Abstract
Induction therapy is used in kidney transplantation to inhibit the activation of donor reactive T cells which are detrimental to transplant outcomes. Choice of induction therapy is decided based on perceived immunological risk rather than by direct measurement of donor T cell reactivity. We hypothesized that immune cellular alloreactivity pre-transplantation can be quantified and that blocking versus deleting therapies have differential effects on the level of donor and third party cellular alloreactivity. We studied 31 kidney transplant recipients treated with either anti-thymocyte globulin (ATG) or an IL-2 receptor blocker. We tested pre- and post-transplant peripheral blood cells by flow cytometry to characterize T cell populations and by IFN-γ ELISPOT assays to assess the level of cellular alloreactivity. CD8+ T cells were more resistant to depletion by ATG than CD4+ T cells. Post-transplantation, frequencies of donor reactive T cells were markedly decreased in the ATG-treated group but not in the IL-2 receptor blocker group, whereas the frequencies of third party alloreactivity remained nearly equivalent. In conclusion, when ATG is used, marked and prolonged donor hyporesponsiveness with minimal effects on non-donor responses is observed. In contrast, induction with the IL-2 receptor blocker is less effective at diminishing donor T cell reactivity.
Keywords: T cell, ELISPOT, donor immunity, induction, thymoglobulin
Introduction
Immune mediated graft injury remains an unresolved problem in human transplantation (1, 2), undermining graft function and promoting the development of graft fibrosis. Alloreactive effector memory T cells are central players in mediating this injury (3–5) and allogeneic (donor and non-donor) reactive memory T cells that exist prior to transplantation are associated with adverse post-transplant outcomes in humans (6–9). When compared with naïve T cells, effector memory T cells mount an accelerated and heightened immune response (10) and are the basis for the use of anti-T cell induction therapy in kidney transplantation. Whereas induction therapy is primarily aimed at preventing the activity of pre-existent donor-reactive T cells, it is also important that donor-reactive T cells remain absent or quiescent following transplantation over time.
The choice of induction immunosuppression in kidney transplantation incorporates consideration of “perceived” immunological risk for cellular rejection by the physician rather than a direct measure of cellular alloreactivity. This perceived immunological risk derives from demographic and clinical data along with assessment of preformed alloantibodies, and assumes that humoral allosensitization is associated with cellular allosensitization. In patients considered to be at high immunological risk, anti-thymocyte globulin (ATG) has superior clinical efficacy when compared with IL-2 receptor blockers (11), although use of the latter may be sufficient to prevent clinical rejection in low risk patients (12). It is possible that knowledge of levels of donor and non-donor reactive T cell immunity at the time of transplantation could facilitate better risk stratification of patients, and therefore, influence the selection of induction therapy. In this regard, whether the immunological risk as perceived by the physician is actually associated with increased T cell reactivity to donor and or third party antigens has not been tested. Ideally, depleting anti-T cell immunosuppressive therapies should be directed at transplant recipients in whom high levels of donor-reactive T cell immunity exists so as to minimize graft injury. At the same time, any therapeutic intervention should preferentially spare non-donor immune reactivity so as to maximize responses to infections and cancers.
In this study we first investigated whether pre-existing cellular alloreactivity correlates with the current approach to immunological risk assessment which eventually dictates the type of induction therapy. We then studied whether induction therapy with rabbit ATG (Thymoglobulin®, Genzyme Corporation) or with an IL-2 receptor blocker, basiliximab (Simulect®, Novartis) has similar or differential effects on donor-reactive T cells, non-donor cellular reactivity and non-allogeneic viral cellular reactivity as measured by the IFN-γ ELISPOT assay. Furthermore, we studied the kinetics and phenotype of the cellular alloresponse over time providing insight into the mechanisms of action of these drugs. We found that pre-existing donor reactivity in the circulating T cell pool was mostly eliminated in subjects treated with ATG but minimally suppressed in the IL-2 receptor blocker treated patients. In contrast, both treatments had minimal effects on non-donor alloreactivity and influenza-reactive T cells. We also showed that current clinical strategies to risk stratify patients are not accurate in segregating transplant candidates on the basis of levels of pre-existing cellular donor-reactivity, thus justifying a need for further refinement of pre-transplant T cell immune monitoring assays to contribute to this decision-making process.
Materials and Methods
We prospectively enrolled 31 kidney transplant recipients of either a living donor or deceased donor between November of 2007 and December 2008 in an observational study. The study was approved by the Cleveland Clinic Institutional Review Board (IRB # 08-549) and subjects provided informed consent prior to participation in the study.
Study population
The studied cohort was divided into two groups based on the induction therapy received: 1) ATG-treated patients (n=14); and, 2) IL-2 receptor blocker (basiliximab)-treated patients (n=17). Demographic and clinical data pertaining to assessment of immunological risk such as donor source, HLA mismatching, panel reactive antibody (PRA), history of sensitizing events and dialysis vintage was collected. Outcomes including acute rejection, infections, and graft function are also presented. All patients received tacrolimus, mycophenolic acid and steroid therapy per protocol. The decision to use either induction regimen was at the discretion of the treating physician and it was based on their perception of risk derived from clinical and laboratory data such as PRA and HLA mismatching. Treatment was not influenced at any time point by the findings of this observational study.
Kidney biopsies were performed mostly for apparent graft dysfunction in 14 out of 31 transplant recipients. One patient (basiliximab group) had biopsy-proven acute cellular rejection (Banff grade 2A) at 5 months post-transplantation and required treatment with ATG. Other diagnoses included various degrees of IF/TA, two showed early evidence of transplant glomerulopathy and one showed BK nephropathy.
ELISPOT assays
Blood samples were obtained prior to transplantation and administration of immunosuppressive therapy, and then at 1, 3 and 6 months post-transplantation. Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll separation and stored in liquid nitrogen. PBMCs were used for ELISPOT assays and flow cytometric studies. Serum was also stored at −80 C for alloantibody detection. Three separate groups of stimulators were used in ELISPOT experiments: donor B cells, third-party B cells, and influenza antigen. Donor stimulator cells were obtained from spleen or lymph nodes from deceased donors or peripheral blood from the living donors. Donor B cells were isolated by magnetic separation, using the Human B cell Enrichment Kit (EasySep, StemCell Technologies, Inc, Vancouver, BC, Canada), and then were grown on a layer of irradiated CD40L-transfected NIH-3T3 fibroblasts in Iscove’s MDM containing 10% heat-inactivated human AB serum, 50 ug/mL human transferrin, 5 ug/mL human insulin, 1% penicillin-streptomycin, and 8 ng/mL human recombinant IL-4(13). Five B cell lines were used as allostimulators to test for non-donor (third party) cellular alloreactivity (9). These B cells were grown using the same procedure used to grow the donor B cells. For the ELISPOT assays, 1×105 B cells per well were used as stimulators. IFN-γ ELISPOT assays were performed as previously described (6, 9, 14). The resulting spots were counted with an Immunospot computer-assisted image analyzer (Cellular Technology, Cleveland, OH). Results were depicted as the mean number of IFN-γ spots per 2×105 recipient PBMCs based on triplicate measurements. Because of the differential lymphopenic effects of each induction therapy used, we then adjusted the IFN-γ producing PBMCs to the percent of T cells in each sample at a particular time point by controlling for CD4+ and CD8+ as measured by flow cytometry. As a non-allogeneic control we also tested transplant recipient PBMCs against influenza antigen (15), to which most transplant candidates would have had prior exposure either through infection or vaccination. Control wells using media was used to assess background cytokine production, and detected spots were subtracted from the total number of spots in wells in which responders and stimulators were mixed. PHA stimulation was used as a positive control.
Flow cytometry
PBMCs were analyzed using flow cytometry for the presence of T cell subtypes. Approximately 5 × 105 PBMCs were stained with anti-human CD4-APC-Cy7 or CD4-PE-Cy5 (both from BD Pharmingen, San Jose, CA), CD8-APC-Cy7 or CD8-PE-Cy5 (both from BD Pharmingen, San Jose, CA), CD45RA-PerCP-Cy5.5 (eBioscience, San Diego, CA) or CD45RA-FITC (BD Pharmingen, San Jose, CA), CD45RO-PerCP-Cy5.5 (BioLegend, San Diego, CA) or CD45RO-PE (BD Pharmingen, San Jose, CA), and CD62L-APC (BD Pharmingen, San Jose, CA). The cells were FC blocked for 20 minutes and then incubated with specific antibody for 30 minutes on ice. After incubation, the cells were washed and read on a BD LSR II or BD FACalibur depending on the antibody used. Isotype controls were used to determine background fluorescence. Frequencies of the following CD4+ and CD8+ T cell subpopulations were determined based on the staining: 1) naïve T cells or TN: CD45RA+, CD62L+, 2) effector memory T cells or TEM: CD45RO+, CD62L−, and 3) central memory T cells or TCM: CD45RO+, CD62L+.
Alloantibody detection
Pre-transplantation PRA was determined using virtual PRA calculations derived from positive antibody binding in the LabScreen Luminex single antigen bead assay (One Lambda, Canoga Park, CA). PRA less than 10% was used to classify patients as non-sensitized. In order to detect de novo donor specific antibodies (DSA) or non-DSA alloantibodies following transplantation, pre-transplant and 6 to 12 month post-transplant sera were tested by Luminex using single antigen beads.
Statistical analysis
Values are shown as mean ± SEM, or percentages. Data was also converted to the logarithmic scale for representation in figures and data analysis with non-parametric tests. Baseline demographic data between patient groups were analyzed using t test for continuous variables and Pearson chi-square test for dichotomous variables. Wilcoxon rank test was used when the continuous data was not normally distributed and matched paired test was used to compare changes over time from baseline (post-transplant to pre-transplant values). Two-sided p values less than 0.05 were considered to indicate statistical significance. All analyses were performed using JMP version 8 (SAS, Carey, NC).
Results
Clinical immunological risk does not necessarily translate into cellular allosensitization
Patients in the ATG- and IL-2 receptor blocker treated groups were comparable with regard to demographic and clinical characteristics (Table 1). Although not statistically different, ATG-treated subjects were more commonly younger females, and had prior allosensitization events such as pregnancies and previous transplants.
Table 1.
Patient characteristics
| IL-2 RB-treated n=17 |
ATG-treated n=14 |
p value | |
|---|---|---|---|
| Recipient age (years old) | 52±10 | 45±13 | 0.09 |
| Female gender | 6/17 (35.3) | 8/14 (57.1) | 0.22 |
| Caucasian race | 11/17 (64.7) | 11/14 (78.6) | 0.40 |
| Prior pregnancies | 2/6 (33.3) | 4/8 (50.0) | 0.63 |
| Re-transplantation | 2/17 (11.8) | 3/14 (21.4) | 0.47 |
| Dialysis duration (months) | 42±26 | 39±30 | 0.75 |
| Pre-transplant PRA class 1 or 2 >10% | 3/17 (17.7) | 5/14 (35.7) | 0.41 |
| Deceased donor | 14/17 (82.4) | 12/14 (85.7) | 0.80 |
| HLA mismatches | 4.0±1.9 | 3.6±1.7 | 0.52 |
| Pre-transplant (weakly) positive XM | 2/17 (11.8) | 2/14 (14.3) | 1.00 |
| Follow up time (months) | 22±5 | 19±8 | 0.23 |
| Estimated GFR at last follow up (ml/min/1.73 m2) | 54±20 | 56±25 | 0.82 |
| Average tacrolimus levels* (ng/ml) | 10.4±2.3 | 9.0±1.7 | 0.18 |
| ACR or AHR | 1/17 (5.9) | 0/14 (0) | 1.00 |
| BK/CMV viremia | 3/17 (17.7) | 4/14 (28.6) | 0.67 |
- Data presented as n (%) for categorical variables or mean±SD for continuous variables;
- PRA is panel of reactive antibodies, CNI is calcineurin inhibitors, GFR is glomerular filtration rate
Average of trough levels at same time points used for study (1, 3 and 6 months post transplant)
The pre-transplant frequencies of donor-reactive T cells producing IFN-γ were not different between groups (161±50 versus 102±60 IFN-γ producing cells/2×105 T cells in IL-2 receptor blocker- and ATG-treated patients, respectively, p=0.13). Likewise, the pre-transplant frequencies of third party reactive T cells were not statistically different (297±49 vs. 320±47 IFN-γ producing cells/2×105 T cells, p=0.68). Cellular reactivity to influenza antigen was not statistically different between groups (data not shown). None of the pre-transplant demographic and clinical variables listed in Table 1 were found to be independently associated with increased donor or third party alloreactive cellular immunity indicating that increased levels of pre-existing cellular alloreactivity, cannot be accurately predicted on clinical grounds.
Effects of induction therapy on peripheral blood lymphocyte counts
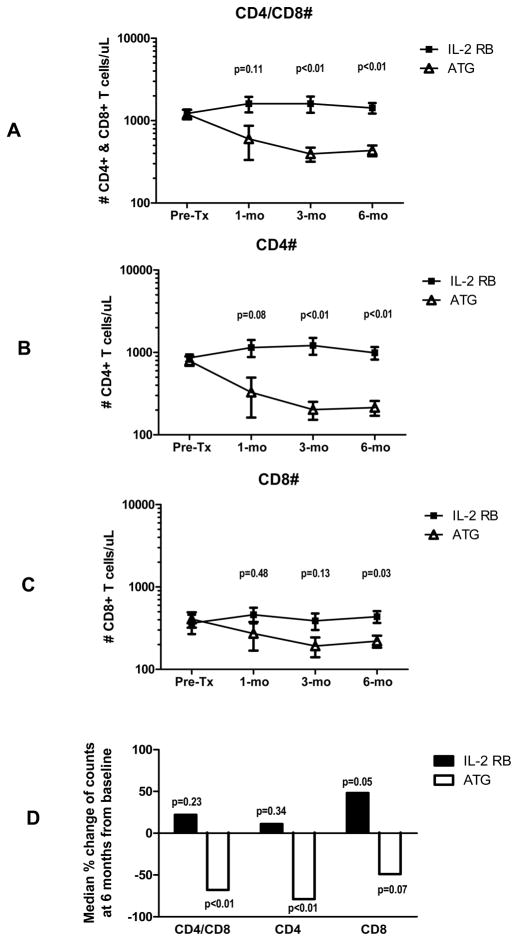
Pre-transplantation, there were no statistical differences between the two groups with regard to numbers of CD4+ and CD8+ lymphocytes in peripheral blood (Figure 1). ATG reduced both CD4+ and CD8+ T cells but this effect was more pronounced in the CD4+ versus the CD8+ population. CD4+ T cells decreased by more than 50% from baseline in the ATG-treated group, but CD4+ T cell numbers were unaffected by the use of the IL-2 receptor blocker. On the other hand, CD8+ T cells were less depleted by ATG and increased from baseline in IL-2 receptor blocker treated patients, an effect that was still present at 6 months post-transplantation. Figure 1D shows the median percent change at 6 months post-transplantation from baseline for each T cell subset population.
Figure 1.
Plots depicting serial frequencies of CD4+/CD8+ T cells (A), CD4+ T cells (B), and CD8+ T cells (C). Figure 1D shows the median percent change of each T cell subpopulation at 6 months post-transplantation from baseline (p values calculated by Wilcoxon rank test).
Effects of induction therapy on circulating T cell subpopulations
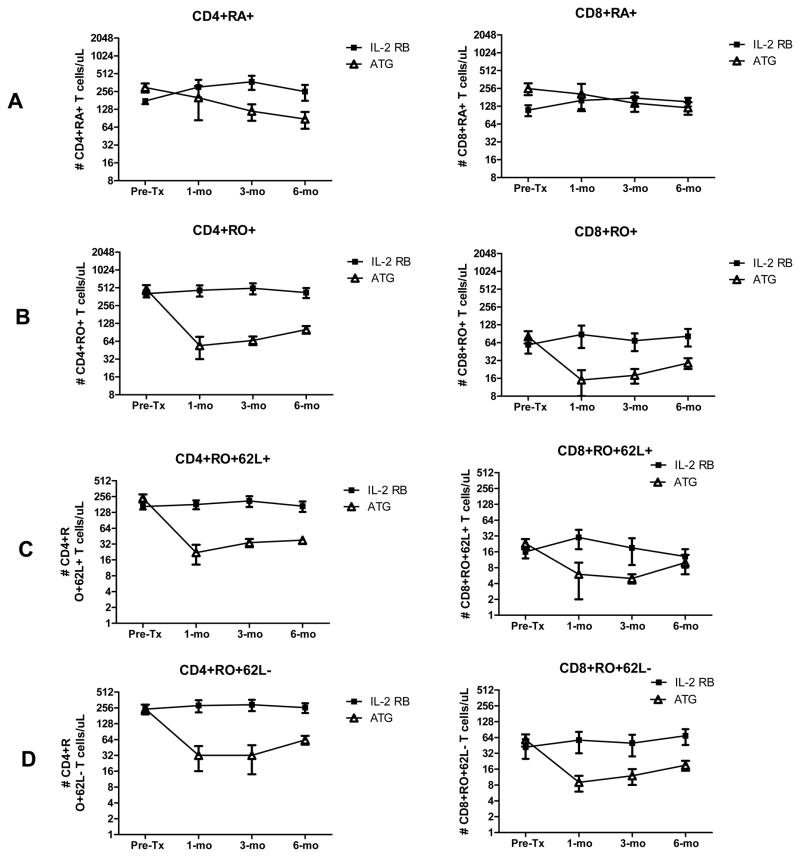
ATG had a significant depleting effect on all CD4+ subtypes (all post-transplant p values are less than 0.05 by Wilcoxon rank test) while the impact on CD8+ T cells was less variable (most p values for post-transplant points are not statistically significant by Wilcoxon rank test) (Figure 2). While numbers of naïve CD8+ T cells (CD8+CD45RAposROneg) were comparable between groups at 6 months post-transplantation, numbers of memory effector CD8+ T cells (CD8+CD45RAnegROpos) remained lower, though not statistically different, between ATG and IL-2 receptor blocker treated subjects. This difference was more pronounced for the CD8+CD45ROposCD62Llow subpopulation when compared to the CD8+CD45ROposCD62Lhigh subset. To better understand the variable depleting effects of ATG on antigen specific cellular reactivity, we then studied how each therapy affected cellular reactivity to donor and non-donor HLA antigen.
Figure 2.
Plots depicting serial frequencies of CD4+ and CD8+ naïve T cells (A), memory T cells (B), central memory T cells (C) and peripheral memory T cells (D).
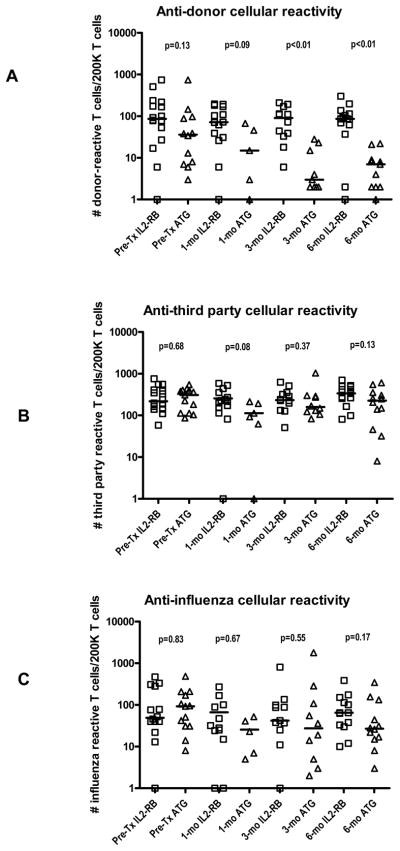
Effects of induction therapies on donor and third party cellular reactivity
Frequencies of IFN-γ producing cells were determined by adjusting for peripheral monocyte counts in view of the differential depleting effects of induction drugs. Both treatment groups showed equal pre-transplant frequencies of cellular reactivity to both donor and third party (Figure 3). When tested at 3 months post-transplantation, ATG treatment had a marked effect on donor cellular reactivity when compared to IL-2 receptor blocker treated patients (9±4 versus 93±20 IFN-γ producing cells/2×105 T cells, p<0.01), while both groups had similar frequencies of third party cellular reactivity (266±89 versus 275±77 IFN-γ producing cells/2×105 T cells, p=0.37). In the ATG-treated group the donor hyporesponsiveness remained evident at 6 months post-transplantation but not in the IL-2 receptor blocker-treated group (7±2 versus 98±21 IFN-γ producing cells/2×105 T cells, p<0.01). Third party cellular alloreactivity was stable post-transplantation irrespective of the induction therapy used, however the sample size is too small to ascertain that there was no differences between groups. Analogous to the third party alloreactivity, frequencies of cellular reactivity to influenza antigen was not different between treatment groups (p>0.10), however, there was a trend to higher infections by BK or CMV virus in patients with low anti-viral cellular immune reactivity (data not shown).
Figure 3.
Scatter plots showing the frequencies of donor reactive T cells (A), third party HLA antigen reactive T cells (B) and T cells reactive to influenza antigen.
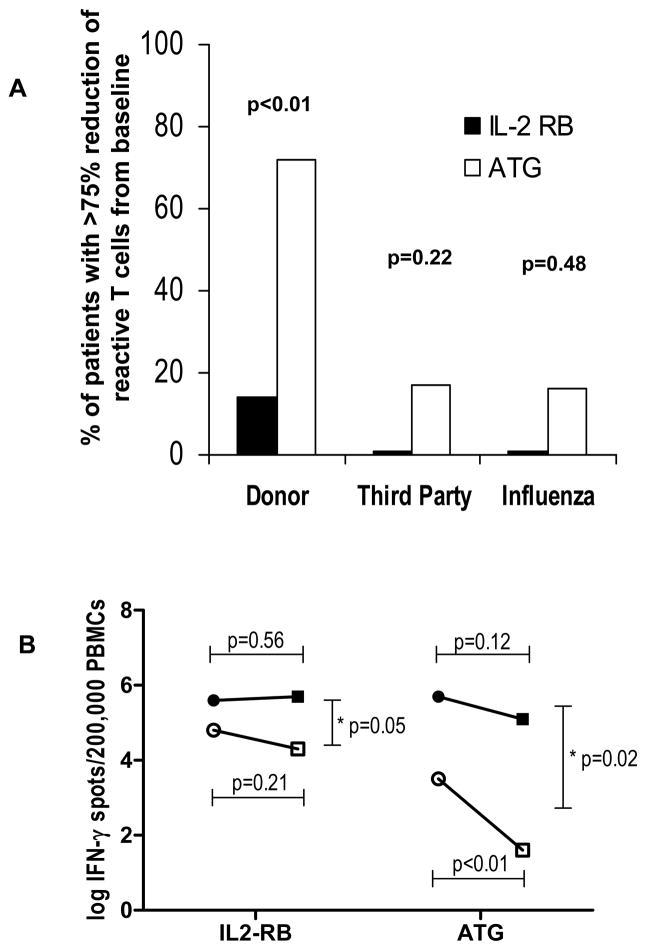
We then compared the ratios between the frequencies of donor and third party alloreactive T cells pre-transplant in relation to six months post-transplant. A ratio close to 1 indicates that there was minimal change of IFN-γ frequencies over time. The post- to pre-transplant anti-donor ratio were 0.21±0.06 versus 1.20±0.37 for the ATG and IL-2 receptor blocker groups respectively (p<0.01 by Wilcoxon rank test). On the other hand, the post- to pre-transplant anti-third party ratio were 0.76±0.15 versus 1.24±0.20 for the ATG and the IL-2 receptor blocker groups respectively (p=0.12 by Wilcoxon rank test). The ratios for influenza virus reactivity closely resembled those observed against the third party stimulators (data not shown). When assessed at six months, ATG-treated patients were more likely to have at least a 75% reduction in the pre-transplant donor frequencies when compared to the IL-2 receptor blocker treated group (Figure 4A). In contrast, the difference in frequencies at the two time points was not statistically significant when considering third party HLA and influenza T cell reactivity. Figure 4B shows the change over time of the anti-donor and anti-third party alloreactivity in each group and demonstrates a marked and significant decrease in anti-donor alloreactivity in the ATG group, but only a slight and statistically insignificant decrease in anti-third party alloreactivity.
Figure 4.
Graph showing the percent of transplanted subjects with at least a 75% decline in frequencies of donor HLA, third party HLA and influenza reactive T cells from baseline at 6 month follow up (Figure 4A). Change of IFN-γ producing PBMCs from baseline (circles) to 6 months post-transplant (squares) for anti-donor (clear marks) and third party (solid marks). *Mean change between anti-donor and anti-third party alloreactivity.
De novo donor and non-donor specific alloantibodies
We asked whether the induction therapy used had any effects on the de novo appearance of non-donor-specific alloantibodies (as measured by any new increase in PRA of greater than 10%) and/or de novo DSA. Six patients developed a PRA>10% by 1 year post-transplantation with three of them developing DSA. Of the patients with increases in total PRA percentages, one patient was treated with ATG and five patients were treated with IL-2 receptor blocker. For DSA, two of the three received IL-2 receptor blocker and one ATG.
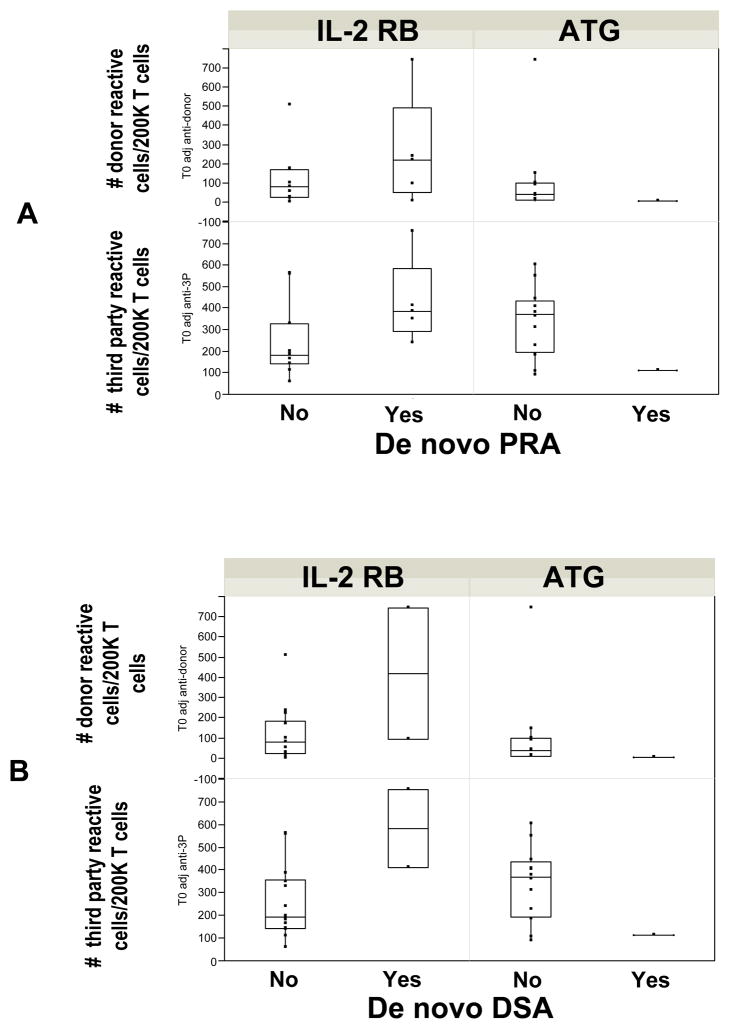
We then looked at whether pre-transplant donor and/or third party cellular alloreactivity predicted de novo formation of alloantibodies. As shown in Figure 5, both donor and third party T cell reactivity was more evident in subjects treated with IL-2 receptor blocker who eventually developed a de novo alloantibody when compared to those who remained PRA negative. The only patient who developed DSA (weak positive) in the ATG group had a low anti-donor and anti-third party cellular response pre-transplant, but none of the ATG treated patients with high donor or anti-third party alloreactivity developed antibody.
Figure 5.
Box plots showing the relationship between pre-transplant anti-donor and anti-third party cellular alloreactivity and the development of de novo non-donor (A) and donor specific alloantibodies (B).
Discussion
Gaining better understanding of the effects of commonly used induction therapies on circulating donor and non-donor reactive T cells has become a matter of biological and clinical interest due to the increasing use of these strategies in kidney transplantation (16, 17). In this study, we show that: 1) cellular allosensitization cannot be predicted on clinical grounds without the use of non-invasive immune monitoring techniques; 2) in contrast to induction with IL-2 receptor blockade that shows minimal lympho-depleting effects, ATG treatment has a marked depleting effect on CD4+ T cells (regardless of phenotype) but a lower effect on CD8+ T cells; and, 3) ATG and IL-2 receptor blockade have differential effects on donor specific and non-donor specific cellular reactivity. This novel finding of our study is supported by the observation that in contrast to IL-2 receptor blocker-treated patients, those receiving ATG demonstrate greater hyporesponsiveness to donor antigens, while the effects on third party alloreactivity and non-allogeneic (anti-influenza) cellular immunity were lower in the patients evaluated. Those with high pre-transplant cellular alloreactivity may also be more susceptible to future alloantibody formation, especially if they have received an IL-2 receptor blocker. The presented data provides further insight into the in vivo effects of T cell antibody therapies not only on peripheral T cell subpopulation numbers but more importantly on the level of donor- and third party-specific cellular alloreactivity.
It is accepted that induction protocols with ATG have marked lymphopenic effects due to its binding to the TCR and other T cell expressed determinants resulting in subsequent induction of apoptosis and/or anergy (18). So called “non-depleting” agents such as IL-2 receptor blockers like basiliximab have less impact on the circulating T cells numbers but yet provide protection against immune-mediated injury through the inhibition of IL-2 dependent T cell proliferation (12). It is also recognized that ATG is a more potent immunosuppressive drug than the IL-2 receptor blockers and thus it is used in higher risk populations (11, 16, 17). Despite the use of ATG, however graft rejection still occurs. This outcome has been postulated to be due to higher resistance of effector memory T cells to ATG. Pearl et al showed that either alemtuzumab or ATG have significant depleting effects on most T cells populations (greater than 99% in the first few weeks post-induction) but less depletion of effector memory T cells (up to 90% depleting effect), and consequently leading to a higher relative predominance of circulating effector memory T cells (19). Notably, CD4+ effector memory T cells were more resistant than CD8+ to depletion in treated patients. Donor specific cellular reactivity was not tested in their study. Unlike this study, we show that ATG does have a significant and consistent depleting effect on all CD4+ subtypes with the nadir at one month post-transplantation followed by a slow but progressive recovery and preferentially sparing of CD8+ T cells, primarily of the central memory phenotype. A plausible explanation for the different results in the two studies is the use of a full immunosuppression regimen in our study, including a calcineurin inhibitor, a drug that showed an inhibiting effect on CD4+ T cell proliferation in their study. A recent study that uses ATG and calcineurin inhibitors also reports similar findings to our study (20).
In contrast to rodent models in which the memory repertoire is not dominant, the memory T cell pool represents a significant percentage of circulating lymphocytes in adult humans. Moreover, the diversity of specific immune reactivity is vast but may include alloreactive T cells in a significant number of individuals irrespective of prior allosensitization events (5, 10, 15, 21). This alloreactivity is assumed to derive from either prior exposure to antigens (for example viral antigens) that may cross-react with alloantigens (heterologous immunity) or by activation of low frequencies of alloreactive T cells through by-stander effects (21, 22). Regardless of the origin, understanding the effects of lympho-depletion on donor reactivity has clinical importance in transplantation.(23) In this study we show that lymphocyte depletion by ATG is not uniform in regards to the allospecificity, with the magnitude and duration of donor-reactivity being depressed to a greater degree than third party alloreactivity and influenza virus-specific reactivity. While the effect of ATG on anti-donor reactivity is robust, the effect on anti-third party alloreactivity can also be appreciated albeit at a lower intensity. On the other hand, the use of IL-2 receptor blocker shows a more uniform effect across the three types of specificities, with minimal on cellular donor-reactivity. A potential hypothesis may relate to the implication of continuous antigen exposure (i.e. the graft) during the reconstitution of peripheral lymphocytes following depletion (homeostatic proliferation) (23, 24). During the reconstitution of the peripheral immune repertoire in lympho-depleted transplant patients, donor-reactive T cells may not be reconstituted, while non-donor reactive memory T cells repopulate the circulation. Factors that could promote this depletion might include the presence of graft antigens and immunoregulation, as ATG has been shown to promote the proliferation of T regulatory cells that are specific to donor antigens (20, 25–27). Understanding the mechanisms of the observed donor hyporesponsiveness is of interest during the development of minimization and or tolerogenic immunosuppressive regimens.
We also show that immune alloreactivity and cellular specificities cannot be predicted based on clinical grounds, suggesting that non-invasive immune monitoring techniques will be important in the future to customize immunosuppression. For example, a question to be studied in clinical trials is whether lower (or higher) doses of specific immunosuppressant drugs will lead to immune profiles more compatible with “tolerogenic states” rather than “immunosuppressive states”. For example, when donor-reactivity is not evident, IL-2 receptor blocker may be of use to minimize naïve T cells from differentiating to donor-reactive T cells, while depleting agents can be dosed to target sustained elimination of circulating donor reactive T cells. At present it is not feasible to provide information on donor reactivity in patients receiving a deceased donor transplant. However, future clinical trials could be developed to test whether a change of induction therapy as soon as donor and third party cellular alloreactivity becomes available (within 24–48 hours) is clinically warranted.
A final but not less important observation of this study is the propensity of subjects with higher pre-transplant cellular alloreactivity to develop de novo alloantibodies after transplant. It is interesting that alloantibodies were more likely to develop in subjects treated with IL-2 receptor blocker despite both groups showing no differences in cellular alloreactivity pre-transplantation. ATG is more likely to deplete T cells with specificity for donor allopeptides presented with class II HLA molecules which would provide the help required for alloantibody responses. If confirmed, pre-transplant cellular monitoring may also be useful to identify candidates at high risk for developing alloantibodies post-transplant allowing for more tailored immunosuppression.
Future studies should focus on understanding mechanisms of de novo and maintenance of humoral and cellular alloresponses following lympho-depletion. It is also important to further understand the role of T regulatory cells on the development of donor hyporesponsivenes but also the roles of other T cell subpopulations. A limitation of our study is the small sample size and lack of statistical power to ascertain that third party immune reactivity indeed is not suppressed by ATG treatment. The small sample size also prevents us from associating the laboratory findings with clinical outcomes. Nevertheless, we believe that by using post-transplant cellular reactivity as well as de novo alloantibody formation as surrogate markers for immune mediated graft injury, our findings retain clinical value.
In conclusion, we show that there is a marked depleting effect of rabbit ATG on most T cells populations but that the specificity of the emerging immune response post-ATG is diminished towards alloantigens. Furthermore, pre-transplant immune monitoring may also facilitate individualization of post-transplant care or study design in the future.
Acknowledgments
American Society of Nephrology - Medical Student Grant (2009) (L.C.), NIAID K23 AI068824 (E.D.P.), National Kidney Foundation of Ohio (E.D.P.) and a reserch grant from Genzyme Transplant Corporation.
Abbreviations
- ATG
rabbit anti-thymoglobulin
- IFN-γ ELISPOT
Interferon gamma enzyme immunosorbent spot assay
- PBMC
peripheral blood mononuclear cell
- DSA
donor specific antibody
- PRA
panel of reactive antibodies
- IF/TA
Interstitial fibrosis/tubular atrophy
Footnotes
Conflict of interest: One of the authors of this manuscript has conflicts of interest to disclose as described by the American Journal of Transplantation – Dr. Poggio has received consultation fees from Genzyme Transplant Corp. for an Advisory Board Meeting in December of 2009.
Preliminary data of this work was presented as a poster presentation at the American Transplant Congress, 2009 and 2010.
Disclosures
Commercial organizations: This work was partially funded by a research grant from Genzyme Transplant Corporation. This manuscript or parts of it were not prepared by a commercial organization. Only the listed authors participated in conducting the research experiments and the manuscript preparation and editing.
References
- 1.Bromberg JS, Heeger PS, Li XC. Evolving paradigms that determine the fate of an allograft. Am J Transplant. 10(5):1143–1148. doi: 10.1111/j.1600-6143.2010.03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Womer KL, Kaplan B. Recent developments in kidney transplantation--a critical assessment. Am J Transplant. 2009;9(6):1265–1271. doi: 10.1111/j.1600-6143.2009.02639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant. 2003;3(5):525–533. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 4.Issa F, Schiopu A, Wood KJ. Role of T cells in graft rejection and transplantation tolerance. Expert Rev Clin Immunol. 6(1):155–169. doi: 10.1586/eci.09.64. [DOI] [PubMed] [Google Scholar]
- 5.Valujskikh A. Memory T cells in allograft rejection. Adv Exp Med Biol. 2007;601:247–256. doi: 10.1007/978-0-387-72005-0_26. [DOI] [PubMed] [Google Scholar]
- 6.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-Transplant IFN-gamma ELISPOTs Are Associated with Post-Transplant Renal Function in African American Renal Transplant Recipients. Am J Transplant. 2005;5(8):1971–1975. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 7.Hricik DE, Rodriguez V, Riley J, Bryan K, Tary-Lehmann M, Greenspan N, et al. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am J Transplant. 2003;3(7):878–884. doi: 10.1034/j.1600-6143.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 8.Najafian N, Salama AD, Fedoseyeva EV, Benichou G, Sayegh MH. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol. 2002;13(1):252–259. doi: 10.1681/ASN.V131252. [DOI] [PubMed] [Google Scholar]
- 9.Poggio ED, Augustine JJ, Clemente M, Danzig JM, Volokh N, Zand MS, et al. Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation. 2007;83(7):847–852. doi: 10.1097/01.tp.0000258730.75137.39. [DOI] [PubMed] [Google Scholar]
- 10.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31(6):859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 12.Lebranchu Y, Bridoux F, Buchler M, Le Meur Y, Etienne I, Toupance O, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am J Transplant. 2002;2(1):48–56. doi: 10.1034/j.1600-6143.2002.020109.x. [DOI] [PubMed] [Google Scholar]
- 13.Zand MS, Bose A, Vo T, Coppage M, Pellegrin T, Arend L, et al. A renewable source of donor cells for repetitive monitoring of T- and B-cell alloreactivity. Am J Transplant. 2005;5(1):76–86. doi: 10.1111/j.1600-6143.2003.00637.x. [DOI] [PubMed] [Google Scholar]
- 14.Augustine JJ, Poggio ED, Heeger PS, Hricik DE. Preferential benefit of antibody induction therapy in kidney recipients with high pretransplant frequencies of donor-reactive interferon-gamma enzyme-linked immunosorbent spots. Transplantation. 2008;86(4):529–534. doi: 10.1097/TP.0b013e31818046db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danziger-Isakov L, Cherkassky L, Siegel H, McManamon M, Kramer K, Budev M, et al. Effects of influenza immunization on humoral and cellular alloreactivity in humans. Transplantation. 89(7):838–844. doi: 10.1097/TP.0b013e3181ca56f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahan BD. Individuality: the barrier to optimal immunosuppression. Nature reviews. 2003;3(10):831–838. doi: 10.1038/nri1204. [DOI] [PubMed] [Google Scholar]
- 17.Kirk AD. Induction immunosuppression. Transplantation. 2006;82(5):593–602. doi: 10.1097/01.tp.0000234905.56926.7f. [DOI] [PubMed] [Google Scholar]
- 18.Hardinger KL, Schnitzler MA, Miller B, Lowell JA, Shenoy S, Koch MJ, et al. Five-year follow up of thymoglobulin versus ATGAM induction in adult renal transplantation. Transplantation. 2004;78(1):136–141. doi: 10.1097/01.tp.0000132329.67611.3f. [DOI] [PubMed] [Google Scholar]
- 19.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 20.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 10(9):2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor DK, Neujahr D, Turka LA. Heterologous immunity and homeostatic proliferation as barriers to tolerance. Current opinion in immunology. 2004;16(5):558–564. doi: 10.1016/j.coi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19(5):318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Lechler RI. The role of the allograft in the induction of donor-specific T cell hyporesponsiveness. Transplantation. 2001;72(3):480–485. doi: 10.1097/00007890-200108150-00020. [DOI] [PubMed] [Google Scholar]
- 25.De Serres SA, Sayegh MH, Najafian N. Immunosuppressive drugs and Tregs: a critical evaluation! Clin J Am Soc Nephrol. 2009;4(10):1661–1669. doi: 10.2215/CJN.03180509. [DOI] [PubMed] [Google Scholar]
- 26.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17(10):2844–2853. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 27.Sewgobind VD, Kho MM, van der Laan LJ, Hendrikx TK, van Dam T, Tilanus HW, et al. The effect of rabbit anti-thymocyte globulin induction therapy on regulatory T cells in kidney transplant patients. Nephrol Dial Transplant. 2009;24(5):1635–1644. doi: 10.1093/ndt/gfn778. [DOI] [PubMed] [Google Scholar]