Abstract
Neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD) and spinocerebellar ataxias (SCAs), present an enormous medical, social, financial and scientific problem. Recent evidence indicates that neuronal calcium (Ca2+) signaling is abnormal in many of these disorders. Similar, but less severe, changes in neuronal Ca2+ signaling occur as a result of the normal aging process. The role of aberrant neuronal Ca2+ signaling in the pathogenesis of neurodegenerative disorders is discussed here. The potential utility of Ca2+ blockers for treatment of these disorders is also highlighted. It is reasoned that Ca2+ blockers will be most beneficial clinically when used in combination with other disease-specific therapeutic approaches.
Ca2+ blockers and a combination approach to the treatment of neurodegenerative disorders
Neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD) and spinocerebellar ataxias (SCAs), present an enormous medical, social, financial and scientific problem. Despite intense research into the causes of these disorders, only marginal clinical progress has been made and they remain incurable. The medications approved for the treatment of these disorders result in limited relief, primarily by temporarily alleviating disease-related symptoms or by modestly delaying the progression of disease (Table 1). The main progress in understanding these disorders has been related to the identification of disease-causing mutations. HD and SCAs are genetic disorders and the genes responsible for most of these disorders were cloned 15 years ago (Table 1). Most cases of AD, PD and ALS are sporadic but, in approximately 5% of patients, the disease is inherited. Most genes responsible for the familial forms of these disorders have also been cloned (Table 1). Studies of disease-causing genes have enabled the formulation of mechanistic hypotheses and the generation of mouse models for these diseases.
Table 1.
Neurodegenerative disorders and US FDA-approved drugs
| Disorder | Affected neurons | Age of onset |
Sporadic or genetic |
Familial disease genes |
Drug | Mechanism of action or target | Date of US FDA approval |
Effect |
|---|---|---|---|---|---|---|---|---|
| AD | Cortical and hippocampal neurons | >65 | 95% sporadic, 5% familial |
APP PSEN1 PSEN2 |
Memantine (Namenda) Donepezil (Aricept), Galantamine (Razadyne), Rivastigmine (Exelon). |
Blocks NMDA receptors, reduces excitotoxicity Acetylcholinesterase inhibitors. Increase concentration of Ach in the brain |
2003 1996 2001 2000 |
Mild improvement in cognitive measures Mild improvement in cognitive measures |
| PD | Dopaminergic neurons in substantia nigra pars compacta | >65 | 95% sporadic, 5% familial |
Synuclein LRRK2 Parkin PINK1 DJ-1 |
L-Dopa (Levodopa) |
Increases amount of dopamine in substantia nigra neurons | 1970 | Symptomatic benefit |
| ALS | Motor neurons | 40–60 | 95% sporadic, 5% familial |
SOD1 | Riluzole (Rilutek) |
Antiglutamate (activator of glutamate uptake, inhibitor of NMDAR and Na+ channels) | 1995 | Extends survival by several months |
| HD | Striatal medium spiny neurons | 40–50 | 100% familial |
Huntingtin | Tetrabenazine (Xenazine) |
Antidopamine (VMAT2 inhibitor, reduces amount of released dopamine) | 2008 | Reduction in chorea |
| SCAs | Various brain regions involved in motor control | 40–50 | 100% familial |
Ataxins | N/A | N/A | N/A | N/A |
Most of the scientific effort is focused on identification of the major causes of these diseases and on developing ways to target them. For example, for AD, the major cause of disease has been proposed to be amyloid accumulation. Thus, most of the research is directed towards finding ways to prevent accumulation of amyloid by blocking its production or by facilitating its clearance from the brain. In the case of HD, the major cause of the disease is expression of mutant huntingtin protein. The main effort here is focused on trying to reduce the expression of mutant huntingtin in the brain, for example, by using antisense or RNAi knockdown. Despite excellent scientific rationales, these approaches have been difficult to translate to the clinic. The AD clinical trials of the amyloid-binding compound tramiprosate (Alzhemed) and γ-secretase inhibitor tarenflurbil (Flurizan) failed. Furthermore, the AD clinical trial of amyloid-binding monoclonal antibodies (Bapineuzumab) yielded limited benefit, if any. For HD clinical trials, the current obstacle is the development of an RNAi or an antisense brain-delivery system that can be used in humans. Until this is achieved, antisense or RNAi approaches cannot be initiated in HD clinical trials.
Although targeting amyloid and mutant huntingtin makes perfect sense for developing eventual cures for AD and HD, respectively, the lessons learned so far indicate that these are difficult targets and that it will take significant time and effort to develop successful therapies based on these approaches. In addition to developing ‘cures’, we might consider therapies that would lead to a delay in the age of onset of symptoms and/or a reduction in the rate of disease progression. Here, I discuss the idea that proteins involved in neuronal Ca2+ signaling (Box 1) constitute an attractive target for developing ‘disease-onset delaying’ therapies for neurodegenerative disorders. I reason that Ca2+ blockers will be most beneficial in the clinic when used in combination with disease-specific therapeutic approaches, such as ‘amyloid-targeting’ therapies for AD or ‘huntingtin-targeting’ therapies for HD.
Box 1. Neuronal Ca2+ signaling.
Ca2+ signaling connects membrane excitability and cell biological functions of neurons [87]. By acting at the interface between ‘electrical’ and ‘signaling’ worlds, Ca2+ channels have a key role in multiple aspects of neuronal function. Ca2+ signaling is essential for short- and long-term synaptic plasticity. Owing to its vital importance, neurons use multiple ways of controlling intracellular Ca2+ concentration, most often within local signaling microdomains. Several Ca2+ channels are involved in neuronal Ca2+ signaling, such as plasma membrane voltage-gated Ca2+ channels (VGCCs), NMDA receptors, AMPA receptors, TRP channels and store-operated Ca2+ entry (SOC) channels. Ca2+ release from intracellular stores in the endoplasmic reticulum (ER) is supported by inositol 1,4,5-trisphosphate receptors (InsP3R) and ryanodine receptors (RyanR). SERCA pump in the ER, plasma membrane Ca2+ pump (PMCA) and Na+/Ca2+ exchanger (NCE) in the plasma membrane tightly control cytosolic Ca2+ levels in a narrow range. Mitochondria have an important role in shaping cytosolic Ca2+ signals. Mitochondrial Ca2+ uniporter (MCU) is an ion channel that is involved in potent and rapid Ca2+ uptake into the mitochondria. Several Ca2+-binding proteins (CaBPs) are involved in buffering Ca2+ levels in neuronal cytosol [such as calbindin-D28 (CALB1), calretinin (CALB2) and parvalbumin (PVALB)] and in the ER lumen [such as calreticulin (CALR) and calnexin (CANX)].
Neurons are extremely sensitive to changes in intracellular Ca2+ and use a range of Ca2+-responsive elements, including proteins involved in synaptic vesicle fusion (such as synaptotagmins), Ca2+-dependent kinases and phosphatases [such as Ca2+/CaM kinases and Ca2+-dependent phosphatase calcineurin (CaN)], Ca2+-dependent signaling enzymes [such as Ca2+-dependent adenylate cyclases and Ca2+-dependent nitric oxide synthase (nNOS)] and Ca2+-dependent transcription factors [such as cAMP response element-binding protein (CREB), calcineurin B-controlled nuclear factor of activated T cells (NFAT) and Ca2+-binding downstream regulatory element modulator (DREAM)]. The diversity of these Ca2+-responsive elements provides a means for Ca2+-dependent regulation of neuronal function in the time scale ranging from microseconds (as in the case of Ca2+-dependent synaptic-vesicle fusion) to seconds and minutes (as in the case of Ca2+-dependent phosphorylation and dephosphorylation), to days and years (as in the case of Ca2+-dependent changes in neuronal gene expression). These Ca2+-dependent processes lead to short- and long-term changes in neuronal excitability (by affecting ion-channel activity and expression pattern) and synaptic transmission (by modifying synaptic machinery and facilitating formation or disassembly of synaptic connections). Because of the extreme sensitivity of neurons to variation in Ca2+ signals, even relatively subtle defects and abnormalities in Ca2+ signaling machinery might lead to devastating consequences over a long time period [1].
Neuronal Ca2+ signaling and aging
Our neurons are as old as we are. Thus, it is not surprising that the risk of neurodegenerative disorders increases with age (Table 1). Comparative studies performed with neurons from young and old rodents have indicated that neuronal Ca2+ signaling machinery undergoes significant age-dependent changes. This subject has recently been reviewed [1]. An integrative model of age-dependent changes in hippocampal Ca2+ handling has also recently been proposed [2]. The predominant changes in aging neurons include increased Ca2+ release from intracellular stores through inositol(1,4,5)-trisphosphate receptors (InsP3R) and ryanodine receptors (RyanR), increased Ca2+ influx through L-type voltage-gated calcium channels (VGCCs), increased slow after-hyperpolarization (sAHP, a tranisent membrane hyperpolarization that often occurs after a train of action potentials) due to activation of Ca2+-dependent K+ channels, reduced contribution of N-methyl D-aspartate receptor (NMDAR)-mediated Ca2+ influx, reduced cytosolic Ca2+ buffering capacity and activation of calcineurin and calpains. The resulting changes in neuronal Ca2+ dynamics lead to augmented susceptibility to induction of long-term depression (LTD) and an increase in the threshold frequency for induction of long-term potentiation (LTP) in aging neurons [3]. LTD and LTP refer to activity-dependent and persistent changes in synaptic strength, which are widely considered to form a basis for formation and storage of memories in the brain. The importance of these changes for age-related memory decline has been discussed elsewhere [3].
The mechanisms responsible for age-related alterations in neuronal Ca2+ signaling are not clearly understood. One potential explanation is related to age-induced defects in mitochondrial function due to cumulative oxidative damage to mitochondria. The mitochondria from aged neurons are depolarized and less efficient in handling Ca2+ load [1]. Age-related changes in the transcription of Ca2+ signaling genes have been observed in microarray studies [1]. Some of these changes are directly caused by aging and some are compensatory, however, the overall picture is consistent with age-related alterations in neuronal Ca2+ signaling at multiple levels. In the following sections, the changes in calcium signaling that have been detected in the neurodegenerative diseases AD, PD, ALS, HD and SCAs will be discussed.
Neuronal Ca2+ signaling and AD
The dominant model for pathogenesis in the AD field is the ‘amyloid hypothesis’, which states that increased production of amyloidogenic Aβ42 peptide (or an increase in Aβ42:Aβ40 ratio) is a major cause of neuronal and synaptic loss in AD [4]. The experimental support for the ‘amyloid hypothesis’ comes from (i) accumulation of amyloid plaques in the brains of AD patients; (ii) the familial AD (FAD) cases that result from missense mutations in amyloid-precursor protein (APP); and (iii) the FAD cases that result from missense mutations in presenilins, which form a catalytic subunit of the APP-cleaving enzyme γ-secretase. The ‘amyloid-targeting’ therapies have been the main focus of AD drug development. Recent clinical trial results have suggested that additional targets beyond amyloid need to be seriously considered for AD treatment [5]. A body of evidence has also suggested that neuronal Ca2+ dyshomeostasis has an important role in AD. The arguments supporting a ‘Ca2+ hypothesis’ of AD have recently been reviewed [6] and are summarized briefly below.
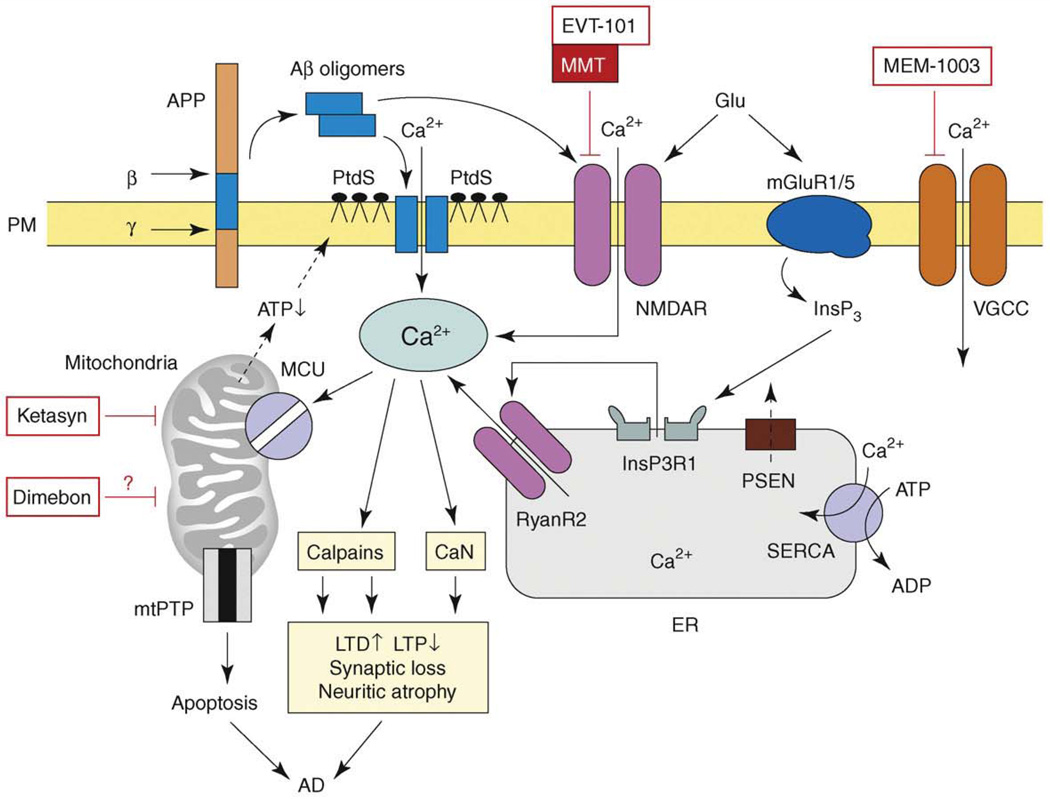
One potential connection between AD pathogenesis and Ca2+ comes from the observation that Aβ oligomers can form Ca2+-permeable channels in membranes [7]. Exposure of phosphatidylserine (PtdS) on the cell surface, which is usually indicative of cells in conditions of energy deficit, enhances the ability of Aβ to associate with the membrane [8]. Age-related mitochondrial impairments might increase surface PtdS levels in affected neurons and set them up for Aβ-mediated pore formation, Ca2+ influx and cell death (Figure 1). Indeed, neurons with reduced cytosolic ATP levels and elevated surface PtdS levels are particularly vulnerable to Aβ toxicity [9]. The ability of Aβ oligomers to form Ca2+-permeable channels in neuronal plasma membranes is consistent with recent in vivo Ca2+-imaging experiments performed with APP transgenic mice [10]. These studies showed that resting Ca2+ levels were significantly elevated in approximately 35% of neurites located in the immediate vicinity of Aβ plaques. The probable explanation for these results is that a high local concentration of Aβ oligomers in the area surrounding amyloid plaques causes the formation of Ca2+-permeable ion channels in the neuronal plasma membrane. The neurites with elevated Ca2+ levels lacked spines and displayed an abnormal morphology [10]. The morphological changes in these neurites could be reduced by treatment with the calcineurin (CaN) inhibitor FK-506 [10], suggesting that CaN has an important role in pathological responses to elevated Ca2+ levels in the APP transgenic mouse. In addition to the direct effects of Aβ on plasma membrane Ca2+ permeability, Aβ oligomers also affect neuronal Ca2+ homeostasis by modulating the activity of NMDARs [11,12], alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) [13] and P/Q-type VGCCs [14] (Figure 1).
Figure 1.
The model of Ca2+ dysregulation in AD. Sequential cleavages of β-amyloid precursor protein (APP) by β-secretase (β) and γ-secretase (γ) generate amyloid β-peptide (Aβ). Aβ forms oligomers, which can insert into the plasma membrane and form Ca2+-permeable pores. The association of Aβ oligomers with the plasma membrane is facilitated by binding to surface phosphatidylserine (PtdS); age and Ca2+-related mitochondrial impairment leads to ATP depletion and might trigger flipping of PtdS from the inner portion of the plasma membrane to the cell surface. Reduction in ATP levels and loss of membrane integrity causes membrane depolarization, which leads to facilitation of Ca2+ influx through NMDAR and VGCC. Aβ oligomers can also affect activity of NMDAR, AMPAR and VGCC directly. Glutamate stimulates activation of mGluR1/5 receptors, production of InsP3 and InsP3-mediated Ca2+ release from the ER. Presenilins (PS) function as an ER Ca2+-leak channels and many FAD mutations impair Ca2+-leak-channel function of PS, resulting in excessive accumulation of Ca2+ in the ER. Increased ER Ca2+ levels result in enhanced Ca2+ release through InsP3-gated InsP3R1 and Ca2+-gated RyanR2. PS might also modulate activity of InsP3R, RyanR and SERCA pump directly. Elevated cytosolic Ca2+ levels result in the activation of calcineurin (CaN) and calpains and lead to facilitation of LTD, inhibition of LTP, modification of neuronal cytoskeleton, synaptic loss and neuritic atrophy. Excessive Ca2+ is taken up by mitochondria through mitochondrial Ca2+ uniporter (MCU), eventually leading to opening of mitochondrial permeability-transition pore (mtPTP) and apoptosis. The NMDAR inhibitor memantine (MMT) is approved for the treatment of AD and the NR2B-specific antagonist EVT-101 was recently developed for AD treatment. ‘CNS-optimized’ L-type VGCC inhibitor MEM-1003, putative ‘mitochondrial agent’ Dimebon and ‘mitochondrial energizer’ Ketasyn are in clinical trials for AD. Adapted from [6].
Another potential connection between Ca2+ signaling and AD comes from the observation that many FAD mutations in presenilins result in abnormal Ca2+ signaling. The connection between presenilins and Ca2+ signaling was initially uncovered when it was reported that fibroblasts from FAD patients release supranormal amounts of Ca2+ in response to InsP3 [15]. Similar results were obtained in experiments with cells expressing FAD mutant presenilins [16] and cortical neurons from FAD presenilin-mutant knock-in mice [17,18]. To explain these results, it has been suggested that mutant presenilins affect store-operated Ca2+ influx [19,20], increase activity and/or expression of intracellular Ca2+-release channels, such as RyanR [18,21,22] and InsP3R [23,24], or modulate the function of the sarcoplasmic and endoplasmic reticulum calcium ATPase (SERCA) ER Ca2+ pump [25]. Presenilins themselves have been reported to function as ER Ca2+-leak channels (Figure 1) and many FAD mutations in presenilins result in loss of ER Ca2+-leak function, leading to ER Ca2+ overload and supranormal Ca2+ release from the ER [26,27]. Although they differ in the details of the proposed mechanisms, the majority of these studies concluded that many FAD mutations in presenilins result in excessive Ca2+ release from the ER through InsP3R and RyanR.
There are several potentially toxic downstream effects resulting from Ca2+ influx through Aβ channels and excessive Ca2+ release from the ER. As discussed earlier, elevated cytosolic Ca2+ might lead to activation of CaN and hence to neurite atrophy [10] (Figure 1). Excessive Ca2+ levels activate calpains, which degrade signaling enzymes involved in learning and memory [28,29] (Figure 1). Old neurons are sensitized to cytosolic Ca2+ toxicity because Ca2+-buffering capacity declines with advancing age (see earlier). Indeed, a tight correlation has been observed between the reduction in expression of Ca2+-binding proteins (CaBPs) in the dentate gyrus region of the hippocampus and onset of cognitive symptoms in AD [30]. The supranormal cytosolic Ca2+ signals might cause excessive Ca2+ handling by mitochondria and induction of apoptotic cell death (Figure 1). The known neuroprotective effects of non-steroidal anti-inflammatory drugs (NSAIDs) might be related to the ability of these drugs to reduce mitochondrial Ca2+ uptake [31].
In summary, several studies point to exaggerated neuronal Ca2+ signals resulting from the accumulation of Aβ oligomers or expression of FAD mutants in presenilins. Further support for the connection between Ca2+ signaling and AD was provided by a recent report that mutation in a novel Ca2+-influx channel, calcium homeostasis modulator 1 (CALHM1), might increase the risk of late-onset AD [32] (but see [33]). The proposed model (Figure 1) offers a variety of potential therapeutic targets for AD treatment. The Aβ-formed Ca2+ channels themselves are an extremely attractive target [34]. Memantine is a non-competitive NMDAR inhibitor that is already approved by the US FDA for AD treatment (Table 1). Potentially more specific NMDAR inhibitors, such as nitro-memantines, can be developed [35]. The NR2B-specific antagonist EVT-101 was recently developed by Evotec AG (Hamburg, Germany; http://www.evotec.com/en/) for AD treatment (Table 2). The L-type VGCC inhibitor MEM-1003 (Memory Pharmaceuticals, Montvale, New Jersey, USA; http://www.memorypharma.com/) has been tested in a Phase II AD clinical trial (Table 2). Other potential and largely unexplored targets for AD include intracellular Ca2+-release channels (RyanR and InsP3R), the SERCA pump, CaN and the mitochondrial Ca2+-handling system (Figure 1).
Table 2.
Latest neurodegeneration clinical trials of Ca2+ inhibitors and mitochondrial stabilizers
| Disorder | Drug | Target or mechanism of action |
Latest trial |
ClinicalTrials ID | Information provided by | Status/comments |
|---|---|---|---|---|---|---|
| AD | Dimebon | Mitochondria (?) | Phase III | NCT00675623 | Medivation, Inc, San Francisco, CA, USA; http://www.medivation.com/ | Recruiting, Phase II published [86] |
| Ketasyn (AC-1202) | Mitochondria | Phase II | NCT00142805 | National Institute on Aging (NIA), Bethesda, Maryland, USA; http://www.nia.nih.gov/ | Completed | |
| MEM-1003 | L-type VGCC | Phase II | NCT00257673 | Memory Pharmaceuticals, Montvale, New Jersey, USA; http://www.memorypharma.com/ | Completed | |
| EVT-101 | NR2B NMDAR | Phase I | NCT00526968 | Evotec AG, Hamburg, Germany; http://www.evotec.com/ | Completed, Phase II planning | |
| PD | Creatine | Mitochondria | Phase III | NCT00449865 | National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, Maryland, USA; http://www.ninds.nih.gov/ | Recruiting |
| CoQ10 + vitamin E | Mitochondria | Phase III | NCT00740714 | NINDS, Bethesda, Maryland, USA; http://www.ninds.nih.gov/ | Recruiting soon | |
| CoQ10 Nanodispersion (Nanoquinone) | Mitochondria | Phase III | NCT00180037 | Dresden University of Technology, Dresden, Germany; http://tu-dresden.de/ | Completed | |
| MitoQ | Mitochondria | Phase II | NCT00329056 | Antipodean Pharmaceuticals, San Francisco, CA, USA; http://www.antipodeanpharma.com/ | Completed | |
| Memantine | NMDAR | Phase II | NCT00294554 | Johns Hopkins University, Baltimore, MD, USA; http://www.hopkinsmedicine.org/som/ | In progress | |
| Riluzole | Antiglutamate | Phase II | NCT00013624 | National Institutes of Health Clinical Center (NIHCC), Bethesda, Maryland, USA; http://clinicalcenter.nih.gov/ccc/crc/ | Completed | |
| Isradipine (Dynacirc CR) | L-type VGCC | Phase II | NCT00753636 | Northwestern University School of Medicine, Chicago, IL, USA; http://www.medschool.northwestern.edu/ | Recruiting | |
| ALS | CoQ10 | Mitochondria | Phase II | NCT00243932 | NINDS, Bethesda, Maryland, USA; http://www.ninds.nih.gov/ | Completed |
| Creatine | Mitochondria | Phase II | NCT00005766 | National Center for Research Resources (NCRR), Bethesda, MD, USA; http://www.ncrr.nih.gov/ | Completed | |
| Memantine + rilusole | Antiglutamate/NMDAR | Phase II/III | NCT00353665 | University of Lisbon, Lisbon, Portugal; http://www.ul.pt/ | Recruiting | |
| HD | Dimebon | Mitochondria (?) | Phase II | NCT00497159 | Medivation, Inc, San Francisco, CA, USA; http://www.medivation.com/ | Completed |
| Creatine | Mitochondria | Phase III | NCT00712426 | Massachusetts General Hospital (MGH), Boston, MA, USA; http://www.massgeneral.org | Recruting soon | |
| CoQ10 | Mitochondria | Phase III | NCT00608881 | NINDS, Bethesda, Maryland, USA; http://www.ninds.nih.gov/ | Recruiting | |
| Memantine | NMDAR | Phase IV | NCT00652457 | University of California San Diego (UCSD), San Diego, CA, USA; http://health.ucsd.edu/ | Recruiting | |
| Riluzole | Antiglutamate | Phase III | NCT00277602 | Sanofi-Aventis, Paris, France; http://en.sanofi-aventis.com/ | Completed, published [66] | |
Neuronal Ca2+ signaling and PD
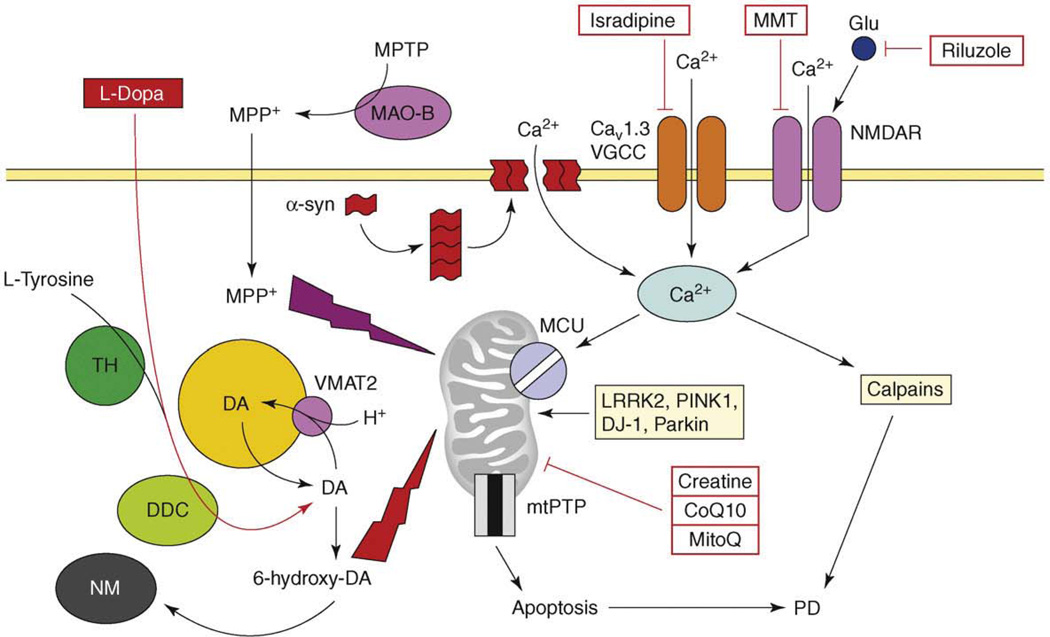
PD results from the selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNc). Most of the genes implicated in familial PD (e.g. PINK1, DJ-1, LRRK2 and Parkin) (Table 1) encode proteins associated with certain aspects of mitochondrial function, which points to mitochondria as a crucial locus in PD pathogenesis [36] (Figure 2). The prevalent idea in the PD field is the ‘dopamine hypothesis’, which states that dopamine (DA) acts as a natural toxin and oxidation of cytosolic DA to 6-hydroxy-DA and other metabolites damages mitochondria (Figure 2) and causes cell death of SNc neurons [37]. Consistent with the continuous oxidative damage, affected SNc neurons accumulate large amounts of neuromelanin (NM), which is a lysosome composed of oxyradical DA derivatives of lipids and proteins (Figure 2). The US FDA-approved treatment for PD is administration of levodopa (L-dopa) (Table 1), which is converted to DA and leads to elevation of DA levels in the cytosol and synaptic vesicles of remaining SNc neurons (Figure 2). In an apparent contradiction to the ‘dopamine hypothesis’, administration of L-dopa does not accelerate disease progression in PD patients despite increasing levels of DA in their brain [37]. Also, PD has low levels of penetrance and most people do not develop PD despite having similar levels of DA in their SNc neurons. These observations point to a ‘multi-hit’ hypothesis of PD, which states that SNc neurons in PD succumb to a combined effect of DA-related oxidative stress and an additional ‘factor X’ [37].
Figure 2.
The model of Ca2+ dysregulation in PD. Continuous Ca2+ influx to SNc neurons is mediated by CaV1.3 L-type voltage-gated Ca2+ channels (CaV1.3). In response to glutamate, Ca2+ influx is mediated by NMDA receptors (NMDAR). Alpha-synuclein forms aggregates (protofibrils) which may form Ca2+-permeable channels in the plasma membrane. Elevated cytosolic Ca2+ is transported into mitochondria through the activity of mitochondrial Ca2+ uniporter (MCU). Dopamine (DA) is generated from L-tyrosine by the action of tyrosine hydroxylase (TH) and is loaded into the synaptic vesicles by the activity of the DA/H+ cotransporter (VMAT2). Cytosolic DA is oxidized to 6-hydroxy-DA, which causes damage to proteins and mitochondria by oxidative stress. The products of DA oxidation accumulate as neuromelanin (NM). Cumulative damage to mitochondria resulting from Ca2+ overload and DA-mediated oxidative stress leads to an opening of mtPTP and apoptotic cell death of SNc neurons in PD. An importance of mitochondria is highlighted by several mitochondria-related genes (e.g. LRRK2, PINK1, DJ-1, Parkin), which are mutated in familial PD. In a chemical model of PD, the toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is converted to the 1-methyl-4-phenylpyridinium (MPP+) by the glial enzyme monoamine oxidase B (MAO-B). MPP+ enters SNc neurons and potently inhibits mitochondrial complex I, causing selective cell death of SNc neurons. The US FDA-approved treatment for PD is levodopa (L-dopa), which is converted to DA by aromatic L-amino acid decarboxylase (DCC) inside SNc neurons. Generated DA is loaded to synaptic vesicles and alleviates symptoms of PD temporarily. The drugs tested or in PD clinical trials currently are ‘mitochondrial stabilizers’ (creatine, CoQ10, MitoQ), NMDAR antagonist memantine (MMT), antiglutamate agent riluzole and L-type VGCC inhibitor isradipine.
Can Ca2+ play the part of ‘factor X’ in this scenario? Midbrain dopaminergic neurons that express high levels of the CaBP calbindin are relatively spared in PD patients and in animal models [38]. Calpain activation has been observed in sporadic PD and in animal models [28]. α-synuclein is a major component of Lewy bodies observed in the brains of sporadic PD patients and mutations in α-synuclein cause autosomal-dominant hereditary PD (Table 1). The probable mechanism of α-synuclein toxicity is related to the formation of small α-synuclein aggregates (protofibrils). Biophysical studies indicated that synuclein protofibrils generate ion pores in synthetic lipid membranes [39] and induce Ca2+ influx in neurons [40,41] (Figure 2). The proposed mechanism of synuclein-mediated Ca2+ influx might be similar to that proposed for Aβ oligomer-formed Ca2+ channels (see earlier).
Additional support for the ‘Ca2+ hypothesis’ of PD [38] was provided by physiological studies. In contrast to most other neurons in the nervous system, the SNc dopaminergic neurons use CaV1.3 L-type Ca2+ channels to drive spontaneous pacemaking activity in the 2–4 Hz range [42]. The continuous Ca2+ influx creates an excessive metabolic load on SNc neurons, making them particularly vulnerable to secondary insults on mitochondrial function [42] (Figure 2). The reliance of SNc neurons on L-type Ca2+ channels to control pacemaking increases with age [42], which might explain why age is such a significant risk factor for developing PD (Table 1). Pharmacological inhibition of CaV1.3 L-type Ca2+ channels with dihydropyridine isradipine restored Ca2+-independent ‘juvenile’ pacemaking activity in SNc neurons [42]. Subcutaneous delivery of isradipine significantly protected SNc neurons in animal models of PD [42]. In support of this linkage to PD, a recent retrospective epidemiological study found that treatment of hypertension with Ca2+-channel antagonists significantly diminished the risk of developing PD [43]. These observations prompted a controlled clinical trial of isradipine in PD patients (Table 2).
Neuronal Ca2+ signaling and ALS
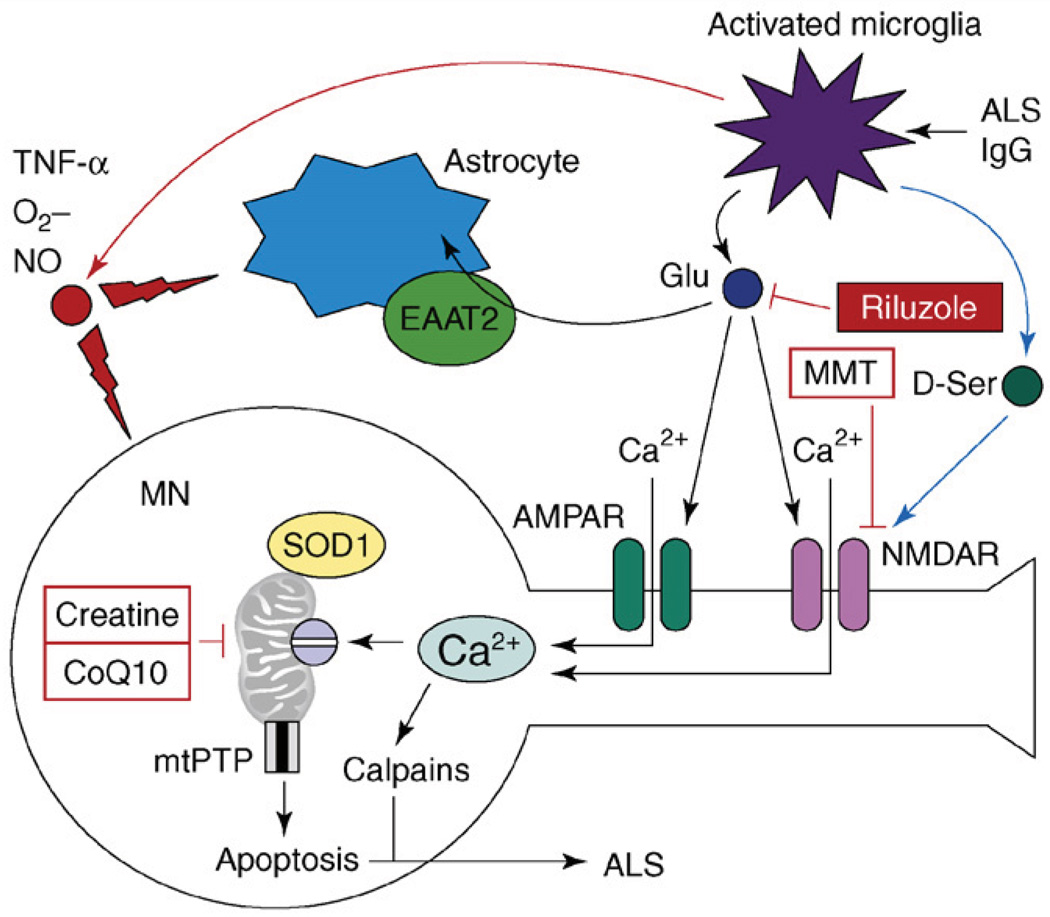
ALS is a disorder that results from selective degeneration of motor neurons (MNs). Most cases of ALS are sporadic, although rare familial cases result from missense mutations in superoxide dismutase 1 (SOD1) [44] (Table 1). The mutations in SOD1 appear to cause disease not by affecting the dismutase activity of SOD1 but rather by inducing aggregation of mutant SOD1 and/or by causing pathological association of mutant SOD1 with mitochondria. Genetic experiments provide strong evidence in support of non-cell autonomous mechanism of neuronal toxicity in ALS [45]. The emerging model suggests that MN degeneration in ALS is caused by neuroinflammatory activation of microglia, which attack MNs and neighboring astrocytes (Figure 3). The activated microglia release proinflammatory agents, such as tumor necrosis factor-alpha (TNF-α), NO and O2−, which damage MN and neighboring astrocytes (Figure 3). Activated microglia also release large amounts of glutamate (Figure 3) and elevated levels of glutamate are found in the cerebrospinal fluid (CSF) of ALS patients [46,47]. The role of excitotoxicity and abnormal neuronal Ca2+ signaling in ALS has been reviewed [46,47] and is briefly summarized here.
Figure 3.
The model of excitotoxicity and Ca2+ dysregulation in ALS. The pathogenic cascade in ALS involves interactions among activated microglia, astrocytes and motor neurons (MNs). In an experimental situation, microglia can be activated by antiserum collected from ALS patients (ALS IgG). Activated microglia release pro-inflammatory factors TNF-α, NO and O2−. Activated microglia also release large amounts of glutamate, which causes activation of AMPA and NMDA receptors on MNs. Activated microglia also release D-serine, which further sensitizes NMDAR to glutamate activation. Astrocytes express glutamate-uptake transporter EAAT2, which is involved in clearing glutamate from the extracellular space. Ca2+ influx by Ca2+-permeable AMPA receptors and NMDA receptors results in mitochondrial Ca2+ overload, mitochondrial swelling, opening of mitochondrial permeability-transition pore (mtPTP) and apoptosis of MNs. Mutant SOD1 binds to MN mitochondria and further impairs their ability to handle Ca2+ load. Antiglutamate agent riluzole is approved by the US FDA for treatment of ALS. NMDAR antagonist memantine (MMT) and ‘mitochondrial stabilizers’ creatine and CoQ10 are in ALS clinical trials currently.
There are several lines of independent evidence supporting an important role for Ca2+ signaling in ALS pathology. Calpain activation was observed in MNs from sporadic ALS patients and in SOD1-mutant mice [28]. Excessive mitochondrial Ca2+ accumulation and mitochondrial swelling has been observed in motor nerve terminals from sporadic ALS patients [47]. The spinal and hypoglossal MNs expressing low levels of CaBPs are most vulnerable in ALS, whereas oculomotor neurons expressing high levels of CaBPs are spared [46,47]. Cross-breeding of SOD1-mutant mice with the mice over-expressing parvalbumin in spinal MNs delayed onset of the symptoms and prolonged survival of these mice [48]. MNs express a high proportion of Ca2+-permeable AMPA receptors, which lack the GluR2 subunit, and pharmacological inhibition of AMPA receptors protected MNs from damage induced by activated microglia in co-culture experiments [49]. D-serine secreted by activated microglia sensitizes NMDAR to glutamate activation and promotes NMDAR-mediated excitotoxicty in ALS [50] (Figure 3). Thus, both AMPAR and NMDAR play a role in glutamate-induced Ca2+ overload in ALS (Figure 3). In contrast to activated microglia, astrocytes have a largely protective role in ALS excitotoxicity (Figure 3). Astrocytes appear to upregulate the expression of GluR2 subunits in MNs, which reduces AMPAR-mediated Ca2+ influx [51]. Astrocytes express the glutamate-uptake transporter excitatory amino acid transporter type 2 (EAAT2), which has a major role in clearing glutamate from the extracellular space (Figure 3). Reduction in the levels of EAAT2 transporting activity was observed in the spinal cord of ALS patients [46,47]. A patient has been identified with a partial loss-of-function mutation in the EAAT2 gene, further supporting an important role of glutamate clearance in ALS pathogenesis [46].
Perhaps the strongest evidence in support for the role of excitotoxicity in ALS comes from the fact that the antiglutamate agent riluzole prolongs life expectancy in ALS clinical trials (Table 1, Figure 3). Riluzole acts by three parallel mechanisms – by inhibiting glutamate release, by blocking NMDAR and by stabilizing an inactivated state of voltage-gated sodium channels. Unfortunately, the benefits offered by riluzole are modest and additional drugs that inhibit Ca2+-signaling targets in MNs need to be evaluated in ALS clinical trials (in combination with riluzole) in the future.
Neuronal Ca2+ signaling and HD
The disorders discussed already – AD, PD and ALS – are mostly sporadic with rare familial forms (Table 1). There are some common themes that emerge from the analysis of the role of neuronal Ca2+ signaling in these sporadic disorders (Box 2). These disorders are ‘multi-hit’ and will probably require a combination therapy, with Ca2+ inhibitors included as a part of the treatment regimen (Box 2). In contrast to these disorders, HD is a purely genetic disorder that is caused by a single mutation – CAG repeat (polyglutamine) expansion in the huntingtin (Htt) gene [52] (Table 1). The medium spiny striatal neurons (MSNs) are most affected in HD. Most researchers agree that mutant Httexp protein acquires a ‘toxic gain of function’ [53]. Destabilization of neuronal Ca2+ signaling is one of the toxic functions of the Httexp protein. Consistent changes in the expression levels of many Ca2+ signaling proteins were observed in microarray studies of the brains from HD patients and also from HD mouse models [54]. The evidence for a ‘Ca2+ hypothesis of HD’ have been reviewed previously [55] and are summarized and updated briefly here.
Box 2. Neuronal Ca2+ signaling and sporadic AD, PD or ALS.
Sporadic AD, PD and ALS are ‘multi-hit’ disorders that are triggered by several pathological factors acting in concert. Some of these factors are common to all three disorders, whereas some are ‘disease specific’. One of the factors common to all of these disorders is ageing. The ‘disease-specific’ factors result in specificity of neuronal populations being affected in these disorders – cortical and hippocampal neurons in AD, dopaminergic SNc neurons in PD and motor neurons in ALS. The major ‘disease-specific’ factor for AD is likely to be accumulation of amyloid aggregates; for PD, it is toxicity resulting from dopamine oxidation; for ALS, it is inflammatory damage induced by activated microglia. Because these disorders are ‘multi-hit’, only combinational therapies can be successful in treating these disorders, with both ‘disease-specific’ and ‘common’ pathways targeted.
Neuronal populations that express high levels of Ca2+-binding proteins (CaBPs) are relatively spared in AD, PD and ALS, whereas neuronal populations with reduced levels of CaBP are severely affected. Reduction in levels of neuronal CaBPs is one of the consequences of the normal ageing process. Reduced ability to buffer cytosolic Ca2+ is likely to be one of the factors that make ageing neurons vulnerable in AD, PD and ALS.
Activation of the calpain family of Ca2+-dependent proteases is observed in aging neurons and in sporadic AD, PD and ALS. The activation of calpains is caused by elevated cytosolic Ca2+ levels. Activated calpains cleave a variety of substrates important for neuronal function, leading to neuronal dysfunction and death.
Mitochondria are significantly impaired in neurons affected in AD, PD and ALS. Mitochondria are partially depolarized, the ability of mitochondria to sequester Ca2+ is diminished, the stoichiometry of electron transfer-chain components is abnormal and mitochondrial DNA is mutated. Similar, but less severe, changes occur in neuronal mitochondria during normal aging. The damage to mitochondria is likely to be caused by excessive Ca2+ load, which leads to the generation of large amounts of ROS and oxidative damage to mitochondrial DNA. Thus, mitochondria are likely to be downstream from Ca2+ signaling in the pathogenic cascade. Nevertheless, it is expected that ‘mitochondrial stabilizers’, such as coenzyme Q10 and creatine, should have some beneficial effect in these disorders. Drugs targeting the mitochondrial-permeability transition pore (mtPTP) should also be extremely valuable as a ‘last defense’ approach separating cell dysfunction and cell death.
Neuronal Ca2+ signaling is affected by ageing and appear to be one of the common factors involved in pathogenesis of sporadic AD, PD and ALS. Thus, Ca2+ signaling blockers are expected to have a beneficial effect in these disorders. NMDA receptor-antagonist memantine demonstrated some clinical efficacy in AD and the antiglutamate agent riluzole has some efficacy in ALS. Additional Ca2+ signaling blockers should be developed and tested in clinical trials for these disorders, alone and as a part of combination therapy together with ‘mitochondrial stabilizers’, mtPTP inhibitors and ‘disease-specific’ approaches.
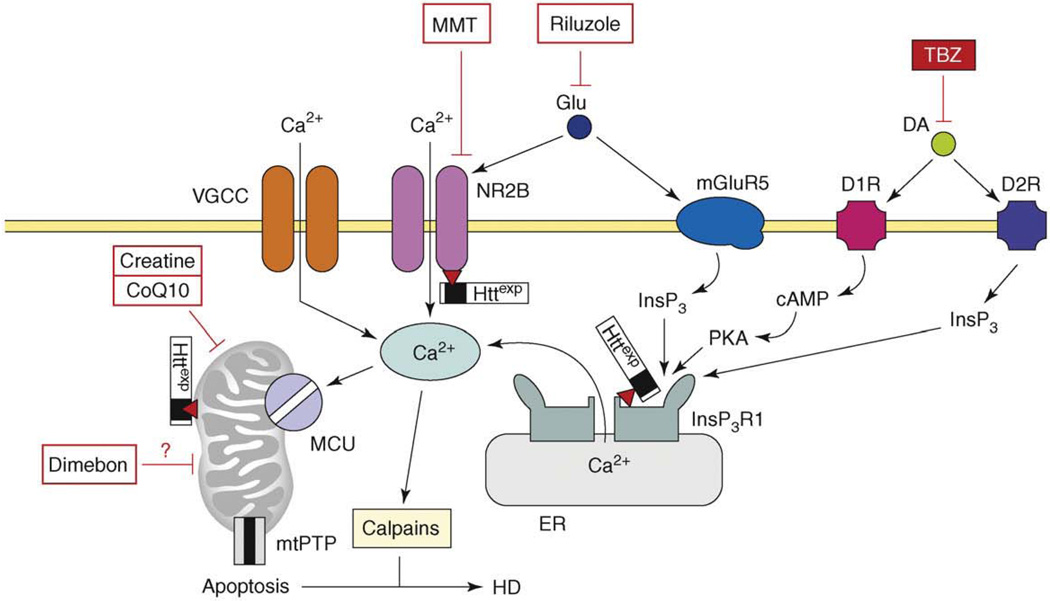
There are several points of Httexp interference with MSN Ca2+ signaling (Figure 4). Httexp binds directly and specifically to the InsP3R1 C-terminal region [56], and the association of Httexp with InsP3R1 was confirmed independently in an unbiased screen [57]. Binding to Httexp increases sensitivity of InsP3R1 to activation by InsP3 [56]. The importance of InsP3R1 activation for Httexp neurotoxicity was validated in pharmacological experiments with MSN cultures from a HD mouse model [58,59] and in genetic experiments with a Drosophila HD model [57]. In recent experiments, viral delivery of a peptide that disrupts Httexp association with InsP3R1 protected MSNs in an HD mouse model in vitro and in vivo [60]. These results supported a role of enhanced InsP3R1 activity in HD pathogenesis.
Figure 4.
The model of Ca2+ dysregulation in HD. In HD MSN, the Httexp perturbs Ca2+ signaling through several synergistic mechanisms. Httexp enhances function of NR2B-containing NMDAR, probably by promoting trafficking to the plasma membrane. Httexp binds strongly to the InsP3R1C terminus and sensitizes the InsP3R1 to activation by InsP3. The low levels of glutamate released from corticostriatal projection neurons lead to supranormal Ca2+ influx by NMDAR and Ca2+ release through the InsP3R1. Additional Ca2+ influx to MSN is mediated by voltage-gated Ca2+ channels (VGCCs). Dopamine released from midbrain dopaminergic neurons stimulates D1-class and D2-class DARs, which are expressed abundantly in MSNs. D1-class DARs are coupled to activation of adenyl cyclase, increase in cAMP levels and activation of PKA. PKA potentiates glutamate-induced Ca2+ signals by facilitating the activity of NMDAR and InsP3R1. D2 receptors are coupled directly to InsP3 production and activation of InsP3R1. Supranormal Ca2+ signals activate calpain, which cleave Httexp and other substrates. Excessive cytosolic Ca2+ signals result in mitochondrial Ca2+ uptake by MCU, which eventually triggers mtPTP opening and apoptosis. The mitochondrial Ca2+ handling is further destabilized by direct association of Httexp with mitochondria. Antidopamine agent tetrabenazine (TBZ) is approved by the US FDA for symptomatic treatment of HD. NMDAR antagonist memantine (MMT), putative ‘mitochondrial agent’ Dimebon and ‘mitochondrial stabilizers’ creatine and CoQ10 are tested in HD clinical trials. Antiglutamate agent riluzole was tested and failed [66]. Adapted from [77].
Expression of Httexp also causes enhancement in activity of NR2B-containing NMDARs [61]. An increase in NMDAR currents results from the effects of Httexp on NMDAR trafficking to the plasma membrane [62]. Striatal MSNs expressing Httexp are sensitized to NMDAR-mediated excitotoxicity and the pharmacological inhibition of NMDAR has a neuroprotective effect in MSN cultures from HD mouse models [58,63]. Both memantine and riluzole were neuroprotective in experiments with HD MSN cultures, with memantine being more effective [64]. Memantine demonstrated some beneficial effects in small-scale pilot evaluation in HD patients [65] and will be tested soon in a Phase IV HD clinical trial (Table 2). Riluzole was tested in a Phase III HD clinical trial but it was not successful [66] (Table 2).
In addition to InsP3R1 and NMDAR, Httexp might also affect the function of VGCCs. Huntingtin binds directly to the α2/δ auxiliary subunit of VGCCs [57] and to the CaV2.2 pore-forming subunit of N-type VGCCs [67]. Genetic removal of Dmca1D (Drosophila L-type calcium channel pore-forming subunit) suppressed photoreceptor neurode-generation in an HD fly model [68]. Electrophysiological analysis of striatal neurons from HD mouse models revealed an initial increase in VGCC density, which was followed by a reduction in VGCC density [69].
Similar to other disorders, the mechanism of Ca2+ toxicity in HD probably involves the activation of calpains and excessive Ca2+ accumulation in mitochondria (Figure 4). Calpain activation occurs in HD and calpain-mediated cleavage of Httexp and NMDARs has an important role in HD pathology [28,70,71]. Multiple evidence also points to mitochondrial dysfunction in HD [72]. Mitochondria isolated from lymphoblasts of HD patients and from brains of HD transgenic mouse models revealed pronounced defects in Ca2+ handling [73]. Mitochondrial function is impaired in HD cellular models [58,59,63,74]. In addition to effects on mitochondria resulting from excessive cytosolic Ca2+ transients, Httexp might also affect mitochondria directly by binding to the mitochondrial outer membrane [73] (Figure 4). Importantly, clinically relevant inhibitors of permeability transition in mitochondria demonstrated neuroprotective effects in cellular and animal models of HD [58,75].
The first drug approved by the US FDA in 2008 for HD treatment was an antidopamine agent, tetrabenazine (TBZ) (Table 1). TBZ is a potent inhibitor of vesicular monoamine transporter (VMAT2) and causes depletion of DA content in the presynaptic vesicles. In clinical trials, TBZ has been shown to significantly reduce chorea symptoms in HD patients [76]. In experiments with HD mouse models, early treatment with TBZ abolished motor coordination deficits and protected striatal neurons from degeneration in vivo [77]. It has been suggested that DA and glutamate act synergistically to induce Ca2+ signals in striatal neurons and that neuroprotective effects of TBZ can be explained by reduced Ca2+ signaling [77] (Figure 4). These findings suggest that TBZ might be considered not only for symptomatic treatment late in the disease but also for presymptomatic treatment. However, in some patients, TBZ causes severe depression [76] and other antidopamine agents, such as DA-specific inhibitors of VMAT2 or blockers of D1 and D2 receptors, should be considered in the future as alternatives to TBZ.
Neuronal Ca2+ signaling and SCAs
Similar to HD, SCAs are autosomal-dominant genetic disorders that are caused by polyglutamine expansion in ataxins [52]. There is some evidence to suggest that abnormal neuronal Ca2+ signaling might contribute to pathogenesis of these disorders. Some of these data are briefly summarized below.
In SCA1, degeneration of cerebellar Purkinje cells (PCs) is caused by polyQ expansion in the nuclear protein ataxin-1 [52]. Cerebellar PCs express extremely high levels of Ca2+-signaling proteins and CaBPs. The reduction of CaBP levels in PCs was reported early in SCA1 pathology in patients and in a SCA1 mouse model [78]. Genetic cross of SCA1 transgenic mice with calbindin-knockout mice resulted in an accelerated phenotype [78]. Early reduction in the expression of several Ca2+-signaling proteins, such as InsP3R1, trp3 Ca2+ channel and SERCA2 Ca2+ pump, was reported in microarray profiling of a SCA1 transgenic mouse model [79]. Although indirect, these results suggest that abnormalities in PC Ca2+ signaling might have a significant role in SCA1.
In SCA2, cerebellar PCs degenerate as a result of polyQ expansion in the cytosolic protein ataxin-2 [52]. The genetic association between polymorphism in the coding sequence of P/Q-type VGCCs and the age of disease onset in SCA2 patients suggests a potential role of Ca2+ signaling in SCA2 pathogenesis [80]. Recently, our laboratory discovered that mutant ataxin-2 specifically binds to and activates InsP3R1, similar to the mutant huntingtin (J. Liu et al., unpublished). We also found that Ca2+-signaling inhibitors protected SCA2 PCs from cell death in vitro and exerted significant beneficial effects in whole-animal studies with a SCA2 transgenic-mouse model (J. Liu et al., unpublished).
In SCA3, the neurons in SNc and in pontine nuclei (PN) degenerate as a result of polyQ expansion in the cytosolic protein ataxin-3 [52]. Calpain-mediated cleavage of ataxin-3 has an important role in SCA3 pathogenesis [81]. Mutant ataxin-3 specifically binds to and activates InsP3R1, similar to mutant huntingtin [82]. Long-term feeding of SCA3-transgenic mice with RyanR inhibitor and Ca2+ stabilizer dantrolene alleviated age-dependent motor coordination deficits in these mice and prevented neuronal loss in SNc and PNs [82].
In SCA6, the cerebellar PCs degenerate as a result of polyQ expansion in the C-terminal region of the CaV2.1 pore-forming subunit of P/Q-type Ca2+ channel [52]. This mutation might enhance P/Q-type Ca2+-channel activity in the expression system [83]. However, recent analysis of a SCA6 knock-in mouse model indicated that pathology might be related to aggregation of mutant CaV2.1 subunits and reduction in the density of dendritic P/Q-type Ca2+ currents [84]. Thus, the exact role of abnormal Ca2+ signaling in SCA6 is an open question.
Abnormal neuronal Ca2+ signaling is not restricted to polyglutamine-expansion ataxias and might be important in other ataxias as well. For example, recent genetic evidence indicated that the cause of SCA15 is deletion of a fragment of a gene encoding InsP3R1 [85].
Concluding remarks
The recurrent theme of this review is that Ca2+-signaling proteins and the mitochondrial Ca2+-handling system constitute attractive targets for the treatment of neurodegenerative disorders. A search of ClinicalTrials.gov (see: http://clinicaltrials.gov) with the keywords ‘calcium’ or ‘mitochondria’ revealed several ongoing or recent clinical trials for AD, PD, ALS and HD with Ca2+ blockers and mitochondrial stabilizers and energizers (Table 2). Detailed information about these trials can be found at the ClinicalTrials.gov site by searching using the unique trial ID number listed in Table 2. The targets of these drugs are shown in Figures 1–4. Based on the postulated target and mechanism of action (MOA), the drugs tested in these trials can be grouped into several categories (Box 3). It is apparent from this information (Table 2, Box 3) that some promising clinical leads are being tested but much more progress is needed.
Box 3. Ca2+ signaling: current prospects for therapeutic targeting.
Mitochondrial stabilizers and energizers
Ketasyn, creatine, CoQ10 and MitoQ are tested in AD, PD, ALS and HD trials (Figures 1–4). Considering the key role played by mitochondria in the pathogenesis of these disorders [88], some beneficial effects are expected in these trails. However, mitochondria are positioned late in the pathological pathway (Figures 1–4) and benefits are thus likely to be limited. Indeed, only modest benefits have been observed so far with this class of compounds in neurodegenerative trials reported so far [88].
Dimebon
Dimebon (Medivation Inc., San Francisco, CA, USA; http://www.medivation.com/) yielded promising results in Phase II AD clinical trails based on cognitive-outcome measures [86] (Figure 1). Dimebon has been also tested in a Phase II HD clinical trial (Figure 4). Dimebon is an old Russian antihistamine compound that has been claimed to exert neuroprotective effects at picomolar concentrations by a novel mitochondrial mechanism of action*. However, significant neuroprotective effects of Dimebon were only observed at 50 µM concentration in studies with HD MSN cultures [89]. Thus the cognitive effects of Dimebon observed in AD clinical trails [86] are likely to be due to the ability of this compound to inhibit α-adrenergic, histamine and serotonin receptors with high affinity [89].
NMDAR antagonists
Memantine is a non-competitive antagonist of NMDAR that is approved by the US FDA for the treatment of AD (Figure 1). Memantine is in clinical trails for PD, ALS and HD (Figures 2–4). The NR2B-specific antagonist EVT-101 (Evotec AG, Hamburg, Germany; http://www.evotec.com/) has been developed for AD treatment (Figure 1) and a Phase II AD trial of EVT-101 is anticipated soon. The same compound should be of great interest for the treatment of HD.
Riluzole
The antiglutamate agent Riluzole is approved by the US FDA for the treatment of ALS (Figure 3). Riluzole has been tested in Phase III HD clinical trails (Figure 4) but did not show significant benefit based on motor-outcome measures [66]. Riluzole was also tested in a Phase II PD trail (Figure 2).
L-type VGCC antagonists
‘CNS-optimized’ L-type VGCC inhibitor MEM-1003 (Memory Pharmaceuticals, Montvale, New Jersey, USA; http://www.memorypharma.com/) has shown some beneficial effects in a Phase II AD clinical trial (Figure 1). L-type VGCC antagonist Isradipine is being tested in a PD clinical trail (Figure 2).
* Bernales, S. et al. Dimebon induces neurite outgrowth and mitochondrial stabilization [abstract]. Program No. 543.29. 2008 Neuroscience Meeting Planner: November 15–19; Washington, DC, Society for Neuroscience, 2008 (http://www.sfn.org/am2008/)
Future translation of the ‘Ca2+ hypothesis of neurodegeneration’ to clinical practice will require a coordinated effort from neurodegenerative disease researchers, drug developers and clinicians. Several key questions need to be resolved for these efforts to be successful (Box 4). One might hope that, in the future, more potent and specific drugs targeting various components of Ca2+-signaling pathways (Figures 1–4) will be developed and tested in neurodegeneration clinical trials – alone and in combination with more specific ‘disease-targeting’ approaches.
Box 4. Outstanding questions.
Fundamental questions
Does neuronal Ca2+ signaling have a similar role in the pathogenesis of all neurodegenerative disorders or it is more important in some of them than in others?
What is the most crucial Ca2+ target?
Is the crucial Ca2+ target the same for all disorders or is it different for different disorders?
Can the lessons about the role of neuronal Ca2+ signaling be extrapolated from familial forms of AD, PD and ALS to sporadic forms?
Is there a crosstalk between ‘deranged neuronal Ca2+ signaling’ and ‘disease-specific’ pathogenic mechanisms?
Drug development questions
Questions about well established Ca2+ targets, such as NMDAR and L-type VGCC
Can more potent and specific drugs against these targets be developed?
Can existing drugs be optimized for neurodegenerative disease applications that will require brain permeability and long-term treatment?
Examples of such efforts are recent developments of NR2B-specific NMDAR antagonist EVT-101 (Evotec AG) and ‘CNS-optimized’ L-type VGCC inhibitor MEM-1003 (Memory Pharmaceuticals).
Questions about novel Ca2+ targets, such as InsP3R, RyanR, SERCA pump and SOC Ca2+ influx channels
Can specific inhibitors and modulators of these novel Ca2+ targets be developed?
Can these compounds be optimized for neurodegenerative disease applications?
Questions about potential Ca2+ targets, such as Aβ Ca2+ channels, ER Ca2+ leak channels, MCU and mtPTP
Are these druggable targets?
Can they be targeted for neurodegenerative disease applications?
Questions about compensatory Ca2+ targets, such as neuronal Ca2+ buffering and extracellular glutamate clearance mechanisms
Can these mechanisms be potentiated by pharmacological approaches?
Will these be therapeutically useful?
Clinical questions
Does the importance of neuronal Ca2+ signaling change with the age of the patients or with progression of the disease?
How much is it influenced by variability among different patients?
Can the variability be predicted or ascertained from genetic and biomarker analysis of these patients?
Are there ‘Ca2+ biomarkers’ that can provide insight into Ca2+-signaling abnormalities in individual patients?
Will it be possible to test ‘Ca2+ blockers’ at presymptomatic stages of the disease?
Which biomarkers and readouts should be used in presymptomatic neurodegeneration trails of ‘Ca2+ blockers’?
Should ‘Ca2+ blockers’ be tested alone or in combination with ‘disease-specific’ therapies and/or ‘mitochondrial stabilizers’?
Acknowledgements
I.B. would like to thank members of his laboratory, his colleagues and collaborators for insightful discussions that helped to formulate many ideas expressed in this article and for the comments on this manuscript. I.B. also would like to sincerely apologize to many scientists working in the field of neurodegeneration whose interesting work he could not cite owing to space limitations. I.B. is a holder of Carla Cocke Francis Professorship in Alzheimer’s Research and supported by the McKnight Neuroscience of Brain Disorders Award and NINDS R01 NS056224.
Footnotes
Disclosure statement
The author has no conflicts of interest to declare.
References
- 1.Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell. 2007;6:267–273. doi: 10.1111/j.1474-9726.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 2.Gant JC, et al. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J. Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 4.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 5.Seabrook GR, et al. Beyond amyloid: the next generation of Alzheimer’s disease therapeutics. Mol. Interv. 2007;7:261–270. doi: 10.1124/mi.7.5.8. [DOI] [PubMed] [Google Scholar]
- 6.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arispe N, et al. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. U. S. A. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee G, et al. Annexin 5 and apolipoprotein E2 protect against Alzheimer’s amyloid-beta-peptide cytotoxicity by competitive inhibition at a common phosphatidylserine interaction site. Peptides. 2002;23:1249–1263. doi: 10.1016/s0196-9781(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 9.Simakova O, Arispe NJ. The cell-selective neurotoxicity of the Alzheimer’s Aβ peptide is determined by surface phosphatidylserine and cytosolic ATP levels. Membrane binding is required for Aβ toxicity. J. Neurosci. 2007;27:13719–13729. doi: 10.1523/JNEUROSCI.3006-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuchibhotla KV, et al. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Felice FG, et al. Aβ oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 12.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh H, et al. AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimmrich V, et al. Amyloid beta oligomers (A β(1–42) globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J. Neurosci. 2008;28:788–797. doi: 10.1523/JNEUROSCI.4771-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito E, et al. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leissring MA, et al. Alzheimer’s presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J. Neurochem. 1999;72:1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- 17.Stutzmann GE, et al. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J. Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stutzmann GE, et al. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J. Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leissring MA, et al. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J. Cell Biol. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo AS, et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 21.Chan SL, et al. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 22.Rybalchenko V, et al. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int. J. Biochem. Cell Biol. 2008;40:84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Cai C, et al. The presenilin-2 loop peptide perturbs intracellular Ca2+ homeostasis and accelerates apoptosis. J. Biol. Chem. 2006;281:16649–16655. doi: 10.1074/jbc.M512026200. [DOI] [PubMed] [Google Scholar]
- 24.Cheung KH, et al. Mechanismof Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP(3) receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green KN, et al. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J. Cell Biol. 2008;181:1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu H, et al. Presenilins form ER calcium leak channels, a function disrupted by mutations linked to familial Alzheimer’s disease. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson O, et al. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J. Clin. Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vosler PS, et al. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trinchese F, et al. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J. Clin. Invest. 2008;118:2796–2807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palop JJ, et al. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanz-Blasco S, et al. Mitochondrial Ca2+ overload underlies Aβ oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One. 2008;3:e2718. doi: 10.1371/journal.pone.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreses-Werringloer U, et al. A polymorphism in CALHM1 influences Ca2+ homeostasis, Aβ levels, and Alzheimer’s disease risk. Cell. 2008;133:1149–1161. doi: 10.1016/j.cell.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertram L, et al. No association between CALHM1 and Alzheimer’s disease risk. Cell. 2008;135:993–994. doi: 10.1016/j.cell.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arispe N, et al. Aβ ion channels. Prospects for treating Alzheimer’s disease with Aβ channel blockers. Biochim. Biophys. Acta. 2007;1768:1952–1965. doi: 10.1016/j.bbamem.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 36.Abou-Sleiman PM, et al. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 37.Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Surmeier DJ. Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. Lancet Neurol. 2007;6:933–938. doi: 10.1016/S1474-4422(07)70246-6. [DOI] [PubMed] [Google Scholar]
- 39.Volles MJ, et al. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 40.Danzer KM, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furukawa K, et al. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. J. Neurochem. 2006;97:1071–1077. doi: 10.1111/j.1471-4159.2006.03803.x. [DOI] [PubMed] [Google Scholar]
- 42.Chan CS, et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’ disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 43.Becker C, et al. Use of antihypertensives and the risk of Parkinson disease. Neurology. 2008;70:1438–1444. doi: 10.1212/01.wnl.0000303818.38960.44. [DOI] [PubMed] [Google Scholar]
- 44.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 45.Monk PN, Shaw PJ. ALS: life and death in a bad neighborhood. Nat. Med. 2006;12:885–887. doi: 10.1038/nm0806-885. [DOI] [PubMed] [Google Scholar]
- 46.Van Den Bosch L, et al. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim. Biophys. Acta. 2006;1762:1068–1082. doi: 10.1016/j.bbadis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Appel SH, et al. Calcium: the Darth Vader of ALS. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2001;(2 (Suppl. 1)):S47–S54. [PubMed] [Google Scholar]
- 48.Beers DR, et al. Parvalbumin overexpression alters immune-mediated increases in intracellular calcium, and delays disease onset in a transgenic model of familial amyotrophic lateral sclerosis. J. Neurochem. 2001;79:499–509. doi: 10.1046/j.1471-4159.2001.00582.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhao W, et al. Activated microglia initiate motor neuron injury by a nitric oxide and glutamate-mediated mechanism. J. Neuropathol. Exp. Neurol. 2004;63:964–977. doi: 10.1093/jnen/63.9.964. [DOI] [PubMed] [Google Scholar]
- 50.Sasabe J, et al. D-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J. 2007;26:4149–4159. doi: 10.1038/sj.emboj.7601840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Damme P, et al. Astrocytes regulate GluR2 expression in motor neurons and their vulnerability to excitotoxicity. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14825–14830. doi: 10.1073/pnas.0705046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gusella JF, MacDonald ME. Molecular genetics: unmasking polyglutamine triggers in neurodegenerative disease. Nat. Rev. Neurosci. 2000;1:109–115. doi: 10.1038/35039051. [DOI] [PubMed] [Google Scholar]
- 53.Li S, Li XJ. Multiple pathways contribute to the pathogenesis of Huntington disease. Mol. Neurodegener. 2006;1:19. doi: 10.1186/1750-1326-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuhn A, et al. Mutant huntingtin’s effects on striatal gene expression in mice recapitulate changes observed in human Huntington’s disease brain and do not differ with mutant huntingtin length or wild-type huntingtin dosage. Hum. Mol. Genet. 2007;16:1845–1861. doi: 10.1093/hmg/ddm133. [DOI] [PubMed] [Google Scholar]
- 55.Bezprozvanny I, Hayden MR. Deranged neuronal calcium signaling and Huntington disease. Biochem. Biophys. Res. Commun. 2004;322:1310–1317. doi: 10.1016/j.bbrc.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 56.Tang T-S, et al. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaltenbach LS, et al. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 2007;3:e82. doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang T-S, et al. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’ disease. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, et al. Full length mutant huntingtin is required for altered Ca2+ signaling and apoptosis of striatal neurons in the YAC mouse model of Huntington’s disease. Neurobiol. Dis. 2008;31:80–88. doi: 10.1016/j.nbd.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang T-S, et al. Neuroprotective effects of inositol 1,4,5-trisphosphate receptor carboxy-terminal fragment in Huntington’s disease mouse model. J. Neuroscience. 2009;29:1257–1266. doi: 10.1523/JNEUROSCI.4411-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeron MM, et al. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington’s disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 62.Fan MM, et al. Altered NMDA receptor trafficking in a yeast artificial chromosome transgenic mouse model of Huntington’s disease. J. Neurosci. 2007;27:3768–3779. doi: 10.1523/JNEUROSCI.4356-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shehadeh J, et al. Striatal neuronal apoptosis is preferentially enhanced by NMDA receptor activation in YAC transgenic mouse model of Huntington disease. Neurobiol. Dis. 2006;21:392–403. doi: 10.1016/j.nbd.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Wu J, et al. Evaluation of clinically-relevant glutamate pathway inhibitors in in vitro model of Huntington’s disease. Neurosci. Lett. 2006;407:219–223. doi: 10.1016/j.neulet.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 65.Ondo WG, et al. A pilot study of the clinical efficacy and safety of memantine for Huntington’s disease. Parkinsonism Relat. Disord. 2007;13:453–454. doi: 10.1016/j.parkreldis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Landwehrmeyer GB, et al. Riluzole in Huntington’s disease: a 3-year, randomized controlled study. Ann. Neurol. 2007;62:262–272. doi: 10.1002/ana.21181. [DOI] [PubMed] [Google Scholar]
- 67.Swayne LA, et al. Crosstalk between huntingtin and syntaxin 1A regulates N-type calcium channels. Mol. Cell. Neurosci. 2005;30:339–351. doi: 10.1016/j.mcn.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 68.Romero E, et al. Suppression of neurodegeneration and increased neurotransmission caused by expanded full-length huntingtin accumulating in the cytoplasm. Neuron. 2008;57:27–40. doi: 10.1016/j.neuron.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cepeda C, et al. The corticostriatal pathway in Huntington’s disease. Prog. Neurobiol. 2007;81:253–271. doi: 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gafni J, et al. Inhibition of calpain cleavage of huntingtin reduces toxicity: accumulation of calpain/caspase fragments in the nucleus. J. Biol. Chem. 2004;279:20211–20220. doi: 10.1074/jbc.M401267200. [DOI] [PubMed] [Google Scholar]
- 71.Cowan CM, et al. Polyglutamine-modulated striatal calpain activity in YAC transgenic Huntington disease mouse model: impact on NMDA receptor function and toxicity. J. Neurosci. 2008;28:12725–12735. doi: 10.1523/JNEUROSCI.4619-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bossy-Wetzel E, et al. Mutant huntingtin and mitochondrial dysfunction. Trends Neurosci. 2008;31:609–616. doi: 10.1016/j.tins.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panov AV, et al. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat. Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 74.Lim D, et al. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J. Biol. Chem. 2008;283:5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, et al. Inhibitors of cytochrome c release with therapeutic potential for Huntington’s disease. J. Neurosci. 2008;28:9473–9485. doi: 10.1523/JNEUROSCI.1867-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savani AA, Login IS. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2007;68:797. doi: 10.1212/01.wnl.0000259143.52138.5c. [DOI] [PubMed] [Google Scholar]
- 77.Tang TS, et al. Dopaminergic signaling and striatal neurodegeneration in Huntington’s disease. J. Neurosci. 2007;27:7899–7910. doi: 10.1523/JNEUROSCI.1396-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vig PJ, et al. Calcium homeostasis and spinocerebellar ataxia-1 (SCA-1) Brain Res. Bull. 2001;56:221–225. doi: 10.1016/s0361-9230(01)00595-0. [DOI] [PubMed] [Google Scholar]
- 79.Lin X, et al. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat. Neurosci. 2000;3:157–163. doi: 10.1038/72101. [DOI] [PubMed] [Google Scholar]
- 80.Pulst SM, et al. Spinocerebellar ataxia type 2: polyQ repeat variation in the CACNA1A calcium channel modifies age of onset. Brain. 2005;128:2297–2303. doi: 10.1093/brain/awh586. [DOI] [PubMed] [Google Scholar]
- 81.Haacke A, et al. Calpain inhibition is sufficient to suppress aggregation of polyglutamine-expanded ataxin-3. J. Biol. Chem. 2007;282:18851–18856. doi: 10.1074/jbc.M611914200. [DOI] [PubMed] [Google Scholar]
- 82.Chen X, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J. Neurosci. 2008;28:12713–12724. doi: 10.1523/JNEUROSCI.3909-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piedras-Renteria ES, et al. Increased expression of alpha 1A Ca2+ channel currents arising from expanded trinucleotide repeats in spinocerebellar ataxia type 6. J. Neurosci. 2001;21:9185–9193. doi: 10.1523/JNEUROSCI.21-23-09185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watase K, et al. Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11987–11992. doi: 10.1073/pnas.0804350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van de Leemput J, et al. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 2007;3:e108. doi: 10.1371/journal.pgen.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Doody RS, et al. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 87.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 88.Chaturvedi RK, Beal MF. Mitochondrial approaches for neuroprotection. Ann. N. Y. Acad. Sci. 2008;1147:395–412. doi: 10.1196/annals.1427.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu J, et al. Evaluation of Dimebon in cellular model of Huntington’s disease. Mol. Neurodegener. 2008;3:15. doi: 10.1186/1750-1326-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]