Abstract
T cell upregulation of B7 molecules CD80 and CD86 limits T cell expansion in immunodeficient hosts; however, the relative roles of CD80 separate from CD86 on CD4 versus CD8 T cells in a normal immune system are not clear. To address this question, we used the parent-into-F1 (P→F1) murine model of graft-versus-host disease and transferred optimal and suboptimal doses of CD80 and/or CD86 knockout (KO) T cells into normal F1 hosts. Enhanced elimination of host B cells by KO T cells was observed only at suboptimal donor cell doses and was greatest for CD80 KO→F1 mice. Wild-type donor cells exhibited peak CD80 upregulation at day 10; CD80 KO donor cells exhibited greater peak (day 10) donor T cell proliferation and CD8 T cell effector CTL numbers versus wild-type→F1 mice. Fas or programmed cell death-1 upregulation was normal as was homeostatic contraction of CD80 KO donor cells from days 12–14. Mixing studies demonstrated that maximal host cell elimination was seen when both CD4 and CD8 T cells were CD80 deficient. These results indicate an important role for CD80 upregulation on Ag-activated CD4 and CD8 T cells in limiting expansion of CD8 CTL effectors as part of a normal immune response. Our results support further studies of therapeutic targeting of CD80 in conditions characterized by suboptimal CD8 effector responses.
The CD28/B7 family of costimulatory molecules has a primary role in regulating initial T cell expansion (1, 2). Although CD28 and its homolog CTLA4 (CD152) are both expressed on T cells, they exhibit opposing actions in that CD28 promotes and CD152 inhibits T cell responses. A similar functional dichotomy is not well recognized for their ligands B7-1 (CD80) and B7-2 (CD86) expressed on APCs. Both B7 molecules exhibit low-affinity binding to CD28 and a much higher-affinity binding to CD152; however, due to different dissociation rates, CD80 exhibits >200-fold greater binding to CD152 than does CD86 (3, 4).
Despite these in vitro binding differences, it has been postulated that CD80 and CD86 are interchangeable in their in vivo costimulatory roles and differ primarily in their kinetics of expression and cellular distribution (5). Nevertheless, some studies have demonstrated that inhibition of CD80 function can enhance immune responses consistent with a loss of CD152-mediated contraction (6–11), supporting the idea that CD80 is the preferential in vivo ligand for CD152.
Complicating our understanding of the biological role of CD80/ CD86 are reports of CD80 upregulation on activated T cells both in vitro (12, 13) and in diseases characterized by persistent T cell activation (14–19). Although in vitro studies clearly demonstrate that T cells can acquire shed CD80 that can in turn costimulate other cells (20, 21), it has become increasingly accepted that activated T cells also express endogenous B7-costimulatory molecules (22– 24). Moreover, Taylor et al. (25) reported that CD80/CD86 knockout (KO) donor cells exhibit an enhanced ability to induce graft-versus-host disease (GVHD) in irradiated recipients, whereas GVHD severity using CD86 transgenic donor T cells was reduced compared with wild-type (WT) donor cells. Additionally, Paust et al. (26) demonstrated that transmission of a suppressive signal by CD4+CD25+ regulatory cells requires engagement of CD80/ CD86 molecules expressed on target T cells. Thus, in immunodeficient hosts, T cell upregulation of B7 molecules can limit T cell expansion by interaction with CTLA4 on Tregs. Functional differences between CD80 and CD86 in limiting T cell expansion were not addressed.
To determine the role of T cell-expressed B7 molecules in the setting of a normal immune system, we used the parent-into-F1 (P→F1) murine model of GVHD, in which homozygous parental strain T cells are injected i.v. into normal unirradiated F1 mice. This form of adoptive transfer is useful for studying in vivo alloantigen-driven T cell responses (27, 28). Donor T cell activation is initiated by recognition of host alloantigens and results in an antihost response that is either predominantly cell-mediated (acute GVHD) or Ab-mediated (chronic GVHD) (29, 30). Previous work in this model has shown that complete interruption of the CD28–B7 signaling prevents donor T cell activation and subsequent disease expression. For example, both acute and chronic GVHD can be prevented by combined blockade of CD80 and CD86 using either CTLA4-Ig or anti-CD80/anti-CD86 mAb treatment (11, 31, 32). Selective CD86 blockade, although less effective, also inhibits both forms of GVHD expression (11). Paradoxically, selective CD80 blockade promotes CD8+ donor T cell engraftment and converts chronic GVHD to acute GVHD in the DBA→B6D2F1 model by enhancing donor CD8+ T cell engraftment (11). A similar enhancement of donor CD8+ T cell engraftment in the P→F1 model is seen with CD152 blockade (33). Taken together, these results support the hypothesis that CD80 is the preferential ligand for CD152 and that CD80 blockade enhances T cell expansion by blocking CD152- mediated contraction.
To test the hypothesis that T cell-expressed CD80 and CD86 have important and possibly different roles in T cell regulation, we examined the ability of T cells deficient in CD80 and/or CD86 to induce acute GVHD in the P→F1 model. Our results indicate that T cell-expressed CD80, more so than CD86, plays an important role in limiting expansion of effector CD8 CTL.
Materials and Methods
Mice
Male mice aged 6–8 wk were purchased from The Jackson Laboratory (Bar Harbor, ME). WT C57BL/6 (B6) and the following KO mice, B6.129S4-Cd80tm1Shr/J (CD80KO), B6.129S4-CD86tm1Shr/J (CD86KO), and CD80tm1ShrCD86tm1Shr/J (CD80/86 KO), were used as a source of donor splenocytes, and B6D2F1 (BDF1) mice were used as recipients. All animal procedures were preapproved by the Institutional Animal Care and Use Committee at the Uniformed Services University of Health Sciences and at the University of Maryland School of Medicine.
Induction of GVHD
Single-cell suspensions of splenocytes were prepared in RPMI 1640 medium from the spleens of donor strain mice. Cell suspensions were filtered through sterile nylon mesh, washed, and diluted to the desired concentration of viable (trypan blue excluding) cells per milliliter. Cells were injected by tail vein at the indicated dosage. Negative controls consisted of age- and sex-matched uninjected F1 mice. In some experiments, the number of donor CD4 and CD8 T cells were first quantified by flow cytometry, and the donor inocula was adjusted so that the content of CD8 and CD4 T cells in the CD80 KO donor inocula was approximately equal to that of WT donor cells. In some experiments, acute GVHD was induced using purified donor T cells achieved through negative selection using a Dynal mouse T cell negative isolation kit (Invitrogen, Carlsbad, CA), which depletes B cells, NK cells, monocytes/macrophages, dendritic cells (DCs), granulocytes, and erythrocytes using mixture of rat mAbs for mouse CD45R, CD11b, Ter-119, and CD16/32. Further depletion of donor CD4+ or CD8+ T lymphocytes was carried out using Dynabeads (Dynal, Oslo, Norway) coated with antimouse CD4 or CD8, respectively. Purity was confirmed by flow cytometry and was typically >95%.
Flow cytometry
Spleen cells were incubated with anti-murine FcγRII/III mAb 2.4G2 for 10 min and stained with saturating concentrations of FITC-conjugated, biotin-conjugated, or PE-conjugated mAb against CD4, CD8, B220, H-2Kd, I-Ad, CD80, CD86, KI-67, CD107, CD11c, CD11b, killer cell lectin-like receptor G1 (KLRG-1), and CD127 (IL-7R) were purchased from BD Pharmingen (San Diego, CA), Invitrogen, Biolegend (San Diego, CA), or Caltag Laboratories (Burlingame, CA). Intracellular staining for perforin, granzyme B, IFN-γ, and TNF was performed using Abs and reagents purchased from BD Pharmingen or Biolegend and staining performed according to the manufacturer’s instructions. Importantly, for intracellular studies there was no in vitro restimulation or use of Golgi blocking agents. Following completion of the staining protocol, cells were analyzed by flow cytometry immediately. Multicolor flow cytometric analyses were performed using an LSRII flow cytometer (BD Biosciences, San Diego, CA). Lymphocyte and monocyte/ macrophage populations were identified by characteristic forward and side scatter using a broad gate and fluorescence data were collected for 10,000 cells. Studies of donor T cells were performed on 5000 gated cells that were either CD4+ or CD8+ and did not stain positively for the MHC class I of the uninjected parent. Host DCs and macrophages were identified as I-Ad positive and CD11c or CD11b positive, respectively.
BrdU incorporation
Donor T cell proliferation was assessed as BrdU uptake on days 10 and 12 after donor cell transfer as previously described (11). Mice received two doses of 2 mg BrdU i.p. separated by a 2-h interval. Two hours after the second dose, mice were sacrificed and splenocytes stained with anti-CD4 PE and anti-H2Kd CyChrome (BD Pharmingen). BrdU-positive cells were identified using the BrdU Flow Kit according to the manufacturer’s instructions (BD Pharmingen). Proliferating donor and host T cells were defined as staining positively for BrdU and CD4 or CD8 and either negatively or positively respectively for H-2Kd.
Statistical analysis
Statistical analysis was performed using Prism 4.0 (GraphPad, San Diego, CA). For all assays shown, mice are tested individually and group mean and SE calculated. Student t test was used to compare mean values between two groups.
Results
CD80-defective donor T cells exhibit enhanced elimination of host cells in P→F1 acute GVHD mice
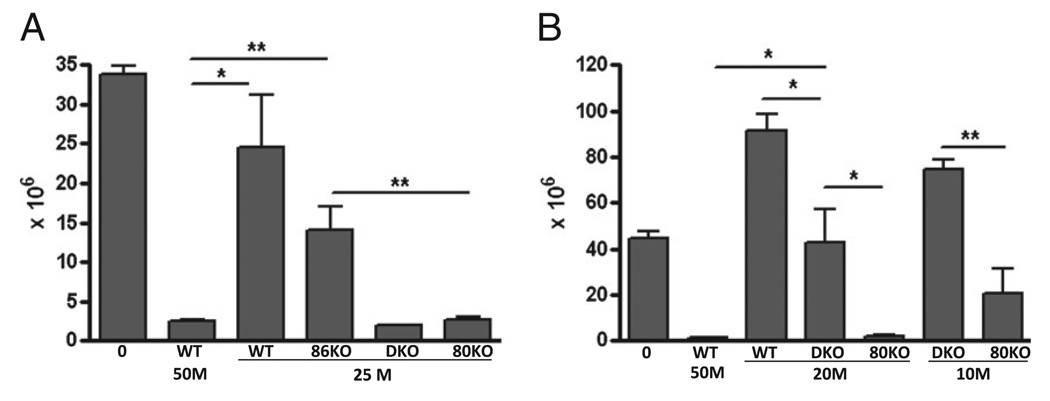
The transfer of homozygous, parental strain CD4+ and CD8+ T cells into normal unirradiated semiallogeneic F1 mice results in an acute GVHD phenotype characterized by maturation of donor CD8 effector CTL specific for host allogeneic MHC class I (maximal at approximately day 10) that eliminate host splenocytes, particularly B cells (maximal by day 14) (27). If CD80 and/or CD86 expression contributes to T cell downregulation, then the absence of these molecules on donor T cells could promote the donor antihost response such that fewer donor cells would be required to achieve host B cell elimination comparable to that of WT donor cells. Typically, 50 × 106 unfractionated WT B6 splenocytes induce an acute GVHD phenotype characterized by near complete elimination (i.e., <10% of control values) of host B cells at day 14, whereas doses of ≤30 × 106 B6 splenocytes are suboptimal, resulting in significantly greater host B cell survival and large mouse-to-mouse variability in host B cell numbers, particularly for donor cell doses close to the threshold (34). To address the role of donor T cell CD80 and/or CD86 expression, we tested the ability of a suboptimal dose (i.e., 25 × 106) of WT donor cells or donor cells defective in CD80 and/or CD86 KO expression to induce acute GVHD. An optimal dose (50 × 106) of B6 WT donor cells served as the positive control. In Fig. 1A, acute GVHD phenotype is shown as the number of surviving host B cells at day 14 posttransfer and is based on previous work demonstrating that host B cell elimination at day 14 is a surrogate marker for the strength of in vivo donor antihost CTL activity and is more sensitive than ex vivo [51Cr] release assessment (35, 36).As shown in Fig. 1A, an optimal dose of B6 WT donor cells resulted in near complete host B cell elimination (7% of control F1 values), where as a suboptimal dose of B6 WT donor T cells resulted in significantly impaired (73% of control F1) elimination of host B cells (i.e., greater surviving host B cell numbers) compared with that seen with an optimal dose of B6 WT donor cells (p < 0.05). Similarly, a suboptimal dose of CD86 KO donor T cells resulted in impaired elimination of host B cells (42% of control F1) that did not differ significantly from a suboptimal dose of WT donor cells and was significantly less than that seen for optimal dose WT donor cells. By contrast, a suboptimal dose of CD80 KO donor cells (either single CD80 KO or CD80/CD86 double KO [DKO]) resulted in near complete (~ 8% of control F1) host B cell elimination that was significantly different from that seen with a suboptimal dose of either WT or CD86 KO donor cells and was not significantly different from that seen for an optimal dose of WT donor cells. These results demonstrate potentiation of acute GVHD using either CD80 KO or DKO donor T cells versus WT and possibly versus CD86 KO donor T cells.
FIGURE 1.
Potentiation of acute GVHD is greatest using CD80 KO donor cells. GVHD was induced as described in Materials and Methods using unfractionated donor splenocytes. A, Normal BDF1 mice received no donor cells (0), 50 × 106 (50M) B6 WT splenocytes, or 25 × 106 (25M) splenocytes from WT, CD86 KO (86KO), CD80/CD86 DKO, or CD80 KO (80KO) donors. B, Normal BDF1 mice received no donor cells (0), 50 × 106 WT splenocytes (50M), 20 × 106 (20M) splenocytes from WT, CD80/CD86 DKO, or CD80 KO (80KO) donors, or 10 × 106 (10M) splenocytes from either CD80/CD86 DKO or CD80 KO (80KO) donors. For A and B, values represent host B cells at day 14 posttransfer (group mean ± SEM × 106; n = 5 mice/group). Host B cells were identified by flow cytometry as described in Materials and Methods. *p < 0.05; **p < 0.01.
To address whether CD80 KO donor cells differ from CD80/ CD86 DKO donor cells in their ability to promote acute GVHD-mediated host B cell elimination, two additional suboptimal doses of donor T cells were tested (20 × 106 and 10 × 106 donor cells). As shown in Fig. 1B, a dose of 20 × 106 WT donor cells resulted in host B cell expansion (207% of control F1) rather than elimination, consistent with complete failure of CD8 CTL effector cells to eliminate donor CD4-driven host B cell expansion and representing a conversion to a chronic GVHD phenotype (27, 28). Similarly, F1 mice receiving 20 × 106 of CD80/CD86 DKO donor cells exhibit no significant reduction in surviving host B cells compared with uninjected control F1 mice, although they are significantly reduced compared with mice receiving the same dose of WT donor cells. By contrast, near complete elimination of host B cells (5% of control F1) is observed in F1 mice receiving a dose of 20 × 106 CD80 KO donor cells and values are significantly reduced compared with the same dose in WT→F1 or CD80/CD86 KO→F1 mice and do not differ significantly from optimal dose WT→F1 mice. At a dose of 10 × 106 donor cells, host B cell elimination is still significantly reduced in CD80KO→F1 mice compared with CD80/CD86 KO→F1 mice, the latter now exhibiting an increase in host B cells compared with uninjected F1 mice consistent with conversion to a chronic GVHD phenotype. These results indicate that although potentiation of host B cell elimination is seen with both CD80 KO and to a lesser extent with CD80/86 KO donor cells, this effect is strongest for CD80 single KO donor cells.
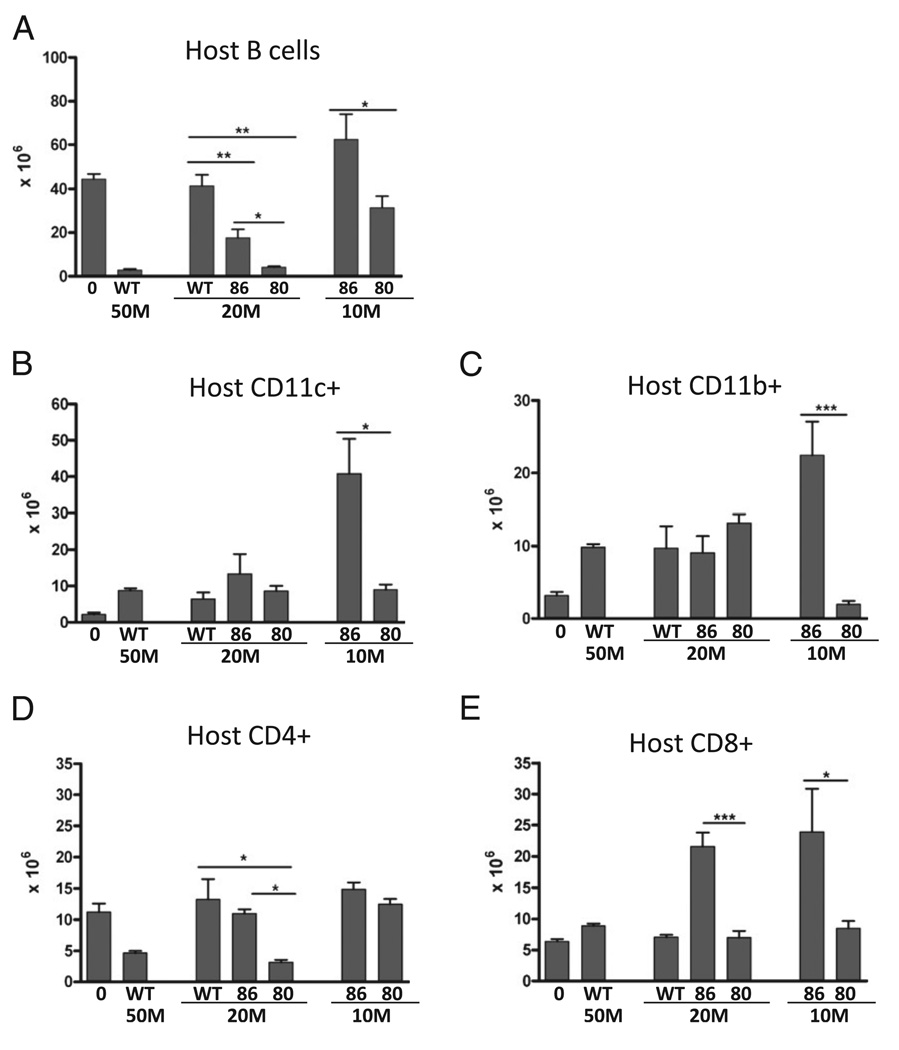
To further clarify the relative effects of donor T cell expression of CD80 versus CD86, dose-response studies were performed using unfractionated donor splenocytes normalized for CD8 T cell numbers prior to in vivo transfer. As shown in Fig. 2A, a subthreshold dose of 20 × 106 WT donor cells (containing ~2 × 106 CD8 T cells) results in impaired host B cell elimination compared with 50 × 106 WT donor cells (p < 0.01), and host B cells were not significantly different from normal uninjected F1 mice. In contrast, F1 mice receiving a subthreshold dose of 20 × 106 (or the equivalent) of either CD86 or CD80 KO donor cells both exhibited a significantly greater reduction in host B cells compared with the same dose in WT→F1 mice; however, host B cell elimination was significantly greater (i.e., fewer B cells remaining) for CD80 KO→F1 versus CD86 KO→F1 mice. Similarly, at the 10 × 106 dose containing 1.2–1.3 × 106 donor CD8 T cells, host B cell elimination was significantly greater for CD80 KO→F1 mice versus CD86 KO→F1 mice; however, at this dose, both groups exhibit impaired killing of host B cells.
FIGURE 2.
Significantly greater B cell elimination is seen using CD80 KO versus CD86 KO donor cells. GVHD was induced using unfractionated donor splenocytes containing roughly equal numbers of donor CD8 T cells. BDF1 recipient mice received either 50 × 106 unfractionated WT splenocytes or unfractionated splenocytes from WT, CD80 KO (80), or CD86 KO (86) donors that were normalized for donor CD8 T cell numbers pre-transfer as described in Materials and Methods. 20M designates F1 mice receiving 20 × 106 WT or CD80KO splenocytes (containing 1.8 × 106 CD8 T cells) or 28 × 106 CD86 KO (containing 2.5 × 106 CD8 T cells) splenocytes. 10M designate F1 mice receiving 107 CD80 KO or 14 × 106 (containing 1.2–1.3 3 106 CD8 T cells) unfractionated splenocytes. Recipient F1 spleens were examined at 14 d after donor cell transfer for the following host populations as described in Materials and Methods: B cells (A), CD11c+ DCs (B), Cd11b+ macrophages (C), CD4 T cells (D), and CD8 T cells (E). Values represent group mean ± SEM (n = 5 for each group). Over three additional experiments comparing CD80 KO to CD86KO and/or CD80/86 KO donor cells have been performed yielding similar results. *p < 0.05; **p < 0.01; ***p < 0.005.
In P→F1 acute GVHD, all host lymphocytes and APCs are targets for elimination by donor CD8 CTLs; however, there is a hierarchy of elimination, with host B cells being the most sensitive, followed by host CD4 T cells, and lastly, host APCs and CD8 T cells (27). Compared to CD86 KO→F1 mice, CD80 KO→F1 mice exhibit significantly greater elimination of: 1) both host DCs (Fig. 2B) and macrophages (Fig. 2C) at the 10 × 106 dose; 2) host CD4 at the 20 × 106 dose (Fig. 2D); and 3) host CD8 T cells at both the 10 and 20 × 106 dose (Fig. 2E). Together, these results and the results of Fig. 1 demonstrate that potentiation of acute GVHD induction is seen for CD86 KO, CD80 KO, and CD80/CD86 DKO donor cells; however, this effect is strongest for CD80 single KO donor cells and is seen as an ~60% reduction in the number of donor cells required to induce an acute GVHD phenotype (i.e., host B cell elimination) at 2 wk compared with B6 WT donor cells. These results are consistent with a major augmentation of CTL effector function using CD80 KO donor cells. Accordingly, the following studies focus on the mechanism involved in acute GVHD potentiation for CD80 KO→F1 mice.
Enhanced acute GVHD in suboptimal CD80 K→F1 mice is associated with greater donor T cell engraftment
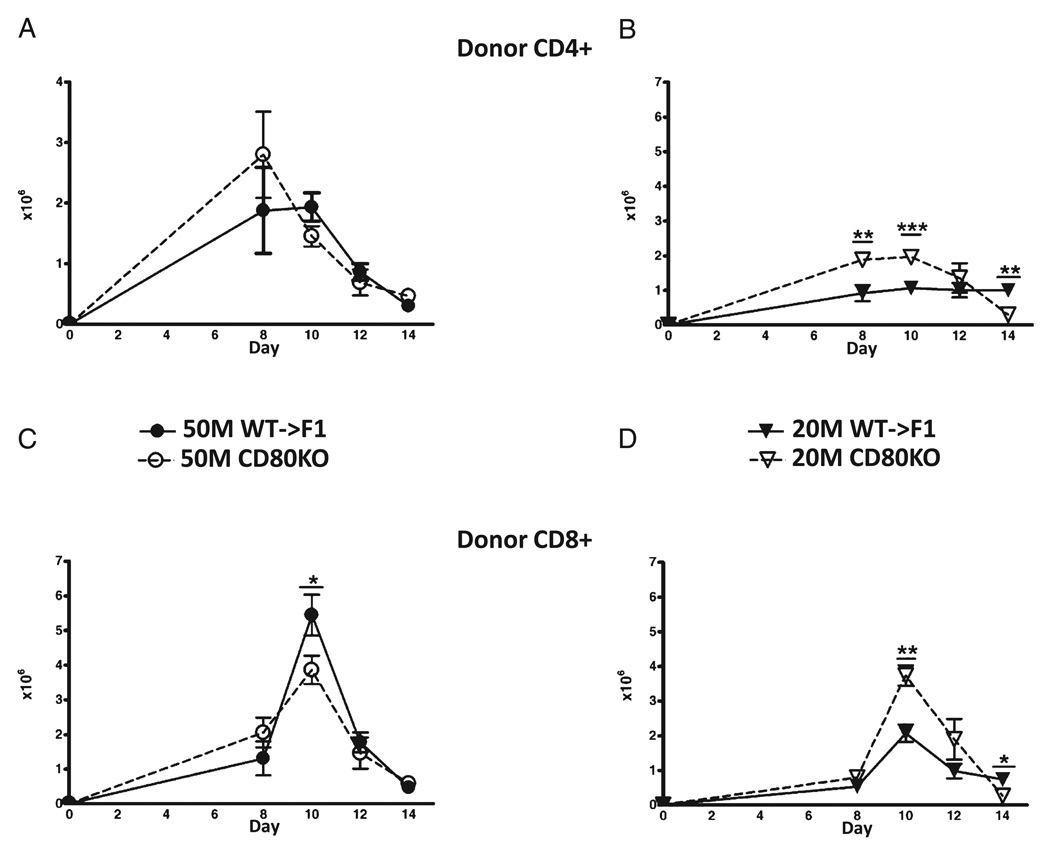
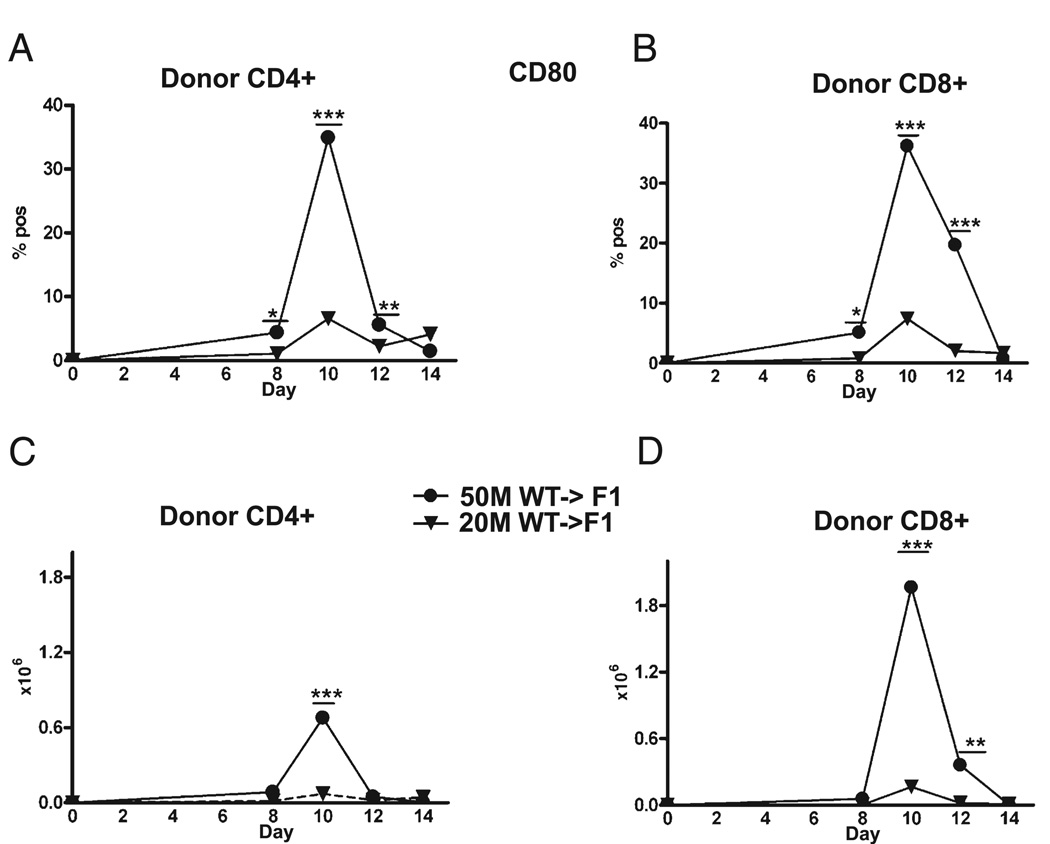
In B6→BDF1 mice, days 0–10 are characterized by an expansion of donor T cells and host splenocytes followed from days 10–14 by a contraction phase as mature donor CD8 CTL effectors eliminate host lymphocytes and then undergo contraction (27, 28). To determine the mechanism of enhanced day 14 elimination of host cells seen with suboptimal doses of CD80 KO→F1 mice, the kinetics of donor T cell engraftment were assessed in F1 mice receiving either an optimal (50 × 106) or suboptimal (20 × 106) dose of WTor CD80 KO unfractionated donor splenocytes. Donor inocula were normalized for T cell subsets as described in Materials and Methods to ensure that the potentiation of acute GVHD in CD80 KO→F1 mice was not due to the transfer of significantly greater numbers donor CD8 T cells. Donor CD4 and CD8 T cell engraftment was assessed in separate independent experiments at days 8, 10, 12, and 14 after donor cell transfer (Fig. 3). At the optimal dose, engraftment of CD4 T cells in CD80 KO→F1 mice is not significantly greater than that of WT at any time (Fig. 3A); however, at the suboptimal dose, CD80 KO→F1 mice exhibit significantly greater donor CD4 T cell engraftment at days 8 and 10 (Fig. 3B). Similarly, CD80 KO→F1 mice do not exhibit significantly greater engraftment of donor CD8 T cells at the optimal dose (Fig. 3C); however, at the suboptimal dose, CD80 KO→F1 mice exhibit significantly greater peak CD8 T cell numbers at day 10 versus WT (Fig. 3D). These results support the idea that potentiation of acute GVHD (i.e., enhanced day 14 host B cell elimination) at suboptimal dosing in CD80 KO→F1 mice is a consequence of greater numbers of effector CD8 CTL.
FIGURE 3.
CD80-defective donor T cells exhibit greater engraftment at suboptimal doses versus WT. BDF1 mice received unfractionated splenocytes from WT (closed symbols) or CD80 KO (open symbols) donors at one of two doses: an optimal dose (circles) of 50 × 106 splenocytes (A, C) or a suboptimal dose (triangles) of 20 × 106 splenocytes (B, D) and the number of donor CD4 T cells (A, B) and donor CD8 T cells (C, D) determined by flow cytometry at the indicated times. The CD8 T cell content of donor splenocytes was determined by flow cytometry pretransfer and the donor cell inocula adjusted such that the CD8 content of CD80 KO inocula was approximately equal to that of WT inocula. Each time point represents a separate independent experiment in which both optimal and suboptimal groups were tested simultaneously. At the 50 × 106 and 20 × 106 doses, the number of donor CD8 T cells transferred was ~3.5–5.0 × 106 and 1.4–2.0 × 106 donor CD8 T cells, respectively, depending on the time point tested. Values represent group mean ± SEM (n = 5 mice/group), and p values compare WT→F1 versus KO→F1 at a given time point. *p < 0.05; **p < 0.01; ***p < 0.005.
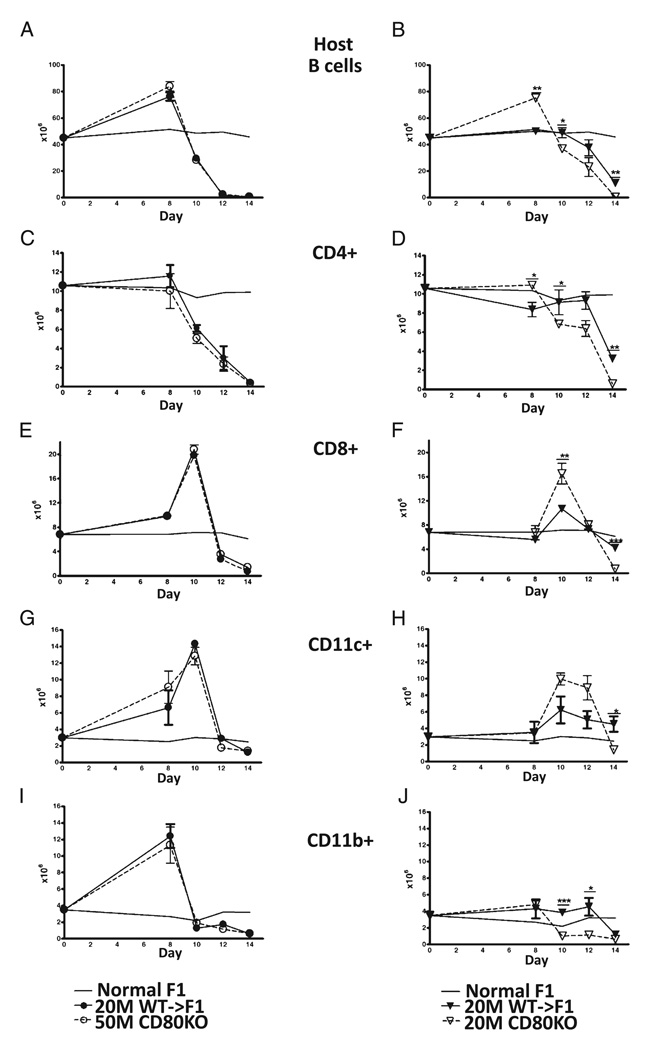
CD80 KO donor cells exhibit enhanced elimination of host cells
The kinetics of elimination of host splenocyte subpopulations were assessed by flow cytometry in the cohort shown in Fig. 3. As mentioned previously, host splenocyte subpopulations exhibit a differential sensitivity to donor CD8 CTL elimination that can be seen in Fig. 4 as differing expansion and contraction kinetics for each subpopulation. At an optimal donor cell dose, host B cells (Fig. 4A) exhibit expansion at day 8 followed by elimination (days 8–14) that did not differ significantly for CD80 KO→F1 versus WT→F1 mice. By contrast, at the suboptimal dose (Fig. 4B), CD80 KO donor cells exhibited significantly greater day 8 host B cell expansion consistent with greater CD80 KO CD4 T cell help (27), followed by a shifting of the B cell elimination curve to the left consistent with enhanced donor CD80 KO CD8 effector function resulting in accelerated host B cell elimination (i.e., significantly greater B cell elimination at days 10 and 14). By day 14, suboptimal CD80 KO→F1 exhibited near complete elimination of host B cells (Fig. 4B), and values were significantly less than those of day 14 suboptimal WT→F1 mice consistent with the results shown in Figs. 1 and 2A. However, host B cell values for day 14 suboptimal WT→F1 mice in Fig. 4B were lower than those seen for this same group in Figs. 1 and 2A and likely reflect the possible transfer of a slightly greater number of donor T cells when using unfractionated splenocytes as the inoculum. This issue is addressed using purified donor T cell subsets (see below).
FIGURE 4.
CD80 KO→F1 mice exhibit enhanced acute GVHD phenotype only at a suboptimal donor cell dose. Host splenocyte populations were determined by flow cytometry on the same F1 cohorts described in Fig. 3. Results are shown for: host B cells (A, B), host CD4+ cells (C, D), host CD8 T cells (E, F), host DCs (CD11c+) (G, H), and host macrophages (CD11b+) (I, J). Results for optimal dosing are shown in A, C, E, G, and I and suboptimal dosing in B, D, F, H, and J. Symbols are as described in Fig. 3. For comparison, the mean values of uninjected age-and sex-matched BDF1 mice (n = 5) tested simultaneously with the experimental groups are shown for each time point (solid line, no symbols), and SEMs have been omitted from this line. p values compare the same dose of WT→F1 versus KO→F1 at a given time point. *p < 0.05; **p < 0.01; ***p < 0.005.
Similar results were observed for other splenic host populations. At the optimal dose, CD80 KO→F1 mice did not differ significantly from WT→F1 mice in the kinetics of expansion and elimination of host CD4 T cells (Fig. 4C), host CD8 T cells (Fig. 4E), host CD11c+ cells (Fig. 4G), or host CD11b+ cells (Fig. 4I). However, at the suboptimal dose, CD80 KO→F1 mice exhibited significantly greater elimination of: host CD4 T cells at days 10 and 14 (Fig. 4D); host CD8 T cells at day 14 (Fig. 4F); host DCs at day 14 (Fig. 4H); and host macrophages at days 10 and 12 (Fig. 4J). We have previously demonstrated that a stronger donor CD8-mediated GVH response elicits a stronger, albeit temporary, reciprocal CD8 host-versus-graft response (27, 28). This effect can be seen at the suboptimal dose at which CD80 KO→F1 mice exhibit a stronger GVH response compared with WT→F1 mice seen as greater day 10 donor CD8 T cell expansion (Fig. 3D) that in turn elicits stronger GVH response seen as greater day 10 host CD8 T cell expansion in KO→F1 mice (Fig. 4F). Together, these results demonstrate that at the suboptimal dose only, elimination of all host splenocyte subpopulations is enhanced and in some cases accelerated in CD80 KO→F1 versus WT→F1 mice indicative of a stronger GVH response.
An additional independent kinetic analysis was performed at days 7, 10, 12, and 14 using equal numbers of optimal and suboptimal donor splenocytes that were not normalized by flow cytometry for donor CD8 T cells pretransfer. Despite this, similar results were seen (data not shown). Specifically, no significant differences were observed in donor T cell engraftment or host cell elimination at the optimal donor cell dose. At the suboptimal dose, there was a trend toward greater peak donor CD80 KO CD8 T cell engraftment; however, unlike the results in Fig. 3B and 3D, this did not reach statistical significance. Nevertheless, CD80KO→F1 mice exhibited significantly greater day 14 elimination of host B cells, macrophages, CD4 T, and CD8 T cells in this cohort. Thus, despite the lack of significantly greater peak donor CD8 CTL engraftment in CD80 KO→F1 mice in this kinetic series, there was significantly greater host B cell, T cell, and APC elimination consistent with significantly greater CD8 CTL effector function in suboptimal CD80 KO→F1 mice and consistent with the results shown in Figs. 3 and 4.
Taken together, our results indicate that the absence of CD80 expression on donor T cells potentiates the acute GVHD phenotype in the setting of suboptimal donor cell transfers and is likely a consequence of the greater expansion of donor CD8 T cells and possibly donor CD4 T cells.
CD8 T cell effector cell numbers are increased in CD80 KO→F1 mice
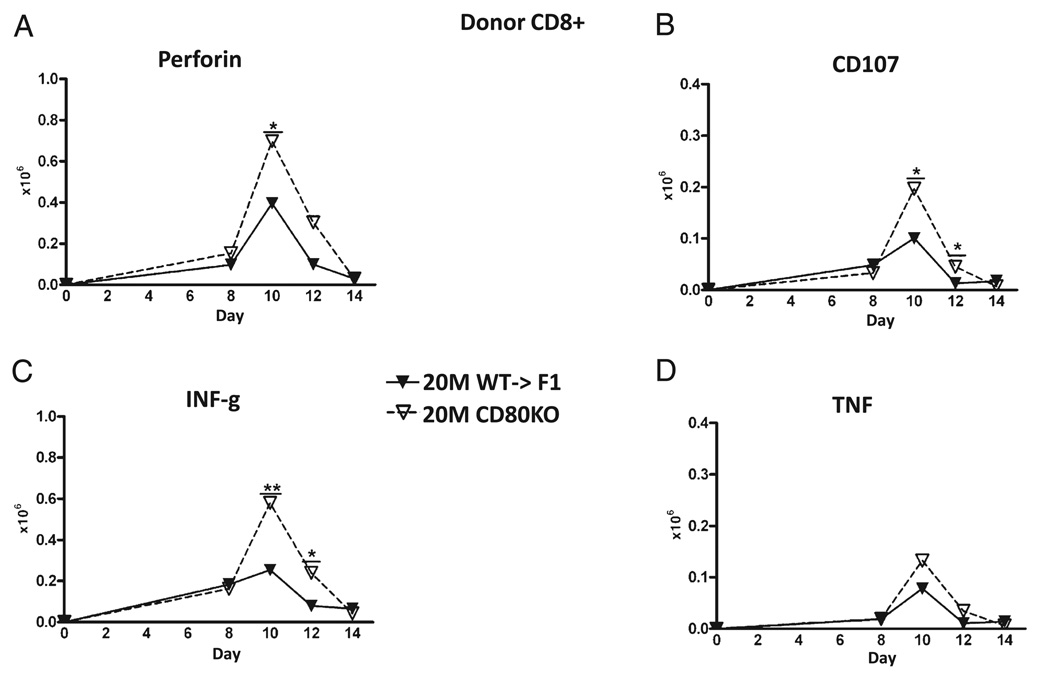
Ex vivo antihost CTL activity in B6→F1 mice has been shown to be maximal on or about day 10 (37), supporting the idea that the greater day 10 engraftment of donor CD8 T cells (Fig. 3D) and greater host elimination in suboptimal CD80 KO versus WT→F1 mice (Fig. 4) is due to greater numbers of donor CD8 effector CTLs. To confirm this, the kinetics of donor CD8 effector populations were assessed by standard and intracellular flow cytometry for typical effector function markers on the same cohort shown in Figs. 3 and 4. No significant increases in donor CD80 KO donor cells versus WT were observed at any time point at the optimal dose (data not shown). In contrast, suboptimal CD80 KO→F1 mice exhibited greater day 10 peak effector numbers versus WT→F1 mice as shown by significantly greater numbers of perforin-positive cells at day 10 (Fig. 5A), CD107a–positive cells (days 10 and 12) (Fig. 5B), and IFN-γ–secreting cells at days 10 and 12 (Fig. 5C). The increase in TNF-secreting CD80 KO donor CD8 T cells was not significant (Fig. 5D), and we were unable to detect a significant difference in granzyme B-positive donor CD8 T cells (data not shown).
FIGURE 5.
CD80 KO→F1 mice exhibit significantly greater peak numbers of effector CD8 T cells. The experimental protocol is as outlined for Fig. 3 and results shown for mice receiving 20 × 10 WT or CD80 KO splenocytes at the times indicated. Donor CD8 T cells were examined for expression of perforin (A), CD107a (B), IFN-γ (C), or TNF (D) as described in Materials and Methods. *p < 0.05; **p < 0.01.
As further confirmation, we assessed the kinetics of cytokine genes important in CD8 CTL responses in the same cohort. At the optimal donor cell dose, CD80 KO→F1 mice exhibited no significant increases versus WT→F1 mice for IFN-γ (Supplemental Fig. 1A), Mx-1 (Supplemental Fig. 1C), or oligoadenylate synthetase (OAS) (Supplemental Fig. 1E) with the exception of a small (<2-fold) increase at day 14 OAS expression. By contrast, at the suboptimal dose, CD80 KO→F1 mice exhibited significantly greater day 10 levels of IFN-γ, Mx-1, and OAS (Supplemental Figs. 1B, 1D, 1F, respectively). These results further support the conclusion that enhanced elimination of host cells in suboptimal CD80 KO→F1 mice is due to greater peak numbers of CD80 KO CD8 effector CTLs.
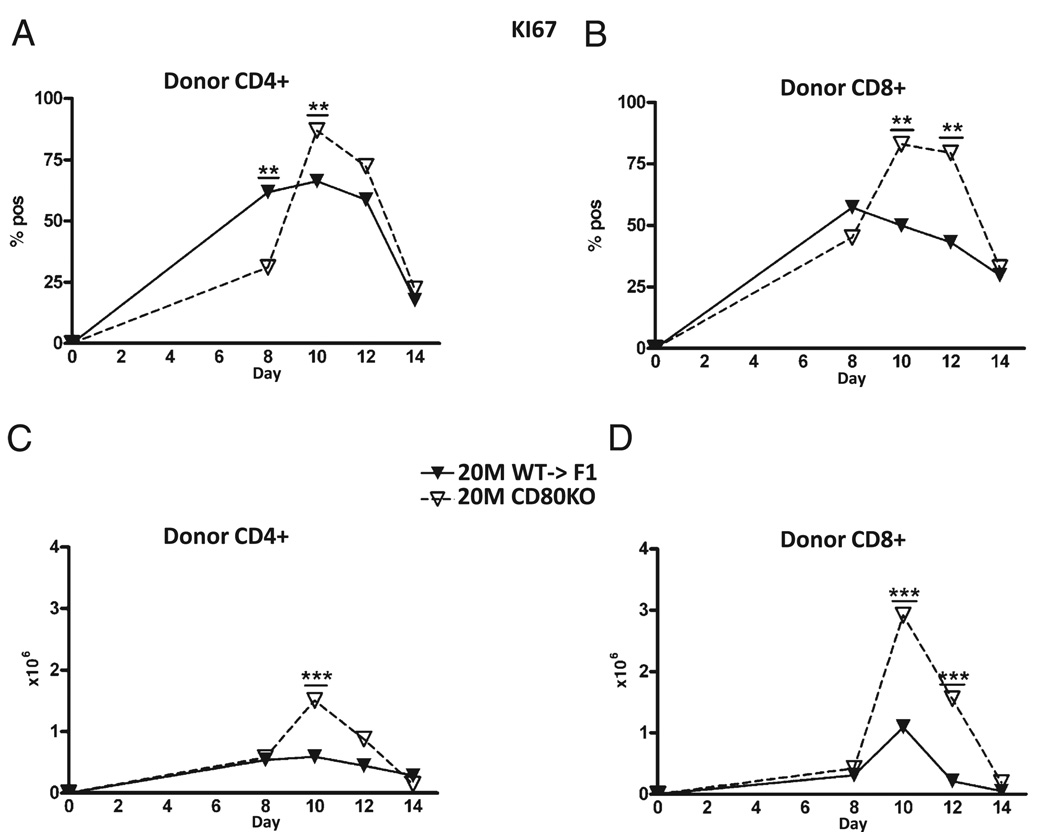
Peak proliferation is increased in donor CD80 KO donor T cells
To address the mechanism of greater numbers of peak donor effector cells for suboptimal CD80 KO→F1 mice, we examined the kinetics of KI-67 expression, a marker of cellular proliferation, on donor CD4 and CD8 T cells from the same injection cohort described in Fig. 3. At the suboptimal dose, CD80 KO→F1 mice exhibited greater peak percentages (Fig. 6A, 6B) and numbers (Fig. 6C, 6D) of KI-67–positive cells for both donor CD4 T cells at day 10 (Fig. 6A, 6C) and CD8 T cells at days 10 and 12 (Fig. 6B, 6D). There were no significant differences in donor T cell KI-67 expression at the optimal dose (data not shown).
FIGURE 6.
CD80 KO→F1 mice exhibit significantly greater numbers of proliferating donor CD4 and CD8 T cells at the suboptimal dose. The experimental protocol is as outlined for Fig. 3. F1 mice received 20 × 106 WT or CD80 KO splenocytes, and the percentage (A, B) or numbers (C, D) of KI-67 positive cells shown for donor CD4 (A, C) and CD8 (B, D) T cells are shown as group mean ± SE. **p < 0.01; ***p < 0.005.
Similar results were observed using in vivo BrdU incorporation in a separate study using 25 × 106 donor splenocytes that were not normalized for donor T cells pretransfer and examined at days 7, 10, 12, and 14. As described in Materials and Methods, two pulses of BrdU separated by 4 h were given on the day of assay to detect only recent, ongoing proliferation. At day 10, BrdU incorporation was significantly greater in both donor CD4 (Supplemental Fig. 2A) and CD8 (Supplemental Fig. 2B) T cells in suboptimal CD80 KO→F1 mice compared suboptimal WT→F1 mice. There was no significant difference for KO versus WT at the optimal dose for donor CD4 or CD8 T cell BrdU incorporation. Thus, by both parameters and in two different cohorts, the absence of CD80 on donor T cells is associated with significantly greater proliferation at day 10 versus WT donor T cells.
Effector CD8 T cells are increased in subthreshold CD80 KO→F1 mice
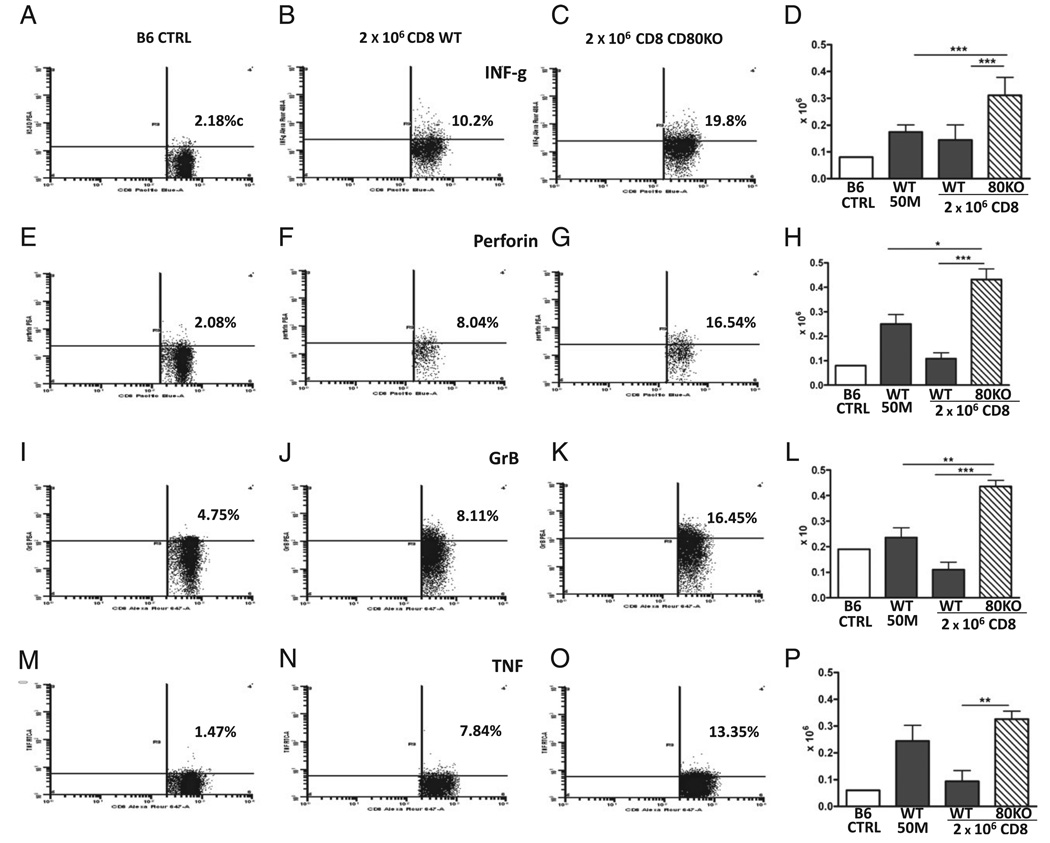
A separate independent experiment was performed to fully characterize the ability of donor T cell CD80 expression to limit peak (day 10) expansion of CD8 effector T cells. Based on the results from the cohorts shown in Figs. 2 and 3, in which enhanced CD8 effector function is seen for CD80 KO versus WT CD8 T cells at a subthreshold dose of ~2 × 106 donor CD8 T cells, we therefore transferred WT or CD80 KO splenocytes determined by flow cytometry to contain 2 × 106 CD8 T cells into WT F1 mice and examined recipient F1 spleens at day 10 for standard parameters of CD8 effector phenotype (Figs. 7, 8). Importantly, for Figs. 7 and 8, staining parameters reflect values directly ex vivo (as described in Materials and Methods) without a secondary in vitro restimulation phase. Controls consisted of optimal-dose (50 × 106) WT→F1 mice or uninjected normal B6 mice. For each parameter, the first three panels show representative splenocyte tracings and percentages, and the fourth panel shows the numbers of effector donor CD8 T cells. For all parameters measured in Figs. 7 and 8, both suboptimal WT→F1 and KO→F1 mice exhibited elevated percentages of donor CD8 effector markers compared with control B6 mice, indicating that activated effector donor CD8 T cells are detectable in both P→F1 combinations. Importantly, compared with suboptimal WT→F1 mice, suboptimal CD80 KO→F1 mice exhibited significantly greater numbers of donor CD8 T cell effectors as shown by greater numbers of IFN-γ–positive cells (Fig. 7D) and perforin-positive cells (Fig. 7H), confirming the results of Fig. 5. In this experiment, we also observed significantly greater numbers of donor CD8 T cells positive for granzyme B (Fig. 7L) and TNF (Fig. 7P). These latter two trends were present in the cohort shown in Fig. 5 but did not reach statistical significance. We did not detect a significant difference in donor CD8 T cell expression of Fas ligand at day 10 comparing suboptimal WT→F1 to KO→F1.
FIGURE 7.
Effector CD8 T cells are increased in suboptimal CD80 KO→F1 versus WT→F1 mice. BDF1 mice received unfractionated splenocytes from WT or CD80 KO donors examined by flow cytometry and adjusted such that each suboptimal inoculum contained 2 × 106 donor CD8 T cells. Controls consisted of optimal dose (50 × 106) WT→F1 mice. Mice were assessed at day 10 by intracellular flow cytometry of splenocytes for donor CD8 T cells expressing the following effector markers: IFN-γ (A–D), perforin (E–H), granzyme B (I–L), and TNF (M–P). As noted in the Materials and Methods, splenocytes were stained directly ex vivo without an in vitro restimulation phase or Golgi blockers. For each parameter, the first three panels show representative splenocyte tracings from: 1) control uninjected WT B6 CD8 T cells; 2) engrafted B6; or 3) engrafted CD80 KO donor CD8 T cells in F1 mice receiving suboptimal inoculum. The rightmost panel shows the numbers of positive CD8 T cells in B6 WT control splenocytes followed by the number of engrafted donor CD8 T cells the experimental groups: optimal WT→F1; suboptimal WT→F1; and suboptimal CD80 KO→F1 mice (values shown for experimental groups as mean ± SE [n = 5]). Similar results were observed in an additional day 10 experiment. *p < 0.05; **p < 0.01; ***p < 0.005.
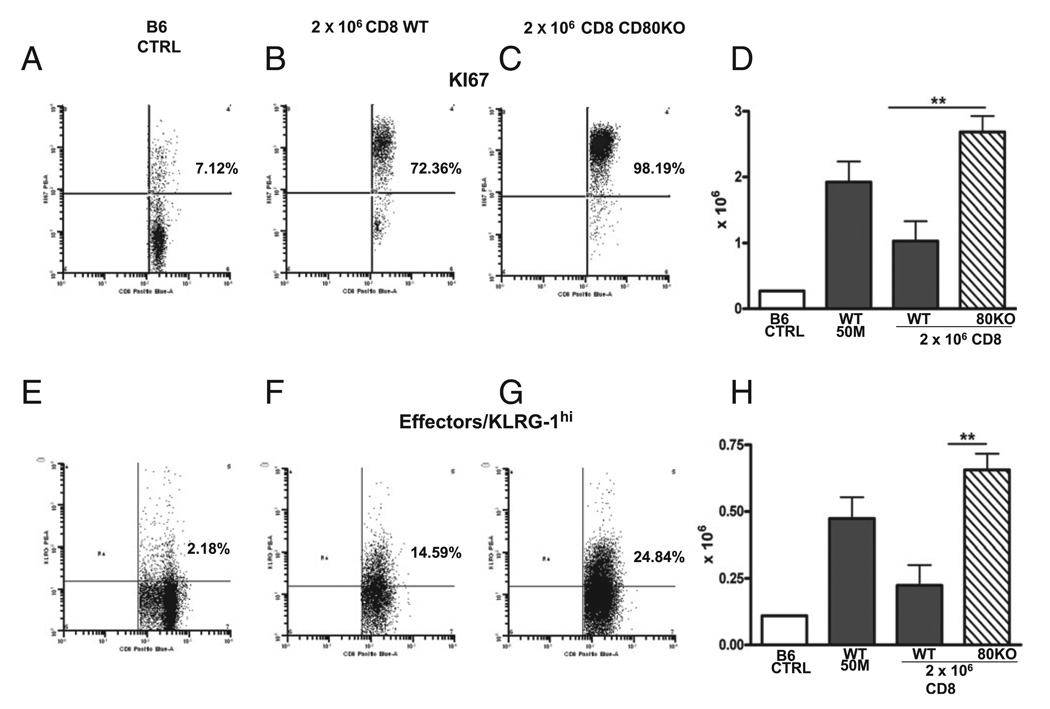
FIGURE 8.
Greater donor effector CD8 T cells in subthreshold CD80 KO→F1 are seen in conjunction with greater proliferation. Experimental groups, staining protocol, and data presentation are as described for Fig. 7 and represent the same cohort. KI-67 staining (A–C) and number of positive engrafted donor CD8 T cells (D). KLRG-1 staining (E–G) and number of positive engrafted donor CD8 T cells (H). Bar graphs are shown as group mean ± SE (n = 5). Similar results were observed in an additional day 10 experiment. **p < 0.01.
Suboptimal CD80 KO→F1 mice also exhibit significantly greater numbers of proliferating (KI-67 bright) donor CD8 T cells (Fig. 8D), confirming the results of Fig. 6. Lastly, suboptimal CD80 KO→F1 mice exhibited significantly greater numbers of effector (KLRG-1 bright) donor CD8 T cells versus WT→F1 mice (Fig. 8H). Thus, at the time of peak donor CD8 effector function (day 10), suboptimal CD80 KO→F1 mice exhibit significantly greater donor CD8 T cell proliferation and greater donor CD8 T effector numbers versus suboptimal WT→F1 mice.
Expression of downregulatory molecules Fas and programmed cell death-1 is not impaired on CD80 KO donor T cells
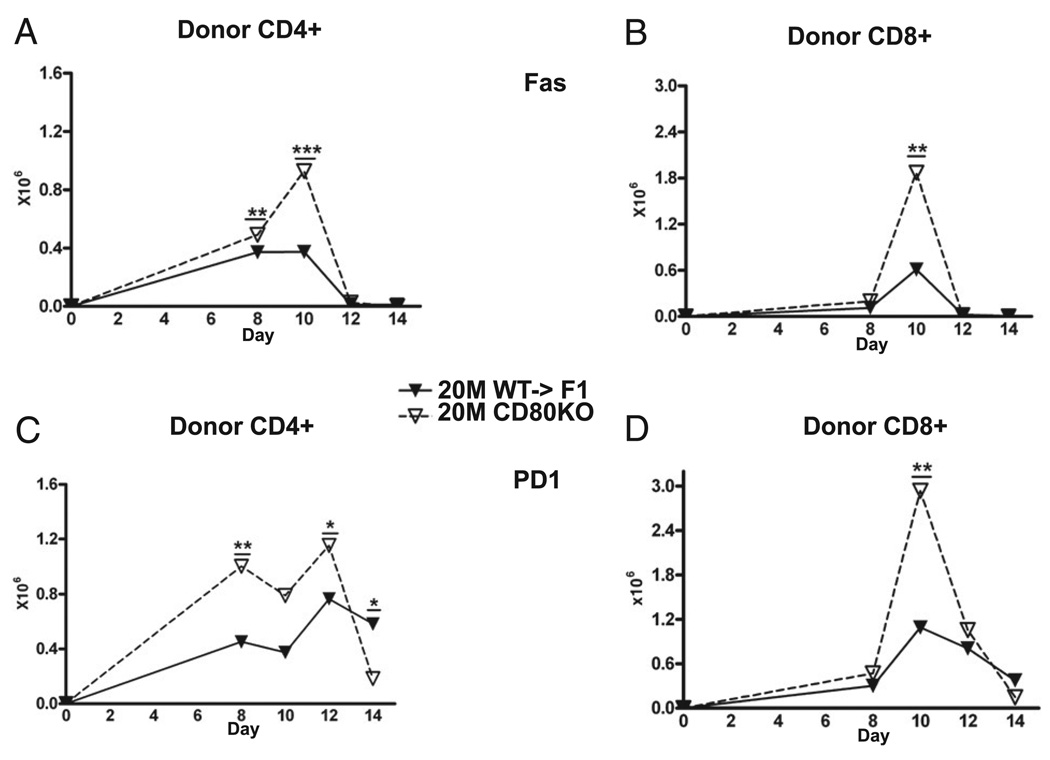
To address whether prolonged proliferation of CD80 KO donor T cells reflects impaired upregulation of molecules important in homeostatic contraction, the expression kinetics of Fas and programmed cell death-1 (PD-1) were measured on donor T cells from the cohort described in Fig. 3. We have previously demonstrated that donor CD8 T cell upregulation of Fas is a major but not exclusive event in effector contraction in B6→BDF1 mice (28). At the suboptimal dose, CD80 KO→F1 mice exhibited significantly greater numbers of Fas expressing CD4 (Fig. 9A) at days 8 and 10 and CD8 (Fig. 9B) donor T cells at day 10 consistent with the increased numbers of effector T cells. A similar pattern was seen for PD-1 expression, with CD80 KO donor cells exhibiting significantly greater numbers of PD-1 high CD4 T cells at days 8 and 10 (Fig. 9C) and CD8 T cells at day 10 (Fig. 9D). Moreover, as seen in Fig. 9, CD80 KO donor CD4 and CD8 T cells both undergo contraction from days 10–14, supporting the idea that greater engraftment of CD80 KO donor T cells at day 10 results from enhanced proliferation rather than impaired contraction.
FIGURE 9.
CD80 KO donor T cell upregulation of PD-1 and Fas is not defective. Experimental protocol is described in Fig. 3. The number of Fas-positive (A, B) or PD-1–positive (C, D) donor CD4 (A, C) or CD8 (B, D) T cells was determined by flow cytometry at the indicated time points for F1 mice receiving 50 × 106 WT or CD80 KO splenocytes. Results are shown as group mean ± SE. *p < 0.05; **p < 0.01; ***p < 0.005.
CD80 upregulation is maximal at the time of peak proliferation and just prior to contraction
The greater proliferation at day 10 in suboptimal CD80 KO versus WT donor T cells supports the idea that donor T cell expression of CD80 has a role in limiting effector proliferation and expansion of both CD4 and CD8 T cells. Further support for this idea is seen in the kinetics of donor T cell upregulation of CD80 on WT donor T cells shown in Fig. 10. For either the optimal or suboptimal dose, maximal upregulation of CD80 on both CD4 (Fig. 10A, 10C) and CD8 (Fig. 10B, 10D) WT T cells is seen at day 10 and followed by downregulation at a time when donor T cells contract. Maximal CD80 upregulation is significantly greater at the optimal dose. In at least two experiments, significant donor T cell expression of CD80 was still present at day 14 at the optimal dose, indicating that downregulation of donor T cell CD80 expression is complete on or about day 14. Together, the kinetics of donor T cell proliferation and CD80 upregulation support the idea CD80 upregulation limits the expansion of Ag-activated CD4 and CD8 T cells and does not alter homeostatic contraction.
FIGURE 10.
Maximal upregulation of CD80 on donor T cells is seen at day 10. Experimental protocol is described in Fig. 3, and CD80 expression was determined by flow cytometry for donor CD4 (A, C) and CD8 (B, D) T cells for F1 mice receiving 50 × 106 WT splenocytes at the indicated times. Results are shown as percent (A B) or cell numbers (C, D), group mean ± SE. CD80 downregulation was complete in at least three experiments but remained elevated in two experiments. *p < 0.05; **p < 0.01; ***p < 0.005.
Maximal enhancement of host cell elimination in CD80 KO→F1 mice is seen when both donor CD4 and CD8 T cells are CD80 defective
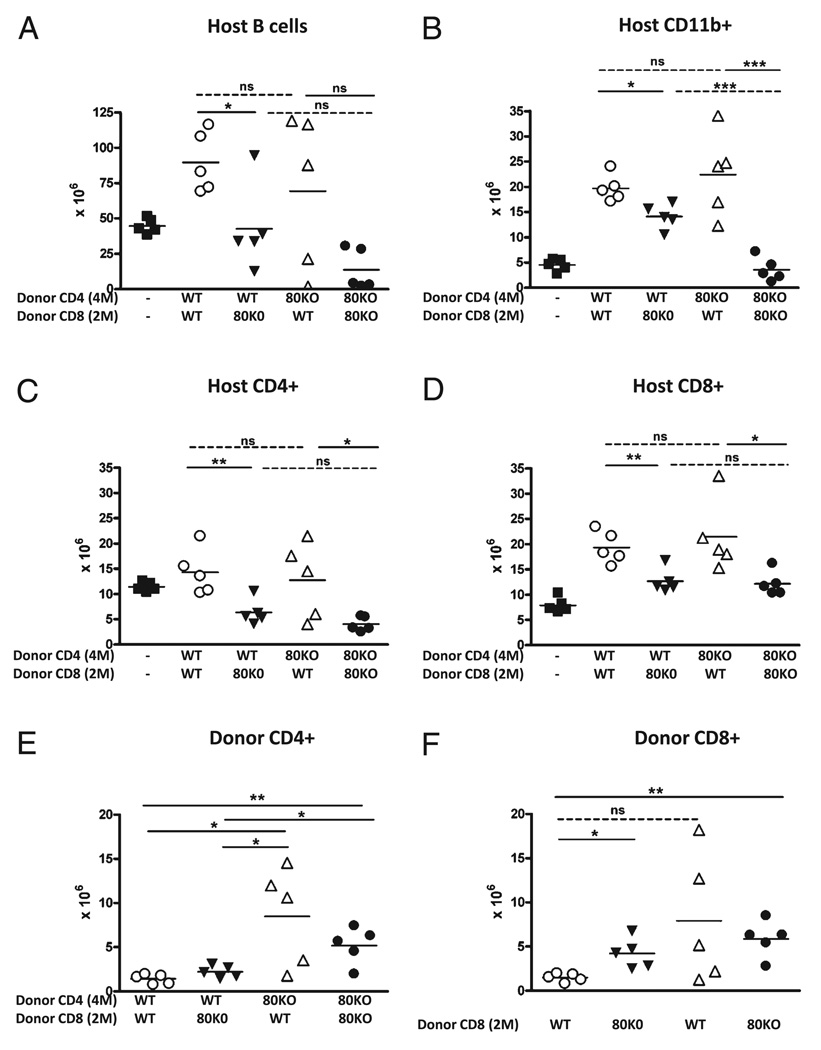
The foregoing data demonstrate that the absence of CD80 on donor CD4 and CD8 T cells results in prolonged proliferation of both subsets resulting in greater numbers of effector CTL and greater elimination of host cells. It is not clear whether one or both donor T cell subsets must be CD80 deficient for this effect. To determine the relative contributions of CD80 on CD4 versus CD8 donor T cells, both T cell subsets were purified from WT and CD80KO mice and then recombined preinjection with all four possible combinations tested. We have previously demonstrated that using separately purified and recombined B6 WT CD4 and CD8 T cells, a dose of 8 × 106 CD4 and 5 × 106 CD8 T cells results in near maximal elimination of host B cells at day 14 (28). In Figs. 2, 3, and 7, we demonstrate that potentiation of acute GVHD in subthreshold CD80 KO →F1 mice is seen at ~2 × 106 CD8 T cells and is lost at ~1 × 106 CD8 T cells when unfractionated splenocytes are transferred. Because unseparated donor T cells are more efficient than purified subsets at inducing acute GVHD phenotype (38), dose-response experiments were begun at the low end (~1 × 106 CD8 T cells) of the dose range. The transfer of 4 × 106 CD4 and 1 × 106 CD8 T cells was below the threshold for GVHD induction (i.e., complete elimination of host B cells was not seen in any group) (Supplemental Fig. 3A), demonstrating that despite the presence of 4-fold greater donor CD4 T cells, 1 × 106 donor CD8 T cells is insufficient to induce acute GVHD even with purified T cell subsets. Donor CD8 T cells were then increased and paired with a 3-fold excess of CD4 T cells (i.e., 6 × 106 CD4 and 2 × 106 CD8 T cells). At this dosage, the combination of CD80 KO CD4 + CD80 KO CD8 was the most potent, resulting in complete host B cell elimination in five out of five mice versus two out of five mice in WT CD4 + WT CD8→F1 mice (p < 0.05). Intermediate values were seen for WT CD4 + KO CD8→F1 (four out of five mice) and KO CD4 + WT CD8→F1 (three out of five mice); however, these trends were not statistically significant (Supplemental Fig. 3B). These two experiments demonstrate that at a CD4/CD8 ratio of 3 to 4:1, transferring 2 × 106 CD8 T is sufficient to eliminate host B cells when both donor T cell subsets are CD80 deficient, whereas 1 × 106 CD8 T is not. Complete host B cell elimination in two out of five WT→F1 mice raises the possibility that CD4 T cell help is not rate limiting.
We therefore tested an intermediate dose using 4 × 106 CD4 and 2 × 106 CD8 T cells. As shown in Fig. 11A, a significant (p < 0.0001) reduction in host B cells is seen for control CD80KO CD4 + CD80KO CD8→F1 mice compared with uninjected control F1 mice consistent with the presence of mature donor CD8 CTL effectors. In contrast, host B cells in control WT CD4 + WT CD8→F1 mice are not reduced below uninjected F1 levels and are significantly (p < 0.005) increased (Fig. 11A), indicating that this dose is well below the threshold for acute GVHD induction for WT→F1, and although mice have an intact initial expansion phase, there is a failure of the CTL-mediated elimination phase consistent with conversion to chronic GVHD phenotype.
FIGURE 11.
Optimal enhancement of host cell elimination in CD80 KO→F1 mice requires that both CD4 and CD8 donor T cells be CD80 defective. WT and CD80 KO CD4 and CD8 T cells were purified as described in Materials and Methods, and normal BDF1 mice received 4 × 106 CD4 and 2 × 106 CD8 T cells from either WT or CD80 KO donors. All four possible combinations were injected into groups of F1 mice (n = 5/group), and at day 14 posttransfer, F1 spleens were analyzed by flow cytometry for: host B cells (A), host macrophages (CD11b+) (B), host CD4 T cells (C), host CD8 T cells (D), donor CD4 T cells (E), and donor CD8 T cells (F). Values are shown as cell numbers × 106 for individual mice, and bars represent group mean. The source of donor CD4 and CD8 T cells is shown on the x-axis. For simplicity, additional p values comparing WT + WT→F1 versus KO + KO→F1 are stated in the text. Purified CD4 T cells had ≤0.6% contamination with CD8 T cells, and purified CD8 T cells had ≤1.4% contamination with CD4 T cells. *p < 0.05; **p < 0.01; ***p < 0.005.
Host T cells and APCs are typically less sensitive to elimination in acute GVHD than are host B cells; however, the results for these cell populations further support the concept that CD80 KO donor cells exhibit enhanced elimination of host cells. For example, control WT CD4 + WT CD8→F1 mice exhibit an increase in host CD4 T cells in some mice (Fig. 11C) and a significant increase in host CD8 T cells (Fig. 11D) and macrophages (Fig. 11B) (p < 0.001 for both), consistent with persistent expansion phase and a failure of CD8 CTLs to mediate their elimination typical of chronic GVHD. In contrast, control KO CD4 + KO CD8→F1 exhibit significant reductions in host CD4 T cells (p < 0.001) (Fig. 11C) and CD8 T cells (p < 0.05) ( Fig. 11D) compared with control F1. Importantly, compared with WT CD4 + WT CD8→F1 mice, KO CD4 + KO CD8→F1 exhibit significant reductions in host B cells (p < 0.001), CD4 T cells (p < 0.05), CD8 T cells (p < 0.005), and macrophages (p < 0.001). Thus, at this donor cell dose, matching of purified donor T cell subsets (i.e., WT + WT→F1 or KO + KO→F1) reproduces the results in Figs. 1 and 2 and demonstrates greater elimination of host cells using CD80 KO donor cells.
When donor T cell subsets are mixed rather than matched (WT + KO or KO + WT), results for host cell elimination are intermediate between WT CD4 + WT CD8→F1 and KO CD4 + KO CD8→F1 control groups, with some mice exhibiting host cells that are above, below, or equal to that of uninjected control F1 mice. These results raise the possibility that the reduction in threshold for acute GVHD induction using CD80 defective donor T cells has both a CD4 and CD8 component.
The role of CD80 on donor CD4 T cells can be seen by comparing the ability of either WT or KO CD4 T cells to promote elimination of host cells while holding constant the source of CD8 effector T cells (dotted lines, Fig. 11A–D). For example, the ability of either WT or KO CD4 T cells to promote killing by WT CD8 T cells is seen by comparing WT CD4 + WT CD8→F1 to KO CD4 + WT CD8→F1 (top left dotted line, Fig 11A–D). In this comparison, KO CD4 + WT CD8→F1 exhibit reductions host B cells (Fig. 11A) and host CD4 T cells (Fig. 11C) below control F1 values in two out of five mice versus zero out of five mice in WT CD4 + WT CD8→F1. Group differences are not statistically significant. These results are consistent with a mildly enhanced helper function for CD80 KO CD4 T cells. A similar mild enhancement of CD80 KO CD4 helper function is also seen when WT or KO CD4 T cells are each paired with KO CD8 T cells (bottom right dotted line, Fig. 11A–D). Compared to WT CD4 + KO CD8→F1, KO CD4 + KO CD8→F1 exhibit a nonsignificant trend toward greater elimination of host B cells and host CD4 T cells and significantly greater reduction in host macrophages (Fig. 11B). Thus, CD80 KO CD4 T cells exhibit a mild trend toward boosting effector function of either WT or KO CD8 T cells, but this trend only reaches statistical significance for host macrophages.
A similar analysis demonstrates a stronger effect of CD80 on donor CD8 T cells. By comparing host cell elimination when the source of CD4 help is held constant, significantly greater elimination is seen using CD80 KO CD8 T cells versus WT CD8 T cells (solid lines, Fig. 11A–D). For example, comparing the effects of WT CD4 T cells paired with either KO CD8 T cells (WT CD4 + KO CD8→F1) or WT CD8 T cells (WT CD4 + WT CD8→F1) (bottom left solid line for each figure), significantly greater elimination of host B cells (Fig. 11A), CD4 T cells (Fig. 11C), CD8 T cells (Fig. 11D), and macrophages (Fig. 11B) is seen when WT CD4 T cells are paired with KO CD8 T cells. The CTL-enhancing effect of CD80 KO CD8 T cells is also seen when KO CD4 T cells are used as a constant source of help and the source of CD8 T cells varied (top right solid line). Compared to KO CD4 + WT CD8→F1 mice, KO CD4 + KO CD8→F1 mice exhibit significant reductions in all host cell types except B cells (Fig. 11A–D), which, due to their greater sensitivity to elimination, exhibit near maximal B cell elimination in two out of five mice in KO CD4 + WT CD8→F1 group versus five out of five KO CD4 + KO CD8→F1.
Engraftment data confirms these trends. Greater day 14 engraftment of CD80 KO donor CD4 T cells is seen regardless of whether they are mixed with WT CD8 or matched with KO CD8 T cells compared with WT CD4 paired either with WT or KO CD8 T cells (Fig. 11E). Similarly, donor CD8 engraftment (Fig. 11F) is significantly greater for KO CD8 T cells whether matched with KO CD4 or mixed WT CD4 donor T cells when compared with WT CD8 T cells paired with WT CD4 (WT CD4 + WT CD8→F1). A variable increase in WT CD8 engraftment is seen when paired with KO CD4 T cells (KO CD4 + WT CD8→F1 mice) (Fig. 11F), with two out of five mice exhibiting strong elevations in donor CD8 engraftment. These two mice also exhibited near maximal B cell elimination (Fig. 11A), supporting the idea that CD80 KO CD4 T cells exhibit a nonsignificant trend toward greater help to WT CD8 than do WT CD4 (dotted line compares CD8 engraftment for KO CD4 + WT CD8→F1 to WT CD4 + WT CD8→F1 in Fig. 11F). The low-level engraftment of CD8 T cells observed in WT CD4 + WT CD8→F1 control mice confirms that the donor cell dose is subthreshold as indicated by a complete lack of host B cell elimination and conversion to chronic GVHD phenotype. Taken together, the data in Fig. 11 indicate that enhancement of acute GVHD seen in CD80 KO→F1 mice in Figs. 1 and 2 has both a CD4 and CD8 component, and, although the CD8 component may be relatively stronger than the CD4 component, maximal host elimination is seen only when both donor CD4 and CD8 T cells are CD80 defective.
Discussion
The P→F1 model is useful for analyzing in vivo activation and maturation of Ag-specific T cells in immunocompetent hosts (27, 28). Advantages of this model are: 1) the high frequency of alloreactive donor T cells that allows tracking by flow cytometry without the need for tetramer staining; and 2) the use of a normal semiallogeneic F1 recipient that allows donor T cell engraftment without the need to render the host immunodeficient pretransfer. Importantly, the immunodeficiency (and lethality) that can result in this model is a consequence of a normal adaptive immune response by donor T cells targeted to host MHC and not a consequence of conditioning regimens. Thus, greater donor T cell activation typically results in greater host immunodeficiency (34).
It has been previously demonstrated that activated T cells upregulate B7 molecules CD80 and CD86 (25, 26); however, the exact in vivo biologic function of these molecules is unclear. In this study, we assessed the in vivo biological function of CD80 and CD86 expression on Ag-activated T cells by transferring donor T cells deficient in one or both of these molecules into CD80/ CD86-intact hosts. These studies clearly demonstrate that CD80, more so than CD86, has an important but not exclusive role in limiting donor CD8+ T cell effector function by limiting peak proliferation and numbers. Specifically, CD80 KO donor T cells exhibited potentiation of acute GVHD phenotype as shown by ~60% reduction in the number of donor cells required for near complete elimination of host B cells at 2 wk of disease. The role of CD80 upregulation on Ag-activated donor T cells was seen only when subthreshold numbers of donor T cells were transferred. Specifically, at subthreshold doses, CD80 KO donor T cells exhibited significantly greater peak (day 10) engraftment and proliferation for both CD4 and CD8 T cells compared with the same dose of WT donor cells. An increase in donor effector T cells for suboptimal CD80 KO→F1 versus WT→F1 mice was shown: 1) directly, as greater numbers of CD8 T cells expressing IFN-γ, perforin, granzyme B, CD107a, and KLRG-1; and 2) functionally, as greater elimination of host splenocyte subpopulations and greater expression of cytokine genes important in CTL function. These latter results confirm that the statistically significant greater numbers of CD80 KO donor CD8 effector cells are also biologically significant and mediate greater host elimination through greater effector function. Both WT and CD80 KO donor CD8 T cells exhibited contraction from days 12–14, and there was no defect in upregulation of Fas or PD-1. Thus, greater peak day 10 numbers of donor CD80 KO T cells reflects enhanced proliferation rather than impaired Fas/PD-1–mediated contraction. Importantly, peak upregulation of CD80 on WT donor T cells occurred at about day 10, the time at which CD80 KO donor T cells first exhibit significantly greater peak proliferation and numbers of donor T cells. Taken together, these results strongly support the idea that CD80 upregulation on activated donor T cells limits their proliferation and expansion. Using purified donor T cell subsets, mixing studies demonstrated that optimal enhancement of antihost CTL effector function (i.e., host cell elimination) was observed when both CD4 and CD8 T donor T cells were CD80 deficient, indicating that CD80 upregulation on WT donor CD4 T cells and greater donor CD4 T cell proliferation and engraftment seen in CD80 KO→F1 mice is functionally significant.
Previous work has shown an important role for upregulation of B7 molecules on activated T cells in limiting their expansion in vivo. For example, Taylor et al. (25) used a murine model of bone marrow transplantation involving lethally irradiated allogeneic hosts and demonstrated that donor CD4+ T cells present in thoracic duct lymph exhibited upregulation of both CD80 and CD86. Moreover, GVHD-induced mortality was accelerated with the use of donor T cells doubly deficient in CD80 and CD86, whereas mortality was attenuated using donor T cells that constitutively overexpressed CD86. Based on alterations in GVHD mortality, the authors concluded that upregulation of B7 (both CD80 and CD86) on T cells is part of a normal in vivo T cell immune response that functions to prevent uncontrolled T cell proliferation. The role of CD80 separate from CD86 on CD4 T cells separate from CD8 T cells was not further characterized.
Similarly, Paust et al. (26) demonstrated that CD4 T cells defective in both CD80 and CD86 were resistant to suppression by CD4+CD25+ T cells. Moreover, when CD80/CD86 DKO CD4 T cells were transferred into syngeneic immunodeficient (Rag-2 KO) hosts, these donor cells induced a lethal wasting disease consistent with a lack of suppression. The in vivo role of B7 molecules on CD8 T cells or the role of CD80 separate from CD86 on in vivo CD4 T cell contraction was not examined.
These studies seemingly contrast with in vitro work demonstrating that constitutive T cell expression of CD80 and 4-1BBL amplifies effector function (39) and that cross-linking of CD80 on myelin-specific CD4 T cells in the presence of Th1 cytokines increases the level of activation and effector function (40). Clearly, these experimental systems contain many differences from the in vivo system studied here.
Our results confirm the importance of B7 molecules on activated T cells and extend previous studies by demonstrating that in the setting of a normal host immune system: 1) upregulated B7 molecules limit expansion of activated CD8 T and CD4 T cells; 2) up-regulated CD80 plays a significantly greater role in limiting T cell expansion than does upregulated CD86; and 3) optimal CD80-induced CD8 CTL limitation of expansion requires CD80 on both CD4 and CD8 T cells. The role of T cell-expressed CD80 in limiting expansion of activated T cells does not prevent subsequent homeostatic contraction of CD8 effector T cells in the setting of a normal host immune system; however, as shown by Paust et al. (26), such may not be the case in an immunodeficient host.
The dichotomy seen in our study between CD80 and CD86 on donor T cells is reminiscent of the dichotomy seen with in vivo CD80 or CD86 blockade in the P→F1 model. In those studies, CD80 blockade alone promoted CD8+ T cell expansion and host B cell elimination, whereas selective CD86 blockade attenuated donor T cell activation and subsequent acute or chronic GVHD phenotype similar to that seen with complete costimulatory blockade using CTLA4-Ig (11). These results suggest that CD86 blockade preferentially inhibits CD28-mediated costimulation, whereas CD80 blockade preferentially inhibits CTLA4-mediated contraction, supporting previous studies suggesting that at least in vitro, CTLA4/CD80 and CD28/CD86 pairing is preferred (4). Together, our results demonstrating enhancement of in vivo CD8 CTL effector responses using either CD80 KO donor T cells or CD80 mAb blockade support the targeting of CD80 in diseases in which promoting suboptimal CD8 CTL effector responses is therapeutically desirable. Clinical interruption of the CTLA4-B7 pathway by targeting of CTLA4 has been shown to remove the inhibitory effects of CTLA4 and results in reduced T cell contraction, enhanced T cell effector responses, and improved outcome in the treatment of poorly immunogenic tumors (41). Our results expand this approach and support the direct targeting of the preferential CTLA4 ligand (i.e., CD80), with the goal of enhancing effector CD8 T cell responses. Although CD80 is expressed on non-T cells, our previous results demonstrating potentiation of CD8 CTL with CD80 mAb blockade begun early in an immune response suggest that it may not necessarily be critical to synchronize mAb administration with T cell upregulation of CD80 (11). Thus, targeting of CD80 may be a clinically useful approach to enhancing subtherapeutic antiviral responses (e.g., to EBV, HIV), tumor responses, and other conditions in which CD8+ T cells play a protective role but are suboptimally activated. Because, as our results demonstrate, other downregulatory mechanisms eventually induce contraction of the T cell effector response, CD80 targeting may be a useful adjunct as part of a combined therapeutic approach in conditions in which CD8+ T cells play a protective role but are suboptimally activated.
Supplementary Material
Acknowledgments
We thank Dr. Cliff Snapper (Pathology Department, Uniformed Services University of Health Sciences, Bethesda, MD) for helpful discussions and Drs. Dragana Trakovic (Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) and Stephen Davies (Microbiology Department, Uniformed Services University of Health Sciences) for insights and discussions regarding intracellular flow cytometry staining.
This work was supported by National Institutes of Health Grant AI047466 and a Veterans Affairs Merit Review (to C.S.V.). T.L. is a recipient of a Veterans Affairs Career Development award, and R.P. is a recipient of an Engelticheff Fellowship.
Abbreviations used in this paper
- B6
C57BL/6
- BDF1
B6D2F1
- CD80KO
B6.129S4-CD80tm1Shr/J
- CD86KO
B6.129S4-CD86tm1Shr/J
- CD80/86 KO
CD80tm1ShrCD86tm1Shr/J
- DC
dendritic cell
- DKO
double knockout
- GVHD
graft-versus-host disease
- KLRG-1
killer cell lectin-like receptor G-1
- KO
knockout
- OAS
oligoadenylate synthetase
- PD-1
programmed cell death-1
- P→F1
parent-into-F1
- WT
wild-type
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sharpe AH, Latchman Y, Greenewald RJ. Accessory Molecules and Co-Stimulation. In: Paul WE, editor. Fundamental Immunology. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 393–417. [Google Scholar]
- 2.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 3.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 4.Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 5.Sansom DM, Manzotti CN, Zheng Y. What’s the difference between CD80 and CD86? Trends Immunol. 2003;24:314–319. doi: 10.1016/s1471-4906(03)00111-x. [DOI] [PubMed] [Google Scholar]
- 6.Judge TA, Wu Z, Zheng XG, Sharpe AH, Sayegh MH, Turka LA. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J. Immunol. 1999;162:1947–1951. [PubMed] [Google Scholar]
- 7.Fallarino F, Fields PE, Gajewski TF. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J. Exp. Med. 1998;188:205–210. doi: 10.1084/jem.188.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaBelle JL, Hanke CA, Blazar BR, Truitt RL. Negative effect of CTLA-4 on induction of T-cell immunity in vivo to B7-1+, but not B7-2+, murine myelogenous leukemia. Blood. 2002;99:2146–2153. doi: 10.1182/blood.v99.6.2146. [DOI] [PubMed] [Google Scholar]
- 9.Martín-Fontecha A, Moro M, Crosti MC, Veglia F, Casorati G, Dellabona P. Vaccination with mouse mammary adenocarcinoma cells coexpressing B7-1 (CD80) and B7-2 (CD86) discloses the dominant effect of B7-1 in the induction of antitumor immunity. J. Immunol. 2000;164:698–704. doi: 10.4049/jimmunol.164.2.698. [DOI] [PubMed] [Google Scholar]
- 10.Yamada A, Kishimoto K, Dong VM, Sho M, Salama AD, Anosova NG, Benichou G, Mandelbrot DA, Sharpe AH, Turka LA, et al. CD28-independent costimulation of T cells in alloimmune responses. J. Immunol. 2001;167:140–146. doi: 10.4049/jimmunol.167.1.140. [DOI] [PubMed] [Google Scholar]
- 11.Lang TJ, Nguyen P, Peach R, Gause WC, Via CS. In vivo CD86 blockade inhibits CD4+ T cell activation, whereas CD80 blockade potentiates CD8+ T cell activation and CTL effector function. J. Immunol. 2002;168:3786–3792. doi: 10.4049/jimmunol.168.8.3786. [DOI] [PubMed] [Google Scholar]
- 12.Azuma M, Yssel H, Phillips JH, Spits H, Lanier LL. Functional expression of B7/BB1 on activated T lymphocytes. J. Exp. Med. 1993;177:845–850. doi: 10.1084/jem.177.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prabhu Das MR, Zamvil SS, Borriello F, Weiner HL, Sharpe AH, Kuchroo VK. Reciprocal expression of co-stimulatory molecules, B7-1 and B7-2, on murine T cells following activation. Eur. J. Immunol. 1995;25:207–211. doi: 10.1002/eji.1830250134. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub JP, Cohen PL. Ectopic expression of B7-1 (CD80) on T lymphocytes in autoimmune lpr and gld mice. Clin. Immunol. 1999;91:302–309. doi: 10.1006/clim.1999.4717. [DOI] [PubMed] [Google Scholar]
- 15.Weintraub JP, Eisenberg RA, Cohen PL. Up-regulation of Fas and the costimulatory molecules B7-1 and B7-2 on peripheral lymphocytes in autoimmune B6/gld mice. J. Immunol. 1997;159:4117–4126. [PubMed] [Google Scholar]
- 16.Köchli C, Wendland T, Frutig K, Grunow R, Merlin S, Pichler WJ. CD80 and CD86 costimulatory molecules on circulating T cells of HIV infected individuals. Immunol. Lett. 1999;65:197–201. doi: 10.1016/s0165-2478(98)00107-2. [DOI] [PubMed] [Google Scholar]
- 17.Ranheim EA, Kipps TJ. Elevated expression of CD80 (B7/BB1) and other accessory molecules on synovial fluid mononuclear cell subsets in rheumatoid arthritis. Arthritis Rheum. 1994;37:1637–1646. doi: 10.1002/art.1780371113. [DOI] [PubMed] [Google Scholar]
- 18.Tompkins MB, Bull ME, Dow JL, Ball JM, Collisson EW, Winslow BJ, Phadke AP, Vahlenkamp TW, Tompkins WA. Feline immunodeficiency virus infection is characterized by B7+CTLA4+ T cell apoptosis. J. Infect. Dis. 2002;185:1077–1093. doi: 10.1086/339847. [DOI] [PubMed] [Google Scholar]
- 19.Verwilghen J, Lovis R, De Boer M, Linsley PS, Haines GK, Koch AE, Pope RM. Expression of functional B7 and CTLA4 on rheumatoid synovial T cells. J. Immunol. 1994;153:1378–1385. [PubMed] [Google Scholar]
- 20.Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, Sabzevari H. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J. Immunol. 2002;169:6162–6169. doi: 10.4049/jimmunol.169.11.6162. [DOI] [PubMed] [Google Scholar]
- 21.Sabzevari H, Kantor J, Jaigirdar A, Tagaya Y, Naramura M, Hodge J, Bernon J, Schlom J. Acquisition of CD80 (B7-1) by T cells. J. Immunol. 2001;166:2505–2513. doi: 10.4049/jimmunol.166.4.2505. [DOI] [PubMed] [Google Scholar]
- 22.Blazar BR, Sharpe AH, Taylor PA, Panoskaltsis-Mortari A, Gray GS, Korngold R, Vallera DA. Infusion of anti-B7.1 (CD80) and anti-B7.2 (CD86) monoclonal antibodies inhibits murine graft-versus-host disease lethality in part via direct effects on CD4+ and CD8+ T cells. J. Immunol. 1996;157:3250–3259. [PubMed] [Google Scholar]
- 23.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions [corrections] J. Immunol. 2004;172:34–39. doi: 10.4049/jimmunol.172.1.34. [DOI] [PubMed] [Google Scholar]
- 26.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc. Natl. Acad. Sci. USA. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puliaev R, Puliaeva I, Welniak LA, Ryan AE, Haas M, Murphy WJ, Via CS. CTL-promoting effects of CD40 stimulation outweigh B cell-stimulatory effects resulting in B cell elimination and disease improvement in a murine model of lupus. J. Immunol. 2008;181:47–61. doi: 10.4049/jimmunol.181.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puliaeva I, Puliaev R, Shustov A, Haas M, Via CS. Fas expression on antigen-specific T cells has costimulatory, helper, and down-regulatory functions in vivo for cytotoxic T cell responses but not for T cell-dependent B cell responses. J. Immunol. 2008;181:5912–5929. doi: 10.4049/jimmunol.181.9.5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gleichmann E, Gleichmann H. Pathogenesis of graft-versus-host reactions (GVHR) and GVH-like diseases. J. Invest. Dermatol. 1985;85(1 Suppl):115s–120s. doi: 10.1111/1523-1747.ep12275619. [DOI] [PubMed] [Google Scholar]
- 30.Via CS, Shearer GM. T-cell interactions in autoimmunity: insights from a murine model of graft-versus-host disease. Immunol. Today. 1988;9:207–213. doi: 10.1016/0167-5699(88)91215-7. [DOI] [PubMed] [Google Scholar]
- 31.Via CS, Rus V, Nguyen P, Linsley P, Gause WC. Differential effect of CTLA4Ig on murine graft-versus-host disease (GVHD) development: CTLA4Ig prevents both acute and chronic GVHD development but reverses only chronic GVHD. J. Immunol. 1996;157:4258–4267. [PubMed] [Google Scholar]
- 32.Hakim FT, Cepeda R, Gray GS, June CH, Abe R. Acute graft-versus-host reaction can be aborted by blockade of costimulatory molecules. J. Immunol. 1995;155:1757–1766. [PubMed] [Google Scholar]
- 33.Sakurai J, Ohata J, Saito K, Miyajima H, Hirano T, Kohsaka T, Enomoto S, Okumura K, Azuma M. Blockade of CTLA-4 signals inhibits Th2-mediated murine chronic graft-versus-host disease by an enhanced expansion of regulatory CD8+ T cells. J. Immunol. 2000;164:664–669. doi: 10.4049/jimmunol.164.2.664. [DOI] [PubMed] [Google Scholar]
- 34.Rus V, Nguyen V, Puliaev R, Puliaeva I, Zernetkina V, Luzina I, Papadimitriou JC, Via CS. T cell TRAIL promotes murine lupus by sustaining effector CD4 Th cell numbers and by inhibiting CD8 CTL activity. J. Immunol. 2007;178:3962–3972. doi: 10.4049/jimmunol.178.6.3962. [DOI] [PubMed] [Google Scholar]
- 35.Puliaev R, Nguyen P, Finkelman FD, Via CS. Differential requirement for IFN-gamma in CTL maturation in acute murine graft-versus-host disease. J. Immunol. 2004;173:910–919. doi: 10.4049/jimmunol.173.2.910. [DOI] [PubMed] [Google Scholar]
- 36.Shustov A, Luzina I, Nguyen P, Papadimitriou JC, Handwerger B, Elkon KB, Via CS. Role of perforin in controlling B-cell hyperactivity and humoral autoimmunity. J. Clin. Invest. 2000;106:R39–R47. doi: 10.1172/JCI8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shustov A, Nguyen P, Finkelman F, Elkon KB, Via CS. Differential expression of Fas and Fas ligand in acute and chronic graft-versus-host disease: up-regulation of Fas and Fas ligand requires CD8+ T cell activation and IFN-gamma production. J. Immunol. 1998;161:2848–2855. [PubMed] [Google Scholar]
- 38.Rolink AG, Gleichmann E. Allosuppressor- and allohelper-T cells in acute and chronic graft-vs.-host (GVH) disease. III. Different Lyt subsets of donor T cells induce different pathological syndromes. J. Exp. Med. 1983;158:546–558. doi: 10.1084/jem.158.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, Sadelain M. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat. Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 40.Podojil JR, Kohm AP, Miller SD. CD4+ T cell expressed CD80 regulates central nervous system effector function and survival during experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:2948–2958. doi: 10.4049/jimmunol.177.5.2948. [DOI] [PubMed] [Google Scholar]
- 41.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin. Cancer Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.