chem. 2005;280:26533–26542. doi: 10.1074/jbc.M502921200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frampton AR, Jr, Uchida H, von Einem J, Goins WF, Grandi P, Cohen JB, Osterrieder N, Glorioso JC. Equine herpesvirus type 1 (EHV-1) utilizes microtubules, dynein, and ROCK1 to productively infect cells. Vet Microbiol. 2010;141:12–21. doi: 10.1016/j.vetmic.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dehmelt L, Nalbant P, Steffen W, Halpain S. A microtubule-based, dynein-dependent force induces local cell protrusions: implications for neurite initiation. Brain Cell Biol. 2006;35:39–56. doi: 10.1007/s11068-006-9001-0. [DOI] [PubMed] [Google Scholar]
- 88.He H, Yim M, Liu KH, Cody SC, Shulkes A, Baldwin GS. Involvement of G proteins of the Rho family in the regulation of Bcl-2-like protein expression and caspase 3 activation by Gastrins. Cell Signal. 2008;20:83–93. doi: 10.1016/j.cellsig.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 89.Gauthaman K, Fong CY, Subramanian A, Biswas A, Bongso A. ROCK inhibitor Y-27632 increases thaw-survival rates and preserves stemness and differentiation potential of human Wharton's jelly stem cells after cryopreservation. Stem Cell Rev. 2010;6:665–676. doi: 10.1007/s12015-010-9184-8. [DOI] [PubMed] [Google Scholar]
- 90.Gauthaman K, Fong CY, Bongso A. Effect of ROCK inhibitor Y-27632 on normal and variant human embryonic stem cells (hESCs) in vitro: its benefits in hESC expansion. Stem Cell Rev. 2010;6:86–95. doi: 10.1007/s12015-009-9107-8. [DOI] [PubMed] [Google Scholar]
- 91.Wang YX, Martin-McNulty B, da Cunha V, Vincelette J, Lu X, Feng Q, Halks-Miller M, Mahmoudi M, Schroeder M, Subramanyam B, Tseng JL, Deng GD, Schirm S, Johns A, Kauser K, Dole WP, Light DR. Fasudil, a Rho-kinase inhibitor, attenuates angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice by inhibiting apoptosis and proteolysis. Circulation. 2005;111:2219–2226. doi: 10.1161/01.CIR.0000163544.17221.BE. [DOI] [PubMed] [Google Scholar]
- 92.Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde WA, Chambers TC. Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J Biol Chem. 2000;275:29980–29985. doi: 10.1074/jbc.M003776200. [DOI] [PubMed] [Google Scholar]
- 93.Ongusaha PP, Qi HH, Raj L, Kim YB, Aaronson SA, Davis RJ, Shi Y, Liao JK, Lee SW. Identification of ROCK1 as an upstream activator of the JIP-3 to JNK signaling axis in response to UVB damage. Sci Signal. 2008;1:ra14. doi: 10.1126/scisignal.1161938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsai NP, Wei LN. RhoA/ROCK1 signaling regulates stress granule formation and apoptosis. Cell Signal. 2010;22:668–675. doi: 10.1016/j.cellsig.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shibata R, Kai H, Seki Y, Kato S, Morimatsu M, Kaibuchi K, Imaizumi T. Role of Rho-associated kinase in neointima formation after vascular injury. Circulation. 2001;103:284–289. doi: 10.1161/01.cir.103.2.284. [DOI] [PubMed] [Google Scholar]
- 96.Matsumoto Y, Uwatoku T, Oi K, Abe K, Hattori T, Morishige K, Eto Y, Fukumoto Y, Nakamura K, Shibata Y, Matsuda T, Takeshita A, Shimokawa H. Long-term inhibition of Rho-kinase suppresses neointimal formation after stent implantation in porcine coronary arteries: involvement of multiple mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:181–186. doi: 10.1161/01.ATV.0000105053.46994.5B. [DOI] [PubMed] [Google Scholar]
- 97.Furuyama T, Komori K, Shimokawa H, Matsumoto Y, Uwatoku T, Hirano K, Maehara Y. Long-term inhibition of Rho kinase suppresses intimal thickening in autologous vein grafts in rabbits. J Vasc Surg. 2006;43:1249–1256. doi: 10.1016/j.jvs.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 98.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 99.Xu EZ, Kantores C, Ivanovska J, Engelberts D, Kavanagh BP, McNamara PJ, Jankov RP. Rescue treatment with a Rho-kinase inhibitor normalizes right ventricular function and reverses remodeling in juvenile rats with chronic pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;299:H1854–H1864. doi: 10.1152/ajpheart.00595.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ziino AJ, Ivanovska J, Belcastro R, Kantores C, Xu EZ, Lau M, McNamara PJ, Tanswell AK, Jankov RP. Effects of rho-kinase inhibition on pulmonary hypertension, lung growth, and structure in neonatal rats chronically exposed to hypoxia. Pediatr Res. 2010;67:177–182. doi: 10.1203/PDR.0b013e3181c6e5a7. [DOI] [PubMed] [Google Scholar]
- 101.Koshikawa S, Nishikimi T, Inaba C, Akimoto K, Matsuoka H. Fasudil, a Rho-kinase inhibitor, reverses L-NAME exacerbated severe nephrosclerosis in spontaneously hypertensive rats. J Hypertens. 2008;26:1837–1848. doi: 10.1097/HJH.0b013e328305086c. [DOI] [PubMed] [Google Scholar]
- 102.Nishikimi T, Akimoto K, Wang X, Mori Y, Tadokoro K, Ishikawa Y, Shimokawa H, Ono H, Matsuoka H. Fasudil, a Rho-kinase inhibitor, attenuates glomerulosclerosis in Dahl salt-sensitive rats. J Hypertens. 2004;22:1787–1796. doi: 10.1097/00004872-200409000-00024. [DOI] [PubMed] [Google Scholar]
- 103.Ishikawa Y, Nishikimi T, Akimoto K, Ishimura K, Ono H, Matsuoka H. Long-term administration of rho-kinase inhibitor ameliorates renal damage in malignant hypertensive rats. Hypertension. 2006;47:1075–1083. doi: 10.1161/01.HYP.0000221605.94532.71. [DOI] [PubMed] [Google Scholar]
- 104.Nishikimi T, Koshikawa S, Ishikawa Y, Akimoto K, Inaba C, Ishimura K, Ono H, Matsuoka H. Inhibition of Rho-kinase attenuates nephrosclerosis and improves survival in salt-loaded spontaneously hypertensive stroke-prone rats. J Hypertens. 2007;25:1053–1063. doi: 10.1097/HJH.0b013e3280825440. [DOI] [PubMed] [Google Scholar]
- 105.Kanda T, Wakino S, Hayashi K, Homma K, Ozawa Y, Saruta T. Effect of fasudil on Rho-kinase and nephropathy in subtotally nephrectomized spontaneously hypertensive rats. Kidney Int. 2003;64:2009–2019. doi: 10.1046/j.1523-1755.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 106.Gojo A, Utsunomiya K, Taniguchi K, Yokota T, Ishizawa S, Kanazawa Y, Kurata H, Tajima N. The Rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2007;568:242–247. doi: 10.1016/j.ejphar.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 107.Park K, Kim SW, Rhu KS, Paick JS. Chronic administration of an oral Rho kinase inhibitor prevents the development of vasculogenic erectile dysfunction in a rat model. J Sex Med. 2006;3:996–1003. doi: 10.1111/j.1743-6109.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 108.Wingard CJ, Moukdar F, Prasad RY, Cathey BL, Wilkinson L. Reversal of voltage-dependent erectile responses in the Zucker obese-diabetic rat by rosuvastatin-altered RhoA/Rho-kinase signaling. J Sex Med. 2009;6 Suppl 3:269–278. doi: 10.1111/j.1743-6109.2008.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wingard CJ, Johnson JA, Holmes A, Prikosh A. Improved erectile function after Rho-kinase inhibition in a rat castrate model of erectile dysfunction. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1572–R1579. doi: 10.1152/ajpregu.00041.2003. [DOI] [PubMed] [Google Scholar]
- 110.Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, Zandi S, Almulki L, Tayyari F, Shimokawa H, Hafezi-Moghadam A, Ishibashi T. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes. 2009;58:215–226. doi: 10.2337/db08-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 113.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 114.Man JH, Liang B, Gu YX, Zhou T, Li AL, Li T, Jin BF, Bai B, Zhang HY, Zhang WN, Li WH, Gong WL, Li HY, Zhang XM. Gankyrin plays an essential role in Ras-induced tumorigenesis through regulation of the RhoA/ROCK pathway in mammalian cells. J Clin Invest. 2010;120:2829–2841. doi: 10.1172/JCI42542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 116.Vemula S, Shi J, Hanneman P, Wei L, Kapur R. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood. 2010;115:1785–1796. doi: 10.1182/blood-2009-08-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hisamoto K, Ohmichi M, Kanda Y, Adachi K, Nishio Y, Hayakawa J, Mabuchi S, Takahashi K, Tasaka K, Miyamoto Y, Taniguchi N, Murata Y. Induction of endothelial nitric-oxide synthase phosphorylation by the raloxifene analog LY117018 is differentially mediated by Akt and extracellular signal-regulated protein kinase in vascular endothelial cells. J Biol Chem. 2001;276:47642–47649. doi: 10.1074/jbc.M103853200. [DOI] [PubMed] [Google Scholar]
- 118.Hamid SA, Bower HS, Baxter GF. Rho kinase activation plays a major role as a mediator of irreversible injury in reperfused myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H2598–H2606. doi: 10.1152/ajpheart.01393.2006. [DOI] [PubMed] [Google Scholar]
- 119.Song P, Zhang M, Wang S, Xu J, Choi HC, Zou MH. Thromboxane A2 receptor activates a Rho-associated kinase/LKB1/PTEN pathway to attenuate endothelium insulin signaling. J Biol Chem. 2009;284:17120–17128. doi: 10.1074/jbc.M109.012583. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Radisavljevic Z. Nitric oxide suppression triggers apoptosis through the FKHRL1 (FOXO3A)/ROCK kinase pathway in human breast carcinoma cells. Cancer. 2003;97:1358–1363. doi: 10.1002/cncr.10081. [DOI] [PubMed] [Google Scholar]
- 121.Wang YZ, Feng ZQ. Induction of apoptosis by L-NMMA, via FKHRL1/ROCK pathway in human gastric cancer cells. Biomed Environ Sci. 2006;19:285–291. [PubMed] [Google Scholar]
- 122.Chandra S, Romero M, Shatanawi A, Alkilany A, Caldwell R. Oxidative species increase arginase activity in endothelial cells through RhoA/Rho Kinase pathway. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01584.x. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kobune M, Chiba H, Kato J, Kato K, Nakamura K, Kawano Y, Takada K, Takimoto R, Takayama T, Hamada H, Niitsu Y. Wnt3/RhoA/ROCK signaling pathway is involved in adhesion-mediated drug resistance of multiple myeloma in an autocrine mechanism. Mol Cancer Ther. 2007;6:1774–1784. doi: 10.1158/1535-7163.MCT-06-0684. [DOI] [PubMed] [Google Scholar]
- 124.Igishi T, Mikami M, Murakami K, Matsumoto S, Shigeoka Y, Nakanishi H, Yasuda K, Gutkind JS, Hitsuda Y, Shimizu E. Enhancement of cisplatin-induced cytotoxicity by ROCK inhibitor through suppression of focal adhesion kinase-independent mechanism in lung carcinoma cells. Int J Oncol. 2003;23:1079–1085. [PubMed] [Google Scholar]
- 125.Chen J, Guerriero E, Lathrop K, SundarRaj N. Rho/ROCK signaling in regulation of corneal epithelial cell cycle progression. Invest Ophthalmol Vis Sci. 2008;49:175–183. doi: 10.1167/iovs.07-0488. [DOI] [PubMed] [Google Scholar]
- 126.Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- 127.Zhao Z, Rivkees SA. Rho-associated kinases play an essential role in cardiac morphogenesis and cardiomyocyte proliferation. Dev Dyn. 2003;226:24–32. doi: 10.1002/dvdy.10212. [DOI] [PubMed] [Google Scholar]
- 128.Croft DR, Olson MF. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol Cell Biol. 2006;26:4612–4627. doi: 10.1128/MCB.02061-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng Y, Liu M, Chen J, Liu S, Qiu M, Liao Z, Li Z, Luo D, Shi F, Zheng Y, Bi F. RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol Cancer Res. 2009;7:570–580. doi: 10.1158/1541-7786.MCR-08-0248. [DOI] [PubMed] [Google Scholar]
- 130.Han S, Sidell N, Roman J. Fibronectin stimulates human lung carcinoma cell proliferation by suppressing p21 gene expression via signals involving Erk and Rho kinase. Cancer Lett. 2005;219:71–81. doi: 10.1016/j.canlet.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 131.Ma J, Liang S, Wang Z, Zhang L, Jiang J, Zheng J, Yu L, Zheng X, Wang R, Zhu D. ROCK pathway participates in the processes that 15-hydroxyeicosatetraenoic acid (15-HETE) mediated in the pulmonary vascular remodeling induced by hypoxia in rat. J Cell Physiol. 2010;222:82–94. doi: 10.1002/jcp.21923. [DOI] [PubMed] [Google Scholar]
- 132.Ma J, Zhang L, Li S, Liu S, Ma C, Li W, Falck JR, Manthati VL, Reddy DS, Medhora M, Jacobs ER, Zhu XD. 8,9-Epoxyeicosatrienoic acid analog protects pulmonary artery smooth muscle cells from apoptosis via ROCK pathway. Exp Cell Res. 2010;316:2340–2353. doi: 10.1016/j.yexcr.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen XY, Dun JN, Miao QF, Zhang YJ. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses 5-hydroxytryptamine-induced pulmonary artery smooth muscle cell proliferation via JNK and ERK1/2 pathway. Pharmacology. 2009;83:67–79. doi: 10.1159/000178814. [DOI] [PubMed] [Google Scholar]
- 134.Koyanagi M, Takahashi J, Arakawa Y, Doi D, Fukuda H, Hayashi H, Narumiya S, Hashimoto N. Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res. 2008;86:270–280. doi: 10.1002/jnr.21502. [DOI] [PubMed] [Google Scholar]
- 135.Xiao L, Eto M, Kazanietz MG. ROCK mediates phorbol ester-induced apoptosis in prostate cancer cells via p21Cip1 up-regulation and JNK. J Biol Chem. 2009;284:29365–29375. doi: 10.1074/jbc.M109.007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- 137.Danen EH, Sonneveld P, Sonnenberg A, Yamada KM. Dual stimulation of Ras/mitogen-activated protein kinase and RhoA by cell adhesion to fibronectin supports growth factor-stimulated cell cycle progression. J Cell Biol. 2000;151:1413–1422. doi: 10.1083/jcb.151.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Benoit YD, Lussier C, Ducharme PA, Sivret S, Schnapp LM, Basora N, Beaulieu JF. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol Cell. 2009;101:695–708. doi: 10.1042/BC20090060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 140.Huang ZY, Wu Y, Hedrick N, Gutmann DH. T-cadherin-mediated cell growth regulation involves G2 phase arrest and requires p21(CIP1/WAF1) expression. Mol Cell Biol. 2003;23:566–578. doi: 10.1128/MCB.23.2.566-578.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levenberg S, Yarden A, Kam Z, Geiger B. p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene. 1999;18:869–876. doi: 10.1038/sj.onc.1202396. [DOI] [PubMed] [Google Scholar]
- 142.Mueller S, Cadenas E, Schonthal AH. p21WAF1 regulates anchorage-independent growth of HCT116 colon carcinoma cells via E-cadherin expression. Cancer Res. 2000;60:156–163. [PubMed] [Google Scholar]
- 143.Zhong Y, Lopez-Barcons L, Haigentz M, Jr, Ling YH, Perez-Soler R. Exogenous expression of H-cadherin in CHO cells regulates contact inhibition of cell growth by inducing p21 expression. Int J Oncol. 2004;24:1573–1579. [PubMed] [Google Scholar]
- 144.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 145.Huang S, Chen CS, Ingber DE. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang S, Ingber DE. A discrete cell cycle checkpoint in late G(1) that is cytoskeleton-dependent and MAP kinase (Erk)-independent. Exp Cell Res. 2002;275:255–264. doi: 10.1006/excr.2002.5504. [DOI] [PubMed] [Google Scholar]
- 147.Bhadriraju K, Yang M, Alom Ruiz S, Pirone D, Tan J, Chen CS. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp Cell Res. 2007;313:3616–3623. doi: 10.1016/j.yexcr.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kozai T, Eto M, Yang Z, Shimokawa H, Luscher TF. Statins prevent pulsatile stretch-induced proliferation of human saphenous vein smooth muscle cells via inhibition of Rho/Rho-kinase pathway. Cardiovasc Res. 2005;68:475–482. doi: 10.1016/j.cardiores.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 149.Liu WF, Nelson CM, Tan JL, Chen CS. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res. 2007;101:e44–e52. doi: 10.1161/CIRCRESAHA.107.158329. [DOI] [PubMed] [Google Scholar]