Normal Development of The Reproductive Axis Activity in Humans
Developmentally, the fetal GnRH pulse generator and its activation of the downstream gonadotrope and gonadal axis in humans is fully operational in both sexes by the end of the 1st trimester of gestation (Grumbach MM 1994). Subsequently, serum LH and FSH levels are suppressed and at the time of delivery are undetectable levels in umbilical cord blood, presumably secondary to the inhibitory effects of the pharmacological levels of placentally-derived sex steroids (Grumbach MM 1990; Massa, de Zegher et al. 1992) and possibly the high levels of circulating kisspeptin (Seminara, Dipietro et al. 2006; Ramaswamy, Seminara et al. 2007). Within minutes of birth, male infants exhibit a brief surge of LH and testosterone that persists for up to 12 hours (Corbier, Dehennin et al. 1990; de Zegher, Devlieger et al. 1992). In the subsequent 1–2 weeks of the neonatal period, the declining levels of placentally derived inhibitory factors then reveal a reawakened secretion of the hypothalamic GnRH pulse generator that results in the characteristic burst of GnRH-induced activity of the reproductive axis that is a hallmark of this period.
This neonatal burst of secretory activity of the hypothalamic-pituitary-gonadal axis, termed the “mini-puberty of infancy” (Fig 1), differs in its duration between the two sexes. In males, LH, FSH, and testosterone levels peak between 4–10 weeks following which GnRH quiescence eventually is complete by 6 months (Forest 1990; Andersson, Toppari et al. 1998). In contrast, female infants show longer persistence of their GnRH secretory activation of their reproductive axes' activity for up to 3 years, with hormonal values being highly variable during this time and characterized by an FSH predominance in striking contrast to the male (Waldhauser, Weissenbacher et al. 1981; Andersson, Toppari et al. 1998). In both sexes, this neonatal window of reproductive activation is driven by GnRH secretion and appears to serve at least two critical developmental agendas. In males, the first is a rapid expansion of the Sertoli cell population (Sharpe, Fraser et al. 2003; Sharpe, McKinnell et al. 2003) with a subsequent increase in the germ cell number that provides the underpinnings for subsequent fertility during adulthood (Muller and Skakkebaek 1984). The second is exposure of all sex steroid dependent target organs (especially the brain) to a key developmental period of adult levels of sex steroids. This exposure has important organizational consequences for both immediate and subsequent development such as phallic development and testicular descent. Consequently, microphallus or micropenis (infantile penile size < 2.5cm) and cryptorchidism represent two phenotypic features that are the biological sequelae in boys when this brief window of in utero/neonatal activation of the HPG axis is defective in cases of human Isolated GnRH Deficiency (Fig 1) (see below). Following this flurry of neonatal activity in both sexes, this activation of the HPG axis that is driven by GnRH secretion, is mysteriously dampened by unknown developmental `brakes' that usher in a prolonged period of reproductive quiescence lasting throughout childhood. During this period of reduced sex steroids characteristic of childhood, a very low amplitude secretion of GnRH, gonadotropins and sex steroids continues that results in this nadir of reproductive activity that permits linear growth of the skeletal system to occur in the absence of epiphyseal fusion (Conte, Grumbach et al. 1975; Wu, Butler et al. 1990; Wu, Butler et al. 1991).
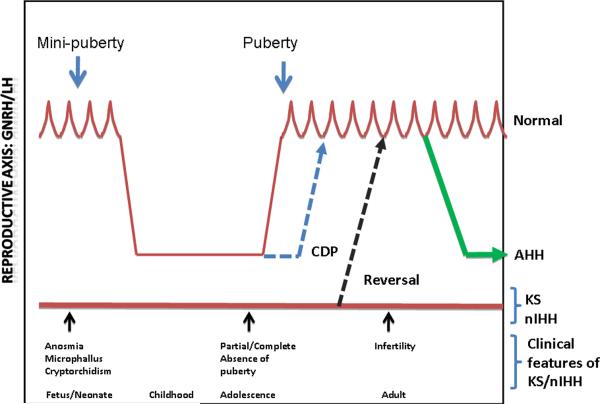
Figure 1. Clinical presentations of isolated GnRH deficiency in humans.
Pulsatile GnRH secretion is initiated during the late fetal/early neonatal period (“mini-puberty”), followed by quiescence during childhood and reawakening of the pulsatile secretion in mid-childhood. Presence of anosmia signals a developmental defect in GnRH neuronal migration while, microphallus or cryptorchidism signal fetal/neonatal lack of GnRH secretion. Constitutional delay of puberty (CDP) represents a late activation of the HPG axis while KS and nIHH represent partial or complete failure of pulsatile GnRH secretion. Recovery of pulsatile GnRH secretion in KS/nIHH subjects in adulthood is termed “Reversal”, while, AHH (adult-onset hypogonadotropic hypogonadism) refers to the onset of isolated GnRH deficiency during adulthood following a normal mini-puberty and puberty.
The gradual reawakening of the GnRH pulse generator during adolescence, initially during sleep and then equally during day and night, then heralds the onset of puberty (Fig 1). In boys, this onset of puberty is initially signalled externally by isolated testicular enlargement. This selective activation of the germ cell compartment of the testes reflects a secondary wave of GnRH-induced FSH secretion that is largely unrestrained by gonadal secretion of Inhibin B and, in fact, initiates Inhibin B biosynthesis and secretion during this `feed forward' phase of FSH's driving of Inhibin B production from the Sertoli cells. These relatively modest increases in FSH that occur during early puberty induce a second wave of seminferous tubular proliferation, maturation of germ cells, and eventually the appearance of sperm in the ejaculate which is termed spermarche (Nielsen, Skakkebaek et al. 1986; Nielsen, Skakkebaek et al. 1986). This period of active gametogenesis in the seminiferous tubular component of the testes is driven by FSH secretion. It is followed by penile and pubic hair growth driven by GnRH-induced LH's activation on the Leydig cells and consequent secretion of testosterone. Eventually, the levels of Inhibin B secretion rise sufficiently to provide important negative feedback upon FSH secretion.
In girls, the appearance of public hair (pubarche) is initially driven by adrenal androgen secretion (adrenarche) and represents the typical first external sign of sexual maturation. This pubarche is soon followed by breast budding (thelarche), a spurt in linear growth, and much later menarche (Marshall and Tanner 1969). This cascade of maturational events is again initiated at the hypothalamic level by GnRH secretion and marks the second important biological and behavioral reproductive transition in humans whose control mechanisms have eluded scientists. In fact, Science magazine viewed the control of this process as one of the “125 great unanswered biological mysteries” in the current century (Kennedy and Norman 2005). Given the fundamental elusiveness of this genetic control, the study of the human disease model of isolated GnRH deficiency has served as an important key to unlocking the secrets of puberty.
ISOLATED GnRH DEFICIENCY IN HUMANS
The advent of specific urinary and then serum assays for gonadotropins permitted classification of hypogonadal patients for the first time (Klinefelter 1943). Whereas most hypogonadal patients exhibited elevated levels of gonadotropins (hypergonadotropic hypogonadism), a much rarer subset of hypogonadal patients' had normal or low gonadotropin levels with otherwise normal anterior pituitary function and CNS anatomy. While this condition was initially referred to as Isolated Gonadotropin Deficiency prior to the discovery of GnRH (Heller and Nelson 1948), once this hypothalamic decapeptide became available for human investigation in 1971, increases in the serum levels of LH and FSH following administration of a single bolus of GnRH in these subjects suggested that the defect was hypothalamic (Naftolin, Harris et al. 1971; Hashimoto, Miyai et al. 1972; Roth, Kelch et al. 1972). Eventually, administration of a physiologic pattern and dosing of exogenous GnRH to these patients complete restored a physiologic pattern and levels of gonadotropins and sex steroids, thus unequivocally demonstrating the hypothalamic nature of their defect (Crowley and McArthur 1980; Hoffman and Crowley 1982). Therefore, isolated GnRH deficiency is a more appropriate term to refer to this phenotype, as this nomenclature reflects the true pathophysiologic defect in the vast majority of these patients.
Phenotypic features
Isolated GnRH deficiency presents a significant clinical and investigative challenge as well as an opportunity for new discovery because of its heterogeneous clinical presentations and complex genetics. In both sexes, a broad phenotypic spectrum of baseline clinical presentations is seen that varies along several lines including the: (1) presence or absence of associated loss of smell (anosmia); (2) degree, severity, and timing of their reproductive defects; (3) patterns of endogenous GnRH secretory defects; and (4) critical presence or absence of non-reproductive defects.
(1) Olfactory function
Historically, two forms of isolated GnRH deficiency have been clinically discernible: Kallmann Syndrome (KS) in which isolated GnRH deficiency is accompanied by anosmia (lack of sense of smell) and its normosmic variant, idiopathic hypogonadotropic hypogonadism (nIHH). This developmental nexus between olfaction and reproduction was first appreciated in 1856 when Maestre de San Juan first described absent olfactory structures during the autopsy of an anosmic hypogonadal male (Maestre de San Juan 1856). This report was followed by several pathologic and clinical reports of subjects with defective olfactory structures/function and hypogonadism (Weidenreich 1914; Altmann 1930; Kallmann, Schoenfeld et al. 1944; de Morsier 1954; Gauthier 1960; De Morsier and Gauthier 1963). In 1944, Franz Kallmann (Kallmann, Schoenfeld et al. 1944) provided a more elaborate description of this syndrome that initiated the clinical investigation into this phenotype. Kallmann presented three pedigrees with multiple family members suffering from hypogonadism and anosmia; the anosmic variant has been referred to as Kallmann Syndrome (KS) ever since. In addition to hinting at a possible genetic etiology by its familial pattern of occurrence, this seminal report also described other hypogonadal individuals who exhibited a normal sense of smell and other congenital anomalies. Although a precise anatomic and functional relationship between the olfactory structures and GnRH neurons was initially unclear, subsequent evidence from rodents and the detailed study of two human fetuses with a KAL1 gene deletion eventually confirmed the common embryonic origins of GnRH neurons and olfactory axons and documented the failure of these neurons to migrate into the CNS when the olfactory systems fails to develop in subjects with KS (Bick, Curry et al. 1989; Schwanzel-Fukuda, Bick et al. 1989). Thus, from this seminal report, it became clear from the various clinical phenotypes that 2 different types of underlying defects could be anticipated. In the first, developmental defects in olfactory system that result in anosmia were associated with the GnRH deficiency. In others, no such developmental or neuroanatomic abnormality was presumably present; however, some neuroendocrine defect in GnRH regulation could be anticipated.
The KS variant of this syndrome accounts for nearly 60% of most series of isolated GnRH deficiency in the human (Seminara, Hayes et al. 1998; Quinton, Duke et al. 2001; Seminara and Crowley 2002) and is now viewed as a medical disorder that results from a developmental failure of GnRH neuronal migration (cf. below). Subjects with a normal sense of smell (nIHH), on the other hand, represent a pure neuroendocrine defect in either secretion or action of GnRH. However clear such a dichotomous clinical distinction may seem between these two phenotypic variants, it is now increasingly evident that KS, nIHH or isolated anosmia can all occur within a given affected family and thus represent some degree of phenotypic heterogeneity occurring within families. Indeed, this was evident in Kallmann's original report (Kallmann, Schoenfeld et al. 1944). Given that Mendelian inheritance was assumed in this familial disorder, this incomplete penetrance and variable expressivity of phenotypes within KS/nIHH pedigress has long puzzled geneticists and clinician investigators. Several pedigrees of GnRH deficiency have now uncovered the role of gene/gene interactions (oligogenicity- cf below) in this condition and thus demonstrates that these phenotypic variants of GnRH deficiency line a much broader and more complex continuous spectrum of clinical defects in isolated GnRH deficiency rather than representing single unique phenotypes. Such genetic and clinical heterogeneity sets the stage for a modern discovery period using genetic tools, the Human Genome Project's reference materials, and genomic and bioinformatic tools. Together, these new techniques have now provided the next and most interesting chapters in this biologic mystery of the genetic control of GnRH in the human as reviewed in this Special Edition of MCE.
(2) The Clinical Spectrum of Reproductive Defects in Humans with Isolated GnRH deficiency
Constitutional delay of puberty (CDP) is a common and psychologically challenging adolescent phenotype that represents the mildest of reproductive defects in GnRH neuronal maturation in which the onset of GnRH secretion is delayed albeit normal once it occurs. CDP is defined statistically as the failure to initiate puberty beyond 2 standard deviations from the mean for a given population. CDP subjects, typically 5:1 male to female ratio, eventually enter puberty spontaneously albeit late but characteristically remain reproductively normal thereafter (Fig 1). In contrast, subjects with the more complete form of isolated GnRH deficiency fail to either initiate or complete puberty resulting in either KS or nIHH and these subjects display varying degrees of pubertal impairment. For example, in some males with KS and nIHH, disruption of GnRH neuronal function and presumably pulsatile GnRH secretion occurred during the window of late fetal/early infancy (mini-puberty) leaving the phenotypic sequelae of micropenis and/or cryptorchidism that persist into adulthood and speak to this earlier deficit in GnRH secretion (Fig 1). Such a picture is typical of subjects with Neurokinin B deficiency as outlined in Chapter XX (Gianetti, Tusset et al. 2010). This combination of micropenis and cryptorchidism thus offers a potential clinical investigative opportunity to detect the consequences of impaired mini-puberty of the neonatal period thereby enabling the identification and diagnosis of those patients with GnRH deficiency at the earliest possible period in their post-natal life. If this window of opportunity to make the diagnosis is missed early in life and no other non-reproductive feature (eg., anosmia, cleft lip/palate, renal agenesis, mental retardation, hearing defect etc) is present, the identification of subjects with isolated GnRH deficiency must await their hallmark failure to undergo a spontaneous and normally timed puberty when they ultimately will declare themselves to their physicians.
Complete absence of puberty is the next developmental window when GnRH deficiency becomes apparent clinically (Fig 1). These GnRH deficient subjects present with a lack of any pubertal signs and symptoms of sexual maturation. They display frank hypogonadism with low gonadotropins, normal neuroanatomic studies, an isolated defect in gonadotropin secretion with other endocrine functions being intact, and infertility in both sexes. In a small percentage of such men, a partial GnRH deficient phenotype can be observed as the “fertile eunuch” variant of this syndrome. These cases, testicular steroidogenesis is nearly completely absent as evidenced by their frankly hypogonadal testosterone levels yet spermatogenesis is somewhat paradoxically preserved (McCullagh, Beck et al. 1953; Faiman, Hoffman et al. 1968; Makler, Glezerman et al. 1977). Similar to their male counterpart, females with IHH subjects can vary from complete absence of puberty with infertility to partial defects often when isolated thelarche or menarche may occur but fail to progress (Shaw, Seminara et al. 2011).
(3) Time of onset of reproductive deficit and duration of reproductive failure
Adult Onset Isolated GnRH Deficiency
The most severe form of GnRH deficiency derives from either the absence of GnRH secretion during either the “mini-puberty” of the neonatal period (with microphallus and cryptorchidism) or more commonly, simply with absence of puberty. However, a more delayed presentation is now well-recognized in men who have undergone an otherwise completely normal sexual development at puberty but in whom hypothalamic secretion of GnRH subsequently ceased during adulthood (Nachtigall, Boepple et al. 1997). This variant of isolated GnRH deficiency is termed adult-onset idiopathic hypogonadotropic hypogonadism whose responsiveness to exogenous GnRH administration in 9/10 cases demonstrates the hypothalamic nature of their defect (AHH) (Fig 1). Long term follow of this syndrome has indicated these men fail to ever recover suggesting a permanent defect in GnRH secretion (45) as opposed to what happens in women.
Hypothalamic Amenorrhea
A milder adult onset but reversible form of functional GnRH deficiency occurs in otherwise normal adult females and was first referred to as `hypothalamic amenorrhoea' (HA) by Albright and his colleagues (Moldawer, Albright et al. 1958). Hypothalamic amenorrhea is the most common reproductive disorder occuring in 2–3% of the normal female population. Whereas most of these subjects had previously experienced a normal puberty and sexual development, they subsequently develop transient hypogonadotropic hypogonadism due to defects in endogenous GnRH secretion that appears to be precipitated by excessive stress, under-nutrition, and/or over-exercise (Santoro, Filicori et al. 1986). A key to this diagnosis and a hallmark of this syndrome attesting to the functional nature of this condition is that the HPG axis generally recovers in most upon removal of the precipitant. Whereas earlier thinking about women with hypothalamic amenorrhea stressed the role of these environmental factors such as exercise and nutrition, recent studies from our group have indicated that even this mildest form of functional GnRH deficiency as seen in women with hypothalamic amenorrhea has a strongly underlying genetic basis (Caronia, Martin et al. 2011).
Reversible GnRH Deficiency
Finally, although isolated GnRH deficiency was initially thought to be a life-long condition, spontaneous reversals of well-established GnRH deficiency have now been demonstrated to occur in approximately 10–15% of men with GnRH deficiency following therapy with a variety of treatments having in common elevation of sex steroids to pubertal levels (Raivio, Falardeau et al. 2007) (Fig 1). This unique observation hints at a previously unrecognized plasticity of the GnRH neuronal network that can override developmental and neuroendocrine defects in GnRH neuronal ontogeny. It also implies that the GnRH neuronal network is in place anatomically so that it can subsequently secrete GnRH in a pulsatile fashion and awaken the pituitary-gonadal axis later in adulthood but that a failure of this mechanism occurs at the time of normal puberty. Since all of these patients received some degree of elevations of their gonadal sex steroids as part of their treatment, it is also suggested that exposure to sex steroid hormones is a key element to the milieu in which these `reversals' occur.
(4) Patterns of GnRH secretory defects
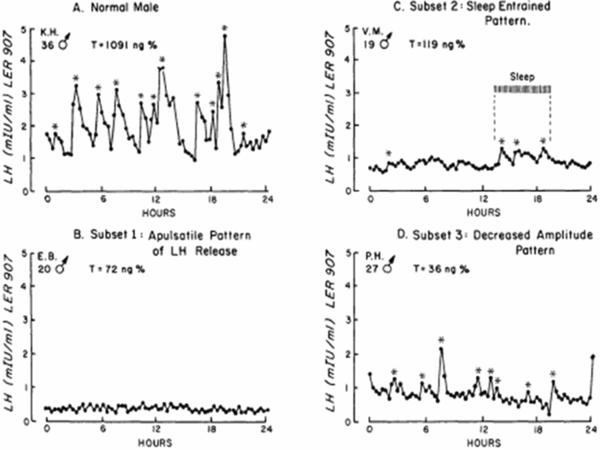
In keeping with the variable degree and timing of reproductive defects, the pattern of GnRH-induced gonadotropin secretion in subjects with isolated GnRH deficiency similarly displays a wide spectrum of neuroendocrine defects in both sexes (Santoro, Filicori et al. 1986; Spratt, Carr et al. 1987; Shaw, Seminara et al. 2011). A normal GnRH-induced LH secretion pattern in adult male is shown in Fig 2A. Although majority of subjects display an undetectable LH pulse profile (termed an apulsatile pattern, Fig 2B), some GnRH deficiency subjects show preserved sleep-augmented LH pulse secretion (developmental arrest pattern, Fig 2C) while yet another minority of patients demonstrate the presence of an enfeebled, low amplitude LH secretion occurring at a normal frequency (Fig 2D). In females with HA, frequent sampling studies during the follicular phase show a similar spectrum of defects in GnRH-induced LH secretion (Santoro, Filicori et al. 1986).
Figure 2. GnRH secretory patterns in normal male and males with isolated GnRH deficiency.
A. Normal adult male pattern of LH secretion: High amplitude LH pulses at ¬2h interval intervals with normal testosterone levels. B. Apulsatile pattern of LH secretion in a GnRH deficient male subject with frank hypogonadal T levels. C. Developmental arrest or sleep entrained (hatched bar) pattern of LH secretion in a GnRH deficient male. D. Normal frequency but low amplitude pattern of LH secretion in a GnRH deficient male subject.
(5) Non-reproductive Phenotypes
Several non-reproductive features are now well-recognized to accompany GnRH deficiency and often provide the phenotypic clues as to the underlying gene defect. Therefore, these phenotypic features should be sought and used to assist in targeting genetic testing more effectively (Genetic Counseling Chapter XX). These well-described associations include: cranio-facial defects (cleft lip/palate, high arched palate, coloboma, choanal atresia); renal agenesis, horseshoe kidney and GU duplications; skeletal defects - digital anomalies (short 4th metacarpals; campylodactyly, syndactyly, clinodactyly), scoliosis; sensorineural deafness, dental agenesis, oculomotor abnormalities, bimanual synkinesia and cerebellar ataxia. Going forward, detailed phenotyping of GnRH deficient subjects with regards to these associated features is imperative to allow targeted diagnostic genetic testing.
AN EVOLVING UNDERSTANDING OF THE GENETIC ARCHITECTURE OF GnRH DEFICIENCY
With the mounting genetic evidence and identification of genetic mutations outlined in this Special Edition, several overview features about the genetic architecture of human GnRH deficiency have become evident:
(1) Only half of the genes causing isolated GnRH deficiency have been identified
Given the impact of genes causing GnRH deficiency on family size prior to any treatment, most cases of isolated GnRH deficiency occurs in the sporadic form (Seminara and Crowley 2002). Even in familial cases, the pedigrees of this disorder are characteristically small, thus hindering the ability to apply traditional linkage analysis as would be possible in larger families. Despite these hurdles, in the last two decades, several independent groups have documented and mapped 14 genes that lead to human GnRH deficiency. This rapid progress has been possible in a large part by the use of targeted clinical investigation, the evolving power of human genetics, combined with the tools and information derived from mapping the human genome. However, striking allelic heterogeneity is evident in each of the discovered genes and almost all of the genetic variants uncovered to date have been private mutations without any obvious mutational hot spots. Moreover, these currently known genes account for only half of the genetic basis of this disorder with each gene contributing only a minor percentage (Fig 3). The role of each gene in the GnRH neuronal ontogeny varies from purely neurodevelopmental genes that impair GnRH development and migration (KAL1, NELF) to purely neuroendocrine genes (GNRH1, GNRHR, KISS1, KISS1R, TAC3, TACR3) while certain genes are implicated in both development and neuroendocrine function (FGF8, FGFR1, PROK2, PROKR2) (Fig 4).
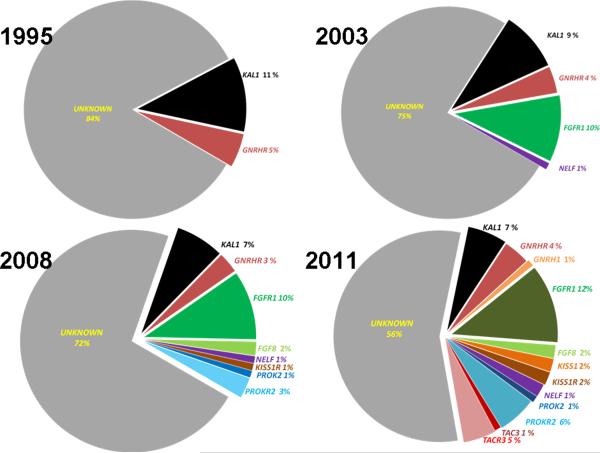
Figure 3. Genetic causes of isolated GnRH deficiency: A historical perspective.
Genetic etiology of isolated GnRH deficiency with relative percentage contribution from each identified gene to the heritability of the syndrome from 1995 – 2011. From only 2 genes that were known in 1995, the number of genes discovered in subjects with isolated GnRH deficiency has steadily increased through 1995–2011. In 2011, the genetic cause of a nearly half of the subjects is known while in the remaining half, the genes are yet to be identified.
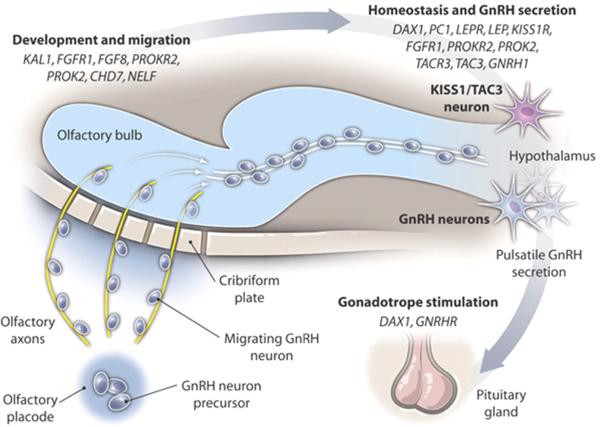
Figure 4. GnRH neuronal ontogeny in humans.
Specific gene mutations in patients with GnRH deficiency result in failure in GnRH development and migration (KAL1, Kallmann syndrome 1 sequence, MIM: 308700; NELF, nasal embryonic LHRH factor, MIM: 608137) while other gene mutations impair hypothalamic GnRH homeostasis and GnRH secretion (DAX1, dosage-sensitive sex reversal, adrenal hypoplasia congenital critical region on the X chromosome, gene 1, MIM: 300473; PC1, prohormone convertase 1, MIM: 162150; LEPR, leptin receptor, MIM: 601007; LEP, leptin, MIM: 164160; KISS1R, KISS1 receptor, MIM: 604161; TACR3, tachykinin receptor 3, MIM: 162332; TAC3, tachykinin 3, MIM: 162330; GNRH1, gonadotropin-releasing hormone 1, MIM: 152760; GNRHR, GnRH receptor, MIM: 138850). Some gene mutations affect both migration/development as well as neuroendocrine secretion of GnRH (FGFR1, fibroblast growth factor receptor 1, MIM: 136350; FGF8, fibroblast growth factor 8, MIM: 600483; PROKR2, prokineticin receptor 2, MIM: 607123; PROK2, prokineticin 2, MIM: 607002; CHD7, chromodomain helicase DNA binding protein 7, MIM: 608765).
Thus, the significant challenge that lies ahead is to utilize the evolving genetic techniques to identify the “missing heritability” in those familial cases of GnRH deficiency. In addition, it is likely that the genes yet to be discovered may be extremely rare variants; there may be several more of them; and thus each will account for only a minor percentage of the etiology of GnRH deficiency. Hence, large numbers of patients, extensive phenotypic databases, and collaborations across multiple groups with well-phenotyped cohorts of these rare patients will be the key to enhance gene discovery in the future. A more intriguing question is to whether the novel missing genes will map to the existing systems biology of the GnRH neurons or if they will unearth completely novel systems biology pathways. To the delight of basic and clinical investigators, the recent discovery of the novel kisspeptin pathway (de Roux, Genin et al. 2003; Seminara, Messager et al. 2003) has opened up an entirely new chapter in the study of the neurobiology of GnRH neuronal function and highlighted the critical role of the kisspeptin signaling system to control sexual maturation in all mammals studied to date (Seminara and Crowley 2008; Oakley, Clifton et al. 2009). It is tempting to speculate that similar novel insights may be expected from future investigation of this unique human phenotype.
(2) Monogenic vs Oligogenic Genetic Architecture to Human GnRH Deficieny
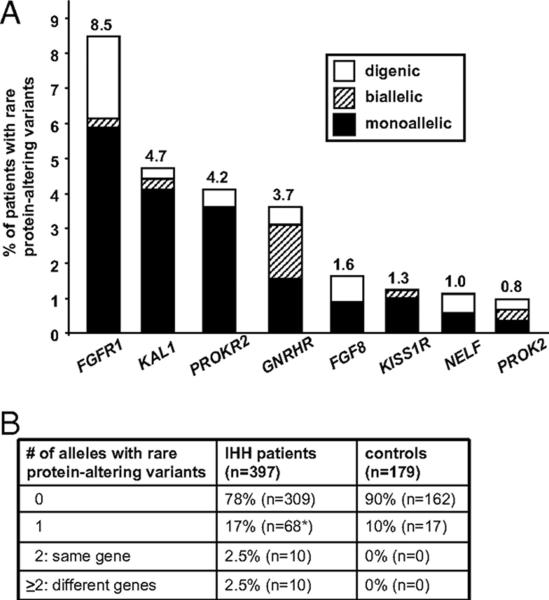
The mode of inheritance in several GnRH deficient families with identified genetic variants poses an interesting genetic puzzle whose perspective has been dramatically changed by recent clinical investigations. For example, most pedigrees of male subjects harboring KAL1 mutations display a fully penetrant expression of their reproductive and olfactory phenotypes (Hardelin, Levilliers et al. 1993; Izumi, Tatsumi et al. 2001; Bhagavath, Xu et al. 2007; Versiani, Trarbach et al. 2007; Hardelin and Dode 2008; Salenave, Chanson et al. 2008) (see Chapter X). Similarly, in subjects with homozyogous mutations in presumed autosomal recessive genes (eg GNRH1, GnRHR, TAC3, TAC3R, PROK2, PROKR2, KISS1R), segregation analysis generally reveals concordance of reproductive and non-reproductive phenotypes amongst mutation carriers (de Roux, Young et al. 1999; Beranova, Oliveira et al. 2001; Dode, Teixeira et al. 2006; Seminara and Crowley 2008; Bouligand, Ghervan et al. 2009; Chan, de Guillebon et al. 2009; Gianetti, Tusset et al. 2010; Martin, Balasubramanian et al. 2010; Sarfati, Guiochon-Mantel et al. 2010). However, in pedigrees with heterozygous mutations in several of the known GnRH deficiency genes (eg FGFR1, FGF8, PROK2, PROKR2, GNRHR), multiple family members harboring identical mutant alleles display considerable phenotypic variability within a pedigree in terms of both their reproductive and nonreproductive features (Dode, Teixeira et al. 2006; Pitteloud, Zhang et al. 2007; Cole, Sidis et al. 2008; Hardelin and Dode 2008; Kim, Hu et al. 2008; Martin, Balasubramanian et al. 2010; Sarfati, Guiochon-Mantel et al. 2010). Similarly, the identical mutation in several different families can equally vary widely in their phenotype (Dode, Teixeira et al. 2006; Pitteloud, Zhang et al. 2007; Martin, Balasubramanian et al. 2010). Moreover, in some of these pedigrees, the mutation can occur in a phenotypically normal individual (Dode, Teixeira et al. 2006; Martin, Balasubramanian et al. 2010). Although these observations were initially puzzling, apparently violating the rules of strict Mendelian segregation analyses, the subsequent identification of digenic pedigrees where two individually rare pathogenic DNA sequence variants synergize or act in concert (digenicity or oligogenicity) to produce the phenotype being observed began to at least explain the observed variability encountered in many of these families (Pitteloud, Quinton et al. 2007). Though initially discovered in only 2 families (Pitteloud et al), subsequent larger studies of nearly 400 GnRH deficient subjects have documented that digenicity occurs in nearly a quarter of all of those in which rare sequence variants could be identified (Sykiotis, Plummer et al. 2010). This systematic evaluation of pathogenic rare variants in a large number of GnRH deficient patients has now shown that the prevalence of oligogenicity within human GnRH deficient subjects is at least 15% of patients with demonstrable coding sequence mutations (Sykiotis, Plummer et al. 2010). Given that only 40–50% of the genetic causes are currently known (Fig. 5) and extrapolating this frequency of oligogenicity to unidentified genes, it is likely that GnRH deficiency in humans will ultimately prove to be an oligogenic human disorder rather than a purely monogenic condition as previously assumed. This oligogenic architecture is now known to occur in several other human disease models such as the ciliopathies (Badano, Kim et al. 2003). Thus, it is fairly likely that similar oligogenic mechanisms may partly explain the hitherto unexplained variable expressivity that has been puzzled clinicians in several monogenic Mendelian disorders.
Figure 5. Oligogenicity in isolated GnRH deficiency.
(A) Frequency of monoallelic, biallelic, and digenic rare protein-altering variants in 8 genes known to cause isolated GnRH deficiency (B) Number of alleles with rare protein-altering variants in isolated GnRH deficient patients (IHH subjects) and healthy controls.
(3) Epigenetic interactions
Although the quest for the missing heritability in many genetic disorders has been focused on protein altering DNA sequence variants, it is increasingly clear that epigenetic changes may well contribute to the phenotypic heterogeneity in several human disease. The notion that epigenetic interactions may play a role in the genetic architecture of GnRH deficiency comes from two observations. Despite a shared genetic background, monozygotic twins discordant for GnRH deficiency have been described (Hipkin, Casson et al. 1990; Seminara and Crowley 2002). Additionally, siblings sharing a large deletion in KAL1 gene exhibit considerable phenotypic heterogeneity (Massin, Pecheux et al. 2003). These observations strongly suggest the existences of potential modifying influences above and beyond mere sequence variations that somehow shape the eventual clinical phenotype of this condition. In addition, despite carrying clearly pathogenic mutations, several GnRH deficient individuals show reversal of their GnRH deficiency later in adulthood following exposure to sex steroids (Raivio, Falardeau et al. 2007). This recovery of GnRH neuronal function, seen in 10–15% of these patients, is highly suggestive of significant gene/environment/hormonal interactions (Raivio, Falardeau et al. 2007). In addition, it is highly likely that the GnRH neuronal network is not fixed in its capacity during adulthood as evidenced by both the reversal phenotype and the occurrence of AHH in otherwise normal individuals (Nachtigall, Boepple et al. 1997).
CONCLUDING REMARKS
The scientific impetus for studying the molecular genetics of human GnRH deficiency is pinned on the hopes of using these unique insights to understand the biological basis of this disorder and to translate these insights into an improved understanding of the complex dynamics of common reproductive disorders as well as to design improved diagnostic and therapeutic strategies in these patients who have been such important partners in the discovery efforts of their condition. The physiologic and genetic studies in humans with isolated GnRH deficiency in the last two decades have just begun to chart the ontogenic determinants of the development and neuro-secretory functioning of GnRH neurons in a unique and powerful fashion. These studies have highlighted a key role of oligogenicity and gene/environment interactions, both of which are likely to underlie the phenotypic diversity in this disorder and guide our understanding in several other genetic diseases. The recent discovery that GnRH deficiency related genes also underlie the susceptibility to HA, the commonest reproductive disorder affecting 2–3% of otherwise normal women, provides an important proof of principle that detailed study of rare disorders such as GnRH deficiency can translate directly to common reproductive diseases.
Several challenges remain with a large number of genetic variants yet to discovered, their expanding clinical diversity, the expensive phenotyping requirements of studying these patients, and the availability of new tools with which to probe this disorder. All of these opportunities and challenges strongly argue that this investigatory journey must continue and that collaborations and consortia will be the key to its future. The forthcoming years promise further novel insights with the advent of large-scale deep sequencing using next generation techniques. This opportunity begets an important question for the field. Are we ready to tackle the tsunami of the genetic variants that will emerge by the use of these advanced techniques? Can we collaborate effectively to do this as increasingly the `numbers game' aspect of this will be important? Are we able to conduct the careful and comprehensive phenotyping of both reproductive and non-reproductive features of GnRH deficient subjects that will now be important than ever? Are we able to develop sufficiently robust functional assays to evaluate the role of these alleleic variations singly and in combination with other mutations as only then can we begin to translate the newly acquired genomic information into the ight clinical context?
There has never been a better time to assemble an international network of clinical investigators to collaborate and work in unison to help solve one of the 21st century's unanswered puzzles: What triggers puberty?
Acknowledgments
Funding Sources: This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD28138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and R01 HD15788.
REFERENCES
- Altmann F. Uber Eunuchoidismus. Virchows Arch [Pathol Anat] 1930;276:455. [Google Scholar]
- Andersson AM, Toppari J, et al. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab. 1998;83(2):675–681. doi: 10.1210/jcem.83.2.4603. [DOI] [PubMed] [Google Scholar]
- Badano JL, Kim JC, et al. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet-Biedl patients with two mutations at a second BBS locus. Hum Mol Genet. 2003;12(14):1651–1659. doi: 10.1093/hmg/ddg188. [DOI] [PubMed] [Google Scholar]
- Beranova M, Oliveira LM, et al. Prevalence, phenotypic spectrum, and modes of inheritance of gonadotropin-releasing hormone receptor mutations in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2001;86(4):1580–1588. doi: 10.1210/jcem.86.4.7395. [DOI] [PubMed] [Google Scholar]
- Bhagavath B, Xu N, et al. KAL1 mutations are not a common cause of idiopathic hypogonadotrophic hypogonadism in humans. Mol Hum Reprod. 2007;13(3):165–170. doi: 10.1093/molehr/gal108. [DOI] [PubMed] [Google Scholar]
- Bick D, Curry CJ, et al. Male infant with ichthyosis, Kallmann syndrome, chondrodysplasia punctata, and an Xp chromosome deletion. Am J Med Genet. 1989;33(1):100–107. doi: 10.1002/ajmg.1320330114. [DOI] [PubMed] [Google Scholar]
- Bouligand J, Ghervan C, et al. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med. 2009;360(26):2742–2748. doi: 10.1056/NEJMoa0900136. [DOI] [PubMed] [Google Scholar]
- Caronia LM, Martin C, et al. A genetic basis for functional hypothalamic amenorrhea. N Engl J Med. 2011;364(3):215–225. doi: 10.1056/NEJMoa0911064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YM, de Guillebon A, et al. GNRH1 mutations in patients with idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0903449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LW, Sidis Y, et al. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab. 2008;93(9):3551–3559. doi: 10.1210/jc.2007-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte FA, Grumbach MM, et al. A diphasic pattern of gonadotropin secretion in patients with the syndrome of gonadal dysgenesis. J Clin Endocrinol Metab. 1975;40(4):670–674. doi: 10.1210/jcem-40-4-670. [DOI] [PubMed] [Google Scholar]
- Corbier P, Dehennin L, et al. Sex differences in serum luteinizing hormone and testosterone in the human neonate during the first few hours after birth. J Clin Endocrinol Metab. 1990;71(5):1344–1348. doi: 10.1210/jcem-71-5-1344. [DOI] [PubMed] [Google Scholar]
- Crowley WF, Jr., McArthur JW. Simulation of the normal menstrual cycle in Kallman's syndrome by pulsatile administration of luteinizing hormone-releasing hormone (LHRH) J Clin Endocrinol Metab. 1980;51(1):173–175. doi: 10.1210/jcem-51-1-173. [DOI] [PubMed] [Google Scholar]
- de Morsier G. Etudes sur les dysraphies cranio-encephaliques. Schweiz Arch Neurol Psychiatr. 1954;74:309–361. [PubMed] [Google Scholar]
- De Morsier G, Gauthier G. Olfacto-Genital Dysplasia. Pathol Biol (Paris) 1963;11:1267–1272. [PubMed] [Google Scholar]
- de Roux N, Genin E, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Young J, et al. Loss of function mutations of the GnRH receptor: a new cause of hypogonadotropic hypogonadism. J Pediatr Endocrinol Metab. 1999;12(Suppl 1):267–275. [PubMed] [Google Scholar]
- de Zegher F, Devlieger H, et al. Pulsatile and sexually dimorphic secretion of luteinizing hormone in the human infant on the day of birth. Pediatr Res. 1992;32(5):605–607. doi: 10.1203/00006450-199211000-00025. [DOI] [PubMed] [Google Scholar]
- Dode C, Teixeira L, et al. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006;2(10):e175. doi: 10.1371/journal.pgen.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiman C, Hoffman DL, et al. The “fertile eunuch” syndrome: demonstration of isolated luteinizing hormone deficiency by radioimmunoassay technique. Mayo Clin Proc. 1968;43(9):661–667. [PubMed] [Google Scholar]
- Forest M. Control of the onset of puberty. Williams and Wilkins; Baltimore: 1990. Pituitary gonadotropins and sex steroid secretion during the first two years of life; pp. 451–477. S. P. Grumbach MM, Aubert ML. [Google Scholar]
- Gauthier G. Olfacto-genital dysplasia (agenesis of the olfactory lobes with absence of gonadal development at puberty. Acta Neuroveg (Wien) 1960;21:345–394. doi: 10.1007/BF01228269. [DOI] [PubMed] [Google Scholar]
- Gianetti E, Tusset C, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857–2867. doi: 10.1210/jc.2009-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumbach MM GP. Maternal-fetal endocrinology. WB Saunders; Philadelphia: 1994. The human fetal hypothalamus and pituitary gland: the maturation of neuroendocrine mechanisms controlling the secretion of fetal pituitary growth hormone, prolactin, gonadotropin, adrenocorticotropin-related peptides, and thyrotropin; pp. 193–261. L. A. Tulchinsky D. [Google Scholar]
- Grumbach MM KS. Control of the onset of puberty. Williams, Wilkins; Baltimore: 1990. The neuroendocrinology of human puberty: An ontogenetic perspective; pp. 1–62. S. P. Grumbach MM, Aubert ML. [Google Scholar]
- Hardelin JP, Dode C. The complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et al. Sex Dev. 2008;2(4–5):181–193. doi: 10.1159/000152034. [DOI] [PubMed] [Google Scholar]
- Hardelin JP, Levilliers J, et al. Heterogeneity in the mutations responsible for X chromosome-linked Kallmann syndrome. Hum Mol Genet. 1993;2(4):373–377. doi: 10.1093/hmg/2.4.373. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Miyai K, et al. Isolated gonadotropin deficiency with response to luteinizing-hormone-releasing hormone. N Engl J Med. 1972;287(21):1059–1062. doi: 10.1056/NEJM197211232872102. [DOI] [PubMed] [Google Scholar]
- Heller CG, Nelson WO. Classification of male hypogonadism and a discussion of the pathologic physiology, diagnosis and treatment. J Clin Endocrinol Metab. 1948;8(5):345–366. doi: 10.1210/jcem-8-5-345. [DOI] [PubMed] [Google Scholar]
- Hipkin LJ, Casson IF, et al. Identical twins discordant for Kallmann's syndrome. J Med Genet. 1990;27(3):198–199. doi: 10.1136/jmg.27.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AR, Crowley WF., Jr. Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307(20):1237–1241. doi: 10.1056/NEJM198211113072003. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Tatsumi K, et al. Analysis of the KAL1 gene in 19 Japanese patients with Kallmann syndrome. Endocr J. 2001;48(2):143–149. doi: 10.1507/endocrj.48.143. [DOI] [PubMed] [Google Scholar]
- Kallmann F, Schoenfeld W, et al. The genetic aspects of primary eunuchoidism. American Journal of Mental Deficiency. 1944;48:203–236. [Google Scholar]
- Kennedy D, Norman C. What don't we know? Science. 2005;309(5731):75. doi: 10.1126/science.309.5731.75. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hu Y, et al. Diversity in fibroblast growth factor receptor 1 regulation: learning from the investigation of Kallmann syndrome. J Neuroendocrinol. 2008;20(2):141–163. doi: 10.1111/j.1365-2826.2007.01627.x. [DOI] [PubMed] [Google Scholar]
- Klinefelter HAF, Griswold GC. Experience with a Quantitative Test for Normal or Decreased Amounts of Follicle Stimulating Hormone in the Urine in Endocrinological Diagnosis. J Clin Endocrinol Metab. 1943;3:529–544. [Google Scholar]
- Maestre de San Juan A. Teratologia: Falta total de los nervios olfactorios con anosmia en un individuo en quien existia una atrofia congénita de los testículos y miembro viril. Siglo Medico. 1856;3:211–221. [Google Scholar]
- Makler A, Glezerman M, et al. The fertile eunuch syndrome. An isolated leydig-cell failure? Andrologia. 1977;9(2):163–170. doi: 10.1111/j.1439-0272.1977.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Balasubramanian R, et al. The Role of the Prokineticin 2 Pathway in Human Reproduction: Evidence from the Study of Human and Murine Gene Mutations. Endocr Rev. 2010 doi: 10.1210/er.2010-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa G, de Zegher F, et al. Serum levels of immunoreactive inhibin, FSH, and LH in human infants at preterm and term birth. Biol Neonate. 1992;61(3):150–155. doi: 10.1159/000243737. [DOI] [PubMed] [Google Scholar]
- Massin N, Pecheux C, et al. X chromosome-linked Kallmann syndrome: clinical heterogeneity in three siblings carrying an intragenic deletion of the KAL-1 gene. J Clin Endocrinol Metab. 2003;88(5):2003–2008. doi: 10.1210/jc.2002-021981. [DOI] [PubMed] [Google Scholar]
- McCullagh EP, Beck JC, et al. A syndrome of eunuchoidism with spermatogenesis, normal urinary FSH and low or normal ICSH: (fertile eunuchs) J Clin Endocrinol Metab. 1953;13(5):489–509. doi: 10.1210/jcem-13-5-489. [DOI] [PubMed] [Google Scholar]
- Moldawer MP, Albright F, et al. Eunuchoidism with low urinary follicle-stimulating hormone in the female: comparison with this syndrome in the male and with the premenarchal menopause. J Clin Endocrinol Metab. 1958;18(1):1–14. doi: 10.1210/jcem-18-1-1. [DOI] [PubMed] [Google Scholar]
- Muller J, Skakkebaek NE. Fluctuations in the number of germ cells during the late foetal and early postnatal periods in boys. Acta Endocrinol (Copenh) 1984;105(2):271–274. doi: 10.1530/acta.0.1050271. [DOI] [PubMed] [Google Scholar]
- Nachtigall LB, Boepple PA, et al. Adult-onset idiopathic hypogonadotropic hypogonadism--a treatable form of male infertility. N Engl J Med. 1997;336(6):410–415. doi: 10.1056/NEJM199702063360604. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Harris GW, et al. Effect of purified luteinizing hormone releasing factor on normal and hypogonadotrophic anosmic men. Nature. 1971;232(5311):496–497. doi: 10.1038/232496a0. [DOI] [PubMed] [Google Scholar]
- Nielsen CT, Skakkebaek NE, et al. Longitudinal study of testosterone and luteinizing hormone (LH) in relation to spermarche, pubic hair, height and sitting height in normal boys. Acta Endocrinol Suppl (Copenh) 1986;279:98–106. doi: 10.1530/acta.0.112s098. [DOI] [PubMed] [Google Scholar]
- Nielsen CT, Skakkebaek NE, et al. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair, and height. J Clin Endocrinol Metab. 1986;62(3):532–535. doi: 10.1210/jcem-62-3-532. [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, et al. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Quinton R, et al. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117(2):457–463. doi: 10.1172/JCI29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, et al. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 2007;104(44):17447–17452. doi: 10.1073/pnas.0707173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton R, Duke VM, et al. Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clin Endocrinol (Oxf) 2001;55(2):163–174. doi: 10.1046/j.1365-2265.2001.01277.x. [DOI] [PubMed] [Google Scholar]
- Raivio T, Falardeau J, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357(9):863–873. doi: 10.1056/NEJMoa066494. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Seminara SB, et al. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;148(7):3364–3370. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- Roth JC, Kelch RP, et al. FSH and LH response to luteinizing hormone-releasing factor in prepubertal and pubertal children, adult males and patients with hypogonadotropic and hypertropic hypogonadism. J Clin Endocrinol Metab. 1972;35(6):926–930. doi: 10.1210/jcem-35-6-926. [DOI] [PubMed] [Google Scholar]
- Salenave S, Chanson P, et al. Kallmann's syndrome: a comparison of the reproductive phenotypes in men carrying KAL1 and FGFR1/KAL2 mutations. J Clin Endocrinol Metab. 2008;93(3):758–763. doi: 10.1210/jc.2007-1168. [DOI] [PubMed] [Google Scholar]
- Santoro N, Filicori M, et al. Hypogonadotropic disorders in men and women: diagnosis and therapy with pulsatile gonadotropin-releasing hormone. Endocr Rev. 1986;7(1):11–23. doi: 10.1210/edrv-7-1-11. [DOI] [PubMed] [Google Scholar]
- Sarfati J, Guiochon-Mantel A, et al. A comparative phenotypic study of kallmann syndrome patients carrying monoallelic and biallelic mutations in the prokineticin 2 or prokineticin receptor 2 genes. J Clin Endocrinol Metab. 2010;95(2):659–669. doi: 10.1210/jc.2009-0843. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Bick D, et al. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res. 1989;6(4):311–326. doi: 10.1016/0169-328x(89)90076-4. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Crowley WF., Jr. Genetic approaches to unraveling reproductive disorders: examples of bedside to bench research in the genomic era. Endocr Rev. 2002;23(3):382–392. doi: 10.1210/edrv.23.3.0469. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Crowley WF., Jr. Kisspeptin and GPR54: discovery of a novel pathway in reproduction. J Neuroendocrinol. 2008;20(6):727–731. doi: 10.1111/j.1365-2826.2008.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Dipietro MJ, et al. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147(5):2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Hayes FJ, et al. Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19(5):521–539. doi: 10.1210/edrv.19.5.0344. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Fraser HM, et al. Role of the neonatal period of pituitary-testicular activity in germ cell proliferation and differentiation in the primate testis. Hum Reprod. 2003;18(10):2110–2117. doi: 10.1093/humrep/deg413. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, et al. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125(6):769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Shaw ND, Seminara SB, et al. Expanding the phenotype and genotype of female GnRH deficiency. J Clin Endocrinol Metab. 2011;96(3):E566–576. doi: 10.1210/jc.2010-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt DI, Carr DB, et al. The spectrum of abnormal patterns of gonadotropin-releasing hormone secretion in men with idiopathic hypogonadotropic hypogonadism: clinical and laboratory correlations. J Clin Endocrinol Metab. 1987;64(2):283–291. doi: 10.1210/jcem-64-2-283. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Plummer L, et al. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci U S A. 2010;107(34):15140–15144. doi: 10.1073/pnas.1009622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versiani BR, Trarbach E, et al. Clinical assessment and molecular analysis of GnRHR and KAL1 genes in males with idiopathic hypogonadotrophic hypogonadism. Clin Endocrinol (Oxf) 2007;66(2):173–179. doi: 10.1111/j.1365-2265.2006.02702.x. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Weissenbacher G, et al. Pulsatile secretion of gonadotropins in early infancy. European journal of pediatrics. 1981;137(1):71–74. doi: 10.1007/BF00441173. [DOI] [PubMed] [Google Scholar]
- Weidenreich F. Uber partiellen Riechlappendefekt und Eunuchoidismus beim Menschen. Z Morphol Anthropol. 1914;18:157–190. [Google Scholar]
- Wu FC, Butler GE, et al. Patterns of pulsatile luteinizing hormone secretion before and during the onset of puberty in boys: a study using an immunoradiometric assay. J Clin Endocrinol Metab. 1990;70(3):629–637. doi: 10.1210/jcem-70-3-629. [DOI] [PubMed] [Google Scholar]
- Wu FC, Butler GE, et al. Patterns of pulsatile luteinizing hormone and follicle-stimulating hormone secretion in prepubertal (midchildhood) boys and girls and patients with idiopathic hypogonadotropic hypogonadism (Kallmann's syndrome): a study using an ultrasensitive time-resolved immunofluorometric assay. J Clin Endocrinol Metab. 1991;72(6):1229–1237. doi: 10.1210/jcem-72-6-1229. [DOI] [PubMed] [Google Scholar]