Abstract
Small molecules designed to specifically activate or inactivate protein functions have been useful to study biological processes. PROTACS are small molecule chimera which comprise a ligand and a peptide recognition motif for an E3 ligase. These novel reagents exploit the ubiquitin-mediated proteasome degradation pathway to target the ligand-bound protein for intracellular degradation. Here, we report that an estrogen receptor (ER)-targeting PROTACS that causes degradation of ER is able to potently inhibit endothelial cell differentiation in a three-dimensional angiogenic sprouting assay. These findings support the use of ER-targeting PROTACS as probes of angiogenesis.
Keywords: PROTACS, Angiogenesis, Estrogen, Receptor, Endothelial cell differentiation
PROTACS (PROteolysis TArgeting Chimeric MoleculeS) are novel chemical reagents that artificially target proteins of interest to the ubiquitin-proteasome pathway for their destruction.1,2 PROTACS are chemical chimera comprising of a small molecule ligand which is covalently linked to a peptide recognition motif for a specific E3 ubiquitin ligase. The small molecule ligand serves as a ‘bait’ to bind to its intracellular ‘receptor’, which makes available the peptide motif to be recognized by the E3 ubiquitin ligase. When cells are treated with PROTACS, the PROTAC-bound protein is artificially recruited for ubiquitination via the E3 ligase recognition motif that subsequently allows the complex to be signaled for degradation by 26S proteasome. Importantly, because PROTACS can be used to control intracellular levels of specific proteins these novel reagents are providing us with a direct means to probe protein function in chemical genetic studies.3,4
The von Hippel–Lindau tumor suppressor protein (pVHL) functions as an E3 ubiquitin ligase to target hypoxia inducible factor (HIF-1α) for proteasomal degradation.5 The degradation of HIF-1α is triggered by the hydroxylation of a conserved proline residue (Pro564) that allows it to be recognized and multi-ubiquitinated by the pVHL E3 ligase.6,7 Under normoxic conditions, HIF-1α is constitutively ubiquitinated and degraded.8,9 Recently, we and others have shown that the pVHL recognition motif of HIF-1α containing a hydroxyproline residue when incorporated into cell-permeable PROTACS can successfully target a variety of intracellular proteins for degradation using their specific ligands as baits.10–12 Thus far, PROTACS have not yet been employed to perturb biological processes in vivo.
Angiogenesis, which is the growth of new blood vessels from preexisting vasculature, occurs during normal development and wound healing. This process is a pathological manifestation in numerous diseases; angiogenesis supports expansion of solid tumors, the development of pannus in joints of patients with rheumatoid arthritis and it contributes to endometriosis.13 During the angiogenic process, vascular endothelial cells that line the lumen of blood vessels differentiate from their quiescent state and invade the interstitial stroma to form a branched vascular network.14 This morphogenic process is highly complex and involves the coordinated activities of endothelial cell migration, invasion, branching, assembly, and maturation into vessel lumens.15 The three-dimensional (3D) endothelial cell sprouting assay (3D-ECSA) recapitulates many of these processes and features the differentiation of endothelial cells into sprouting structures within a 3D matrix of fibrin or collagen I.16 Thus, the 3D-ECSA is a very useful model to study endothelial cell behavior in 3D matrices.17 We have recently shown that the 3D-ECSA can also be exploited as a screening tool to identify new classes of angiogenesis inhibitors.18
The endogenous small molecule hormone 17β-estradiol (E2) promotes angiogenesis through multifactorial mechanisms.19 E2 induces vascular endothelial cell proliferation and migration.20 E2 also promotes the upregulation of basic fibroblast growth factor21 and vascular endothelial cell growth factor (VEGF) and its receptors. 22,23 More recently, E2 was found to induce the angiogenic switch through downregulation of the expression of soluble decoy receptor for VEGF (sVEGF-R1) in estrogen receptor (ER)-positive but not in ER-negative breast cancer cell lines.24 Taken together, these findings have suggested that ER is a potential target for anti-angiogenesis and understanding its unique role in endothelial cells with the aid of specific antagonists can help us in identifying new treatments for ER-dependent disease mechanisms.
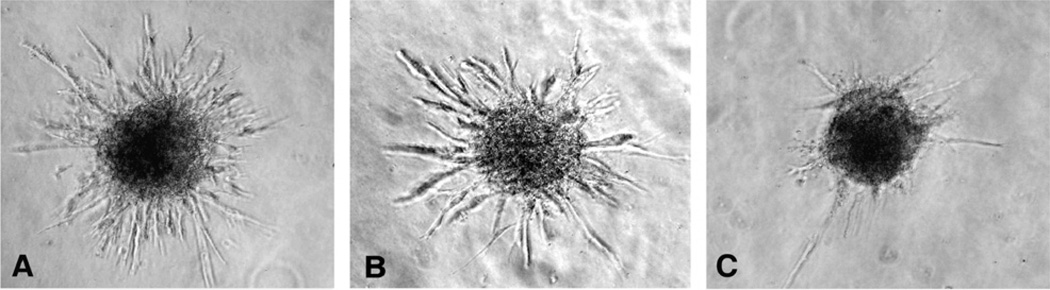
Herein, we report the development of cell-permeable PROTACS that target estrogen receptor-α (ERα) for ubiquitination and degradation (Scheme 1). We previously showed that the first generation ER-targeting PROTAC, E2-octa, can promote ERα degradation in cultured cells.10 In this investigation, we have applied the PROTAC concept to probe angiogenesis by focusing our investigations on the study of endothelial cell differentiation. Toward this end, we tested E2-octa25 and its inactive analog E2-octa-[Ala] (that does not interact with pVHL)10 in the 3D-ECSA.26 We show that the HIF-1α octapeptide by itself and E2-Octa-[Ala] do not interfere with VEGF-induced sprouting of human umbilical vein endothelial cells (HUVECs), whereas E2-Octa is highly efficient in inhibiting angiogenic sprouting (Fig. 1). These findings reveal that the entire chemical chimera comprising E2 ligand-HIF octapeptide needs to be intact to exert biological activity, because if either the E2 ligand is eliminated or the chemical chimera is altered by introducing a ProOH → Ala mutation in the octapeptide the sprouting inhibitory activity is abrogated. This result is in keeping with our previous findings where we showed that neither the octapeptide nor E2-Octa-[Ala] support the degradation of ERα in cultured cells.10
Scheme 1.
Estradiol (E2)-based PROTAC containing a pentapeptide derived from HIF-1α recruits estrogen receptor (ER) to the pVHL complex for ubiquitination and degradation.
Figure 1.
Endothelial cell spheroids were seeded in collagen I gels in a 96 well plate and stimulated with vascular endothelial growth factor (VEGF; 20 ng/ml) to induce angiogenic sprouting. In replicate wells, VEGF-treated spheroids were co-incubated with 12 µM of HIF-1α octapeptide, E2-octa-[Ala] or E2-octa. Representative photographic images of spheroids taken after 20 h show invasive growth of vessel structures with octapeptide (A) or E2-octa-[Ala] (B) co-treatment, but potent inhibitory effect with E2-octa (C) co-treatment.
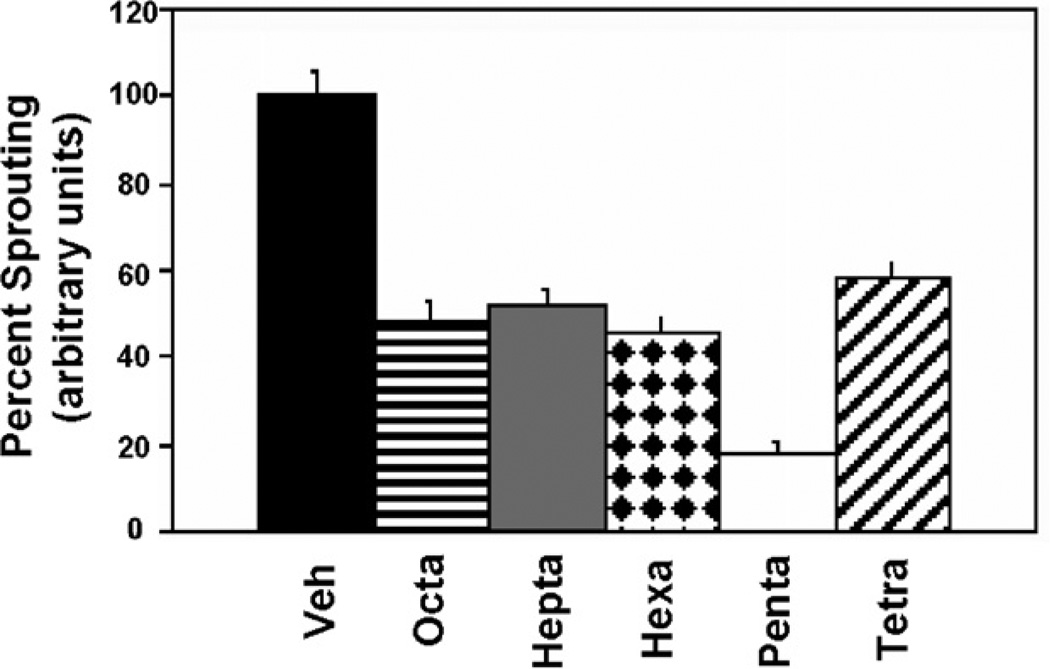
We next wanted to identify the optimal size of the HIF-1α peptide motif that would confer the ERα-mediated anti-angiogenic response. For this study, the length of the HIF-1α peptide was reduced sequentially by one amino acid from both N- and C-terminal ends. These newly derived E2-PROTACS were subsequently tested in the 3D-ECSA and sprouting in response to VEGF was quantified. We discovered that E2-octa, E2-hepta, and E2-hexa inhibited angiogenic sprouting to approximately the same extent (~50%), whereas E2-penta was the most potent inhibitor, decreasing sprouting morphogenesis by about 80% compared to vehicle-treated controls (see Fig. 2).
Figure 2.
E2-penta exerts the most potent inhibition of sprouting. VEGF-stimulated spheroids were co-treated with 3 µM of E2-PROTAC, which contain the HIF-1α peptide of differing length. The percentage of angiogenic sprouting was quantified by measuring total number and length of sprouts as done previously.18
Interestingly, E2-tetra was only as efficient as E2-octa in this assay, suggesting that retention of the pentapeptide core was required for optimal inhibition of angiogenic sprouting.
Finally, to demonstrate that E2-penta indeed retains ERα targeting activity in endothelial cells similar to that of the parental E2-octa PROTAC,10 we investigated the abundance of ERα in E2-penta-treated HUVECs by Western blotting.27 As seen in Figure 3, incubation of HUVECs for 24 h with E2-penta at 2 µM totally abrogates ERα expression, whereas the lower dose of E2-penta does not interfere with intracellular levels of ERα or inhibit angiogenic sprouting (data not shown). It is of interest that E2-penta exerts its biological activity in HUVECs at 10- to 20-fold lower concentrations than in MCF-7 cancer cell lines (data not shown). Collectively, these findings suggest that the endothelial cell-targeting activity of E2-penta, which occurs at low doses has potential for application in diverse angiogenic disorders.
Figure 3.
HUVECs were treated with vehicle (Con) or E2-penta at 0.5 and 2 µM for 24 h. Total cell protein was fractionated on polyacrylamide gels and Western blotted using a polyclonal ERα-specific antibody. The blots were also probed with anti-β-actin antibody to control for protein loading.
Taken together, these results show for the first time that E2-PROTACS are novel chemical genetic probes for studying angiogenic differentiation. The further refinement of E2-penta to a metabolically stable analog, which is currently underway, should allow us to exploit this novel approach to the study of angiogenic mechanisms in animal models of diseases.
Acknowledgements
We are grateful to the Department of Ophthalmology and Visual Sciences (University of Kentucky) for generous start-up funds to R.M. and the Kentucky Lung Cancer Research Program for financial support to K.K.
References and notes
- 1.Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8554. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakamoto KM, Kim KB, Verma R, Ransick A, Stein B, Crews CM, Deshaies RJ. Mol. Cell. Proteomics. 2003;2:1350. doi: 10.1074/mcp.T300009-MCP200. [DOI] [PubMed] [Google Scholar]
- 3.Crews CM, Splittgerber U. Trends Biochem. Sci. 1999;24:317. doi: 10.1016/s0968-0004(99)01425-5. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber SL. Bioorg. Med. Chem. 1998;6:1127. doi: 10.1016/s0968-0896(98)00126-6. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. Nature. 1999;399:271. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 6.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. Cell. 2001;107:43. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 7.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Science. 2001;292:468. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 8.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Nat. Cell Biol. 2000;7:423. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 9.Tanimoto K, Makino Y, Pereira T, Poellinger L. EMBO J. 2000;19:4298. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Baek SH, Ho A, Kim K. Bioorg. Med. Chem. Lett. 2004;14:645. doi: 10.1016/j.bmcl.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D, Baek SH, Ho A, Lee H, Jeong YS, Kim K. Comb. Chem. High Throughput Screening. 2004;7:691. doi: 10.2174/1386207043328364. [DOI] [PubMed] [Google Scholar]
- 12.Schneekloth JS, Jr, Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, Sakamoto K, Crews CM. J. Am. Chem. Soc. 2004;126:3748. doi: 10.1021/ja039025z. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Nat. Med. 1995;1:27. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 14.Risau W. Nature. 1997;386:671. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 15.Jain RK. Nat. Med. 2003;9:685. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 16.Korff T, Augustin HG. J. Cell Biol. 1998;143:1341. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haspel HC, Scicli GM, McMahon G, Scicli AG. Microvasc. Res. 2002;63:304. doi: 10.1006/mvre.2001.2383. [DOI] [PubMed] [Google Scholar]
- 18.Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP, Rougas J, Pribluda VS. Angiogenesis. 2004;7:115. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 19.Rubanyi GM, Johns A, Kauser K. Vasc. Pharmacol. 2002;38:89. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 20.Cid MC, Schnaper HW, Kleinman HK. Ann. N.Y. Acad. Sci. 2002;966:143. doi: 10.1111/j.1749-6632.2002.tb04211.x. [DOI] [PubMed] [Google Scholar]
- 21.Rusnati M, Casarotti G, Pecorelli S, Ragnotti G, Presta M. Gynecol. Oncol. 1993;48:88. doi: 10.1006/gyno.1993.1014. [DOI] [PubMed] [Google Scholar]
- 22.Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Makela S, Chiappetta C, Stancel GM. Environ. Health Perspect. 2000;108:785. doi: 10.1289/ehp.00108s5785. [DOI] [PubMed] [Google Scholar]
- 23.Suzuma I, Mandai M, Takagi H, Suzuma K, Otani A, Oh H, Kobayashi K, Honda Y. Invest. Ophthalmol. Vis. Sci. 1999;40:2122. [PubMed] [Google Scholar]
- 24.Elkin M, Orgel A, Kleinman HK. J. Natl. Cancer Inst. 2004;96:875. doi: 10.1093/jnci/djh140. [DOI] [PubMed] [Google Scholar]
- 25.The peptides used in this study and the synthesis of PROTACS were according to previously described methods.1,10 The amino acid sequences of E2-PROTAC are E2-octa (previously named E2-SMPI):10 NH2-Met-Leu-Ala-ProOH-Tyr-Ile-Pro-Met-COOH; E2-Octa-[Ala] (previously named E2-SMPI[ProOH → Ala]): NH2-Met-Leu-Ala-Ala-Tyr-Ile-Pro-Met-COOH; E2-hepta: NH2-Met-Leu-Ala-ProOH-Tyr-Ile-Pro-COOH; E2-Hexa: NH2-Leu-Ala-ProOH-Tyr-Ile-Pro-COOH: E2-penta: NH2-Leu-Ala-ProOH-Tyr-Ile-COOH: E2-tetra: NH2-Ala-ProOH-Tyr-Ile-COOH.
- 26.Endothelial cell spheroids were generated from HUVECs (Cascade Biologicals, Portland, OR) essentially according to previously published methods of Korff and Augustin.16 The spheroids (4–6/well) were distributed in 96-well plates in collagen I matrix for the 3D-ECSA. Cell culture medium was added to each well along with 20 ng/ml VEGF in the presence and absence of the individual PROTAC. The 3D cultures were incubated in tissue culture chambers at 37 °C in 5% CO2 for 20 h. Photographic images of spheroids were taken using a digital camera attached to an inverted microscope with 10X objective. Sprouting was quantified from digital images according to our previously published method.18
- 27.HUVECs were cultured in 100 mm dishes to 80% confluence in Medium M199 containing 10% fetal bovine serum18 and treated with DMSO (vehicle) or E2-penta for 24 h. Cell lysates were prepared and equal amounts of total protein subjected to Western blotting. The detection of ERα has been described previously.10