Abstract
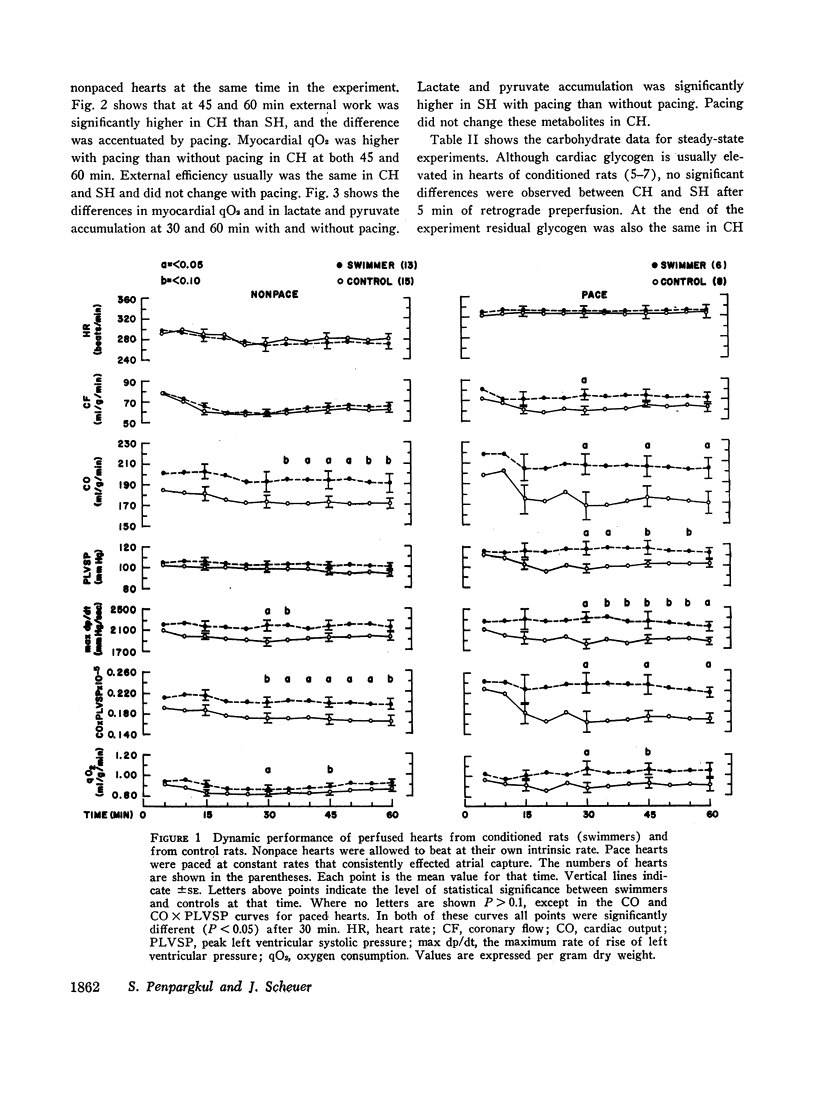
The dynamic and metabolic performance of rats conditioned by a swimming program (CH) and hearts of sedentary rats (SH) was studied in an isolated working rat heart apparatus. Heart rate, filling pressure, and afterload were controlled or kept constant, and heart weights were comparable in both groups.
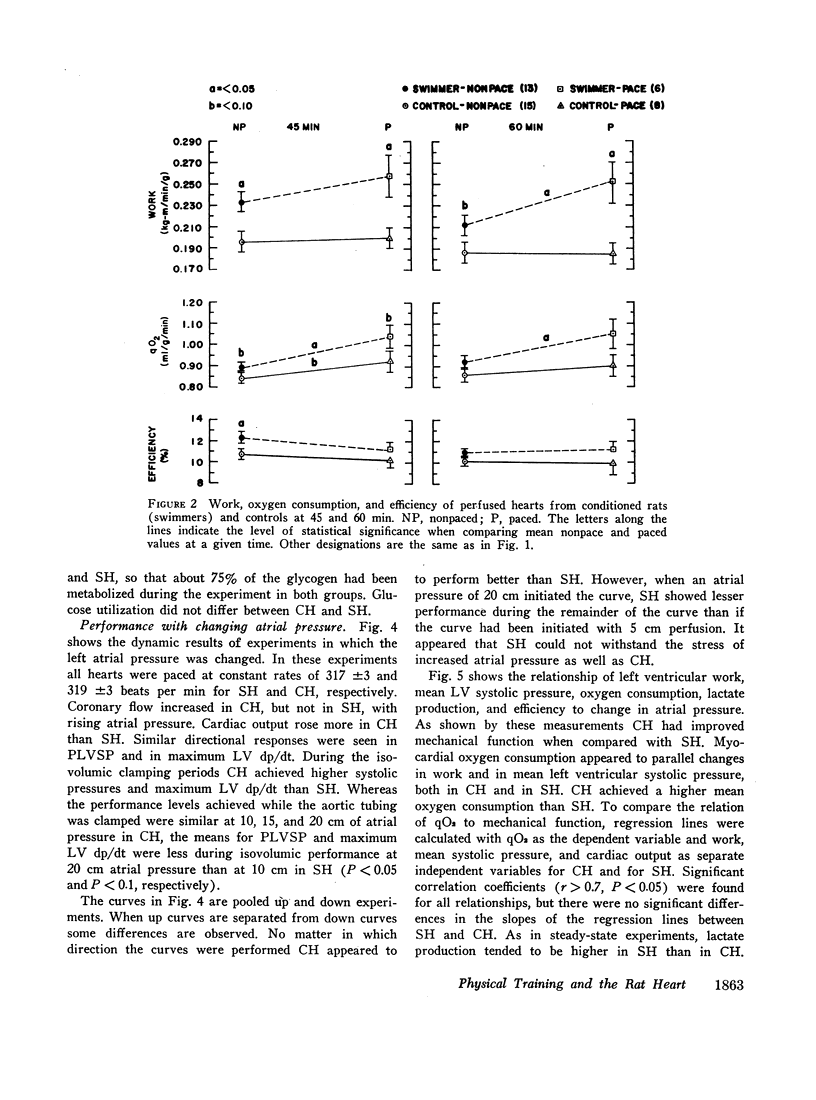
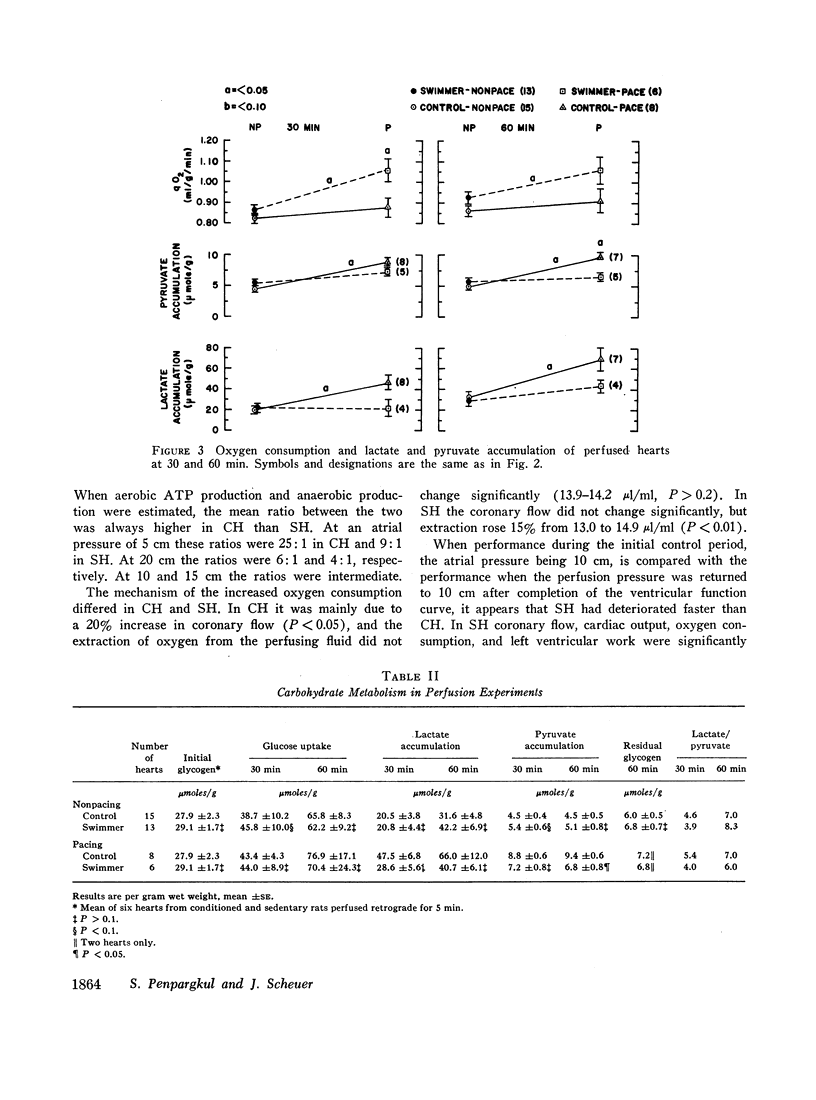
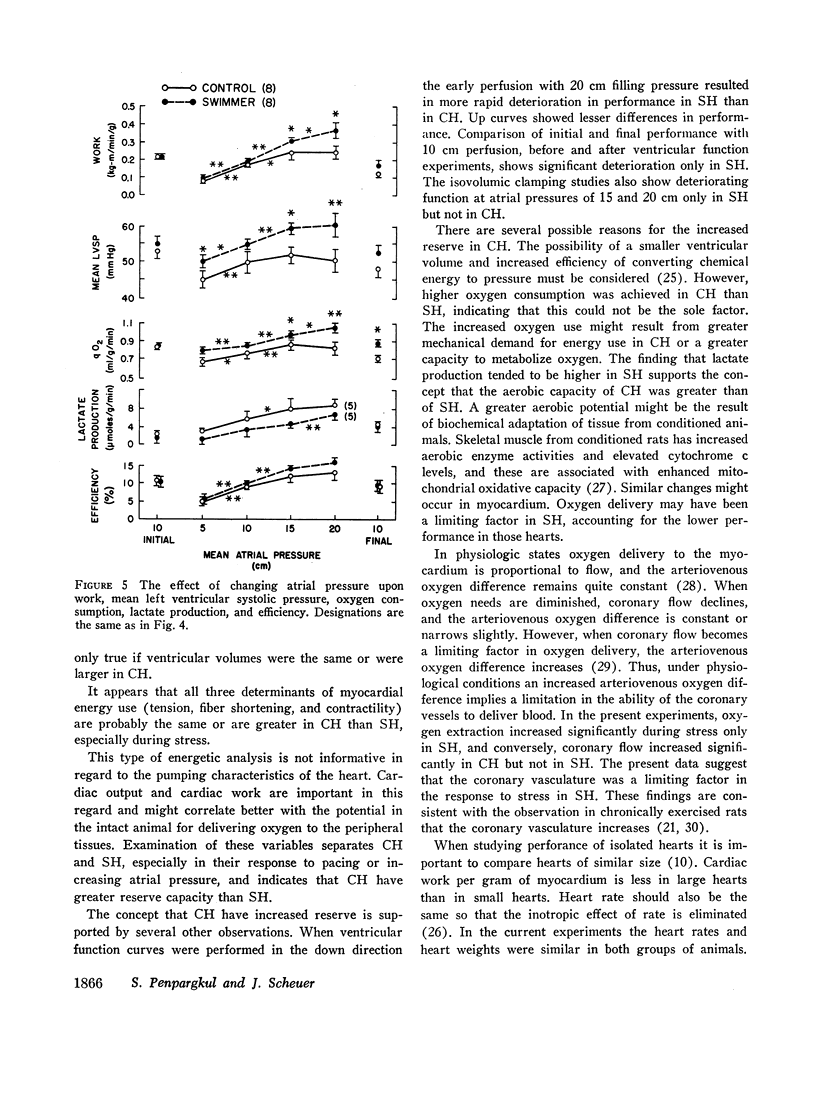
When compared with SH, CH had increased cardiac output and cardiac work. Atrial pacing at more rapid rates caused greater differences in these functions, and left ventricular pressure and maximal rate of pressure rise (dp/dt) became higher in CH than in SH. Atrial pacing was associated in CH with increased oxygen consumption but in SH by increased lactate and pyruvate production.
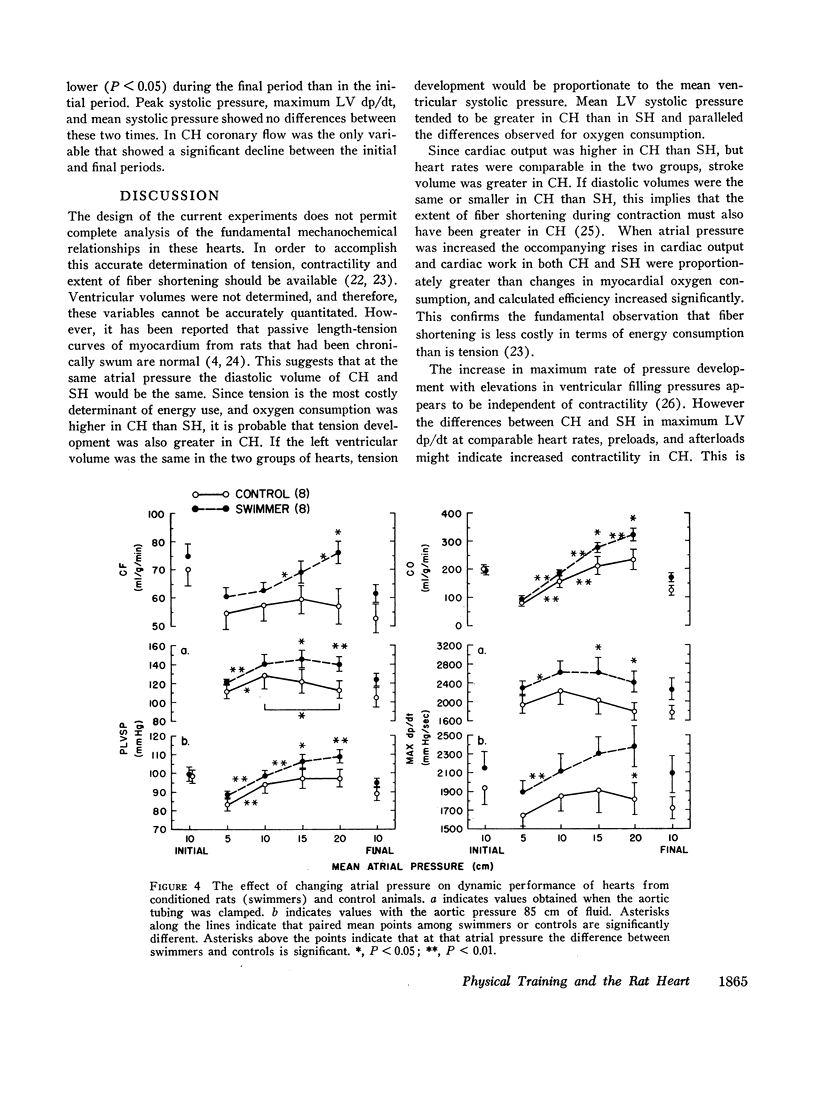
When atrial filling pressure was elevated in order to perform ventricular function curves, CH showed greater dynamic responses than SH. There were also greater increments in oxygen consumption, and the ratio of aerobic to anaerobic energy production was also higher in CH.
The mechanism of increasing oxygen consumption during stress in CH was mainly by improved coronary flow. In SH coronary flow did not change, but extraction of oxygen from the perfusing fluid increased.
The results indicate that in physically trained rats the function of the heart as a pump is improved. These hearts have greater aerobic and mechanical reserve than hearts of sedentary animals. These effects appear to be at least partially due to improved mechanisms of oxygen delivery.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALELLA A., WILLIAMS F. L., BOLENE-WILLIAMS C., KATZ L. N. Interrelation between cardiac oxygen consumption and coronary blood flow. Am J Physiol. 1955 Dec;183(3):570–582. doi: 10.1152/ajplegacy.1955.183.3.570. [DOI] [PubMed] [Google Scholar]
- Crews J., Aldinger E. E. Effect of chronic exercise on myocardial function. Am Heart J. 1967 Oct;74(4):536–542. doi: 10.1016/0002-8703(67)90013-0. [DOI] [PubMed] [Google Scholar]
- De Schryver C., De Herdt P., Lammerant J. Effect of physical training on cardiac catecholamine concentrations. Nature. 1967 May 27;214(5091):907–908. doi: 10.1038/214907b0. [DOI] [PubMed] [Google Scholar]
- FOX S. M., 3rd, SKINNER J. S. PHYSICAL ACTIVITY AND CARDIOVASCULAR HEALTH. Am J Cardiol. 1964 Dec;14:731–746. doi: 10.1016/0002-9149(64)90002-5. [DOI] [PubMed] [Google Scholar]
- GRIMM A. F., KUBOTA R., WHITEHORN W. V. Properties of myocardium in cardiomegaly. Circ Res. 1963 Jan;12:118–124. doi: 10.1161/01.res.12.1.118. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Struck P. J., Bogyo T. P. Lactic dehydrogenase activities of rat heart and skeletal muscle after exercise and training. J Appl Physiol. 1967 Apr;22(4):623–627. doi: 10.1152/jappl.1967.22.4.623. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967 May 10;242(9):2278–2282. [PubMed] [Google Scholar]
- LEVINE H. J., WAGMAN R. J. Energetics of the human heart. Am J Cardiol. 1962 Mar;9:372–383. doi: 10.1016/0002-9149(62)90155-8. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Liebermeister H., Battersby E. J., Morgan H. E. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 1967 Apr;212(4):804–814. doi: 10.1152/ajplegacy.1967.212.4.804. [DOI] [PubMed] [Google Scholar]
- Poland J. L., Blount D. H. The effects of training on myocardial metabolism. Proc Soc Exp Biol Med. 1968 Oct;129(1):171–174. doi: 10.3181/00379727-129-33276. [DOI] [PubMed] [Google Scholar]
- Pool P. E., Chandler B. M., Seagren S. C., Sonnenblick E. H. Mechanochemistry of cardiac muscle. II. The isotonic contraction. Circ Res. 1968 Apr;22(4):465–472. doi: 10.1161/01.res.22.4.465. [DOI] [PubMed] [Google Scholar]
- SAIFER A., GERSTENFELD S. The photometric microdetermination of blood glucose with glucose oxidase. J Lab Clin Med. 1958 Mar;51(3):448–460. [PubMed] [Google Scholar]
- SEGAL S., BLAIR A. E., WYNGAARDEN J. B. An enzymatic spectrophotometric method for the determination of pyruvic acid in blood. J Lab Clin Med. 1956 Jul;48(1):137–143. [PubMed] [Google Scholar]
- STEVENSON J. A., FELEKI V., RECHNITZER P., BEATON J. R. EFFECT OF EXERCISE ON CORONARY TREE SIZE IN THE RAT. Circ Res. 1964 Sep;15:265–269. doi: 10.1161/01.res.15.3.265. [DOI] [PubMed] [Google Scholar]
- Scheuer J., Kapner L., Stringfellow C. A., Armstrong C. L., Penpargkul S. Glycogen, lipid, and high energy phosphate stores in hearts from conditioned rats. J Lab Clin Med. 1970 Jun;75(6):924–929. [PubMed] [Google Scholar]
- Scheuer J., McDonald R. H. Current status of myocardial mechanical-energetic relationships. Mt Sinai J Med. 1970 May-Jun;37(3):311–330. [PubMed] [Google Scholar]
- Scheuer J., Stezoski S. W. Discordance between the electrocardiogram and ATP levels in the isolated rat heart. Am J Med Sci. 1969 Apr;257(4):218–227. doi: 10.1097/00000441-196904000-00002. [DOI] [PubMed] [Google Scholar]
- TEPPERMAN J., PEARLMAN D. Effects of exercise and anemia on coronary arteries of small animals as revealed by the corrosion-cast technique. Circ Res. 1961 May;9:576–584. doi: 10.1161/01.res.9.3.576. [DOI] [PubMed] [Google Scholar]
- Tanz R. D., Marcus S. M. Observations on responses of the heart to catecholamine-depletion produced by reserpine. Proc Soc Exp Biol Med. 1966 Mar;121(3):853–857. doi: 10.3181/00379727-121-30907. [DOI] [PubMed] [Google Scholar]
- Tipton C. M. Training and bradycardia in rats. Am J Physiol. 1965 Dec;209(6):1089–1094. doi: 10.1152/ajplegacy.1965.209.6.1089. [DOI] [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]
- WALLACE A. G., SKINNER N. S., Jr, MITCHELL J. H. Hemodynamic determinants of the maximal rate of rise of left ventricular pressure. Am J Physiol. 1963 Jul;205:30–36. doi: 10.1152/ajplegacy.1963.205.1.30. [DOI] [PubMed] [Google Scholar]


