Abstract
OBJECTIVE
The joint guidelines of the American College of Cardiology and American Heart Association support the use of contrast-enhanced MR angiography (CEMRA) to diagnose the location and degree of stenosis in patients with known or suspected peripheral arterial disease (PAD). The high prevalence of chronic renal impairment in diabetic patients with PAD and the need for high doses of gadolinium-based contrast agents place them at risk for nephrogenic systemic fibrosis. The purpose of our study was to evaluate the accuracy of the rapid technique of quiescent-interval single-shot (QISS) unenhanced MR angiography (MRA) compared with CEMRA for the diagnosis in diabetic patients referred with symptomatic chronic PAD.
SUBJECTS AND METHODS
This prospective two-center study evaluated 25 consecutive diabetic patients with documented or suspected symptomatic PAD. Both centers used identical imaging protocols. Images were independently analyzed by two radiologists. A subgroup analysis was performed of patients who were also assessed with digital subtraction angiography (DSA) as part of the standard-of-care protocol before revascularization.
RESULTS
For this study, 775 segments were analyzed. On a per-segment basis, the mean values of the diagnostic accuracy of unenhanced MRA compared with reference CEMRA for two reviewers, reviewers 1 and 2, were as follows: sensitivity, 87.4% and 92.1%; specificity, 96.8% and 96.0%; positive predictive value, 90.8% and 94.0%; and negative predictive value, 95.5% and 94.6%. Substantial agreement was found when overall DSA results were compared with QISS unenhanced MRA (κ = 0.68) and CEMRA (κ = 0.63) in the subgroup of patients who also underwent DSA. There was almost perfect agreement between the two readers for stenosis scores, with Cohen’s kappa values being greater than 0.80 for both MRA techniques.
CONCLUSION
The results of our study indicate that QISS unenhanced MRA is an accurate noncontrast alternative to CEMRA for showing clinically significant arterial disease in patients with diabetes with symptomatic PAD.
Keywords: contrast-enhanced MR angiography, diabetes, peripheral vascular disease, quiescent-interval single-shot (QISS) MR angiography, unenhanced MR angiography
Diabetes mellitus, a well-known risk factor for progressive atherosclerotic occlusive disease, increases the risk of lower extremity peripheral arterial disease (PAD) by two- to fourfold [1]. It is present in 12–20% of persons with lower extremity PAD [2, 3]. The risk of developing lower extremity PAD is proportional to the severity and duration of diabetes. Furthermore, a susceptibility to infection and the presence of microvascular disease in individuals with diabetes mellitus make it more likely that ischemia in these patients will progress rapidly [4]. The risk of lower extremity amputation is 7–15 times higher in diabetic patients than in nondiabetic patients [5]. Common risk factors for amputation of the foot in diabetic patients include peripheral neuropathy, structural foot deformity, infection, ulceration, and peripheral arterial occlusive disease.
The radiologic approach to the diagnosis of PAD has changed substantially in the past few years [6]. Typically the ankle-brachial index (ABI) is the first noninvasive imaging test performed in the investigation of PAD. However, the ABI may not be accurate in individuals in whom the systolic blood pressure cannot be abolished by inflation of an air-filled blood pressure cuff. The incidence of noncompressible arteries is highest in diabetic and elderly patients. In these individuals, it may be impossible to abolish the systolic pressure signal even with cuff inflation to pressures in excess of 200 mm Hg. Despite the artifactually high systolic pressure, these individuals may have arterial disease [7].
Duplex ultrasound is a well-established noninvasive modality with good sensitivity and specificity for PAD [8]. The performance of this modality can be further improved by the addition of functional (color-flow) imaging [9]. Duplex ultrasound, however, is operator-dependent and does not provide a “road map” of the vascular system that is useful for treatment planning.
CT angiography (CTA) offers the spatial resolution of catheter digital subtraction angiography (DSA) without the risks associated with the invasive procedure [10]. It suffers however from ionizing radiation and the risk of iodinated contrast nephropathy. Nephropathy induced by contrast medium remains one of the most clinically important complications of the use of iodinated contrast medium [11]. In a systematic review of the published literature, contrast-enhanced MR angiography (CEMRA) was shown to be highly accurate for the detection of stenosis greater than 50% or occlusion within the entire lower extremity arterial tree [12].
Until evidence linking the administration of gadolinium-based contrast agents with nephrogenic systemic fibrosis (NSF) was reported [13], many physicians preferred MR angiography (MRA) to CTA and DSA for evaluating patients with chronic kidney disease because of the previously considered safety of these agents. The risks of contrast-induced nephropathy and NSF associated with the prevalence of end-stage chronic renal failure [14] and diabetes [15] in PAD patients have renewed interest in unenhanced MRA techniques for the assessment of symptomatic PAD. Several techniques for unenhanced MRA of the peripheral arteries have been proposed, including gated 2D time of flight; 3D phase contrast [16]; and subtractive techniques such as fresh blood imaging, native spatial and chemical-shift–encoded excitation (SPACE) [17], and flow-sensitive dephasing [18].
Each of the proposed unenhanced MRA techniques has advantages and drawbacks. For instance, the gated 2D time-of-flight technique is simple to implement but scan times are excessive, on the order of an hour; image quality is relatively poor; and artifacts occur from in-plane flow. Subtractive techniques appear promising but are intrinsically sensitive to patient motion. This feature can be problematic given that patients with PAD tend to be elderly and may suffer from restless legs or back pain, which makes it difficult for them to remain motionless. Moreover, subtractive techniques require precise synchronization of the systolic image to the peak velocity flow for each vessel segment, which is both patient- and operator-dependent, and may be difficult to achieve in patients with severe multifocal or bilateral disease. Subtractive techniques may also require calibration of flow-dephasing gradients to achieve optimal image quality.
Compared with subtractive techniques, quiescent-interval single-shot (QISS) MRA is fast, is motion insensitive, and does not require patient-dependent adjustments. The QISS MRA technique evolved because of the clinical necessity and desire to address the practical realities of applying an unenhanced MRA technique to assess patients with PAD. Such a technique must be capable of imaging over a vascular territory more than 1 m long. A short scan time is essential to avoid misregistration artifact. In addition, hemodynamically significant PAD causes large variations in velocity among vessel segments. Patients with PAD often suffer from coexistent cardiac disease and resultant arrhythmias. Finally, imaging of the abdomen and pelvis must contend with respiratory motion. The QISS technique addresses all of these challenges. Scan times to encompass a region from the renal arteries to the feet are less than 7 minutes. In most cases, neither variations in flow velocity nor arrhythmias produce substantial alterations in vascular signal intensity. Because data for each slice are acquired during a single shot extending over only a few hundred milliseconds, QISS is naturally motion resistant. Respiratory motion can be further ameliorated through the use of breath-holding or signal averaging.
The purpose of our study was to evaluate and compare the diagnostic accuracy of the rapid QISS unenhanced MRA technique [19] with that of CEMRA in diabetic patients referred for imaging because of symptomatic chronic PAD.
Subjects and Methods
This prospective two-center study was performed with institutional review board approval.
Patients
Twenty-five consecutive patients (mean age, 67.1 ± 9.9 [SD] years; age range, 47–85 years) with documented or suspected symptomatic chronic PAD underwent peripheral MRA after being referred for evaluation of the lower extremity vessels. The 20 men in the study had a mean age of 66.7 ± 9.2 years (range, 47–84 years), and the five women had a mean age of 68.5 ± 13.8 years (range, 47–85 years). Imaging was performed in both centers on a 1.5-T scanner (Avanto, Siemens Healthcare) equipped with a high-performance gradient system (maximum gradient strength and slew rate of 45 mT/m and 200 T/m/s, respectively). Patients undergoing peripheral MRA studies had point-of-care testing of estimated glomerular filtration rate (GFR) in both centers. Patients with an estimated GFR of less than 30 mL/min/1.73 m2, which precluded CEMRA, were excluded from the study.
MR Angiography Technique
QISS unenhanced MRA is a nonsubtractive thin-slice 2D technique whereby an in-plane pre-saturation pulse is first applied to suppress signal from stationary tissue in the slice. Next, a tracking inferior presaturation pulse is applied to suppress venous signal, followed by an inflow period of several hundred milliseconds. Data are acquired during diastole, when arterial flow is minimal or absent. This approach is substantially different from other currently available unenhanced MRA techniques, such as native SPACE and native true fast imaging with steady-state precession (FISP). Native SPACE is a subtractive 3D technique, whereas native true FISP is a nonsubtractive 3D technique. With both native techniques, preinversion is applied rather than presaturation and data are acquired in a relatively thick slab rather than a thin slice. Native true FISP uses a long inflow time (~ 1 second) and does not attempt to synchronize the period of data acquisition to diastole.
The QISS technique is not currently a commercially available technique. The QISS sequence begins with the application of an in-plane pre-saturation radiofrequency pulse; the longitudinal magnetization always starts at zero irrespective of variations in the length of the cardiac cycle. Signal intensity thus depends on only the inflow time, which is fixed, not on the heart rate or arrhythmias. However, poor ECG gating can sometimes result in artifacts with QISS as well. Gating-related artifacts were insignificant in our patient cohort but are a potential concern in patients with low QRS voltage or poor lead placement. In phantom studies, flow at velocities as low as 2 cm/s could be depicted; however, we have not determined the in vivo lower threshold for the detection of slow flow.
The unenhanced MRA scanning of the lower extremities used the following imaging parameters as outlined in a previous study [19]: TR/TE, 3.0/1.4; quiescent interval, 1.4 ms; inversion time, 228 ms; flip angle, 90°; trigger delay, 100 ms; 2.4-mm effective slice thickness (3.0 mm with 0.6-mm overlap); parallel acceleration factor, 2; bandwidth, 676–694 Hz/pixel; and flip angle, 135° for the fat-suppression pulse. The acquisition matrix ranged from 352 to 400 and, depending on the FOV (34–40 cm), maintained an in-plane spatial resolution of 1 mm (interpolated to 0.5 mm) [19].
Eight groups of 60 slices were acquired to span the peripheral arteries from the level of the distal aorta to the pedal arteries. For a nominal heart rate of 72 beats/min, the total scan time was approximately 6 minutes 40 seconds. A 16-channel peripheral vascular coil, a 6-channel body array coil, and a 4-channel flex coil were placed anterior to the subject and a 24-channel spine array coil was placed posterior to the subject.
The CEMRA protocol consisted of time-resolved CEMRA through the calf and stepping-table MRA for imaging the remainder of the peripheral vascular system. The contrast agent was either gadopentetate dimeglumine (Magnevist, Bayer HealthCare) or gadobenate dimeglumine (MultiHance, Bracco Imaging) administered IV followed by a saline chaser. Images from time-resolved angiography with interleaved stochastic trajectories (TWIST) [20] were obtained through the calf and foot using an injection rate of 2 mL/s for 5–8 mL of contrast agent, a parallel acceleration factor of 2, a TR/TE of 3/1, a spatial resolution of 1.9 (slice thickness) × 1.1 × 1.3 mm, and 9.6 seconds per phase.
For the stepping-table acquisition, a 1- to 2-mL test bolus was used to determine timing. Three stations were acquired before and after contrast administration using a TR/TE of 3/1, flip angle of 20°, FOV of 50 cm, slice thickness of 1.5 mm, in-plane spatial resolution of 1 × 1 mm, parallel acceleration factor of 2, and 22 seconds per station. A dual-phase infusion protocol was used to administer 0.14 mmol/kg of the contrast agent initially at 1.5 mL/s, followed by 0.6 mL/s. The subtracted images were processed using a maximum intensity projection for interpretation. Breath-holding was used for the pelvic station only. Both centers used identical imaging protocols.
Digital Subtraction Angiography Technique
All conventional angiographic examinations were performed using a digital subtraction technique. Nine patients underwent DSA for correlation in 115 segments. The lack of the invasive reference standard for all patients reflects the fact that CEMRA—because of its excellent diagnostic accuracy [21] and noninvasive nature—has in recent years emerged as an alternative to intraarterial DSA [22, 23].
Selective DSA images were obtained via a 5-French catheter positioned in the vessel of interest with the use of a standard angiographic unit for imaging. Anteroposterior and lateral views were chosen to best depict the arteries at the discretion of the interventionalist. Injections were performed using iohexol (Omnipaque, GE Healthcare), a non-ionic contrast agent, or iodixanol (Visipaque, GE Healthcare) in cases of creatinine levels greater than 2.0 mg/dL. DSA images were acquired with a standard angiographic unit (Multistar Polytron, Siemens Healthcare) using a 15.75-inch (40-cm) image intensifier for a 40-cm FOV and a 1024 × 1024 matrix.
The DSA images were read by a board-certified radiologist with 5 years of experience in vascular imaging. From 16 to 20 mL of the contrast agent was injected into each station at a rate of 8–10 mL/s using a power injector (Mark V, Medrad), and sequential DSA images were obtained.
Data Collection and Image Analysis
Two radiologists with 6 years’ experience each in vascular imaging evaluated the QISS unenhanced MRA, CEMRA, and DSA images. The readers evaluated the images independently; a consensus approach was not used. For comparison purposes, each study was divided into 29 segments. Radiologists independently evaluated 2D source partitions, maximum intensity projections, and volume-rendered 3D images for the presence of disease in each of the 29 anatomic segments with no other clinical information provided with the images.
The American College of Radiology grading system [24] that was used in a multiinstitutional trial of peripheral MRA was applied as follows: 0, normal; 1, minimal stenosis of less than 50%; 2, one lesion with 50% or greater stenosis; 3, more than one lesion with 50% or greater stenosis; and 4, occlusion. The diagnostic quality of the images was also graded for all 54 subjects at each of the three imaging stations on a Likert scale of 0 to 4: 0, nondiagnostic; 1, poor quality and observer not confident; 2, fair quality and observer marginally confident; 3, good quality and observer confident; and 4, excellent quality and observer highly confident. A p value < 0.05 was considered to indicate a significant difference.
The MRA studies were read first and were correlated with DSA if available by a third radiologist blinded to the MRA findings. The sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of QISS unenhanced MRA and of standard double-dose intraarterial stepping-table and time-resolved CEMRA were determined for the characterization of significant stenoses (> 50%) or vessel occlusions; 95% CIs were calculated for sensitivity and specificity. All DSA examinations were performed within 3 weeks of the corresponding MRA examinations. For statistical analysis, DSA images were graded for disease on the basis of the same five-category scale used for peripheral MRA.
Statistical Analysis
All statistical analyses were performed using SPSS software (version 18.0, SPSS).
The level of observer agreement in terms of stenosis scores between angiography techniques was calculated using Cohen’s kappa based on the scores assigned by one of the reviewers. Similarly, unenhanced MRA and CEMRA findings and the gold standard, catheter DSA, were compared on a per-segment basis in 25 patients. According to Landis and Koch [25], the following strengths of agreements were assigned to corresponding ranges of kappa: less than 0, poor agreement; 0–0.2, slight agreement; 0.21–0.4, fair agreement; 0.41–0.6, moderate agreement; 0.61–0.8, substantial agreement; and 0.81–1, almost perfect agreement. A 95% CI was calculated for each kappa value.
The sensitivity, specificity, PPV, and NPV of the unenhanced MRA technique compared with reference CEMRA protocol for the determination of insignificant (≤ 50%) versus significant (51–100%) stenosis were calculated for each reviewer on a per-segment basis; 95% CIs were provided for each estimate.
The unenhanced MRA technique was also compared with the gold standard, DSA, and sensitivity, specificity, PPV, and NPV were calculated. Also, the average stenosis scores for these two modalities were compared using a nonparametric Wilcoxon signed rank test (Tables 1 and 2).
TABLE 1.
Average Stenosis Scores for Gold Standard Digital Subtraction Angiography (DSA) and MR Angiography (MRA) Techniques
| Imaging Modality | Stenosis Score (mean ± SD) |
|---|---|
| Contrast-enhanced MRA | 1.28 ± 1.26 |
| Unenhanced MRA | 1.22 ± 1.28 |
| DSA | 1.16 ± 1.19 |
|
| |
| pa | |
| Contrast-enhanced MRA versus DSA | > 0.05 |
| Unenhanced MRA versus DSA | > 0.05 |
Wilcoxon signed rank test.
TABLE 2.
Diagnostic Performance of MR Angiography (MRA) Techniques Compared With Gold Standard Digital Subtraction Angiography (DSA)
| Performance Measure | Mean Value (95% CI)
|
|
|---|---|---|
| DSA Versus CEMRA | DSA Versus Unenhanced MRA | |
| Sensitivity (%) | 90.7 (79.7–96.9) | 96.2 (87.0–99.5) |
| Specificity (%) | 93.4 (85.3–97.8) | 96.1 (89.0–99.1) |
| Positive predictive value (%) | 90.7 (79.7–96.9) | 94.4 (84.6–98.8) |
| Negative predictive value (%) | 93.4 (85.3–97.8) | 97.4 (90.8–99.6) |
Likert scores of image quality were compared on a per-region basis using the Mann-Whitney test and were reported as means ± SD. The mean stenosis scores for different regions were compared using the Mann-Whitney test, whereas the mean scores for the similar region were compared between the MRA techniques using the Wilcoxon signed rank test.
For assessment of interobserver variability, agreement between the readers regarding the stenosis severity was evaluated using Cohen’s kappa for each MRA technique. A p value < 0.05 was considered to indicate statistical significance.
Results
Diagnostic Accuracy of Unenhanced MR Angiography Versus Contrast-Enhanced MR Angiography
For the 775 segments evaluated, substantial agreement was found between the unenhanced MRA and CEMRA techniques for most segments examined. Cohen’s kappa were greater than 0.60 for all segments except the tibioperoneal artery (κ = 0.57) and distal peroneal artery (κ = 0.59), for which the level of agreement was moderate (Table 3).
TABLE 3.
Agreement Between Quiescent-Interval Single-Shot Unenhanced MR Angiography (MRA) and Contrast-Enhanced MRA Techniques by Segment Using Cohen’s Kappa
| Segment | Cohen’s κ | p |
|---|---|---|
| Distal aorta | 0.96 | < 0.001 |
| Common iliac artery | 0.84 | < 0.001 |
| Internal iliac artery | 0.96 | < 0.001 |
| External iliac artery | 0.92 | < 0.001 |
| Common femoral artery | 0.74 | < 0.001 |
| Proximal superficial femoral artery | 0.90 | < 0.001 |
| Distal superficial femoral artery | 0.86 | < 0.001 |
| Profunda femoris artery | 0.85 | < 0.001 |
| Popliteal artery | 0.74 | < 0.001 |
| Tibioperoneal artery | 0.57 | < 0.001 |
| Proximal anterior tibial artery | 0.66 | < 0.001 |
| Distal anterior tibial artery | 0.77 | < 0.001 |
| Proximal posterior tibial artery | 0.70 | < 0.001 |
| Distal anterior tibial artery | 0.72 | < 0.001 |
| Proximal peroneal artery | 0.65 | < 0.001 |
| Distal peroneal artery | 0.59 | < 0.001 |
Note—Level of agreement between unenhanced and contrast-enhanced MRA techniques for the depiction of significant arterial stenosis using Cohen’s kappa: p < 0.05.
On a per-segment basis, the mean values of the diagnostic accuracy of the unenhanced MRA technique compared with the reference CEMRA for reviewers 1 and 2 (Table 4) were as follows: sensitivity, 87.4% and 92.1%; specificity, 96.8% and 96.0%; PPV, 90.8% and 94.0%; and NPV, 95.5% and 94.6% (Figs. 1 and 2).
TABLE 4.
Diagnostic Performance of Unenhanced MR Angiography (MRA) Compared With Conventional Contrast-Enhanced MRA (CEMRA) for the Detection of Significant Stenosisa
| Performance Measure | Mean Value (95% CI)
|
|
|---|---|---|
| Reviewer 1 | Reviewer 2 | |
| Sensitivity (%) | 87.4 (82.6–91.3) | 92.1 (87.8–95.1) |
| Specificity (%) | 96.8 (95.1–97.9) | 96.0 (93.3–97.7) |
| Positive predictive value (%) | 90.8 (86.3–94.1) | 94.0 (90.1–96.6) |
| Negative predictive value (%) | 95.5 (92.7–97.4) | 94.6 (91.7–96.4) |
Note—Per-segment analysis was based on data from a total of 775 segments.
Stenosis ≥ 50%.
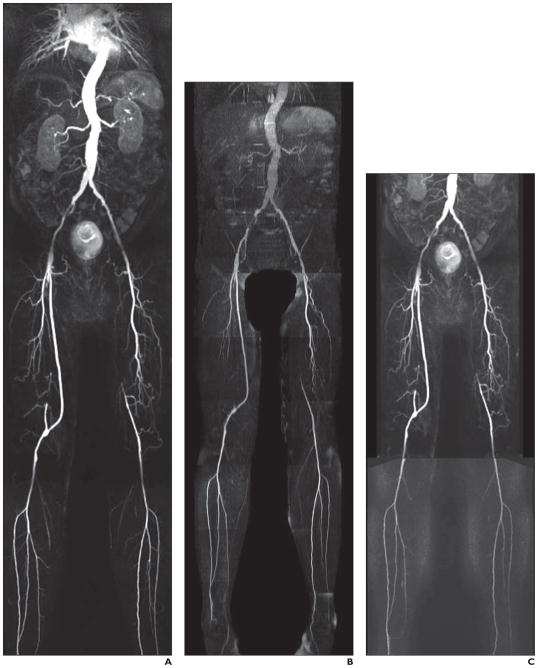
Fig. 1. 61-year-old man with diabetes.
A–C, Contrast-enhanced MR angiography (CEMRA) image (A), quiescent-interval single-shot unenhanced MR angiography image (B), and CEMRA image with superimposed time-resolved angiography with interleaved stochastic trajectories (C) show moderately diseased right external iliac and common femoral artery. Note right external iliac artery stent in situ. There is focal narrowing of left external iliac artery. Femoral-popliteal graft with three-vessel runoff. Occluded left superficial femoral artery with reconstitution above knee and three-vessel runoff.
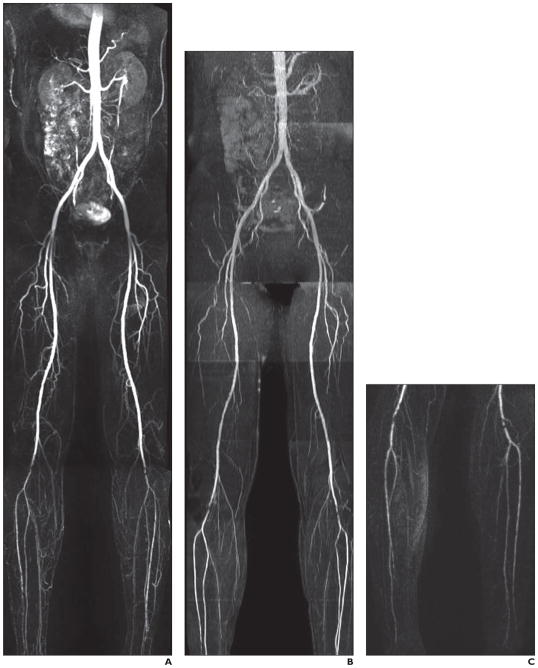
Fig. 2. 59-year-old man with diabetes.
A–C, Contrast-enhanced MR angiography (CEMRA) image (A), quiescent-interval single-shot unenhanced MR angiography image (B), and CEMRA image with superimposed time-resolved angiography with interleaved stochastic trajectories (C). On left side, images show significant focal stenosis of distal popliteal artery and distal occlusive disease of left anterior tibial artery as well as severe occlusive disease of posterior tibial artery. On right, images show short segment of significant stenosis of distal popliteal artery and significant occlusive disease of distal half of anterior tibial artery as well as occlusion of posterior tibial artery at mid leg.
Diagnostic Accuracy of Unenhanced MR Angiography Technique Versus Conventional Catheter Digital Subtraction Angiography
Catheter DSA was compared with unenhanced MRA in 115 segments. Substantial agreement was found when the overall DSA results were compared with the unenhanced MRA (κ = 0.68) and CEMRA (κ = 0.63) techniques.
On a per-segment basis, the mean values of the diagnostic accuracy of the unenhanced MRA technique compared with the gold standard, DSA, were as follows: sensitivity, 96.2%; specificity, 96.1%; PPV, 94.4%; and NPV, 97.4% (Table 4).
No significant difference was found when the average mean stenosis scores for the noncontrast MRA technique (1.22 ± 1.26) were compared with those for catheter DSA (1.16 ± 1.19) (p > 0.05).
Comparison of Stenosis and Likert Scores in Lower Extremity Regions
Stenosis scores showed an incremental pattern toward the distal region in both CEM-RA and unenhanced MRA techniques, with the highest scores noticed in the calf vessel, although the difference was not significant (p > 0.05). There was no significant difference between the two techniques in similar regions in terms of stenosis scores (Table 5).
TABLE 5.
Comparison of Average Stenosis Scores in Lower Extremity by Region on Unenhanced MR Angiography (MRA) and Contrast- Enhanced MRA (CEMRA) Using Nonparametric Tests
| MRA Technique | Stenosis Score (Mean ± SD) | p | |||
|---|---|---|---|---|---|
| Pelvis | Thigh | Calf | Pelvis Versus Thigha | Thigh Versus Calfa | |
| Unenhanced MRA | 1.03 ± 0.92 | 1.20 ± 0.67 | 1.28 ± 1.01 | > 0.05 | > 0.05 |
| CEMRA | 0.98 ± 0.91 | 1.23 ± 0.65 | 1.38 ± 1.05 | > 0.05 | > 0.05 |
|
| |||||
| p for CEMRA versus unenhanced MRAb | > 0.05 | > 0.05 | > 0.05 | ||
Note—An incremental pattern in the mean stenosis scores toward the distal region was shown but did not reach statistical significance (p > 0.05). No statistically significant difference was found in mean stenosis scores between the two MRA techniques in similar regions (p > 0.05).
Wilcoxon signed rank test.
Mann-Whitney signed rank test.
The mean Likert score for CEMRA was significantly higher in the pelvis (3.59 ± 0.59) and thigh (3.54 ± 0.58) than in the calf region (2.86 ± 0.65) (both, p < 0.05). For the noncontrast MRA technique, no significant difference was found when the mean Likert scores in the calf region (3.21 ± 0.64) were compared with those in the pelvis (3.13 ± 0.83) and thigh (3.35 ± 0.65) (both, p > 0.05).
Interreader Agreement
Almost perfect agreement between the two readers was identified for stenosis scores, with Cohen’s kappa values of greater than 0.80 for both MRA techniques (CEMRA, κ= 0.92; unenhanced MRA, κ= 0.90).
Discussion
In our study, we showed that an unenhanced MRA technique is feasible to assess vascular status in patients with diabetes and symptomatic peripheral vascular disease and that this technique is a valuable tool in this setting. The QISS MRA technique shows diagnostic performance for the detection of clinically significant stenoses (> 50%) that is comparable with CEMRA using time-resolved imaging of the calf and stepping-table bolus MRA. The complete vascular tree from the infrarenal aorta to the foot could be depicted with the unenhanced MRA protocol in an average of 6 minutes 40 seconds. The QISS MRA technique showed improved image quality in the calf station compared with time-resolved and stepping-table CEM-RA. QISS unenhanced MRA is currently available only from Siemens as a works-in-progress package; it is not yet available from other vendors.
This study is the first application of an unenhanced MRA technique, to date, solely for the investigation of chronic or suspected PAD in a symptomatic diabetic population. CEMRA has become an attractive option for the noninvasive imaging of peripheral vascular disease because of its proven accuracy for the depiction of significant PAD [26] and its ability to show patent vessel segments of the foot in patients with diabetes and severe arterial occlusive disease not identified with DSA [27]. When compared with CTA, which has benefited significantly from the introduction of fast MDCT scanners, CEMRA provides images without a disturbing overlay from bone or calcified plaques, thereby improving diagnostic confidence in the evaluation of PAD [28]. In previous studies, investigators have reported that lower limb ulceration and infections, particularly in diabetic patients, may cause increased and early venous return in the affected limb, rendering delineation and visualization of patent vessels suboptimal [29]. Additional studies have shown that the presence of advanced PAD or the additive effect of endogenous risk factors including hypertension may predict the likelihood of a nondiagnostic CEMRA study [30].
With the QISS unenhanced MRA sequence, imaging of a full runoff from the infrarenal aorta to the pedal vessels can be performed before contrast administration. This study is particularly useful in patients with advanced PAD, lower limb ulceration, or other risk factors for a nondiagnostic CEM-RA runoff. The ACC/AHA guidelines support the use of CEMRA of the extremities to diagnose the anatomic location and degree of stenosis of PAD (level of evidence: A) and recommend that MRA should be performed with gadolinium enhancement (level of evidence: B), outlining its usefulness in selecting patients with lower extremity PAD as candidates for endovascular intervention (level of evidence: A). The high prevalence of chronic renal impairment in the diabetic population with PAD and of the use of CEMRA studies requiring double-dose (0.2 mmol/kg) IV contrast administration often places diabetic patients with significant renal impairment at increased risk for NSF and limits both dosage options and the ability to repeat nondiagnostic studies [31].
QISS unenhanced MRA should be routinely useful for the evaluation of PAD in patients with impaired renal function or other contraindications to contrast administration. In addition, it could provide a potentially useful alternative to the ABI for some patients who have heavily calcified peripheral arteries given that the accuracy of MRA is not expected to be degraded by the presence of calcifications.
We acknowledge several limitations of our study. The purpose of the study was to compare the diagnostic accuracy of QISS unenhanced MRA with that of CEMRA. Correlation of MRA findings to the gold standard, DSA, was made in small group of patients who underwent DSA, reflecting the fact that DSA is largely reserved for cases in which revascularization is being considered. We acknowledge that our conclusions are tempered by the relatively small number of subjects in this study. The small number of women in our study group reflects the population of diabetic patients with suspected or symptomatic PAD referred to tertiary centers and continuous enrollment is directed at gathering a sufficient study group independent of sex bias.
In conclusion, the results of our study have shown that QISS unenhanced MRA is a potential alternative or complementary sequence to conventional CEMRA for showing clinically significant arterial disease in patients with diabetes with symptomatic chronic lower limb ischemia.
Acknowledgments
This work was supported in part by NIH grant R01HL096916 and by a grant from the Grainger Foundation.
C. B. Glielmi and X. Bi are employees of Siemens Healthcare. The authors who are not employees of Siemens Healthcare had full control of the inclusion of any data or information that might have presented a conflict of interest.
References
- 1.Criqui MH, Denenberg JO, Langer RD, Fronek A. The epidemiology of peripheral arterial disease: importance of identifying the population at risk. Vasc Med. 1997;2:221–226. doi: 10.1177/1358863X9700200310. [DOI] [PubMed] [Google Scholar]
- 2.Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease: the San Luis Valley Diabetes Study. Circulation. 1995;91:1472–1479. doi: 10.1161/01.cir.91.5.1472. [DOI] [PubMed] [Google Scholar]
- 3.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–192. doi: 10.1161/01.atv.18.2.185. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, et al. American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Most RS, Sinnock P. The epidemiology of lower extremity amputations in diabetic individuals. Diabetes Care. 1983;6:87–91. doi: 10.2337/diacare.6.1.87. [DOI] [PubMed] [Google Scholar]
- 6.de Vries M, Ouwendijk R, Flobbe K, et al. Peripheral arterial disease: clinical and cost comparisons between duplex US and contrast-enhanced MR angiography—a multicenter randomized trial. Radiology. 2006;240:401–410. doi: 10.1148/radiol.2402050223. [DOI] [PubMed] [Google Scholar]
- 7.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 8.Koelemay MJ, Legemate DA, van Gurp JA, de Vos H, Balm R, Jacobs MJ. Diagnosis of arterial disease of the lower extremities with duplex ultra-sonography. Br J Surg. 1996;83:404–409. doi: 10.1002/bjs.1800830336. [DOI] [PubMed] [Google Scholar]
- 9.de Vries SO, Hunink MG, Polak JF. Summary receiver operating characteristic curves as a technique for meta-analysis of the diagnostic performance of duplex ultrasonography in peripheral arterial disease. Acad Radiol. 1996;3:361–369. doi: 10.1016/s1076-6332(96)80257-1. [DOI] [PubMed] [Google Scholar]
- 10.Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138:273–281. doi: 10.1148/radiology.138.2.7455105. [DOI] [PubMed] [Google Scholar]
- 11.Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 12.Koelemay MJ, Lijmer JG, Stoker J, Legemate DA, Bossuyt PM. Magnetic resonance angiography for the evaluation of lower extremity arterial disease: a meta-analysis. JAMA. 2001;285:1338–1345. doi: 10.1001/jama.285.10.1338. [DOI] [PubMed] [Google Scholar]
- 13.Prince MR, Zhang H, Morris M, et al. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology. 2008;248:807–816. doi: 10.1148/radiol.2483071863. [DOI] [PubMed] [Google Scholar]
- 14.Bridges MD, St Amant BS, McNeill RB, Cernigliaro JG, Dwyer JP, Fitzpatrick PM. High-dose gadodiamide for catheter angiography and CT in patients with varying degrees of renal insufficiency: prevalence of subsequent nephrogenic systemic fibrosis and decline in renal function. AJR. 2009;192x:1538–1543. doi: 10.2214/AJR.07.3895. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero A, Montes R, Muñoz-Terol J, et al. Peripheral arterial disease in patients with stages IV and V chronic renal failure. Nephrol Dial Transplant. 2006;21:3525–3531. doi: 10.1093/ndt/gfl470. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki M, Lee VS. Nonenhanced MR angiography. Radiology. 2008;248:20–43. doi: 10.1148/radiol.2481071497. [DOI] [PubMed] [Google Scholar]
- 17.Lim RP, Hecht EM, Xu J, et al. 3D nongadolinium-enhanced ECG-gated MRA of the distal lower extremities: preliminary clinical experience. J Magn Reson Imaging. 2008;28:181–189. doi: 10.1002/jmri.21416. [DOI] [PubMed] [Google Scholar]
- 18.Fan Z, Sheehan J, Bi X, Liu X, Carr J, Li D. 3D noncontrast MR angiography of the distal lower extremities using flow-sensitive dephasing (FSD)-prepared balanced SSFP. Magn Reson Med. 2009;62:1523–1532. doi: 10.1002/mrm.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelman RR, Sheehan JJ, Dunkle E, Schindler N, Carr J, Koktzoglou I. Quiescent-interval single-shot unenhanced magnetic resonance angiography of peripheral vascular disease: technical considerations and clinical feasibility. Magn Reson Med. 2010;63:951–958. doi: 10.1002/mrm.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korosec FR, Frayne R, Grist TM, Mistretta CA. Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med. 1996;36:345–351. doi: 10.1002/mrm.1910360304. [DOI] [PubMed] [Google Scholar]
- 21.Bueno A, Acin F, Cañibano C, Fernandez-Casado JL, Castillo E. Diagnostic accuracy of contrast-enhanced magnetic resonance angiography and duplex ultrasound in patients with peripheral vascular disease. Vasc Endovascular Surg. 2010;44:576–585. doi: 10.1177/1538574410377018. [DOI] [PubMed] [Google Scholar]
- 22.Swan JS, Carroll TJ, Kennell TW, et al. Time-resolved three-dimensional contrast-enhanced MR angiography of the peripheral vessels. Radiology. 2002;225:43–52. doi: 10.1148/radiol.2251011292. [DOI] [PubMed] [Google Scholar]
- 23.Dorweiler B, Neufang A, Kreitner KF, Schmiedt W, Oelert H. Magnetic resonance angiography unmasks reliable target vessels for pedal bypass grafting in patients with diabetes mellitus. J Vasc Surg. 2002;35:766–772. doi: 10.1067/mva.2002.119505. [DOI] [PubMed] [Google Scholar]
- 24.Baum RA, Rutter CM, Sunshine JH, et al. Multi-center trial to evaluate vascular magnetic resonance angiography of the lower extremity: American College of Radiology Rapid Technology Assessment Group. JAMA. 1995;274:875–880. [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 26.Dyet JF, Nicholson AA, Ettles DF. Vascular imaging and intervention in peripheral arteries in the diabetic patient. Diabetes Metab Res Rev. 2000;16(suppl 1):S16–S22. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr131>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Kreitner KF, Kalden P, Neufang A, Duber C, Krummenauer F, Kuster E. Diabetes and peripheral arterial occlusive disease: prospective comparison of contrast-enhanced three-dimensional MR angiography with conventional digital subtraction angiography. AJR. 2000;174:171–179. doi: 10.2214/ajr.174.1.1740171. [DOI] [PubMed] [Google Scholar]
- 28.Ouwendijk R, Kock MC, Visser K, Pattynama PM, de Haan MW, Hunink MG. Interobserver agreement for the interpretation of contrast-enhanced 3D MR angiography and MDCT angiography in peripheral arterial disease. AJR. 2005;185:1261–1267. doi: 10.2214/AJR.04.1296. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Chen CZ, Chabra SG, et al. Bolus arterial-venous transit in the lower extremity and venous contamination in bolus chase three-dimensional magnetic resonance angiography. Invest Radiol. 2002;37:458–463. doi: 10.1097/00004424-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Dinter DJ, Neff KW, Visciani G, et al. Peripheral bolus-chase MR angiography: analysis of risk factors for nondiagnostic image quality of the calf vessels—a combined retrospective and prospective study. AJR. 2009;193:234–240. doi: 10.2214/AJR.08.1814. [DOI] [PubMed] [Google Scholar]
- 31.Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148–157. doi: 10.1148/radiol.2431062144. [DOI] [PubMed] [Google Scholar]