Summary
Background
Circadian rhythms regulate physiology and behavior through transcriptional feedback loops of clock genes running within specific pacemaker cells. In Drosophila, molecular oscillations in the small ventral Lateral Neurons (sLNvs) command rhythmic behavior under free-running conditions releasing the neuropeptide PIGMENT DISPERSING FACTOR (PDF) in a circadian fashion. Electrical activity in the sLNvs is also required for behavioral rhythmicity. Yet, how temporal information is transduced into behavior remains unclear.
Results
Here we developed a new tool for temporal control of gene expression to obtain adult-restricted electrical silencing of the PDF circuit, which led to reversible behavioral arrhythmicity. Remarkably, PER oscillations during the silenced phase remained unaltered, indicating that arrhythmicity is a direct consequence of the silenced activity. Accordingly, circadian axonal remodeling and PDF accumulation were severely affected during the silenced phase.
Conclusions
Although electrical activity of the sLNvs is not a clock component it coordinates circuit outputs leading to rhythmic behavior.
Keywords: Drosophila, Kir 2.1, GeneSwitch, PDF, reversible silencing
Introduction
Rhythmic rest/activity cycles are the result of the action of roughly ten proteins that are the essence of the circadian clock and the coherent activity of about 150 neurons in the adult Drosophila brain, which assemble into the circadian network [1]. Clock neurons were originally identified by the expression of bonafide circadian clock components, and were named after their anatomical position, although evidence of heterogeneity within each cluster has been reported [2, 3]. In recent years efforts have been devoted to define the contribution of different clusters. The emerging picture suggests that the concerted action of a molecular clock running within each component of the circadian network is necessary for a plastic biological clock to respond to different environmental stimuli (such as light and temperature, revised in [4]).
Under constant conditions a key circuit for the rhythmic control of behavior is the LNv cluster [5-7], which includes four small (sLNvs) and four large (lLNvs) neurons expressing the PDF neuropeptide, and a 5th neuron which lacks PDF and shares other properties common to the LNds [3]. PDF immunoreactivity in the sLNvs axonal termini changes throughout the day, suggesting that its transport, accumulation or release is under clock control [8]. Since perturbing PDF function is associated with progressive arrhythmicity [5, 9], desynchronization of ventral [10, 11] and dorsal oscillators [11, 12] and direct effects on period length [9, 13, 14] the relevance of this molecule in setting basic properties of rhythmic behavior is granted. However, additional mechanisms are likely to be in place to ensure rhythmic behavior under free running conditions [15]. One possibility to convey time of day information to locomotor centers in the central brain is to control the excitability of different circadian clusters, which could be achieved directly through transcriptional regulation of ion channels [16, 17]; in fact, the LNvs exhibit circadian changes in resting membrane potential [18-20]. This and other electrical properties have been shown to cycle in the neurons within the suprachiasmatic nucleus (SCN), the anatomical location of the central oscillator in the mammalian brain [21-24]. Interestingly, it has been reported that affecting the excitability of the PDF circuit leads to complex rhythmic patterns [15, 25] and behavioral arrhythmicity [26-28]. In addition, circadian remodeling of the PDF axonal terminals, likely resulting in a change in synaptic contacts at different times in the day [29], might offer another relevant link between the molecular oscillator and sustained rhythmic behavior.
A number of years ago it was reported that reducing membrane excitability through the expression of an inward rectifier K+ channel (KIR) within the PDF circuit resulted in behavioral arrhythmicity and loss of rhythmic molecular oscillations [26, 27]. However, no effect on pacemaker function was observed after reversible blockade of action potential firing in cultured mammalian SCN neurons [30] and years earlier in intact animals [31]. Such striking difference in an intrinsic property of otherwise highly similar biological clocks prompted us to look into this matter further.
Considerable understanding of how the circadian network operates in the fly brain has emerged through genetic manipulations based on the GAL4/UAS system, ensuring gene expression during the development of the circadian network. However, altering intrinsic properties –such as membrane excitability- throughout development could potentially trigger compensatory mechanisms or have an irreversible effect on cellular viability. To circumvent such limitations, an inducible GAL4 chimera (termed GeneSwitch) was engineered, allowing the temporal as well as spatial control of gene expression [32]. Thus, we generated a transgenic line pdf-GeneSwitch (pdf-GS) to more precisely define the impact of reducing excitability of the PDF circuit in an adult-specific fashion thus avoiding indirect effects. In this work we report that silencing the PDF circuit through the expression of the inward rectifier K+ channel KIR, acutely in the adult brain, gives rise to arrhythmic locomotor behavior without impairing the molecular oscillations in the sLNvs. Adult-restricted silencing also resulted in a decreased complexity of the arborization pattern of the PDF axonal terminals. Surprisingly, once kir expression was turned off, behavioral rhythmicity was restored to the phase of the initial light-dark (LD) entrainment, underscoring that the molecular clock was not affected throughout the treatment. Likewise, PDF levels were reduced through the silenced phase and its cycling was abolished. Both effects were restored upon deactivation, opening the possibility that PDF accumulation, transport and/or release is coupled to changes in membrane excitability which in turn restores behavioral rhythmicity. Strikingly, affecting excitability since early development on occasion brings the biological clock to a halt, likely as a result of secondary effects.
Results
Adult-restricted silencing of the PDF circuit results in arrhythmic behavior
To inquire about the direct consequences of altering essential properties of the PDF circuit during adult stages, we generated a transgenic line that allows expression of an inducible GAL4 version, termed GeneSwitch [32], under the control of the pdf promoter [5]. GeneSwitch (GS) is a fusion between the GAL4 binding, the NFκb activation and the human progesterone receptor ligand-binding domains, which is expressed in the pattern dictated by the desired promoter but remains transcriptionally silent in the absence of RU486 (RU), an analog of progesterone.
To address the consequences of acutely silencing the PDF circuit, pdf-GS was employed to drive expression of UAS-egfp-kir2.1 [33]. In the absence of RU no GFP was detected (Figure 1A, top panel). Systemic administration (i.e. in the food) of the activator RU resulted in the accumulation of EGFP-KIR as early as 12 h post administration (Figure 1A, middle panel), when the GFP signal was detectable in about 40% of the brains, mostly associated to the somas. By 16 h post induction about 60% of the brains expressed detectable levels of EGFP-KIR (data not shown), and this pattern became widespread both in somas and projections by 24 h (Figure 1A, bottom panel).
Figure 1. The pdf-GS inducible system allows modulation of circadian locomotor activity.
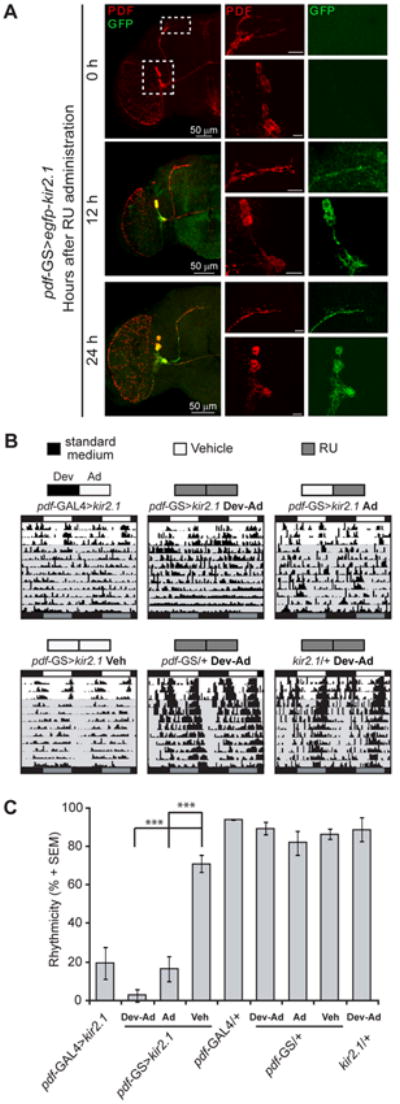
(A) Activation kinetics of the pdf-GS system. Representative confocal images of pdf-GS>egfp-kir2.1 brains dissected 0, 12 and 24 h after transfer to RU containing food and stained with anti-GFP (green) and anti-PDF (red). LNvs somas (upper inset) and dorsal axonal projections (lower inset) are displayed for each timepoint. Note that in shorter inductions (12h in food containing RU) higher laser intensity was used in order to detect EGFP-KIR signal. Unless otherwise indicated the scale bar represents 10 μm. (B-C) Acute silencing effectively impairs locomotor behavior. (B) Representative double-plotted actograms are shown. Flies were entrained for 3 days to 12:12 light-dark cycles (LD) and then monitored in constant darkness (DD) for 9 days. In the actograms, white bars represent day, black bars, night and gray bars, subjective day while gray background indicates constant darkness conditions. Above each actogram a schematic diagram illustrates the treatment performed, highlighting the presence of RU (gray), vehicle (white) or standard medium (black) in the food during development (left bar) and adulthood (right bar). Dev stands for RU present during development and Ad, only in adult stages. (C) Percentage of rhythmicity. Data represents at least 3 independent experiments and a minimum of 28 flies were analyzed. *** indicates p<0.001 (ANOVA with Bonferroni post hoc test). See also Figure S1.
We next compared the effect of the new pdf-GS line driving UAS- egfp-kir2.1 (from now onwards pdf-GS>kir2.1) to that of pdf-GAL4 in terms of its effect on locomotor behavior. Newly eclosed flies were placed in standard food supplemented with either RU or vehicle (ethanol). A single copy of either transgene (control lines pdf-GS or kir 2.1) in the presence of RU as well as pdf-GS>kir2.1 in the absence of RU (vehicle) were clearly rhythmic (Figure 1, B-C and Figure S1).
Silencing the PDF circuit with pdf-GAL4 driving UAS-egfp-kir2.1 rendered about 20% of rhythmic flies under free running conditions, similarly to what was observed after chronic activation of pdf-GS, that is, throughout development and in the adult animal (pdf-GS>kir2.1 “Dev-Ad” (See also [26, 34]). Notably, reducing the excitability of the PDF circuit exclusively in the adult (pdf-GS>kir2.1 “Ad”) also resulted in a highly arrhythmic population, indistinguishable from that of pdf-GAL4>kir2.1, along the lines of what was reported by Nitabach and colleagues, where adult expression of a spider toxin that leads to inactivation of the voltage-gated Na+ channel paralytic results in complex behavioral rhythms and arrhythmicity [28]. Interestingly, the proportion of pdf-GS>kir2.1 flies still displaying behavioral rhythmicity showed a short period phenotype (Figure S1), which is also consistent with previous observations [34].
To confirm that kir2.1 expression was able to silence the LNvs we directly measured the ability of the lLNvs to fire action potentials in an intact brain preparation. We performed cell-attached voltage clamp recordings of extracellular action currents to measure neuronal activity without altering the cytoplasmic milieu. Control (pdf-GS>CD8GFP) lLNvs displayed tonic and bursting action currents, consistent with the modes of firing characteristic of these neurons [19, 20](Figure 2A). However, over-expression of kir2.1 completely abolished neuronal activity. Interestingly, no differences were observed when kir2.1 expression was directed by either the inducible or the constitutive system, indicating that pdf-GS is as effective as pdf-GAL4 in silencing the lLNvs (Figure 2A,C). To determine whether KIR-induced silencing affected neuronal viability, we recorded neuronal activity after blocking KIR channels with 200 μM BaCl2 in the extracellular solution [35]. These recording conditions restored the ability of Kir-expressing neurons to fire action potentials, demonstrating that silenced lLNvs were still functional (Figure 2B, C). Therefore, these results demonstrate that both transient (pdf-GS>kir2.1) and persistent (pdf-GAL4>kir2.1) expression of kir2.1 reliably abolish firing of PDF neurons without affecting their viability.
Figure 2. Expression of kir2.1 results in electrical silencing of PDF neurons.
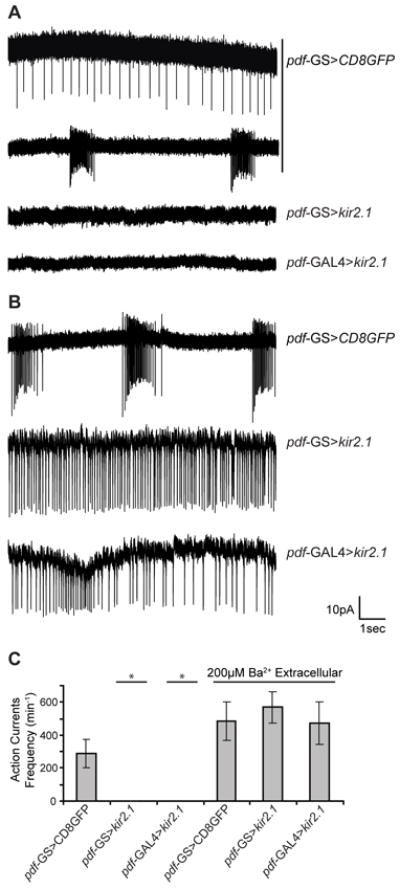
(A) Cell-attached recordings of control (pdf-GS>CD8GFP) lLNvs show action currents in the tonic or bursting firing modes (top two traces). No firing was observed when kir2.1 was expressed with either the inducible (pdf-GS>kir2.1) or constitutive (pdf-GAL4>kir2.1) system (botton traces). (B) The addition of 200 μM BaCl2 to the external solution blocks Kir2.1 channels and restores firing. (C) Quantification of the firing rate for the genotypes and treatments described in (A) and (B). Statistical analysis was performed using the Kruskal-Wallis test followed by pairwise comparisons. Only silenced neurons (Kir2.1 expressing neurons without BaCl2 in the external solution) show a statistically significant difference in the quantification of action potential currents. (*) indicates p<0.05 when compared to the CD8GFP genotype in the absence of Ba2+, n = 5 − 11.
Taken together these results suggest that acutely silencing the PDF circuit during post-developmental stages, after circuit establishment and refinement, results in incoherent network activity which translates into arrhythmic behavior.
Electrical silencing of PDF neurons disrupts circadian locomotor activity in a reversible fashion
The finding that an acute (adult-specific) effect on excitability of the PDF circuit was equally effective as a long-term one to trigger behavioral arrhythmicity prompted us to investigate whether its effect on the behavioral output was sustained in time. Flies were entrained for several days in the presence of RU and then transferred to constant darkness. We noticed that on occasion control pdf-GS>CD8GFP flies (but not pdf-GS/+, Figure 3A, top left) in the presence of RU exhibited a distinct long period phenotype that returned to 24h upon transfer to vehicle-containing food (see also Figure 4A, top left). To the extent examined so far this period phenotype was restricted to this genotype in the presence of RU and did not compromise rhythmicity (Figure 4B and Fig S2B). As shown earlier, flies expressing KIR under the pdf promoter (pdf-GS>kir2.1 Ad in Figure 1B-C) became arrhythmic in the absence of environmental signals (DD1-8, DD stands for constant darkness, Figure 3A-B). When transferred to fresh test tubes containing no RU during the subjective day, under red safe light, flies initially responded with a marked reduction of activity. However, three days after transfer to regular food the RU treated pdf-GS>kir2.1 flies recovered rhythmicity to the levels exhibited during the untreated stage (Figure 3B, compare untreated flies to those in DD9-18 after RU treatment), in concert with the window required to clear most of the KIR associated signal in the axonal projections at the dorsal protocerebrum (Figure S2A). Interestingly, activity was recovered in a phase reminiscent of the one displayed under LD cycles. These results suggest that an acute effect on the excitability of the PDF circuit through the inducible expression of KIR post-developmentally affects the signaling and/or connectivity to downstream targets, and results in behavioral arrhythmicity, which in turn can be reversed upon removal of the activator. These observations are consistent with the work by Schwartz and colleagues on the rat SCN [31].
Figure 3. Electrical silencing of PDF neurons disrupts circadian rhythms in a reversible fashion.
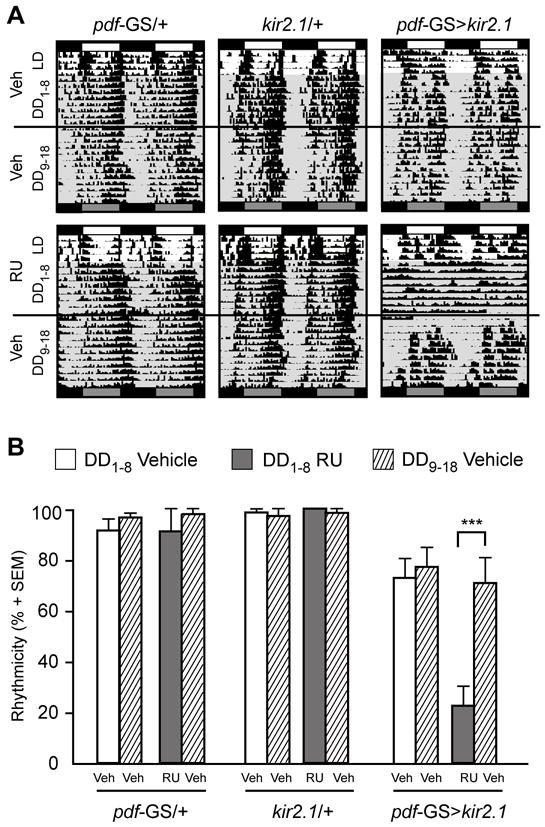
(A) Representative actograms of control and treated animals are shown. Flies were raised in standard medium throughout development, and young adults were transferred to test tubes containing RU or vehicle. Flies were entrained for 4 days, spent 8 days in DD (DD1-8) with or without RU (Veh), following which all experimental groups were transferred back to control medium (Veh) for 10 additional days (DD9-18). (B) Percentage of rhythmicity for the indicated groups across the different treatments. Data obtained around food transfer, on DD9-10, were not taken into account for this analysis. Data represents 3 independent experiments and the number of total flies analyzed per treatment ranged from 45 to 64. *** indicates p<0,001 (ANOVA with Bonferroni post hoc test). See also Figure S2.
Figure 4. Long-term silencing of the PDF circuit triggers irreversible locomotor arrhythmicity.
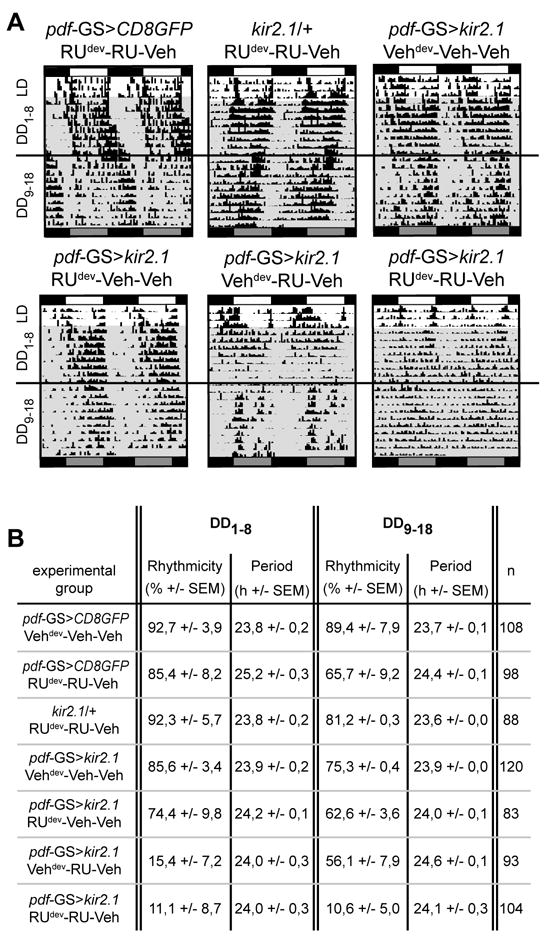
(A) Representative double plotted actograms. Flies were raised in medium with (RU) or without RU (indicated as Veh) throughout development (dev). Young adults were transferred to test tubes containing RU or vehicle and entrained for 3 days before releasing them in constant darkness for 8 days (DD1-8). At this point all experimental groups were transferred during the subjective day to new test tubes with no RU (Veh) and monitored for 10 additional days (DD9-18). Above each representative actogram the genotype and treatment are indicated. (B) Percentage of rhythmicity and average period in control and treated groups calculated for each free running phase. n refers to the number of individuals analyzed per experimental group. Percentage of rhythmicity of pdf-GS>kir2.1 RUdev-Veh-Veh does not differ from control groups (pdf-GS>kir2.1 Vehdev-Veh-Veh and pdf-GS>CD8GFP RUdev-RU-Veh) in any of the DD phases analyzed (Two-way ANOVA and Bonferroni correction). Additional induction during pupation did not increase the arrhythmicity observed in animals maintained in RU during development (data not shown). pdf-GS>kir2.1 RUdev-RU-Veh could not restore rhythmic behavior upon removal of RU (percentage of rhythmicity is statistically different from control groups on DD9-18. p< 0,001 Two-way ANOVA and Bonferroni correction). On the contrary, pdf-GS>kir2.1 Vehdev-RU-Veh restored rhythmic activity to control levels (percentage of rhythmicity does not show statically differences on DD9-18).
Long- term KIR expression triggers irreversible effects on locomotor behavior
To establish the time window when silencing the PDF+ neurons irreversibly affects their output, pdf-GS>kir2.1 flies along with the proper genetic controls were allowed to develop in food containing RU or vehicle. Control pdf-GS>CD8GFP or UAS-kir2.1/+ maintained and tested in RU supplemented food displayed highly rhythmic behavior (Figure 4 A-B).
Surprisingly, silencing the PDF circuit only through early development (Figures 4A, bottom left, and B) resulted in a highly rhythmic population, suggesting that the initial steps in circuit development (i.e. migration and initial establishment of connectivity) are not particularly challenged by a dramatic effect on its excitability. Noteworthy, although larvae had fed on RU-containing food up to third instar stage, a CD8GFP associated signal could be seen in the PDF somas several days later in newly eclosed adults (EA Gorostiza and MF Ceriani, unpublished observations), which could be attributed to an activated expression due to a surplus of RU stored in the fat or, alternatively, to the inherent stability of the GFP protein.
In stark contrast, reducing electrical activity of PDF neurons from early development into adulthood resulted in a highly arrhythmic phenotype that could not be reversed upon transfer to standard food (Figure 4A, bottom panel, on the right), likely indicative that irreversible changes to the physiology of the PDF neurons could have taken place. This observation contrasts with the arrhythmicity observed when restricting the silencing to the adult stage, which can be partially reversed upon removal of the activator (Figures 3 and 4A, bottom middle panel).
These results imply that there is a temporal window in which pacemaker neurons are capable of overcoming potential detrimental effects that result from prolonged and defective excitability.
Molecular oscillations in central pacemaker neurons remain unaltered despite electrical silencing restricted to the adult
Reversible changes in locomotor behavior imply that either adult-restricted silencing of the PDF circuit did not affect the pace of the biological clock or, rather, that the molecular oscillations re-synchronized upon removal of the RU. To examine this possibility we monitored PER accumulation and subcellular distribution on whole mount brains of pdf-GS>kir2.1 flies by immunohistochemistry during the silenced period (Figure 5A-B) as well as after recovery (Figure S3A-B). Brains were dissected at circadian times (CT, indicates the hours in darkness accumulated after the last dark to light transition should have taken place) 5, 11, 17 and 23. Timecourses were performed on DD4 (after 1 week on RU containing food) to reflect the state of the molecular oscillator during the silenced phase, and on DD13, four days after transfer to fresh tubes containing no RU. On DD4 PER expression in the small LNvs of pdf-GS>kir2.1 flies maintained in vehicle-containing food was found primarily in the nucleus at CT5 and also at CT11, albeit at lower levels. By CT17 PER increasingly accumulated in the cytoplasm of the sLNvs, and by CT23 it was found within the nucleus. Surprisingly, robust PER oscillations persisted in the presence of RU resulting in a profile of PER accumulation undistinguishable from that of the non-induced controls, although the overall levels were slightly reduced achieving statistical significance only at CT5 and 11 for the nuclear signal (Figure 5A-B). We also examined PER localization in acutely silenced pdf-GS>kir2.1 individuals during the recovery phase. Flies were kept in RU-containing food through the first week in free running conditions (DD1-8 in Figure 3) and dissected on the fourth day after transfer to vehicle containing food (on DD13, corresponding to days DD9-18 in Figure 3). Not surprisingly, the profile of PER accumulation in the small LNvs of animals kept in RU containing food through DD1-8 paralleled that of the corresponding controls (kept in vehicle containing food throughout the experiment) on DD13, with a clear change in nuclear PER levels and cellular distribution throughout the day even after 13 days under constant conditions; interestingly, no gross changes in the overall dynamics of PER oscillations became evident after the silenced phase; in fact, low amplitude oscillations in cytoplasmic PER levels were observed in both genotypes (Figure S3A-B), which contrasted to the high amplitude cycling observed in DD4 (Figure 5B); this is unlikely the result of defective neural activity since both genotypes displayed similar profiles. These observations suggest that restricting KIR expression to adult stages disorganizes locomotor behavior without affecting the pace of intracellular oscillations in the core pacemaker cells.
Figure 5. Adult-restricted silencing does not affect the pace of the molecular clock while prolonged KIR expression eventually runs down molecular oscillations.
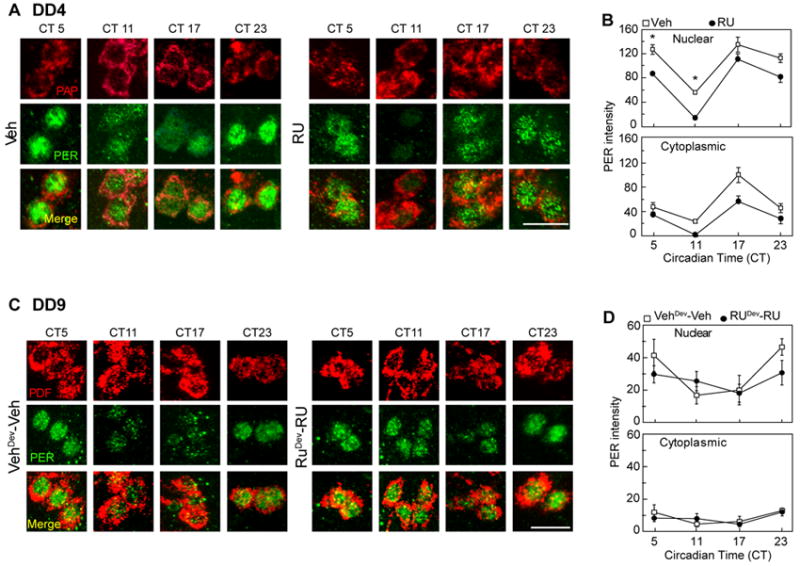
(A) Newly eclosed pdf-GS> kir2.1 adult flies in food containing no inducer (vehicle) or RU were synchronized and samples were taken every 6 hours on DD4. Whole mount brain immunofluorescence was performed to follow PAP (upper panel) and PER (middle) accumulation. (B) Plots display the average change in PER intensity in the nucleus and cytoplasm at each timepoint. Each value represents the average of three independent experiments (n= 2-4 cells per brain, with a minimum of 10 brains per condition/experiment). Both groups display significant changes in PER nuclear localization throughout the day (one-way ANOVA with a Bonferroni post hoc test). PER intensity was significantly different at CT5 and 11 with p<0.05 (*). (C) Newly eclosed pdf-GS>kir2.1 flies kept either in vehicle or RU-containing food since early development (and into adulthood) were synchronized and samples were taken every 6 hours on DD9. The timepoint was selected to examine the state of the molecular oscillator at a point of no return with regards to locomotor behavior (see Figure 4). Whole mount brain immunofluorescence was performed to follow PDF (upper panel) and PER (middle) accumulation. The experiment was repeated six times (Details of the specific experiments are included in Figure S3). Representative ones of the average levels shown in D were included. Scale bar: 10μm. (D) Plots display the average change in PER intensity in the nucleus and cytoplasm at each timepoint. Each value represents the average of six experiments including an average of 10 brains per timepoint/experiment. Controls exhibit significant changes in PER nuclear localization throughout the day (CT17 vs CT23 p< 0.05). Although there were no significant differences between the two treatments, no oscillations in nuclear PER levels were found in the RU treated group in average. See also Figure S3.
To investigate the state of the molecular oscillator in flies whose rhythmicity could not be recovered, we followed PER localization in pdf-GS>kir 2.1 flies induced from early development into adulthood the day they should have been transferred to fresh vials containing no RU (DD9). The time point was selected to ensure evaluation of molecular oscillations at a point of no return with regard to locomotor arrhythmicity. As expected, nuclear PER levels cycled in control brains on DD9 (Figures 5C-D). However, on average, no molecular oscillations could be detected in flies that had been maintained in the presence of RU since early development (Figure 5D). Interestingly, the lack of molecular oscillations resulted from a distinct pattern of PER accumulation in the nucleus among the different experiments within the RU treated group, rather than the absence of molecular oscillations per se (Figure S3C, i-iii). In 3 out of 6 experiments low amplitude to no PER cycling was detected (Figure S3Civ, and v-vi, respectively), suggesting that subtle differences in the effect of the persistent inactivation in turn triggered pleiotropic effects within the sLNvs.
In parallel, no clear sign of PER oscillation was detected in pdf-GAL4>kir2.1 flies (Figure S3D-E), in agreement with previously published data [26]. However, in our hands a constitutive PER nuclear signal was detected throughout the day, in contrasts to the very low expression levels previously reported.
In sum, our data supports the notion that prolonged KIR expression irreversibly affects behavior, likely through indirect effects on second messenger cascades which in turn lead to impaired molecular oscillations.
Electrical silencing of PDF neurons reduces the complexity of their axonal arbor
Altering intrinsic properties of a neuronal circuit such as its excitability could impinge upon the structure of the circuit, especially at times of circuit refinement and maturation. To examine whether adult-restricted kir expression affected the structure of the PDF circuit a membrane tethered GFP version was employed [29]. pdf-GS>CD8GFP and pdf-GS>CD8GFP; kir2.1 flies transferred to RU-containing food at least two days after eclosion were dissected at CT2 and 14 on DD4. In control brains (pdf-GS>CD8GFP) the PDF circuit underwent circadian remodeling of the axonal arborizations at the dorsal protocerebrum as it has been reported [29], exhibiting more numerous and likely higher order processes during the subjective day and fewer branches at subjective night (Figure 6A-B). Interestingly, pdf-GS>CD8GFP; kir2.1 brains displayed grossly normal projections. However, quantification of the degree of arborization [29] indicated that the total number of axonal crosses was reduced in the acutely silenced brains, displaying a degree of complexity during the subjective day which resembled the nighttime configuration of a wild type one. Interestingly, although overall less complex arborizations accompanied the silenced phase, circadian remodeling of the axonal terminals was still taking place (Figure 6A, right panel), as it would be anticipated from a circadian-modulated phenomenon in a condition when the molecular oscillator is still running (Figure 5A-B).
Figure 6. PDF outputs are affected upon acute silencing.
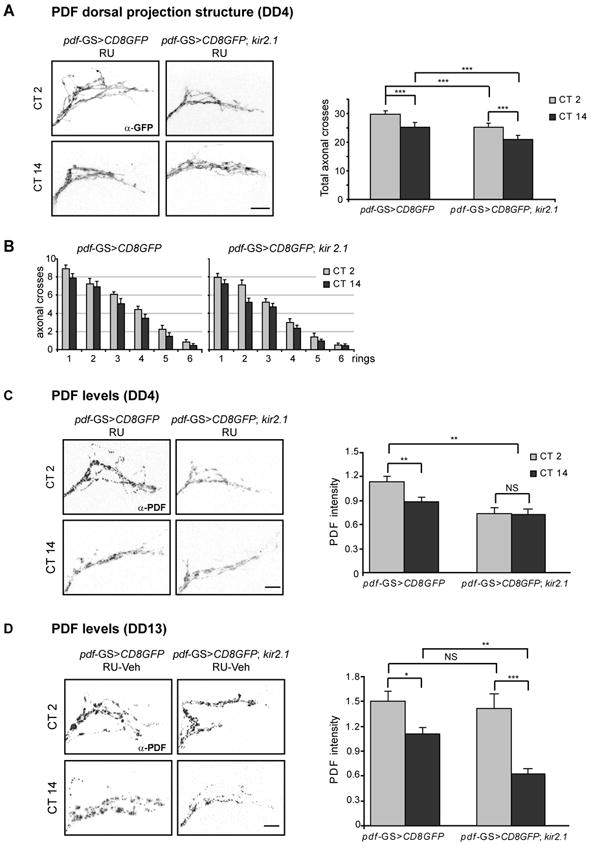
(A-B) The complexity of PDF axonal arborisations is compromised during the silenced phase. (A) Left panel. Representative confocal images of fly brains stained for GFP taken at the early subjective day (CT2) and early subjective night (CT14) on DD4. Right panel. Total number of axonal crosses. Control pdf-GS>CD8GFP flies kept as adults on RU displayed circadian remodelling of the axonal terminals. pdf-GS>CD8GFP;kir2.1 flies displayed reduced complexity throughout the day, although circadian structural plasticity was still evident. Forty to fifty flies were analysed per group. (B) Number of intersections between each concentric ring and the axonal projections in control and electrically silenced flies. The complexity of the axonal arbors is consistently lower in the night-time conformation. (C-D) Analysis of PDF levels. Left panels. Magnified views of the LNvs projections in the dorsal region stained for PDF. Brains were fixed at CT 2 and CT 14. Samples were taken 4 days after the last light-dark transition (DD4, C) and 5 days after RU removal (DD13, D). Experiments were repeated at least 3 times and a minimum of 30 brains were analysed per timepoint. Right panels. Quantitation of the average intensity of the LNvs dorsal projections during the silenced (DD4, C) and recovered (DD13, D) phase was performed blind. Error bars represent the standard error of the mean. Statistical analysis for total axonal crosses and PDF levels included ANOVA followed by a pairwise comparison (Bonferroni test). *** indicates p<0,001, ** indicates p<0,01. NS: Non Significantly different. Scale bar: 10μm.
These observations indicate that electrical activity is required for the structural refinement of the PDF circuit, and strongly suggests that silencing the circuit throughout development could irreversibly affect its structure/properties.
Electrical silencing affects PDF levels at the dorsal terminals
PDF immunoreactivity in the axonal terminals at the dorsal protocerebrum has been shown to oscillate in a circadian fashion both under LD and DD conditions [8]; interestingly, its cycling is affected in mutants with impaired clock function [8] or altered membrane properties [12, 25, 36].
To examine whether the adult-restricted effect on membrane excitability affected PDF levels at the axonal terminals we performed immunohistochemistry on whole mount adult brains dissected at times when PDF levels peak and reach a trough (CT2 and CT 14) on DD4. Control pdf-GS>CD8GFP flies in the presence of RU exhibited a significant difference in PDF immunoreactivity at these two timepoints (Figure 6C). In contrast, PDF immunoreactivity at the dorsal terminals of the sLNvs of pdf-GS>kir2.1 flies kept in the presence of RU did not significantly vary from levels exhibited by control flies during subjective night; in fact they were maintained constitutively low throughout the subjective day and night, thus demonstrating that an acute reduction of the excitability of the PDF circuit directly affected neuropeptide accumulation and/ or release (Figure 6C). Given that rhythmic behavior could be restored upon shutting down KIR expression (Figure 3) we predicted that oscillations in PDF levels should accompany that recovery. As shown in Figure 6D, cycling PDF levels were observed both in control and previously silenced brains, with significant differences between CT2 and CT14 in both groups.
Taken together these results indicate that reducing the excitability of the PDF circuit, specifically in the adult, does not cause long-term effects on two well characterized clock outputs, PDF level and locomotor behavior.
Discussion
Work from many laboratories has shaped the current view of the molecular clockworks. Although the relative contribution of specific molecular mechanisms is still a matter of debate [37] it is clear that a transcriptional/translational negative feed-back loop is key to give rise and sustain molecular oscillations. Years ago it was proposed that circadian oscillations arise from interactions between ion transport systems across the cell membrane and the resulting ion concentration gradients (reviewed in [38]). In fact, in support of such possibility, electrical silencing of a key pacemaker circuit in Drosophila stopped the free-running clock both in the larval [27] and adult brains [26, 34], leading the authors to propose that active ionic conductances are an essential component of this cellular mechanism [38]. One potential caveat of those experiments is that they rely on the long term expression of ion channels from early circuit development, which could not only trigger compensatory mechanisms to avoid net changes in excitability (reviewed in [39]), but also cell death [40].
To more precisely examine the connection between the membrane and the molecular clock we restricted KIR expression to adult stages. Such genetic manipulation rendered the flies as behaviorally arrhythmic as those expressing the channel from early circuit development (Figure 1), and prevented action potential firing to a similar extent (Figure 2). Interestingly, however, no effects were observed in the pace of the molecular oscillations after several days under free-running conditions (i.e., on DD4, Figure 5A, and even in DD9, Fig. S3Ci-iii), which along with the reversibility observed once kir 2.1 expression was turned off in several affected outputs (free-running locomotor behavior, PDF immunoreactivity), strongly support the notion of an unaltered molecular clock during the “silenced” phase. In favor of an alternative interpretation of the original observations, we noticed a run-down in the molecular oscillations –an even no oscillations whatsoever- after prolonged KIR expression (Figure 5B and S3Civ-vi), opening the possibility that long-term changes on intrinsic properties of the neurons likely through the alteration of second messenger cascades, as it has been shown in a different but also extreme condition [41], ultimately impinge upon cell viability, and thus indirectly result in abnormal clock function. In fact, adult-restricted silencing of the PDF circuit triggered morphological changes in second order processes giving rise to a less complex arborization pattern (Figure 6 A-B); it follows that a more severe treatment, such as long term KIR expression, could result in stronger structural phenotypes indicative of defective cell physiology [39].
In addition, constantly low PDF levels could potentially account for the progressive run-down in molecular oscillations. Along this line, Taghert and colleagues showed that, in the absence of PDF, the sLNvs eventually desynchronize, becoming evident by DD6 [11]. Since acute electrical silencing of PDF neurons clamps the neuropeptide to trough levels that are insufficient to sustain synchronicity in dorsal oscillators (data not shown), affecting excitability for longer terms could eventually result in reduced amplitude oscillations and internal desynchronization in central pacemakers. In the mammalian SCN, evidence from different laboratories has lent support to the notion that membrane excitability or, more precisely, a certain degree of depolarization and activation of Ca2+ and cAMP second messenger cascades, may be required for sustained molecular oscillations [42-44]. These observations underscore that intercellular communication is important to reinforce high amplitude molecular oscillations through synchronization of independent cellular oscillators [21, 45], as opposed to be an essential component within the mechanism responsible for the generation of the molecular oscillations. Interestingly, it has been reported that, in a subset of SCN neurons, molecular oscillations of a circadian reporter still takes place even in the absence of synaptic connectivity [46], highlighting the autonomy of the molecular oscillator.
Circadian control of membrane excitability as a regulator of clock outputs
Adult-restricted silencing of the PDF circuit impairs locomotor behavior to a similar extent compared to constitutive silencing them, demonstrating that regardless of the overall levels of KIR achieved through the inducible system, short-term expression effectively prevents communication with other neuronal targets. Such scenario offers the possibility to identify the direct consequences of reducing the excitability of the PDF circuit in a defined temporal window. Surprisingly, despite kir expression being limited to the adult brain, it correlated with axonal arbors of reduced complexity throughout the day in the dorsal protocerebrum, even though the circadian remodeling phenomenon continued to take place (Figure 6A-B). The latter lends further support to the notion that no effect on the pace of the molecular oscillator became evident during the acutely silenced phase.
In addition, adult-restricted silencing correlated with non-cycling PDF levels. PDF is transported along the axonal tract in large dense core vesicles (DCV), which apparently are released outside of the chemical synapse [47]. Although no precise information is available on PDF, it is expected for neuropeptides to be released after high frequency stimulation, suggesting that during the silenced phase the DCV would accumulate in the axonal terminals. Nitabach and colleagues proposed that the trough of PDF accumulation at dusk might represent the depletion of the PDF readily releasable pool, and it correlates with the time of day when the sLNvs are most hyperpolarized [20]. Interestingly, despite no release is expected to occur while KIR is expressed, PDF intensity at the axonal terminals stayed at trough levels throughout the day, underscoring that reduced excitability affected additional steps such as peptide synthesis, processing or transport (Figure 6C). In favor of this possibility, hyperexcitation of the PDF circuit correlates with constantly high (daytime) PDF levels at the dorsal protocerebrum [25]. Moreover, once kir expression was turned off PDF levels resume to cycle (Figure 6D), indicating a direct modulatory effect of membrane excitability on this specific output. In line with a defective output from the sLNvs [12, 36] desynchronization of dorsal oscillators (i.e., the DN1s) became evident as early as in DD4 (data not shown). Gaining more insight into the mechanisms of communication within the circadian network [10, 11, 48], as well as those connecting the cell membrane with the molecular clock [49], will provide a better understanding on how these components interact to sustain temporal and spatial order to shape rhythmic overt behavior.
Conclusions
Taken together these results confirm that in Drosophila altering membrane excitability mainly affects the output of pacemaker cells and thus intercellular communication, as it is the case in the eye of the mollusk Bulla [50] and the rodent SCN [31], highlighting the degree of conservation in the mechanisms underlying the biological clock in distant organisms.
Experimental procedures
Strains and fly rearing
pdf-GAL4 and UAS-CD8GFP were obtained from the Bloomington Stock Center. UAS-egfp-kir2.1 [33] was provided by Dr. Blau (NYU, USA). pdf-GeneSwitch (pdf-GS) was generated in our laboratory (see below). Flies were grown and maintained at 25 °C in vials containing standard cornmeal medium under 12:12 h light: dark cycles, with the exception of those including RU486 (mifepristone, Sigma, USA). In those experiments, food was mixed with RU in 80% ethanol to a final concentration of 200 μg/ml or with the same amount of ethanol (vehicle) in control treatments. To deliver RU to pupae an interior small incision in the pupal case was performed, and then pupae were immersed for 2 minutes in 1 ml of RU (4 μg/μl) as in [32].
Generation of the pdf-GS transgenic line
A 2367 bp fragment containing the pdf promoter region (a generous gift of Dr. J. Park, Univ. of Tennessee, Knoxville, TN) was amplified from the P2.4-pBS plasmid [8] using the oligonucleotides 5’- GCG GCC GCG GAT CCG TGG GTT TCA TCC TTA CC -3’ (to add a NotI restriction site) and antisense 5’- ACG CGT GGA TCC GTG GGT TTC ATC C -3’ (adding the MluI restriction site). The fragment was cloned into Zero blunt TOPO PCR (Invitrogen, USA), then digested with NotI/MluI and subcloned into the pSwitch#1 (Drosophila Genomics Resource Center, Indiana University, IN) vector between NotI (144), and MluI (221), then injected into y w embryos with pUChpsΔ2–3 helper plasmid. The genetic background was changed crossing the pdf-GS transgenic line to w1118 for 6 generations to carry out behavioral analysis.
Locomotor behavior analysis
Flies were entrained to 12 h LD cycles during their entire development, and newly eclosed adult males were placed in glass tubes containing standard food (supplemented with 200 μg/ml RU 486 or vehicle, as indicated in each experiment) and monitored for activity with infrared detectors and a computerized data collection system (TriKinetics, Waltham, MA). Activity was monitored in LD conditions for 3-4 days, followed by constant darkness for at least a week (DD1-8). Phenotype rescue was assessed in flies that after spending one week on DD (DD1-8) were transferred to a fresh test tube under safe red light and monitored for additional ten days (DD9-18). Period and rhythmicity were estimated using ClockLab software (Actimetrics, Evanston, IL). Flies with a single peak over the significance line (p <0.05) in a Chi-Squared analysis were scored as rhythmic, which was confirmed by visual inspection of the actograms. Flies classified as weakly rhythmic as in Ceriani et al. (2002) were not taken into account for average period calculations. Average rhythmicity for independent genotypes was evaluated employing a one-way ANOVA with a Bonferroni post-hoc test. The corresponding p values are included in the figure legends.
Electrophysiology
Flies were kept at 25 °C in 12 h LD cycles. pdf-GS flies were induced with RU486 in the food for 7 days. pdf-GAL4 containing flies were kept on regular food and dissected 7 days after eclosion. Female flies were anesthetized with a brief incubation of the vial on ice; brain dissection was performed on external recording solution which consisted of (in mM): 101 NaCl, 3 KCl, 1 CaCl2, 4 MgCl2, 1.25 NaH2PO4, 5 glucose, and 20.7 NaHCO3, pH 7.2, with an osmolarity of 250 mmol/kg. After removal of the proboscis, air sacks and head cuticle, the brain was glued ventral side up to a sylgard-covered coverslip using a few μl of tissue adhesive 3M Vetbond. All recordings were performed under a drop of external recording solution with no perfusion and between ZT2 and ZT9. LNvs were visualized by GFP fluorescence using a Leica DM LFS upright microscope. The lLNvs were distinguished from the sLNvs by their size and anatomical location. The surface glia directly adjacent to the lLNvs was digested with protease XIV solution (10mg/ml, Sigma-Aldrich P5147) to allow the access of the recording electrode. Once the fluorescent lLNvs were identified, cells were patched under IR-DIC using a Hamamatsu ORCA-ER camera and Wasabi software. Cell-attached recordings were performed using borosilicate glass pipettes (KG-33, King Precision Glass, Inc.) pulled to 8-9 MΩ using a Narishige PP-830 vertical puller. Data were acquired using an Axopatch 200B amplifier, Digidata 1322A and pClamp 9.0 software (Molecular Devices). Cell-attached configuration was achieved by gentle suction and recordings were performed in voltage-clamp mode with no hold. Recording pipettes were filled with patch solution containing (in mM): 102 potassium gluconate, 17 NaCl, 0.085 CaCl2, 0.94 EGTA and 8.5 HEPES, pH 7.2 with an osmolarity of 235 mmol/kg. To prevent a perforated patch effect produced by the high density of Kir2.1 channels in the patch we added 200 μM BaCl2 to the internal solution. Action potential currents were quantified using MiniAnalysis 6.0.3 software (Synaptosoft), for at least 30 sec. No AP currents were obtained in the kir2.1 expressing neurons (in some cases up to 8 min were recorded with no AP currents observed). To confirm that the kir2.1 expressing neurons were viable we recorded firing rate with the addition of 200 μM BaCl2 to the external solution. Traces shown in Figure 2 were digitally filtered after acquisition using a low-pass Bessel filter at 2000Hz.
Generation of rat anti PDF antiserum
An N-terminal amidated synthetic PDF peptide generated by NeoMPS (France) was conjugated to BSA (in a 1:3 and 1:30 ratio) using glutaraldehyde, dialyzed against PBS, and injected in to rats. Specificity of the raised antisera in whole mount adult brain stainings was determined through comparison against rabbit anti-PDF (custom made by NeoMPS, [29]). The pattern is identical although on occasion the rat anti-PDF shows a non-specific labeling in the central and ventral region in the adult brain.
Dissection and Immunofluorescence
For pdf-GS activation or inactivation experiments, animals were sacrificed at different times after being transferred to fresh vials supplemented with RU (0, 12, 16 and 24 h) or after being removed from RU containing food (0, 24, 48 and 72 h), respectively. Adult heads were fixed with 4% formaldehyde in 100mM phosphate buffer pH7.5 for 45 min at room temperature (RT). Brains were dissected and rinsed three times in PBS with 0.6% Triton X-100 (PT) for 15 min. Samples were blocked in 7% normal goat serum for 1 h in PT, and incubated with primary antibody at 4°C overnight. The primary antibodies employed were rabbit anti-GFP 1:500 (Invitrogen, USA), and homemade rat anti-Drosophila-PDF (1:500). Samples were washed 4×15 min in PT, and incubated with secondary antibody at 1:250 for 2h at RT; secondary antibodies were washed 4×15 min in PT and mounted in 80% glycerol in PT. The secondary antibodies used were Cy2-conjugated donkey anti-rabbit and Cy3-conjugated donkey anti-rat (Jackson InmunoResearch, USA). Images were taken either on a Zeiss Pascal LSM or a Zeiss LSM 510 Meta confocal microscope. The proportion of brains with PDF neurons expressing GFP was calculated at each timepoint.
For timecourse analysis, synchronized flies were released into free running conditions and brains were dissected on DD4, DD9 or DD13. Brains were fixed in 15-min windows centered on each reported circadian time. Immunohistochemistry was performed as previously described but the PT buffer contained 0.1% Triton X-100. The primary antibodies were as follows: guinea pig anti-PAP (1:500), rat anti-PDF (1:500), mouse anti-PDF (DSHB, 1:20) and rabbit anti-PER (1:1000), generous gifts of Drs. P. Taghert and R. Stanewsky (for PAP and PER, respectively). The secondary antibodies were Cy5-conjugated donkey anti-mouse, Cy3-conjugated donkey anti-guinea pig, Cy3-conjugated donkey anti-rat and Cy2-conjugated donkey anti-rabbit (Jackson ImmunoResearch, USA). Nuclear and cytoplasmic quantification of PER immunoreactivity in single focal planes was performed blind employing Image J (Downloaded from the NIH website (http://rsbweb.nih.gov/ij/). Statistical analysis included a one-way ANOVA with a Bonferroni post hoc test.
For the analysis of PDF levels and assessment of the structural plasticity of the PDF circuit, immunohistochemistry was performed as described above, except that brains were rinsed 4×5min in PBS plus 0.1% Triton X-100 after dissection. The primary antibodies used were rabbit anti-GFP 1:500 (Invitrogen, USA) and rat anti-PDF 1:500. The secondary antibodies used were Cy2-conjugated donkey anti-rabbit and Cy3-conjugated donkey anti-rat (Jackson InmunoResearch, USA). For the analysis of PDF immunoreactivity at the dorsal projections all pictures were taken employing the same confocal settings and quantification was performed as previously reported [29]. For experiments involving immunohistochemistry quantifications were made blind to the operator. A two way ANOVA with Bonferroni multiple comparison test was used to calculate the significance between the mean PDF intensities for each group. Logarithm transformation was applied to DD13 data to fulfill ANOVA requirements. Structural plasticity was analyzed as reported [29]. The number of total axonal crosses was compared by a two way ANOVA with genotype and circadian time as factors. Normality was tested using Shapiro-Wilks test and the homogeneity of variance was assessed with Levene’s test. p< 0.05 was considered statistically significant. Statistical analyses were performed with InfoStat version 2009 (Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina).
Supplementary Material
Acknowledgments
We are grateful to Jae Park for sharing the plasmid containing the pdf promoter, and to Adriana Perez for advice on statistical analysis. We thank the Bloomington Stock Center for fly stocks, and Paul Taghert, Ralf Stanewsky and the Developmental Studies Hybridoma Bank for antibodies. We are indebted to Alejandro Schinder for critical reading of the manuscript, to Emiliano Merlo for helpful discussion, and to Diego Galagovsky for the art work. MFC and NIM are members of the Argentine Research Council (CONICET). JB, EJA and EJB were/are supported by a graduate fellowship from CONICET. ADC was supported by a graduate fellowship from the Agencia Nacional para la Promoción de Ciencia y Tecnología (ANPCyT, Argentina). JB is currently supported by a postdoctoral EMBO fellowship. This work was supported by a grant from the ANPCyT, Argentina (PICT2006-1249) and by a FIRCA-NIH grant (1R03TW008342) to MFC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–R93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Hamasaka Y, Nassel DR. Mapping of serotonin, dopamine, and histamine in relation to different clock neurons in the brain of Drosophila. J Comp Neurol. 2006;494:314–330. doi: 10.1002/cne.20807. [DOI] [PubMed] [Google Scholar]
- 3.Shafer OT, Helfrich-Forster C, Renn SC, Taghert PH. Reevaluation of Drosophila melanogaster’s neuronal circadian pacemakers reveals new neuronal classes. J Comp Neurol. 2006;498:180–193. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frenkel L, Ceriani MF. Circadian Plasticity: From structure to behavior. International Review of Neurobiology. 2011;99:107–138. doi: 10.1016/B978-0-12-387003-2.00005-7. [DOI] [PubMed] [Google Scholar]
- 5.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 6.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 7.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Helfrich-Forster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci U S A. 2000;97:3608–3613. doi: 10.1073/pnas.070036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helfrich-Forster C, Tauber M, Park JH, Muhlig-Versen M, Schneuwly S, Hofbauer A. Ectopic expression of the neuropeptide pigment-dispersing factor alters behavioral rhythms in Drosophila melanogaster. J Neurosci. 2000;20:3339–3353. doi: 10.1523/JNEUROSCI.20-09-03339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biol. 2003;1:E13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández MP, Chu J, Villella A, Atkinson N, Kay SA, Ceriani MF. Impaired clock output by altered connectivity in the circadian network. Proc Natl Acad Sci U S A. 2007;104:5650–5655. doi: 10.1073/pnas.0608260104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wulbeck C, Grieshaber E, Helfrich-Forster C. Pigment-dispersing factor (PDF) has different effects on Drosophila’s circadian clocks in the accessory medulla and in the dorsal brain. J Biol Rhythms. 2008;23:409–424. doi: 10.1177/0748730408322699. [DOI] [PubMed] [Google Scholar]
- 14.Lear BC, Zhang L, Allada R. The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior. PLoS Biol. 2009;7:e1000154. doi: 10.1371/journal.pbio.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheeba V, Sharma VK, Gu H, Chou YT, O’Dowd DK, Holmes TC. Pigment dispersing factor-dependent and -independent circadian locomotor behavioral rhythms. J Neurosci. 2008;28:217–227. doi: 10.1523/JNEUROSCI.4087-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22:9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW. Circadian regulation of gene expression systems in the Drosophila head. Neuron. 2001;32:657–671. doi: 10.1016/s0896-6273(01)00515-3. [DOI] [PubMed] [Google Scholar]
- 18.Park D, Griffith LC. Electrophysiological and anatomical characterization of PDF-positive clock neurons in the intact adult Drosophila brain. J Neurophysiol. 2006;95:3955–3960. doi: 10.1152/jn.00117.2006. [DOI] [PubMed] [Google Scholar]
- 19.Sheeba V, Gu H, Sharma VK, O’Dowd DK, Holmes TC. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol. 2008;99:976–988. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci. 2008;28:6493–6501. doi: 10.1523/JNEUROSCI.1503-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itri JN, Vosko AM, Schroeder A, Dragich JM, Michel S, Colwell CS. Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol. 2010;103:632–640. doi: 10.1152/jn.00670.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci. 2005;8:650–656. doi: 10.1038/nn1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 27.Nitabach MN, Sheeba V, Vera DA, Blau J, Holmes TC. Membrane electrical excitability is necessary for the free-running larval Drosophila circadian clock. J Neurobiol. 2005;62:1–13. doi: 10.1002/neu.20053. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Cao G, Pavlicek B, Luo X, Nitabach MN. Phase coupling of a circadian neuropeptide with rest/activity rhythms detected using a membrane-tethered spider toxin. PLoS Biol. 2008;6:e273. doi: 10.1371/journal.pbio.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández MP, Berni J, Ceriani MF. Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol. 2008;6:e69. doi: 10.1371/journal.pbio.0060069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz WJ, Gross RA, Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci U S A. 1987;84:1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Cao G, Nitabach MN. Electrical silencing of PDF neurons advances the phase of non-PDF clock neurons in Drosophila. J Biol Rhythms. 2008;23:117–128. doi: 10.1177/0748730407312984. [DOI] [PubMed] [Google Scholar]
- 35.Owen JM, Quinn CC, Leach R, Findlay JB, Boyett MR. Effect of extracellular cations on the inward rectifying K+ channels Kir2.1 and Kir3.1/Kir3.4. Exp Physiol. 1999;84:471–488. [PubMed] [Google Scholar]
- 36.Lear BC, Lin JM, Keath JR, McGill JJ, Raman IM, Allada R. The ion channel narrow abdomen is critical for neural output of the Drosophila circadian pacemaker. Neuron. 2005;48:965–976. doi: 10.1016/j.neuron.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Rosbash M. The implications of multiple circadian clock origins. PLoS Biol. 2009;7:e62. doi: 10.1371/journal.pbio.1000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nitabach MN, Holmes TC, Blau J. Membranes, ions, and clocks: testing the njus-sulzman-hastings model of the circadian oscillator. Methods Enzymol. 2005;393:682–693. doi: 10.1016/S0076-6879(05)93036-X. [DOI] [PubMed] [Google Scholar]
- 39.Hodge JJ. Ion channels to inactivate neurons in Drosophila. Front Mol Neurosci. 2009;2:13. doi: 10.3389/neuro.02.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadeau H, McKinney S, Anderson DJ, Lester HA. ROMK1 (Kir1.1) causes apoptosis and chronic silencing of hippocampal neurons. J Neurophysiol. 2000;84:1062–1075. doi: 10.1152/jn.2000.84.2.1062. [DOI] [PubMed] [Google Scholar]
- 41.Harrisingh MC, Wu Y, Lnenicka GA, Nitabach MN. Intracellular Ca2+ regulates free-running circadian clock oscillation in vivo. J Neurosci. 2007;27:12489–12499. doi: 10.1523/JNEUROSCI.3680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci. 2005;25:7682–7686. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nahm SS, Farnell YZ, Griffith W, Earnest DJ. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J Neurosci. 2005;25:9304–9308. doi: 10.1523/JNEUROSCI.2733-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 45.Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152:165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci U S A. 2009;106:16493–16498. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yasuyama K, Meinertzhagen IA. Synaptic connections of PDF-immunoreactive lateral neurons projecting to the dorsal protocerebrum of Drosophila melanogaster. J Comp Neurol. 2010;518:292–304. doi: 10.1002/cne.22210. [DOI] [PubMed] [Google Scholar]
- 48.Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- 49.Berni J, Beckwith EJ, Fernandez MP, Ceriani MF. The axon-guidance roundabout gene alters the pace of the Drosophila circadian clock. Eur J Neurosci. 2008;27:396–407. doi: 10.1111/j.1460-9568.2007.06010.x. [DOI] [PubMed] [Google Scholar]
- 50.Michel S, Geusz ME, Zaritsky JJ, Block GD. Circadian rhythm in membrane conductance expressed in isolated neurons. Science. 1993;259:239–241. doi: 10.1126/science.8421785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


