Abstract
Purpose
To investigate the feasibility of using time-of-flight (TOF) images as a constraint in the reconstruction of a series of highly undersampled time-resolved contrast-enhanced MR images (HYPR TOF), to allow simultaneously high temporal and spatial resolution and increased SNR.
Materials and Methods
Ten healthy volunteers and three patients with aneurysms underwent a HYPR TOF study, which includes a clinical routine TOF scan followed by a first pass time-resolved contrast-enhanced exam using an undersampled three-dimensional (3D) projection trajectory (VIPR). Image quality, waveform fidelity and signal to background variation ratio measurements were compared between HYPR TOF images and VIPR images without HYPR reconstruction.
Results
Volunteer results demonstrated the feasibility of using the clinical routine TOF as the spatial constraint to reconstruct the first pass time-resolved contrast-enhanced MRA acquired using highly undersampled 3D projection trajectory (VIPR). All the HYPR TOF images are superior to the corresponding VIPR images with the same temporal reconstruction window on both spatial resolution and SNR.
Conclusion
HYPR TOF improves the spatial resolution and SNR of the rapidly acquired dynamic images without losing the temporal information.
Keywords: time-resolved, contrast-enhanced, MRA, constrained reconstruction
FOR SEVERAL DECADES, time-resolved serial imaging using x-ray digital subtraction angiography (DSA) has played a major role in the diagnosis of vascular disease (1). Non–contrast-enhanced MR angiography and computed tomography (CT) angiography are widely used methods that permit evaluation of the cerebrovascular system at reduced cost and low risk compared with DSA but do not provide temporal information (2,3). Time-resolved contrast-enhanced MR angiography is essential for the characterization of high-flow lesions such as arteriovenous malformations and dural fistulas (1,3).
Recently, there has been extensive work directed at simultaneously obtaining high spatial and temporal resolution in contrast-enhanced MRA. Clinical studies have been reported using parallel imaging approaches such as sensitivity encoding (SENSE) (4,5), simultaneous acquisition of spatial harmonics (SMASH) (6) and refinements of these techniques (7,8), which use information from multiple receiver coils to reduce the number of k-space samples required to reconstruct images with significantly fewer artifacts than would be obtained using conventional undersampled Cartesian acquisition. These techniques usually require inversion of a matrix that leads to noise amplification at high accelerations. Some are computationally intensive. Nevertheless, acceleration factors of 2–4 are commercially available and higher accelerations are becoming available with the increasing number of elements in receiver coils.
Undersampled radial acquisitions like two-dimensional (2D) PR (9, 10), VIPR (11), and PC VIPR (12) have been proved to be effective to accelerate MR angiography. However, as the undersampling factor increases, images are degraded by increased under-sampling artifacts and decreased signal to noise ratio (SNR). A recent innovative reconstruction algorithm called HYPR (HighlY Constrained backPRojection) is able to reduce undersampling artifacts and maintain good SNR even at high levels of acceleration (13, 14). HYPR LR (Local Reconstruction) (15) permits the use of a longer constraint window, resulting in increased SNR and quantitative reconstruction accuracy. Phase Contrast (PC) HYPR FLOW (16, 17), which uses post-contrast PC VIPR images as the spatial constraint and reconstructs the first pass time-resolved contrast-enhanced VIPR acquisition using HYPR LR technique, for the first time, splits the tasks of providing spatial resolution/SNR and temporal resolution between two separate scans with the same acquisition geometry and uses HYPR constrained reconstruction to obtain a time series of images with all of these features.
Time-of-flight (TOF) angiography is a more accepted clinical standard for imaging of the intracranial arterial system due to the improved vessel lumen depiction, shorter echo times (TEs), and improved acquisition efficiency (18, 19). The high SNR and large spatial coverage of 3D multi-slab TOF have made this technique one of the clinical routines for intracranial MRA. One drawback is that it does not provide temporal information, which, may be essential for accurate diagnosis and treatment planning for certain cerebral vascular diseases.
In this study, we use TOF images as a constraint in the reconstruction of a time series of undersampled first pass contrast-enhanced angiograms using HYPR processing. The result, HYPR TOF, is a set of time-resolved angiograms with spatial resolution and SNR comparable to TOF and sub-second temporal resolution.
MATERIALS AND METHODS
HYPR TOF has been tested in ten volunteers and three patients with aneurysm in compliance with HIPAA regulations and using a protocol and consent form approved by local IRB. The images were obtained using a 3T MR system (GE Healthcare) with an eight-channel head coil. Each HYPR TOF study involves two scans, a multi-slab 3D TOF before contrast injection as the spatial constraint and a series of time-resolved multi-echo (ME) VIPR images during contrast injection served for the temporal weighting. The acquisition parameters for 3D TOF were as follows: field of view (FOV) = 22 × 22 × 8.8 cm3, repetition time (TR)/TE = 24/2.7 ms, bandwidth (BW) = 41.76 kHz, acquisition matrix: 512 × 256 × 40/slab, flip angle = 20°, flow compensation, and magnetization transfer were used. The acquired spatial resolution for TOF was 0.4 × 0.8 × 1.0 mm3. The imaging parameters for ME VIPR were: FOV = 22 × 22 × 22 cm3, TR/TE = 3.1/0.4 ms, BW = 125 kHz, read out points were 64 per echo covering between the center to the edge of the k-space, frame update time was 0.5 s. The acquired spatial resolution for ME VIPR was 1.7 × 1.7 × 1.7 mm3. A contrast dose of 0.1 mm/kg was injected at a rate of 3 mL/s for each volunteer.
Reconstruction Scheme
The reconstruction of HYPR TOF can be formulated as the temporal weighting image ( ) multiplied by the composite (IC) and is performed using HYPR LR algorithm (15) as following:
where It is a conventionally reconstructed time frame image from the ME-VIPR scan, IC is the composite image, in our case the TOF image, is the reprojected composite image along the same trajectory as the current time frame image It, and K is the convolution kernel. Zero protection is applied to the denominator by rolling off the normalization after a set threshold of 5% of the maximum signal. The convolution operation is performed in k-space and the Gaussian low-pass filter is used as the multiplier. The equivalent kernel size in image space is approximately 10 × 10 × 10 pixels. To compensate the signal variations due to the high undersampling, a tornado filter (11) was used with 0.5 s at the central k-space and 0.75 s at the cutoff frequency of the local kernel being applied. Such temporal weighting images feature of high temporal resolution (the actual data usage is within 0.75 s acquisition window), high SNR but low spatial resolution due to the spatial low pass filtering and low acquired resolution. The composite image is the high resolution TOF image, which provides the vascular map with high spatial resolution and high SNR. When the composite is multiplied by the weighting images, the result is a time series of high spatial resolution MR angiography images (0.4 × 0.8 × 1.0 mm3) with the uptake features of high temporal resolution CE MRA(0.75 s).
RESULTS
Figure 1 shows the representative time series of HYPR TOF images from a healthy volunteer (Fig. 1a–e). Image series demonstrates the contrast arrival in arteries and passing into veins with high spatial resolution, high SNR and sub-second temporal resolution. The sagittal image comparison of HYPR TOF (Fig. 1f), VIPR reconstructed with gridding algorithm (Fig. 1g) and the TOF composite (Fig. 1h) demonstrates the significant improvement of the spatial resolution and SNR of the HYBRID HYPR reconstruction while maintains the temporal resolution. The same tornado filter was used for both the weighting images of HYPR TOF algorithm and the VIPR gridding reconstruction.
Figure 1.
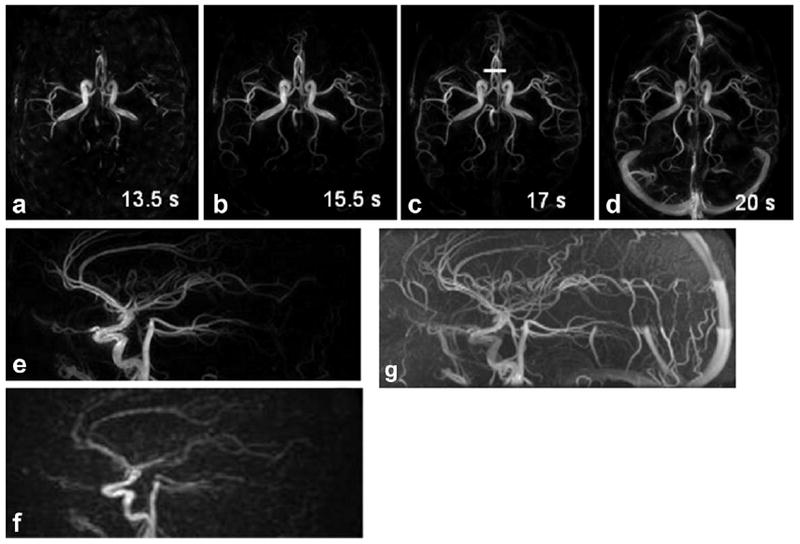
Representative time series of HYPR TOF images from a healthy volunteer (a–d). Numbers on the images are the corresponding time in seconds after the injection. The sagittal image at time frame 17 s after the injection (corresponding to the axial image c) is shown in e and compared with the same time frame VIPR image reconstructed with gridding algorithm (f) and the TOF composite image (g). HYPR TOF image has superior image quality over the VIPR image on both SNR and spatial resolution while preserving the contrast kinetics.
Figure 2 compares the temporal profiles from same regions of interest (ROIs) drawn on the basilar artery and sinus vein. HYPR TOF maintains the waveform fidelity obtained from vastly undersampled dataset.
Figure 2.
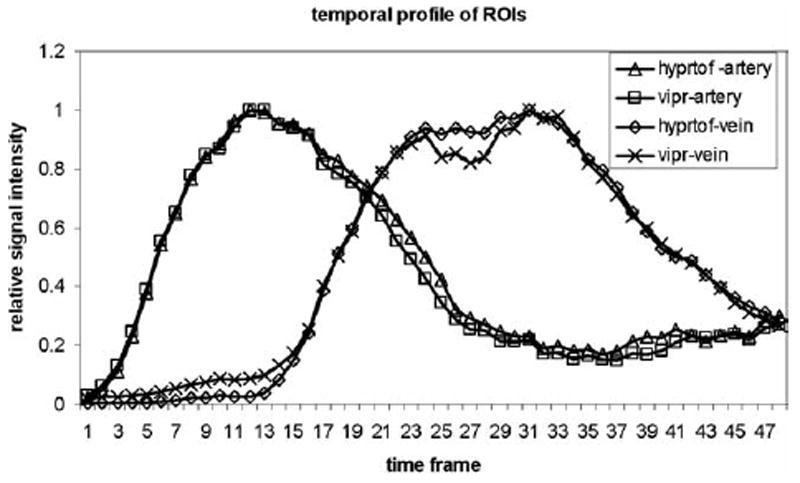
The temporal profiles from the same ROIs are drawn on the basilar artery and the sinus vein. HYPR TOF maintains the waveform fidelity obtained from a vastly undersampled dataset.
Figure 3 compares the vessel profiles selected at A2 segments as shown in the solid line in Figure 1c. With high spatial resolution and high SNR TOF images as constraint, HYPR TOF images are able to distinguish these small vessels in a time-resolved series.
Figure 3.
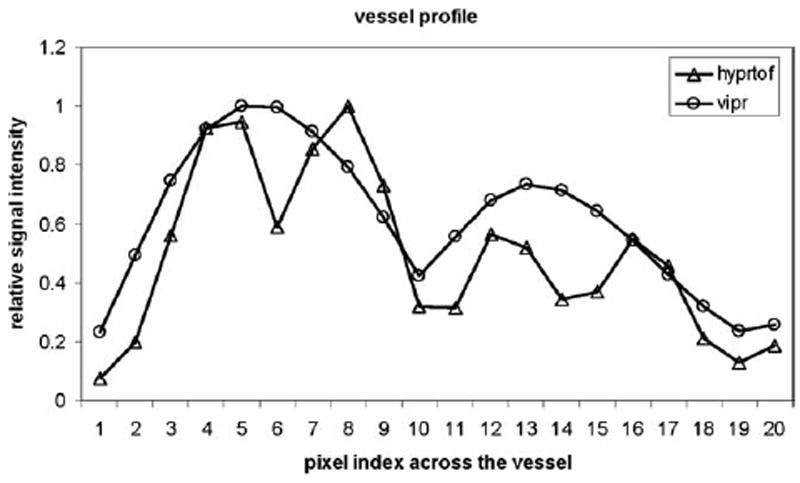
The vessel profiles selected at A2 segments as shown by the solid line in Figure 1c. With high spatial resolution and high SNR TOF images as constraint, HYPR TOF images are able to distinguish these small vessels in a time-resolved series.
Figure 4 shows five selected HYPR TOF images from a patient with aneurysm and the TOF image. HYPR TOF images demonstrate the contrast dynamics, including arrival, passing through, peak phase, and washout of the aneurysm, with the spatial resolution and SNR comparable to the TOF image.
Figure 4.
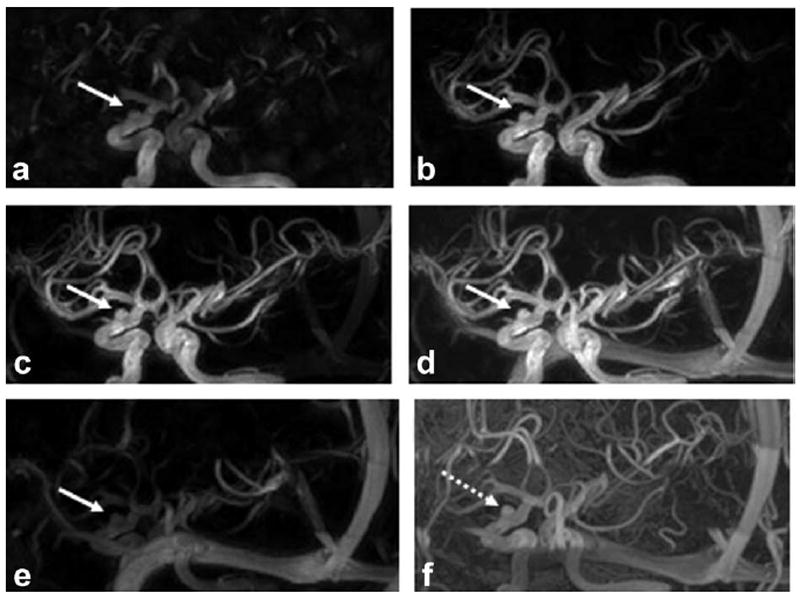
Selected HYPR TOF time frames from a patient with an aneurysm (a–e) and the TOF image (f). HYPR TOF images show comparable spatial resolution and aneurysm delineation as TOF (pointed at by arrows) with additional contrast dynamics.
The HYPR TOF images and the standard TOF images from the normal volunteers and patients with intracranial aneurysms were presented as matched pairs to a clinical neuroradiologist. The delineation of the arterial structures was scored as follows: (i) HYPR TOF quality less than TOF, (ii) HYPR TOF equivalent to TOF or (iii) HYPR TOF superior to TOF. In three instances, the HYPR TOF image quality was considered less than the TOF images (one aneurysm patient, two normals). In the remaining 10 exams, the image quality was equivalent (2 aneurysm patients, 8 normals) to the TOF images with additional contrast dynamics. The small sample size precluded any statistical analysis.
Signal to Background Variation Comparison
To quantitatively compare the signal to background variation improvement of HYPR TOF technique, for each volunteer, three target ROIs from internal carotid artery (ICA), middle cerebral artery (MCA), and sagittal sinus vein (SSV) were drawn at the peak arterial phases and peak venous phase. Corresponding background ROIs were drawn near the target ROIs at the same time frames. Signal to background variation measurements were taken from HYPR TOF images and the ME VIPR images from conventional reconstruction (gridding). A two-tailed paired t-test was performed to determine whether mean values were significantly different between HYPR TOF and conventional ME VIPR. Results are shown in Table 1. SNR of HYPR TOF is significantly higher than that of ME VIPR for all three target ROIs (P < 0.05).
Table 1.
Mean ± Standard Deviation of Signal to Background Variation Ratio of Both ME VIPR (Regular Gridding Reconstruction) and HYPR TOF Images From Volunteer Studies
| ICA | MCA | SSV | |
|---|---|---|---|
| ME VIPR | 18.6 ± 8.5 | 18.0 ± 6.3 | 23.2 ± 12.2 |
| HYPR TOF | 82.8 ± 21.7 | 54.8 ± 18.8 | 102.2 ± 22.0 |
| P-values* | 0.000045 | 0.00029 | 0.000059 |
P values are the result of a two-tailed paired t-test on the SNR difference between ME VIPR and HYPR TOF. P value significant at 5% level.
DISCUSSION
For time-resolved contrast-enhanced MRA, trade-offs between temporal and spatial resolution limit the overall achievement. HYBRID HYPR methods (16, 17,20), for the first time, decouple the high temporal resolution, which requires fast acquisition, from the high spatial resolution and SNR, which demands for longer scan time, achieving high temporal resolution, high spatial resolution and high SNR simultaneously. TOF, as one of the clinical standards for intracranial MRA due to its high spatial resolution, high SNR and relatively large spatial coverage, provides excellent vascular constraint for the HYBRID HYPR reconstruction. Intracranial TOF angiography generally uses multiple overlapping thin slabs which, due to the FOV and lack of sparsity, is poorly suited for radial imaging. We investigated the feasibility of using a TOF composite acquired with a Cartesian trajectory to constrain the reconstructions of a contrast-enhanced exam acquired with a multi-echo VIPR trajectory. Such a method can be generalized to any acquisition trajectory. HYPR TOF adds minimum scan time (~2 min) to the clinical routine, while providing high resolution visualization of rapid filling dynamics.
It should be noted that in HYPR LR reconstruction is dependent on the kernel size. Small kernels can be used to reduce spatiotemporal blurring; however, signal variations in the weighting images will degrade the apparent SNR of the final HYPR images. Large kernels will reduce noise in the weighting images such that noise is dominated by the composite image; however, the spatial resolution of the HYPR images is equivalent to that of the composite, only if the same dynamic changes happen within the local kernel. For cases where different waveforms appear adjacently (i.e., artery and vein are close to each other), it is very important to reduce the kernel size to maintain waveform fidelity. Size of the kernel should be adjusted based on applications. For intracranial MRA, a moderate kernel size (10 × 10 × 10) was chosen because in general, in the brain, the arteries and veins are separated by several millimeters. Such kernel size, although not being optimized, is able to improve the SNR of the HYPR TOF images without misclassifying the dynamic characteristics of vessels. Alternatively, iterative reconstruction methods which have reduced dependence on the composite image can be used. This comes at the risk of apparent noise amplification from residual streaking.
Because HYPR TOF involves two scans, patient motion is a concern. In the case when the subject moves between two scans, image registration between two scans can be applied by using a fully automated linear image registration tool (FLIRT) before HYPR LR reconstruction. Because motion of head is rigid, FLIRT has been tested to be very robust (17).
Another challenge is that TOF images are sensitive to flow. In the HYPR LR reconstruction, the multiplication of the weighting images with the composite does not guarantee data consistency, although the denominator of the weighting images normalizes the composite. However, due to the background and nonzero mean noise of the composite, signal bias appears if there is signal saturation in the composite image and the error level depends on the severity of the saturation effects. If the saturation appears as reduced signal level, for example, the discontinuities of the sinus vein, similar signal reduction will be carried to the HYPR images as visualized in Figure 4. However, if the saturation effect is so severe that signal is close to noise level, HYPR TOF images will lose spatial resolution and SNR in these areas. In such situations, iterative reconstruction methods (21,22) can be used to improve the reconstruction accuracy by forcing the local images to be consistent to the ME-VIPR data. However, for such regions, the SNR and spatial resolution will be similar to that of the ME-VIPR.
In conclusion, HYPR TOF provides time-resolved contrast-enhanced images with sub-millimeter spatial resolution and high SNR, comparable to TOF with visualization of sub second temporal dynamics, all with minimum extra scan time (~2 min) added to the clinical routine MRA protocols.
Acknowledgments
The authors thank Kelli Hellenbrand and Sara Plad-ziewicz for helping the volunteer scans.
Contract grant sponsor: NIH; Contract grant numbers: R21EB009441, R01NS066982, R01 EB006882, R01EB007021.
References
- 1.Kwan ES, Hall A, Enzmann DR. Quantitative analysis of intracranial circulation using rapid-sequence DSA. AJR Am J Roentgenol. 1986;146:1239–1245. doi: 10.2214/ajr.146.6.1239. [DOI] [PubMed] [Google Scholar]
- 2.Pant B, Sumida M, Arita K, Tominaga A, Ikawa F, Kurisu K. Usefulness of three-dimensional phase contrast MR angiography on arteriovenous malformations. Neurosurg Rev. 1997;20:171–176. doi: 10.1007/BF01105560. [DOI] [PubMed] [Google Scholar]
- 3.Marchal G, Bosmans H, Van Fraeyenhoven L, et al. Intracranial vascular lesions: optimization and clinical evaluation of three-dimensional time-of-flight MR angiography. Radiology. 1990;175:443–448. doi: 10.1148/radiology.175.2.2326471. [DOI] [PubMed] [Google Scholar]
- 4.Pruessmann KP, Weiger M, Bornert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001;46:638–651. doi: 10.1002/mrm.1241. [DOI] [PubMed] [Google Scholar]
- 5.Weiger M, Pruessmann KP, Kassner A, et al. Contrast-enhanced 3D MRA using SENSE. J Magn Reson Imaging. 2000;12:671–677. doi: 10.1002/1522-2586(200011)12:5<671::aid-jmri3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Griswold MA, Jakob PM, Chen Q, et al. Resolution enhancement in single-shot imaging using simultaneous acquisition of spatial harmonics (SMASH) Magn Reson Med. 1999;41:1236–1245. doi: 10.1002/(sici)1522-2594(199906)41:6<1236::aid-mrm21>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Tsao J, Boesiger P, Pruessmann KP. k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med. 2003;50:1031–1042. doi: 10.1002/mrm.10611. [DOI] [PubMed] [Google Scholar]
- 8.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized auto-calibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 9.Peters DC, Korosec FR, Grist TM, et al. Undersampled projection reconstruction applied to MR angiography. Magn Reson Med. 2000;43:91–101. doi: 10.1002/(sici)1522-2594(200001)43:1<91::aid-mrm11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Vigen KK, Peters DC, Grist TM, Block WF, Mistretta CA. Undersampled projection-reconstruction imaging for time-resolved contrast-enhanced imaging. Magn Reson Med. 2000;43:170–176. doi: 10.1002/(sici)1522-2594(200002)43:2<170::aid-mrm2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Barger AV, Block WF, Toropov Y, Grist TM, Mistretta CA. Time-resolved contrast-enhanced imaging with isotropic resolution and broad coverage using an undersampled 3D projection trajectory. Magn Reson Med. 2002;48:297–305. doi: 10.1002/mrm.10212. [DOI] [PubMed] [Google Scholar]
- 12.Gu T, Korosec FR, Block WF, et al. PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR Am J Neuroradiol. 2005;26:743–749. [PMC free article] [PubMed] [Google Scholar]
- 13.Mistretta CA, Wieben O, Velikina J, et al. Highly constrained backprojection for time-resolved MRI. Magn Reson Med. 2006;55:30–40. doi: 10.1002/mrm.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Kim N, Korosec FR, et al. 3D time-resolved contrast-enhanced cerebrovascular MR angiography with subsecond frame update times using radial k-space trajectories and highly constrained projection reconstruction. AJNR Am J Neuroradiol. 2007;28:2001–2004. doi: 10.3174/ajnr.A0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson KM, Velikina J, Wu Y, Kecskemeti S, Wieben O, Mistretta CA. Improved waveform fidelity using local HYPR reconstruction (HYPR LR) Magn Reson Med. 2008;59:456–462. doi: 10.1002/mrm.21505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velikina J, Johnson KM, Wieben O, Turski P, Grist TM, Mistretta C. First pass HYPR flow: time-resolved CE-MRA with 322-fold undersampling and A phase contrast HYPR composite providing 4-fold SNR restoration per time frame. Presented at the ISMRM Workshop on Non-Cartesian Trajectories; Sedona AZ. February 25–28, 2007. [Google Scholar]
- 17.Wu Y, Johnson KM, Velikina J, Turski P, Mistretta CA. Clinical experience of HYPR FLOW. Proceedings of the 16th Annual Meeting of ISMRM; Toronto, Canada. 2008. abstract 110. [Google Scholar]
- 18.Huston J, III, Rufenacht DA, Ehman RL, Wiebers DO. Intracranial aneurysms and vascular malformations: comparison of time-of-flight and phase-contrast MR angiography. Radiology. 1991;181:721–730. doi: 10.1148/radiology.181.3.1947088. [DOI] [PubMed] [Google Scholar]
- 19.Oelerich M, Lentschig MG, Zunker P, Reimer P, Rummeny EJ, Schuierer G. Intracranial vascular stenosis and occlusion: comparison of 3D time-of-flight and 3D phase-contrast MR angiography. Neuroradiology. 1998;40:567–573. doi: 10.1007/s002340050645. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Johnson KM, Velikina J, Turski P, Mistretta CA. Hybrid HYPR MRA. Proceedings of the 17th Annual Meeting of ISMRM; Honolulu, Hawaii. 2007. abstract 3257. [Google Scholar]
- 21.O’Halloran RI, Wen Z, Holmes JH, Fain SB. Iterative projection reconstruction of time-resolved images using highly-constrained back-projection (HYPR) Magn Reson Med. 2008;59:132–139. doi: 10.1002/mrm.21439. [DOI] [PubMed] [Google Scholar]
- 22.Griswold M, Barkauskas K, Blaimer M, et al. More optimal HYPR reconstruction using a combination of hypr and conjugate-gradient minimization. Proceedings of the 17th Annual Meeting of ISMRM; Berlin, Germany. 2007. abstract 188. [Google Scholar]


