Abstract
Background
Anatomic studies suggest the deep inferior epigastric artery (DIEA) medial branch perfuses more tissue across the midline than the lateral branch. We hypothesized that unilateral DIEP and MS FTRAM flaps based on medial branch perforators would have fewer perfusion-related complications.
Methods
We evaluated 2043 consecutive free flap breast reconstructions and included unilateral reconstructions where DIEP or MS FTRAM flaps were definitively harvested from a single DIEA branch. We grouped flaps by tissue volume, i.e., Hemiflaps, Cross-Midline Flaps, and Total Flaps. Primary outcome measures were fat necrosis and partial flap necrosis. Logistic regression was used to evaluate the association between patient and reconstruction characteristics and perfusion outcomes.
Results
We included 228 patients: 120 (52.6%) medial and 108 (47.4%) lateral branch flaps. Mean follow-up was 33.2 months. Cross-Midline Flaps (79.8%) were most common, followed by Hemiflaps (15.4%) and Total Flaps (4.8%). Overall fat necrosis and partial flap necrosis rates were 10.5% and 3.1%, respectively. Medial and lateral branch flaps had similar rates of fat necrosis (8.3% vs. 13.0%, respectively; p=0.26) and partial flap necrosis (3.3% vs. 2.8%, respectively; p=1.0). DIEP and MS FTRAM flaps had no difference in the incidence of fat necrosis (10.2% vs. 11.3%; p=0.81) or partial necrosis (3.2% vs. 2.8%; p=1.0). Medial and lateral branch flap perfusion-related complications were also similar among the flap volume classifications.
Conclusions
We suggest that surgeons base their decisions regarding DIEA branch harvest on the clinical assessment of perforator perfusion quality rather than relying on the theoretic benefit of medial branch perforator harvest.
Introduction
Abdominal-based free flaps perfused by the deep inferior epigastric artery (DIEA), such as the deep inferior epigastric perforator flap (DIEP) and the muscle-sparing transverse rectus abdominis musculocutaneous flap (MS FTRAM), remain a popular choice for autologous breast reconstruction.(1-24) Hartrampf defined perfusion zones of the anterior abdominal wall to facilitate decision-making when designing pedicled TRAM (PTRAM) flaps.(25) Fluorescence angiography by Holm demonstrated the Hartrampf zones to be appropriate for the superior epigastric artery (Figure 1a) but demonstrated a reversal of zones 2 and 3 when the perfusion was isolated to the DIEA (Figure 1b).(26, 27) Recent cadaveric anatomic studies using dynamic computed tomographic (CT) scanning have elegantly shown the medial and lateral DIEA branch perforasomes to differ in type II DIEA branching pattern flaps.(28, 29) Medial branch DIEA perforators appear to more often follow the perfusion pattern described by Hartrampf, while lateral branch DIEA perforators appear to resemble the Holm zones.(26, 28-33) On the basis of these findings, it has been suggested that DIEP and MS FTRAM flaps including tissue from the contralateral hemiabdomen should be perfused by medial DIEA branch perforators, and flaps based only on the ipsilateral hemiabdomen should be perfused by lateral DIEA branch perforators. Interestingly, attempts to replicate these cadaveric findings in living patients have not demonstrated significant differences in the medial vs. lateral branch perforasomes.(33) Despite the theoretic advantages of medial DIEA perforator harvest, it has not been determined whether perfusion-related complications such as fat necrosis and partial flap necrosis actually differ between flaps perfused by medial- or lateral-only branch perforators. We hypothesized that unilateral DIEP and MS FTRAM flaps based on medial DIEA branch perforators that include Holm zone 3 tissue across the midline will have a lower rate of perfusion-related complications such as fat necrosis and partial flap necrosis than those based on lateral branch perforators.
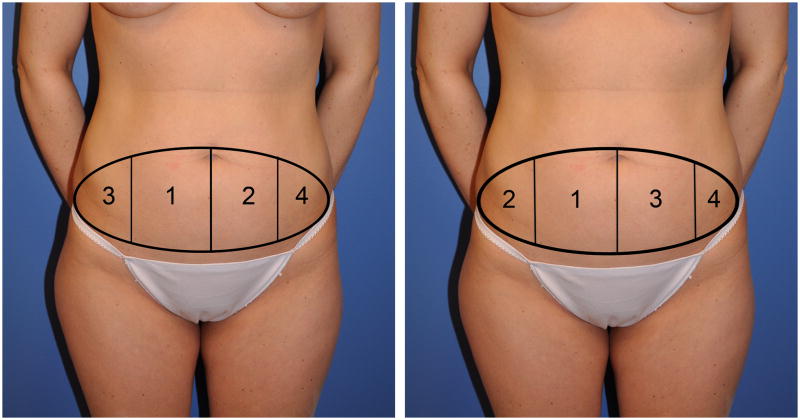
Figure 1.
a) Holm's perfusion zones of the abdomen appear to more reflect the perfusion of lateral DIEA branch perforators. b) Hartrampf's perfusion zones of the abdomen appear to more reflect the perfusion of medial DIEA branch perforators.
Patients and Methods
We evaluated all free-flap, abdominal-based autologous breast reconstructions performed at The University of Texas MD Anderson Cancer Center between January 1, 2000, and April 13, 2010 (2043 consecutive cases). Data collected from a prospectively entered departmental database and patients' medical records were retrospectively reviewed. We included only those cases of post-mastectomy, unilateral DIEP or MS FTRAM flap breast reconstruction in which the flap was perfused by either medial-only or lateral-only type II DIEA branch perforators. We excluded flaps harvested with both the medial and lateral DIEA branches, flaps harvested for one side of a bilateral breast reconstruction, bipedicled flaps for unilateral breast reconstruction, superficial inferior epigastric artery (SIEA) flaps, cases in which the operative report did not clearly communicate from which DIEA branch the flap was harvested, cases with type I or III DIEA branching patterns, and complete flap failures. MD Anderson Cancer Center's Institutional Review Board approved this study.
Patient, treatment, and surgical outcome data were analyzed. The primary outcome measure was the relationship between DIEA branch flap harvest and the occurrence of fat necrosis or partial flap necrosis. Secondary outcome measures included the effects of DIEP vs. MS FTRAM harvest, flap design, and perforator number on the occurrence of fat necrosis or partial flap necrosis.
Flap harvest patterns were classified into three groups--Hemiflaps, Cross-Midline Flaps, or Total Flaps--according to the Holm perfusion zones included with the flap (Fig. 2).(26) Fat necrosis was defined as a palpable firmness ≥1 cm in diameter that persisted beyond 3 months postoperatively. Partial flap necrosis was defined as necrosis of the flap skin island and underlying fat. For the purpose of this evaluation fat necrosis and partial flap necrosis were considered mutually exclusive complications. The presence of fat necrosis or partial flap necrosis was determined by clinical examination and radiographic and/or pathologic confirmation. The decision to image and/or biopsy a palpable firmness was made at the surgeons' and/or oncologists' clinical discretion. Patients were followed postoperatively at least monthly after discharge for 6 months, every 3-6 months until 1 year, and then at least yearly thereafter.
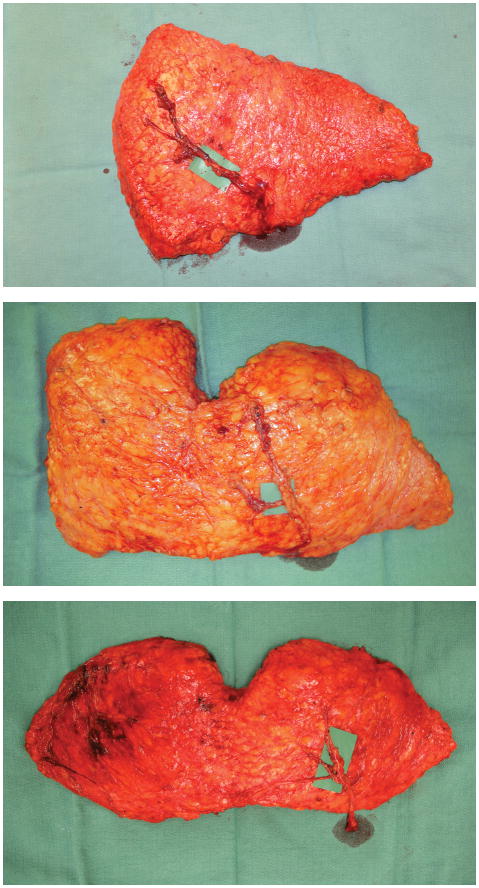
Figure 2.
Flap harvest patterns were classified into three groups according to the Holm perfusion zones included with the flap: a) Example of a Hemiflap, which includes Holm zones 1 and 2; b) Example of a Cross-Midline Flap, which includes Holm zones 1, 2, and 3; and c) Example of a Total Flap, which includes Holm zones 1, 2, 3, and 4.
Surgical Technique
All DIEP and MS FTRAM flaps included in this patient series were harvested on either the medial or lateral DIEA branch through fascial incisions around the individual perforators using a fascia-sparing technique. DIEP flaps were raised by splitting the rectus abdominis muscle. MS FTRAM flaps were raised by harvesting a longitudinal section of muscle around the same-branch perforators.(14) Surgeons tended to harvest single perforators only when the combined arterial and venous perforator diameter was >3 mm.(34-36) Harvesting a flap on combined artery/vein perforators measuring <1.5 mm was typically avoided.
Statistical Analysis
Fisher's exact test or the Chi-square test was used to evaluate the association between categorical variables. Wilcoxon rank sum or Kruskal-Wallis testing was used to compare the distributions of continuous variables between patient groups. The multicovariate logistic regression model was used to determine the effects of patient and reconstructive characteristics on complication status.(37) Values for p ≤ 0.05 were considered statistically significant. SAS statistical software (version 9.1.3, Cary, NC) was used for all the analyses. All statistical analyses were performed by a senior staff biostatistician (L.F.).
Results
We identified 228 patients meeting the strict criteria for study inclusion. There were 120 (52.6%) medial and 108 (47.4%) lateral DIEA branch cases. Reconstruction immediately followed mastectomy in 148 patients (64.9%) and was delayed in 80 patients (35.1%). DIEP flaps were used in 157 (68.9%) cases and MS FTRAM flaps in 71 (31.1%) cases. Of the MS FTRAM flaps, 64 (90.1%) were MS2 FTRAM flaps and 7 (9.9%) were MS1 FTRAM flaps. Flap designs were distributed as follows: Cross-Midline Flaps (79.8%), Hemiflaps (15.4%), and Total Flaps (4.8%). Patient follow-up was 33.2 ± 22.9 months (range 7.6 – 107.0 months).
Overall Flap Outcomes
Of the 228 cases, fat necrosis occurred in 24 (10.5%) and partial flap necrosis occurred in 7 (3.1%), for a total of 31 (13.6%) flaps that developed either fat necrosis or partial flap necrosis. Radiologic and/or pathologic confirmation was available for 61.3% of the cases of fat necrosis. Other flap complications included hematoma/seroma (5.7%), infection requiring antibiotics (2.6%), and anastomotic thrombosis (0.9%). Forty-five flaps (19.7%) developed at least one flap complication. Eight flaps (3.5%) developed two or more complications.
Medial vs. Lateral DIEA Branch Flap Outcomes
Patient clinical characteristics and co-morbidities for the cases stratified by DIEA branch are shown in Table 1. There were no significant differences in body mass index (BMI), smoking status, or adjuvant chemotherapy between the two groups. The lateral branch group had a higher incidence of diabetes mellitus (9.3%) compared to the medial branch group (2.5%; p=0.042).
Table 1. Patient Clinical Characteristics and Co-Morbidities.
| Medial Branch N=120 |
Lateral Branch N=108 |
P-value | |
|---|---|---|---|
| Body Mass Index, mean (mg/kg2) | 26.9 ± 5.2 | 27.3 ± 6.2 | 0.95 |
| Age, mean (years) | 50.8 ± 9.1 | 50.8 ± 9.2 | 0.86 |
| Bra Cup Size, mean | |||
| • A or B | 53 (46.5%) | 34 (37%) | 0.17 |
| • ≥ C | 61 (53.5%) | 58 (63%) | |
| Active Smoker | 9 (7.5%) | 5 (4.6%) | 0.42 |
| Alcohol Consumption | 54 (45%) | 45 (41.7%) | 0.61 |
| Preoperative Chemotherapy | 47 (39.2%) | 49 (45.4%) | 0.34 |
| Preoperative Radiation Therapy | 35 (29.2%) | 32 (29.6%) | 0.94 |
| Postoperative Radiation Therapy | 11 (9.2%) | 11 (10.2%) | 0.79 |
| Any Medical Co-morbidity | 42 (35%) | 45 (41.7%) | 0.30 |
| • Adrenal Disease | 1 (0.8%) | 0 (0%) | 1.0 |
| • Arrhythmias | 6 (5%) | 3 (2.8%) | 0.50 |
| • Cerebrovascular Disease | 2 (1.7%) | 0 (0%) | 0.50 |
| • Coronary Artery Disease | 1 (0.8%) | 0 (0%) | 1.0 |
| • Diabetes Mellitus | 3 (2.5%) | 10 (9.3%) | 0.04 |
| • Gastrointestinal Disease | 14 (11.7%) | 9 (8.3%) | 0.40 |
| • Hypertension | 28 (23.3%) | 28 (25.9%) | 0.65 |
| • Liver Disease | 1 (0.8%) | 0 (0%) | 1.0 |
| • Morbid Obesity | 1 (0.8%) | 3 (2.8%) | 0.35 |
Table 2 shows differences in breast reconstruction characteristics between the medial and lateral branch patients. DIEP and MS FTRAM flaps were similarly distributed (p=0.45), but flap designs differed significantly between the medial and lateral branch groups (p=0.012).
Table 2. Reconstruction Characteristics.
| Medial Branch N=120 |
Lateral Branch N=108 |
P-value | |
|---|---|---|---|
| Immediate Reconstruction | 80 (66.7%) | 68 (63%) | 0.56 |
| Number of Perforators Harvested | |||
| • 1 | 32 (28.3%) | 17 (17.2%) | 0.03 |
| • 2 | 47 (41.6%) | 36 (36.4%) | |
| • ≥ 3 | 34 (30.1%) | 46 (46.5%) | |
| Flap Type | |||
| • DIEP | 80 (66.7%) | 77 (71.3%) | 0.45 |
| • MS FTRAM | 40 (33.3%) | 31 (28.7%) | |
| Recipient Vessels | |||
| • Internal Mammary | 114 (95%) | 98 (90.7%) | 0.21 |
| • Thoracodorsal | 6 (5%) | 10 (9.3%) | |
| Flap Design | |||
| • Hemiflap | 12 (10%) | 23 (21.3%) | 0.012 |
| • Cross-Midline Flap | 99 (82.5%) | 83 (76.9%) | |
| • Total Flap | 9 (7.5%) | 2 (1.9%) |
DIEP, Deep Inferior Epigastric Perforator Flap; MS FTRAM, Muscle-Sparing Free Transverse Rectus Abdominis Musculocutaneous Flap.
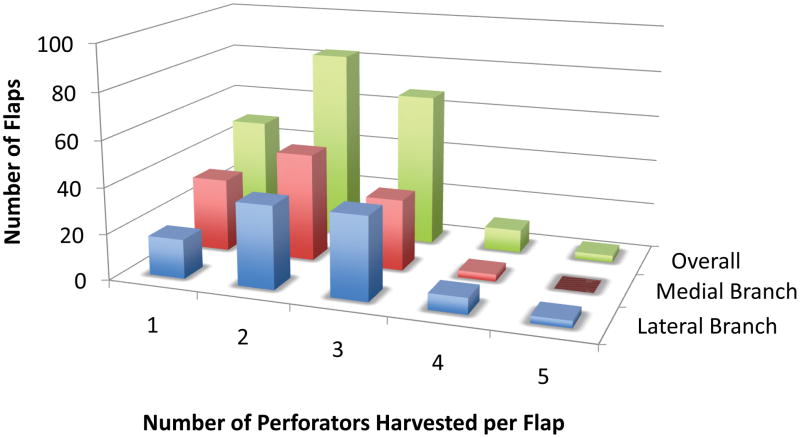
The distribution of the number of perforators included with the flap harvest (1 vs. 2 vs. ≥3) differed significantly between the medial and lateral branch groups (p=0.03) (Figure 3). More of the overall medial branch flaps were harvested on 1 perforator (28.3% vs. 17.2%) and fewer on ≥3 perforators (30.1% vs. 46.5%) compared to the lateral branch flaps. The distribution of the number of perforators included with the flap harvest (1 vs. 2 vs. ≥3) was not statistically different for the Hemiflap and Total Flap groups (p=0.72 and p=0.27, respectively).
Figure 3.
A comparison of the number of perforators included per lateral branch flap and medial branch flap and overall.
A comparison of outcomes for the combined, overall flap design classifications (i.e. Hemiflaps, Cross-Midline Flaps, and Total Flaps) demonstrated no differences in recipient site complications between the medial and lateral branch groups (Table 3). Unicovariate analysis demonstrated similar rates of fat necrosis in the medial (8.33%) and lateral (12.96%) DIEA branch flaps (p=0.26). There were also similar rates of partial flap necrosis between medial (3.3%) and lateral (2.78%) DIEA branch flaps (p=1.0). Multicovariate logistic regression analysis demonstrated no significant independent associations between 1) the presence of any patient co-morbidity or 2) lateral vs. medial perforator harvest and the development of fat necrosis. Unicovariate analysis showed no significant association between the numbers of perforators harvested and the development of fat necrosis in all flaps included in this study (p=0.92). We further attempted to control for the confounding affect of multiple perforators by performing direct comparisons between flaps perfused on the same number of perforators (e.g. one perforator medial branch DIEP flaps vs. 1 perforator lateral branch DIEP flaps). Direct comparison between 1, 2, or ≥3 perforator medial flaps to 1, 2, or ≥3 perforator lateral flaps and 1, 2, or ≥3 perforator DIEP flaps to 1, 2, or ≥3 perforator MS FTRAM flaps also demonstrated no differences in perfusion related complications between the comparison groups (data not shown).
Table 3. Postoperative Outcomes.
| Medial Branch N=120 |
Lateral Branch N=108 |
p-value | |
|---|---|---|---|
| Fat Necrosis | 10 (8.33%) | 14 (12.96%) | 0.26 |
| Partial Flap Necrosis | 4 (3.3%) | 3 (2.78%) | 1.0 |
| Fat Necrosis / Partial Flap Necrosis | 14 (11.7%) | 17 (15.7%) | 0.37 |
| Inpatient Hospital Days, mean | 4.7 ± 1.0 | 4.9 ± 1.4 | 0.37 |
| Follow-Up, mean (Months) | 28.2 ± 24.2 | 33.2 ± 26.1 | 0.09 |
Flap Characteristics and Outcomes by Perfusion Zones
Surgical outcomes based on flap design classification are shown in Table 4. Patient characteristics were similar among the three flap design classifications, with the exception of the Total Flap group having a lower age (p<0.0001), lower BMI (p=0.003), and fewer medical co-morbidities than the other two groups (p=0.032). Rates of fat necrosis and partial flap necrosis were not statistically different among the three groups (Table 4). Table 5 demonstrates the characteristics and outcomes for the Cross-Midline Flap, the most commonly used flap in the study. The distribution of the number of perforators included with the flap harvest (1 vs. 2 vs. ≥3) differed significantly between the Cross-Midline medial and lateral branch groups (p=0.017). More of the Cross-Midline medial branch flaps were harvested on 1 perforator (28.0% vs. 14.5%) and fewer on ≥3 perforators (28.0% vs. 47.4%) compared to the lateral branch flaps. The fat necrosis rates for the medial and lateral branch Cross-Midline Flap patients were similar (9.1% and 14.5%, respectively; p=0.26). The partial flap necrosis rates were also similar between medial (4.0%) and lateral (3.6%) branch Cross-Midline Flap patients (p=1.0).
Table 4. Postoperative Outcomes by Flap Design.
| Hemiflaps N=35 |
Cross-Midline Flaps N=182 |
Total Flaps N=11 |
p-value | |
|---|---|---|---|---|
| Fat Necrosis | 3 (8.6%) | 21 (11.5%) | 0 (0%) | 0.70 |
| Partial Flap Necrosis | 0 (0%) | 7 (3.8%) | 0 (0%) | 0.72 |
| Fat Necrosis / Partial Flap Necrosis | 3 (8.6%) | 28 (15.4%) | 0 (0%) | 0.32 |
| Inpatient Hospital Days, mean | 4.8 ± 1.3 | 4.8 ± 1.2 | 5.0 ± 0.8 | 0.36 |
| Follow-Up, mean (Months) | 28.9 ± 25.7 | 31.0 ± 24.9 | 29.4 ± 30.8 | 0.66 |
Table 5. Cross-Midline Flap Patient and Reconstruction Characteristics and Surgical Outcomes.
| Medial Branch N=99 |
Lateral Branch N=83 |
p-value | |
|---|---|---|---|
| Body Mass Index, mean (mg/kg2) | 26.5 ± 4.9 | 27.1 ± 6.6 | 0.97 |
| Age, mean (years) | 50.7 ± 9.0 | 49.7 ± 8.5 | 0.54 |
| Any Medical Co-morbidity | 37 (37.4%) | 31 (37.3%) | 1.0 |
| Number of Perforators | |||
| • 1 | 26 (28.0%) | 11 (14.5%) | 0.017 |
| • 2 | 41 (44.1%) | 29 (38.2%) | |
| • ≥3 | 26 (28.0%) | 36 (47.4%) | |
| Flap Type | |||
| • DIEP | 69 (69.7%) | 58 (69.9%) | 0.98 |
| • MS FTRAM | 30 (30.3%) | 25 (30.1%) | |
| Complications | |||
| • Fat Necrosis | 9 (9.1%) | 12 (14.5%) | 0.26 |
| • Partial Flap Necrosis | 4 (4.0%) | 3 (3.6%) | 1.0 |
| • Fat Necrosis / Partial Flap Necrosis | 13 (13.1%) | 15 (18.1%) | 0.36 |
| • Infection | 4 (4%) | 2 (2.4%) | 0.69 |
| • Hematoma/Seroma | 4 (4%) | 7 (8.4%) | 0.23 |
| • Anastomotic Thrombosis | 2 (2%) | 0 (0%) | 0.50 |
| • Any Complication | 21 (21.2%) | 19 (22.9%) | 0.79 |
DIEP, Deep Inferior Epigastric Perforator Flap; MS FTRAM, Muscle-Sparing Free Transverse Rectus Abdominis Musculocutaneous Flap.
In the Cross-Midline Flap group, multicovariate logistic regression analysis of lateral vs. medial harvest and the presence of any comorbidity showed a significant association between the presence of any comorbidity and the development of fat necrosis or partial flap necrosis (OR 2.63; 95% CI 1.16-5.98; p=0.021). No significant association was identified between medial vs. lateral perforator harvest and the development of fat necrosis, partial flap necrosis, fat necrosis/partial flap necrosis, or any individual complication. There was also no significant difference in fat necrosis rates or partial flap necrosis rates when medial and lateral branch groups were compared within the Hemiflap and Total Flap groups (data not shown).
DIEP vs. MS FTRAM Patient Characteristics and Flap Outcomes
All patient characteristics and co-morbidities were similar between the DIEP and MS FTRAM patients, with the following exceptions: more of the DIEP patients received preoperative chemotherapy (p=0.0016) and more of the MS FTRAM patients received postoperative radiotherapy (p=0.045). Table 6 demonstrates that the number of perforators (1 vs. 2 vs. ≥3) differed between the MS FTRAM and DIEP flaps (p=0.0001). More of the MS FTRAM flaps had ≥3 perforators (61.8%) compared to the DIEP flaps (29.3%). There were no significant differences in fat necrosis (p=0.81) or partial flap necrosis (p=1.0) rates between the DIEP (10.2%, 3.2%) and MS FTRAM (11.3%, 2.8%) flaps, respectively.
Table 6. DIEP vs. MS FTRAM Patient and Reconstruction Characteristics and Surgical Outcomes.
| DIEP N=157 |
MS FTRAM N=71 |
p-value | |
|---|---|---|---|
| Body Mass Index, mean (mg/kg2) | 26.9 ± 5.5 | 27.5 ± 6.2 | 0.55 |
| Age, mean (years) | 50.9 ± 9.3 | 50.4 ± 8.8 | 0.59 |
| Any Medical Co-morbidity | 62 (39.5%) | 25 (35.2%) | 0.54 |
| Number of Perforators | |||
| • 1 | 44 (28.0%) | 5 (9.1%) | 0.0001 |
| • 2 | 67 (42.7%) | 16 (29.1%) | |
| • ≥3 | 46 (29.3%) | 34 (61.8%) | |
| Harvest Type | |||
| • Medial DIEA Branch | 80 (51.0%) | 40 (56.3%) | 0.45 |
| • Lateral DIEA Branch | 77 (49.0%) | 31 (43.7%) | |
| Complications | |||
| • Fat Necrosis | 16 (10.2%) | 8 (11.3%) | 0.81 |
| • Partial Flap Necrosis | 5 (3.2%) | 2 (2.8%) | 1.0 |
| • Fat Necrosis / Partial Flap Necrosis | 21 (13.4%) | 10 (14.1%) | 0.89 |
| • Any Complication | 31 (19.7%) | 14 (19.7%) | 1.0 |
DIEP, Deep Inferior Epigastric Perforator Flap; MS FTRAM, Muscle-Sparing Free Transverse Rectus Abdominis Musculocutaneous Flap; DIEA, Deep Inferior Epigastric Artery.
Overall Predictors of Fat Necrosis and Partial Flap Necrosis
We assessed whether any patient or reconstructive characteristic was independently predictive of or protective for the development of fat necrosis or partial flap necrosis. Univariate logistic regression analysis did not demonstrate any individual comorbid medical condition to be predictive of complications but did identify that patients with at least one co-morbidity had a significantly greater risk of developing either fat necrosis or partial flap necrosis compared to those without any co-morbidities (20.7% vs. 9.2%; p=0.014). Multivariate logistic regression analysis that included 1) any comorbidity and 2) lateral vs. medial DIEA harvest demonstrated that the presence of at least one pre-existing co-morbid medical condition was the only independent risk factor predictive of fat necrosis/partial flap necrosis (OR 2.52; 95% CI 1.16 – 5.47; p=0.019).
Discussion
We found that DIEP and MS FTRAM flaps harvested on medial and lateral DIEA branch perforators had similar rates of the perfusion-related complications fat necrosis and partial flap necrosis. This was true for all flaps in the cohort combined and also when flaps were stratified on the basis of flap type and design classification. To our knowledge, this study is the first reported patient series comparing fat necrosis and partial flap necrosis rates between strict medial- or lateral-only DIEA branch harvest groups. The only characteristic that consistently predicted the development of postoperative perfusion-related complications in this meticulously controlled group of patients was the presence of any co-morbid medical condition.
This is the first clinical study to quantify the clinical relevance of the perforasome variances of the medial and lateral DIEA branches with respect to actual patient outcomes. We anticipated significantly higher rates of fat necrosis and partial flap necrosis in flaps perfused by lateral DIEA branch perforators in comparison to medial DIEA branch perforators, particularly for flaps that included tissue across the midline. However, our results were contrary to our hypothesis.
We did find a difference in the numbers of perforators included with the medial and lateral DIEA branch flaps, a finding that could explain the similarities in perfusion-related complication rates between these two groups. A recent study by Baumann and co-workers demonstrated the importance of perforator number in the development of fat necrosis.(22) The study did not specify from which DIEA branch the perforators were harvested but was specifically designed to test the effects of perforator number by dividing the flaps into 4 groups: 1) SIEA flaps, 2) one to two perforators, 3) three to five perforators, and 4) greater than five perforators. Although we saw a difference in our study between the distribution of 1, 2, or ≥3 perforators, few of the flaps in our study, irrespective of branch harvest classification, were harvested on more than 3 perforators (Figure 3). This observation is likely owing to our exclusion of flaps that included perforators from both the medial and lateral branches of the DIEA, as a single branch of the DIEA rarely has >3 perforators.(36) Irrespective of the differences in perforator distribution between flap classifications, we saw no differences in perfusion-related complications both with regression analysis and with direct comparison of 1, 2, or ≥3 perforator medial flaps vs. 1, 2, or ≥3 perforator lateral flaps and 1, 2, or ≥3 perforator DIEP flaps vs. 1, 2, or ≥3 perforator MS FTRAM flaps.
It is unclear why the surgeons in our study chose to include more perforators with the lateral branch flaps. One possibility is that this was a strategy to potentially increase perfusion across the midline. However, the more likely possibility is that our surgeons tended to harvest multiple perforators in a longitudinal row. The differences in lateral and medial flap perforator number likely reflect an inherent anatomic difference between the medial and lateral DIEA branches. Although DIEA perforators originate from the lateral branch only 34% of the time, the lateral DIEA branch takes a vertical course through the rectus abdominis complex 79.2% of the time.(36) This vertical orientation allows the rectus abdominis muscle to be split longitudinally so that more same-branch perforators may be included with a lateral branch flap, with minimal muscle damage. In contradistinction, the medial DIEA branch takes an oblique course through more than one intermuscular septum 81.8% of the time.
The longitudinal vs. oblique course of the DIEA branch likely explains why more perforators were included in the MS FTRAM patients. When inclusion of more perforators was felt appropriate in an obliquely oriented, medial DIEA branch, our surgeons may have been more likely to harvest the intervening muscle between perforators as an MS FTRAM flap rather than dissect the muscle from the perforators to create a DIEP flap. Although the observed differences in perforator numbers between the medial vs. lateral DIEA branch groups may explain the observed similarities in perfusion-related complications in our patients, we were not able to demonstrate an association between perforator number and perfusion-related complications among the specific comparative groups in our study.
Another important finding in this study is the effect of DIEP vs. MS FTRAM technique. Prior studies have questioned the durability of perfusion with the DIEP technique in comparison to TRAM.(7, 18, 23, 35, 38) We found the rates of perfusion-related flap morbidity between the DIEP and MS FTRAM flaps to be almost identical in this series of flaps harvested exclusively on perforators from a single DIEA branch. The inclusion or exclusion of rectus abdominis muscle in this highly selected, homogenous group of patients appeared to have had no effect on the development of fat necrosis or partial flap necrosis.
The strengths of this study include the large experience with free flap breast reconstruction by multiple surgeons using similar techniques at a single center, careful study design to isolate and compare perfusion-related flap morbidity between strict medial- or lateral-only branch harvest groups, data obtained from a prospectively entered patient database, and unicovariate and multicovariate regression analyses. Limitations of this study include its retrospective design, imprecise nature of evaluations of fat necrosis and partial flap necrosis, potential variability in the amount of contralateral tissue or Zone 3 sub-Scarpal fat transferred, and potential surgeon bias to harvest a greater number of perforators for larger flaps, lateral DIEA branch flaps, or MS FTRAM flaps.
On the basis of the results of this study we suggest that medial row perforators not be specifically selected on the basis of anticipated reduced flap morbidity. To control for surgeon bias, it is reasonable to hypothesize that a prospective study arbitrarily assigning patients into medial or lateral perforator groups irrespective of the intraoperative findings might demonstrate greater perfusion-related complications among the lateral branch groups. However, unless such a study clearly demonstrates fewer perfusion-related complications among medial branch flaps, we suggest that surgeons not overestimate the clinical relevance of the anatomic variances in medial versus lateral DIEA branch perforasomes when harvesting abdominally based flaps. The selection of perforators should take into account the perforator size, perfusion quality, and DIEA branch orientation. Knowledge of the similar rates of perfusion-related complications for flaps raised on medial or lateral DIEA branch perforators should enable surgeons to make more informed intraoperative decisions when harvesting DIEP and MS FTRAM flaps for breast reconstruction.
Acknowledgments
The authors wish to recognize former and current members of the Department of Plastic Surgery at The University of Texas MD Anderson Cancer Center for their support and/or contribution of patients to this series, Drs. Donald P. Baumann, Elisabeth K. Beahm, David W. Chang, Melissa A. Crosby, Matthew M. Hanasono, Steven J. Kronowitz, Scott D. Oates, Gregory P. Reece, Geoffrey L. Robb, Jesse C. Selber, Roman Skoracki, Mark T. Villa, and Peirong Yu and former colleagues Drs. Bonnie J. Baldwin, Pierre M. Chevray, Mennen T. Gallas, Lior Heller, Stephen S. Kroll, Howard N. Langstein, Michael J. Miller, and Justin M. Sacks. The authors also thank Dawn Chalaire from The University of Texas MD Anderson Cancer Center, Department of Scientific Publications for assistance with scientific editing. Lastly, the authors would like to acknowledge the hard work and dedication of our fellows and residents who helped with these cases.
Financial Support: This research is supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672.
Footnotes
Financial Disclosure: None of the authors has a financial interest associated with this publication.
Products Mentioned: There were no products mentioned in this manuscript.
References
- 1.Schusterman MA, Kroll SS, Weldon ME. Immediate breast reconstruction: Why the free TRAM over the conventional TRAM flap? Plast Reconstr Surg. 1992;90:255–261. [PubMed] [Google Scholar]
- 2.Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32:32–38. doi: 10.1097/00000637-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Schusterman MA, Kroll SS, Miller MJ, et al. The free transverse rectus abdominis musculocutaneous flap for breast reconstruction: One center's experience with 211 consecutive cases. Ann Plast Surg. 1994;32:234–241. doi: 10.1097/00000637-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Kroll SS, Schusterman MA, Reece GP, Miller MJ, Robb G, Evans G. Abdominal wall strength, bulging, and hernia after TRAM flap breast reconstruction. Plast Reconstr Surg. 1995;96:616–619. doi: 10.1097/00006534-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Blondeel PN, Vanderstraeten GG, Monstrey SJ, et al. The donor morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br J Plast Surg. 1997;50:322–330. doi: 10.1016/s0007-1226(97)90540-3. [DOI] [PubMed] [Google Scholar]
- 6.Futter CM, Webster MHC, Hagen S, Mitchell SL. A retrospective comparison of abdominal muscle strength following breast reconstruction with a free TRAM or DIEP flap. Br J Plast Surg. 2000;53:578–583. doi: 10.1054/bjps.2000.3427. [DOI] [PubMed] [Google Scholar]
- 7.Kroll SS. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106:576–583. doi: 10.1097/00006534-200009030-00008. [DOI] [PubMed] [Google Scholar]
- 8.Moran SL, Serletti JM. Outcome comparison betwee free and pedicled TRAM flap breast reconstruction in the obese patient. Plast Reconstr Surg. 2001;108:1954–1960. doi: 10.1097/00006534-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Nahabedian MY, Dooley W, Singh N, Manson PN. Contour abnormalities of the abdomen after breast reconstruction with abdominal flaps: The role of muscle preservation. Plast Reconstr Surg. 2002;109:91–102. doi: 10.1097/00006534-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Allen RJ. DIEP versus TRAM for breast reconstruction. Plast Reconstr Surg. 2003;111:2478. doi: 10.1097/01.PRS.0000063118.22954.20. [DOI] [PubMed] [Google Scholar]
- 11.Bottero L, Lefaucheur JP, Fadhul S, Raulo Y, Collins ED, Lantieri L. Electromyographic assessment of rectus abdominis muscle function after deep inferior epigastric perforator flap surgery. Plast Reconstr Surg. 2004;113:156–162. doi: 10.1097/01.PRS.0000095941.86060.8E. [DOI] [PubMed] [Google Scholar]
- 12.Gill PS, Hunt JP, Guerra AB, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg. 2004;113:1153–1161. doi: 10.1097/01.prs.0000110328.47206.50. [DOI] [PubMed] [Google Scholar]
- 13.Garvey PB, Buchel EW, Pocakj BA, Gray RJ, Samson TD. The deep inferior epigastric perforator flap for breast reconstruction in overweight and obese patients. Plast Reconstr Surg. 2005;115:447–457. doi: 10.1097/01.prs.0000149588.09148.53. [DOI] [PubMed] [Google Scholar]
- 14.Nahabedian MY, Tsangaris T, Momen B. Breast reconstruction with the DIEP flap or the muscle-sparing (MS-2) free TRAM flap: Is there a difference? Plast Reconstr Surg. 2005;115:436–445. doi: 10.1097/01.prs.0000149404.57087.8e. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj AK, Chevray PM, Chang DW. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg. 2006;117:737–747. doi: 10.1097/01.prs.0000200062.97265.fb. [DOI] [PubMed] [Google Scholar]
- 16.Bonde CT, Christensen DE, Elberg JJ. Ten years' experience of free flaps for breast reconstruction in a Danish microsurgical centre: an audit. Scand J Plast Reconstr Surg Hand Surg. 2006;40:8–12. doi: 10.1080/02844310500296016. [DOI] [PubMed] [Google Scholar]
- 17.Garvey PB, Buchel EW, Pockaj BA, et al. DIEP and pedicled TRAM flaps: A comparison of outcomes. Plast Reconstr Surg. 2006;117:1711–1720. doi: 10.1097/01.prs.0000210679.77449.7d. [DOI] [PubMed] [Google Scholar]
- 18.Scheer AS, Novak CB, Neligan PC, Lipa JE. Complications associated with breast reconstruction using a perforator flap compared with a free TRAM flap. Ann Plast Surg. 2006;56:355–358. doi: 10.1097/01.sap.0000201549.83738.42. [DOI] [PubMed] [Google Scholar]
- 19.Vega S, Smartt JM, Jiang S, et al. 500 Consecutive patients with free TRAM flap breast reconstruction: a single surgeon's experience. Plast Reconstr Surg. 2008;122:329–339. doi: 10.1097/PRS.0b013e31817f45cb. [DOI] [PubMed] [Google Scholar]
- 20.Vyas RM, Dickinson BP, Fastekjian JH, Watson JP, Dalio AL, Crisera CA. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg. 2008;121:1519–1526. doi: 10.1097/PRS.0b013e31816b1458. [DOI] [PubMed] [Google Scholar]
- 21.Man LX, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg. 2009;124:752–764. doi: 10.1097/PRS.0b013e31818b7533. [DOI] [PubMed] [Google Scholar]
- 22.Baumann DB, Lin HY, Chevray PM. Perforator number predicts fat necrosis in a prospective analysis of breast reconstruction with free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg. 2010;125:1335–1342. doi: 10.1097/PRS.0b013e3181d4fb4a. [DOI] [PubMed] [Google Scholar]
- 23.Chun YS, Sinha I, Turko A, et al. Comparison of morbidity, functional outcome, and satisfaction following bilateral TRAM versus bilateral DIEP flap breast reconstruction. Plast Reconstr Surg. 2010;126:1133–1142. doi: 10.1097/PRS.0b013e3181ea42d3. [DOI] [PubMed] [Google Scholar]
- 24.Selber JC, Serletti JM. The deep inferior epigastric perforator flap: myth and reality. Plast Reconstr Surg. 2010;125:50–58. doi: 10.1097/PRS.0b013e3181c49770. [DOI] [PubMed] [Google Scholar]
- 25.Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69:216–225. doi: 10.1097/00006534-198202000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Holm C, Mayr M, Hofter E, Ninkovic M. Perfusion zones of the DIEP flap revisited: A clinical study. Plast Reconstr Surg. 2006;117:37–44. doi: 10.1097/01.prs.0000185867.84172.c0. [DOI] [PubMed] [Google Scholar]
- 27.Holm C, Tegeler J, Mayr M, Becker A, Pfeiffer UJ, Muhlbauer W. Monitoring free flaps using laser-induced fluorescence of indocyanine green: A preliminary experience. Microsurgery. 2002;22:278–287. doi: 10.1002/micr.10052. [DOI] [PubMed] [Google Scholar]
- 28.Wong C, Saint-Cyr M, Arbique G, et al. Three- and four-dimensional computed tomographic studies of commonly used abdominal flaps in breast reconstruction. Plast Reconstr Surg. 2009;124:18–28. doi: 10.1097/PRS.0b013e3181aa0db8. [DOI] [PubMed] [Google Scholar]
- 29.Wong C, Saint-Cyr M, Mojallal A, et al. Perforasomes of the DIEP flap: Vascular anatomy of the lateral versus medial row perforators and clinical implications. Plast Reconstr Surg. 2010;125:772–783. doi: 10.1097/PRS.0b013e3181cb63e0. [DOI] [PubMed] [Google Scholar]
- 30.Boyd JB, Taylor GI, Corlett R. The vascular territories of the superior epigastric and the deep inferior epigastric systems. Plast Reconstr Surg. 1984;73:1–16. doi: 10.1097/00006534-198401000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Saint-Cyr M, Wong C, Schaverien M, Mojallal A, Rohrich RJ. The perforasome therory: Vascular anatomy and clinical implications. Plast Reconstr Surg. 2009;124:1529–1544. doi: 10.1097/PRS.0b013e3181b98a6c. [DOI] [PubMed] [Google Scholar]
- 32.Rozen WM, Palmer KP, Suami H, et al. The DIEA branching pattern and its relationship to perforators: the importance of preoperative computed tomographic angiography for DIEA perforator flaps. Plast Reconstr Surg. 2008;121:367–374. doi: 10.1097/01.prs.0000298313.28983.f4. [DOI] [PubMed] [Google Scholar]
- 33.Rahmanian-Schwarz A, Rothenberger J, Hirt B, Luz O, Schaller HE. A combined anatomical and clinical study for quantitative analysis of the microcirculation in the classic perfusion zones of the deep inferior epigastric artery perforator flap. Plast Reconstr Surg. 2011;127:505–513. doi: 10.1097/PRS.0b013e3181fed543. [DOI] [PubMed] [Google Scholar]
- 34.Lindsey J. Integrating the DIEP and muscle-sparing (MS-2) free TRAM techniques optimizes surgical outcomes: presentation of an algorithm for microsurgical breast reconstruction based on perforator anatomy. Plast Reconstr Surg. 2007;119:18–27. doi: 10.1097/01.prs.0000244743.90178.89. [DOI] [PubMed] [Google Scholar]
- 35.Nahabedian MY, Momen B, Galdino G, Manson PN. Breast reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110:466–475. doi: 10.1097/00006534-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Munhoz AM, Ishida LH, Sturtz GP, et al. Importance of lateral row perforator vessels in deep inferior epigastric perforator flap harvesting. Plast Reconstr Surg. 2004;113:517–525. doi: 10.1097/01.PRS.0000100812.37842.A8. [DOI] [PubMed] [Google Scholar]
- 37.Van Belle G. Biostatistics: a methodology for the health sciences. 2nd. Hoboken: Wiley-Interscience; 2004. [Google Scholar]
- 38.Blondeed PN, Arnstein M, Verstraete K, et al. Venous congestion and blood flow in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106:1295–1299. doi: 10.1097/00006534-200011000-00009. [DOI] [PubMed] [Google Scholar]