Abstract
Consistently low rates of physical activity are reported for older adults and there is even lower participation if a chronic disease is present.
Purpose
To explore the predictors of physical capacity and participation in older community-dwelling individuals living with multiple chronic diseases.
Methods
This was a descriptive cross-sectional investigation of physical capacity (physiological potential) and physical activity participation (recorded engagement in physical activity). Multiple regression and odds ratios were used to investigate determinants of physical capacity (6 Minute Walk Test) and physical activity participation (Physical Activity Scale for Individuals with Physical Disabilities Questionnaire; pedometer steps/day).
Results
200 community dwelling ambulatory participants living with 2 or more chronic disease were assessed. Sixty-five percent (65%) were women and the mean age was 74 ± 6 years (range 65–90 years). Mobility (Timed Up and Go) was a consistent determinant across all 3 primary outcomes. For the Six Minute Walk Test, determinants included mobility, BMI, grip strength, number of medications, leg strength, balance and Chronic Disease Management Self Efficacy Scale (r2=0.58; P=.000). The determinants for the self-reported participation measure (Physical Activity Scale for Individuals with Physical Disabilities Questionnaire) was mobility (r2=0.04; P=.007). For the mean daily pedometer steps, the determinants included mobility, body mass index (BMI), age and Chronic Disease Management Self-Efficacy Scale (r2=0.27; P=.000). There were higher risks for inactivity associated with impairments compared with the presence of a chronic disease. In addition, over 1/3 of participants had sufficient physical capacity, but did not meet minimal recommendations of physical activity.
Conclusion
This study suggests that it is easier to predict an individual’s physical capacity than their actual physical participation.
Keywords: older adults, physical activity, performance, chronic conditions
INTRODUCTION
There is great potential to enhance the quality of life for individuals with chronic diseases through physical activity, a broad term that encompasses both leisure-time activity (sports, exercise) and activities of daily living (household living tasks, transportation). Although physical activity is an important health determinant, it is often under-utilized despite numerous studies highlighting its benefits in reducing morbidity and in the secondary prevention of chronic diseases (e.g., obesity, depression, fractures, osteoarthritis and osteoporosis) (21).
Consistently low rates of physical activity are reported for older adults and there is even lower participation if a chronic disease is present (12,15). There are shared characteristics for not meeting physical activity recommendations that exist for individuals living with different chronic diseases states, including advancing age, female gender, lower educational level and income, low self-esteem, social isolation, depressive symptoms, anxiety, obesity, and osteopenia (8), but most previous research has investigated disease-specific determinants and interventions (8,23,14). Common impairments in multiple domains (e.g., functional balance, aerobic capacity, muscle strength and self-efficacy) may limit activity participation in a similar way across different chronic diseases (8), but this has not been widely studied.
Functional capacity measures in older adults [e.g., Six Minute Walk Test (6MWT)](6) can provide an estimate of physical potential and determine what an individual is capable of doing. Participation (engagement in physical activity) in physical activity can be measured either directly or indirectly and consists of three broad areas— direct observation, self-report questionnaires and portable monitors such as pedometers. These tools can assist in determining how much and which activities an individual actually engages or participates in regularly and can assist in understanding the distinction between capacity and participation. Therefore the primary objectives of this cross-sectional study were to i) determine the predictors of physical capacity and physical activity participation in older community-dwelling individuals living with multiple chronic diseases; and i) explore the relation between capacity and participation. The secondary objective was to evaluate the risk of inactivity associated with chronic disease and impairments. We hypothesized that there would be common impairments across different chronic diseases.
METHODS
Participants
We sought to enrol community-dwelling people living with chronic diseases. In this manuscript we define chronic disease as any medical condition or syndrome that was diagnosed by a health professional and expected to last longer than 6 months. We chose chronic diseases that have the potential to lead to physical conditions with the greatest impact on function. Two hundred community-dwelling ambulatory men and women aged 65 years and older participated in this study. Participants were recruited from local pharmacies using “Shelf-talkers” described previously (27). Using this technique, strategically placed recruitment posters were located within community pharmacies. In addition, pharmacists provided study information to participants who presented at the pharmacy counter with multiple prescriptions. This strategy was based on the assumption that potential participants were living in the community and were sufficiently mobile and cognitively intact to purchase their own medications. Inclusion Criteria: Individuals who: 1) were aged 65 years or older, 2) had two or more chronic diseases, 3) were living in their own home; 4) were ambulatory and able to walk for a minimum of 10 metres with/without assistive devices, 5) achieved a score ≥24 on the Folstein Mini Mental Examination (FMME) (9) and 6) were able to follow three step commands in English. Exclusion Criteria: Individuals were excluded if they were unable to communicate with the investigator over the phone and/or had an impairment or health concern that prevented the completion of testing. Pharmacists identified 242 potential participants, of whom 173 met inclusion/exclusion criteria when screened by the investigator (72% recruitment success). The remaining 27 participants were enrolled by contacting disease-specific support groups directly for a total of 200. This study was approved by the local university and hospital ethics review boards, and all eligible participants gave informed, written consent to participate in the study.
Data Collection
We chose select impairment measures to assess key domains known to influence frailty in older adults: balance, strength, respiratory function, mobility, endurance and psychosocial components (depression, cognition, and self-efficacy) (22). In this study we chose measures to assess body systems (e.g. musculoskeletal, respiratory) as they relate to functional status in older adults. For example, the 6MWT is a walking test of cardiovascular endurance and an efficient way to assess functional capacity in older adults (6,7). Table 1 highlights the outcome measures and normative values.
Table 1.
Description of outcome variables.
| List of Measures | Description of Measurement | Available Normative Data |
|---|---|---|
| Activities-Specific Balance Confidence (ABC) Scale (20) | Reported self-efficacy or confidence with performing 16 specific functional activities | Higher scores indicate a greater level of perceived balance self-efficacy. Range from 0–100 |
| Body Mass Index (BMI) | weight in kgs/height in meters2 | <18.5= low BMI; 18.5–24.9= normal; 25–29.9=overweight; 30 and above= obese |
| Centre for Epidemiological Studies–Depression Scale (CESD) (11) | 20-item screen for symptoms of depression | Scores > 16 are indicative of depression (11) |
| Folstein Mini Mental Status Examination (9) | Screen for dementia. | <24 cut-off scores suggest cognitive impairment (26) |
| Instrumental Support Evaluation List (ISEL) (13) | Measures companionship, self-esteem, emotional support and instrumental support. | Higher scores indicate a greater level of perceived social support. |
| National Institutes for Aging Battery of Lower Extremity Function Battery (10) | Measures 3 tasks: standing balance, time to complete 8 foot walk, sit-stand from chair 5 times. | Higher scores indicate better balance measures. Range from 0–12. |
| Pedometer Recording | Daily mean in steps recorded using a pedometer. | <5000 steps/day=sedentary lifestyle; 5000–7499 steps/day= low active; 7500–9999 steps/day = somewhat active; 10000–11999 steps/day= active; 12,000+ steps/day = highly active (28) |
| Physical Activity Scale for Individuals with Physical Disabilities (PASIPD) (30) | Metabolic demands in 3 domain areas (recreation, household and occupational activities) are captured using this tool. | Possible score range from 0.0–199.5 but no normative data available. |
| Pulmonary Airflow Limitation (spirometry) | Forced expiratory volume (FEV) and forced vital capacity (FVC) ratio. | Values less than 70% suggest obstructive lung disease, although FEV/FVC declines with normal aging (4) |
| Self-efficacy for Managing Chronic Disease (17) | Self-efficacy for managing living with a chronic disease. | Higher scores indicate a greater level of perceived disease management self-efficacy; Range from 0–60. |
| Six Minute Walk Test (6MWT) (6,7) | Walking distance in meters completed in 6 minutes. | <400 meters in 6 minutes associated with higher risk of mortality (19) |
| Strength Dynamometry | Grip and quadriceps strength for the both limbs determined by using the mean of 2 trials. | Grip strength pooled results for adults age 50 years+= 14.7kgs for women and 23.3 kgs for men (18) |
| Timed Up and Go Test (TUG) (24) | Time taken to stand from sitting, walk 3-meters, turn, walk back and sit. | >15 seconds identifies participants at higher risk for falling (32) |
Descriptive Variables
The following data were collected: date of birth, gender and marital status and body weight/height. All participants were asked to self-report chronic diseases she/he had that lasted or were expected to last 6 months or more and had been diagnosed by a health professional. Each participant was also asked to self-rate the severity of their disease as mild, moderate or severe. Participants were asked about mobility aids, current medications, and to rate social support using the Instrumental Support Evaluation List (ISEL) (13). Participants were asked to recall if she/he had sustained any falls in the past 12 months.
Primary Outcome Variables (Dependent variables)
Physical capacity was operationalized as an individual’s ability to perform an action or activity and was assessed using the 6MWT. The two measures of participation (defined as physical activity the individual engages in) were the self-reported Physical Activity Scale for Individuals with Physical Disabilities (PASIPD) and the mean daily pedometer steps. The 6MWT is a walking test of cardiovascular endurance or capacity and an efficient way to assess functional capacity in older adults living with multiple chronic diseases (6,7). Participants were screened before undertaking the 6MWT and were excluded from this test only if there was any chest pain, heart attacks, angioplasty or heart surgery in the previous 3 months, resting heart rate was above 110 beats per minute or was excluded at the discretion of the tester if musculoskeletal or balance disturbances were safety issues. All participants followed their regular medication regime as per the American Thoracic Society guidelines for testing (1).
The PASIPD is a 13-item questionnaire that captures metabolic demands in 3-domain areas (recreation, household and occupational activities) (30). The PASIPD captures energy expenditure for both ambulation and/or using a wheelchair or mobility aid. Using the same scale (PASIPD) enabled us to compare across groups of people with similar diseases but different levels of impairment. Respondents were asked to recall the frequency (in days) and amount of time they spent participating in the list of activities over the past 7 days. Kilocalorie per week was obtained by multiplying the PASIPD by the mean metabolic equivalent values for each question. Each participant was asked to record daily steps over 3 consecutive days using a pedometer (New-Lifestyles DigiWalker SW-200, Lee’s Summit, MO.) as this amount has been shown to provide sufficient data to estimate weekly physical activity in adults (29).
Determinant Variables (Independent variables)
Key impairment variables were the National Institutes of Aging Balance Scale (10) which provided an indication of balance and leg strength in sitting and standing. Upper extremity isometric muscle strength was tested using a JAMAR hand-held dynamometer (JLW Instruments, Sammons Preston, Bolingbrook, IL), and lower extremity strength using hand-held myometry (Nicolas MMT, Model 01160, Lafayette Instruments, Lafayette, IN). The mean of 3 trials for bilateral grip and quadriceps muscle strength was used. Leg strength was normalized to body weight. Basic mobility function was assessed using the Timed Up and Go Test (TUG) (24). To detect airflow limitation of the pulmonary system that is associated with many chronic disease states, a portable spirometry system (GPFS/D USB Spirometer; MedGraphics, St. Paul, MN) was used. The ratio of forced expiratory volume and forced vital capacity (FEV1/FVC %) was calculated; values less than 70% were indicative of obstructive lung disease (4).
Falls-efficacy or confidence with balance was measured using the Activities-Specific Balance Confidence (ABC) Scale (20). The Stanford Self-efficacy for Managing Chronic Disease Scale (ESE) was administered; it consisted of six questions, and assessed participants’ confidence in coping with the impact of living with a chronic disease (17). The Folstein Mini Mental Examination (FMME) was used to screen for cognition, and depression was measured using the Centre for Epidemiological Studies – Depression Scale (CESD) (11).
Sample Size Justification
The sample size (N=200) for this study was designed to provide sufficient power to address our primary research hypothesis using multiple linear regression analyses. Up to 17 variables were modeled at what Cohen (5) defines as a moderate effect size (the proportion of variance in the dependent variable - physical activity - accounted for by the other variables f2=.20) at an alpha of 0.001 (to control for the effects of multiple testing) and power of 0.80.
Statistical Analysis
Descriptive characteristics of the cohort were assessed using means, standard deviations and frequencies depending on the measurement tool. The PASIPD was used to calculate the proportion of participants who met the recommended amount of weekly leisure-time physical activity (>1000 kcal/week) (15). We used a scatterplot to create a 2x2 figure with quadrants to describe the relation between capacity (6MWT) and participation (pedometer steps) (16) and compared differences between groups using independent t-tests. For the primary objective the Pearson Correlation Coefficients were calculated to determine the strength of the associations between continuous variables and determine entry into the regression model as a significant p-value ≤ 0.05. Multiple linear regression was used to ascertain predictive models for capacity (6MWT) and for engaging in physical activity participation (PASIPD and pedometer steps). For the secondary objective to determine the effect of chronic disease and impairment on physical capacity (6MWT) and participation (pedometer steps), logistic regression was used to compute odds ratios (3). Established cut-off points (Table 1) were used and variables were made dichotomous in order to establish risk. When normative values were not available, participant variables below the 25th percentile were considered below healthy norms (3). All statistical analyses were performed using SPSS v. 13 software (SPSS Inc., Chicago, Illinois) using a significance level of P≤.05 (2-tailed).
RESULTS
Characteristics of the Participants
Two hundred (n=200) eligible people volunteered to participate in this study and met inclusion/exclusion criteria. Means, standard deviations and ranges are reported in Table 2. Sixty-five (65%) were women and the mean age was 74.4 ± 5.7 years (range 65–90 years). Mean body-mass index (BMI) was 26.4±5.3; 2% of participants were underweight (<18.5), 41% had a normal BMI (18.5–24.9), 39% were overweight (25.0–29.9) and 18% were obese (BMI≥30). The three most frequently reported chronic diseases were high blood pressure (58% of cohort), cataracts (55%) and osteoarthritis (50%). The mean proportion of respondents for self-reported disease-severity categories of mild, moderate and severe across all diseases was 54.3%, 32.9% and 12.8% respectively. The range of disease duration was 6 months to 74 years, and the median time was 13 years. Thirty-four percent (34%) of participants reported a fall in the past 12 months.
Table 2.
Descriptive characteristics of participants for entire cohort and for N=163 who completed the Six Minute Walk Test.
| Mean ± SD/Frequencies (N=200) | Min-Max/% (N=200) | Mean ± SD/Frequencies (N=163) | |
|---|---|---|---|
|
Descriptive Variables
| |||
| Gender: Women | 130 | 65% | 108 (66%) |
| Men | 70 | 35% | 55 (34%) |
| Age (years) | 74.4±5.7 | 65–90 | 74.2±5.7 |
| Number of Chronic Diseases (n) | 6±3 | 2–16 | 6±3 |
| Number of medications | 5±3 | 0–16 | 5±3 |
| Walking aid (n) | 14 | 6.7% | 8 |
| Height (m) | 1.67±1.0 | 1.43–1.92 | 1.67±1.0 |
| Weight (kg) | 74.0±17.0 | 43.7–127.0 | 73.1±16.3 |
| Body Mass Index (kg/m) | 26.4±5.3 | 16.2–49.1 | 26.1±5.1 |
| ISEL | 12.5±2.7 | 2–18 | 12.5±2.8 |
|
| |||
|
Primary Outcome Variables
| |||
| 6MWT (m) | 415±113 | 126–672 | 415±113 |
| PASIPID | 11.1±7.9 | 0.44–39.2 | 11.5±8.4 |
| Pedometer (1 day mean steps) | 6078±4031 | 136–9289 | 6272±3889 |
|
| |||
|
Determinant Variables
| |||
| NIA Balance | 9.0±2.7 | 0–12 | 9.3±2.4 |
| FEV/FVC (%) | 73.4±10.0 | 35–100 | 73.0±10.1 |
| Left grip strength (kg) | 24.2±10.9 | 4–67 | 24.3±10.8 |
| Left leg strength (kg) | 18.2±7.3 | 3–42 | 18.2±7.3 |
| TUG (seconds) | 10.9±3.6 | 5.4–27.9 | 10.1±2.6 |
| ABC Scale | 82±17 | 22–100 | 84±15 |
| Management Self-Efficacy | 44.6±12.8 | 0–60 | 44.9±12.7 |
| CES-Depression Scale | 11.2±8.5 | 0–40 | 11.2±8.5 |
ABC Scale=Activities-Specific Balance Confidence; BMI=body mass index; CES-Depression= Centre for Epidemiological Studies – Depression Scale; FEV/FVC= ratio of forced expiratory volume and forced vital; ISEL=Instrumental Support Evaluation List; Management Self-efficacy= Stanford Chronic Disease Management Self-efficacy Scale; NIA Balance= National Institutes of Aging Balance Scale; TUG=Timed Up and Go; 6MWT=Six Minute Walk Test; PASIPID= Physical Activity Scale for Individuals with Physical Disabilities.
Capacity vs. Participation
Only 163 participants were able to complete the 6MWT as 37 participants were excluded as per the American Thoracic Society (ATS) guidelines for relative contraindications of high blood pressure or recent cardiac event (1). This was consistent with previous work that was unable to have all participants complete the 6MWT for health reasons (3). The characteristics of the 163 participants were similar to the total group (Table 2). Data were obtained for 200 participants for the PASIPD self-report questionnaire and 188 responses for the mean pedometer-daily steps (2 participants were lost to follow-up and 10 participants could either not use the pedometer or the pedometer malfunctioned).
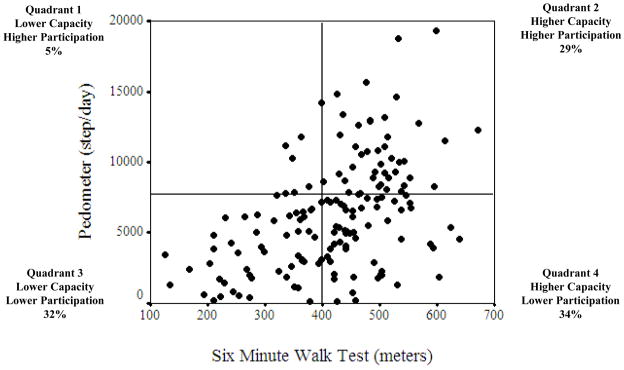
Only 27.5% of participants met the 1000k/cal per week recommended level of physical activity (15). Forty-three percent (43%) of participants were consider sedentary (<5000 steps/day); 25% were low-active (5000–7499 steps); 15% somewhat active (7500–9999); and only 17% of participants recorded >10,000 steps/day [8% active (10,000–11,999 steps) and 9% highly active (>12,000 steps/day)]. Of the participants who completed the 6MWT, 39% were below 400 meters, the threshold associated with higher risk of mortality (19). The mean energy expenditure for reported physical activity was 849.3 ± 644.1 kilocalories/week (median 676.4 and range 106.0–4371.7 kilocalories/week). The Figure highlights the relation between capacity (6MWT) and participation. In this descriptive scatterplot, 34% of participants considered to be above the 400 meters cut-off did not meet 7500 pedometer steps/day (considered somewhat active or higher). For the group that had sufficient capacity (>400 meters; N=106) there were statistically significant differences between the two groups (those who reported >7500 steps and who did not); the group who did not record >7500 steps were slower on the TUG and had lower confidence with chronic disease management and balance confidence; TUG (9.6 vs. 8.2 seconds; P=.013), ESE (45 vs. 53/60; P=.000) and ABC (87.4 vs. 92.3/100; P=.000).
Determinants of physical capacity and participation
Table 3 reports associations between primary outcomes and determinant variables to highlight those variables that were used in the regression models. Using multiple linear regression, there was a moderate association for capacity (6MWT) and the determinants were the TUG, left leg strength normalized to mass, number of medications, ESE, balance, BMI and grip strength (r2=0.58;P=.000). For the mean number of pedometer steps, a positive low association was found with the TUG, BMI and age (r2=0.27;P=.000). Only one variable provided a very low determinant for the self-reported PASIPD [TUG (r2=0.04; P=.007] (Table 4) while no other variables were significantly correlated with the PASIPD. Risk of Inactivity: Using odds ratios, there were greater risks for being inactive associated with impairments and restrictions than for the presence of a chronic disease (Table 5). For the 6MWT, the risk for not achieving 400 meters was increased if there was depression, lower grip strength, balance problems, BMI indicating either overweight or obese, and the presence of arthritis and/or diabetes mellitus. In contrast, for the pedometer, the presence of cataracts, being underweight or obese, having depression or balance challenges increased the risk for not being sufficiently active. Self-efficacy measures provided the highest odds ratios of not being active for both capacity and participation.
Table 3.
Significant bivariate correlations using Pearson Product Correlation Coefficient. These measures were entered into the multiple regression models for the 3 primary outcome variables (6MWT; pedometer steps and PASIPD).
| Six Minute Walk Test (N=163) | r-value | Pedometer Mean Steps (N=188) | r-value | PASIPD (N=200) | r-value |
|---|---|---|---|---|---|
| ABC | 0.52*** | ABC | 0.32*** | CES-Depression Scale | −0.15* |
| Age | −0.19* | Age | −0.29*** | Management Self- | 0.18** |
| Body Mass Index | −0.27*** | Body Mass Index | −0.25*** | Left leg strength | 0.16* |
| CES-Depression Scale | −0.35*** | CES-Depression Scale | −0.22** | NIA Balance Test | 0.17* |
| ISEL | 0.28*** | ISEL | 0.151* | Number of medications | −0.16* |
| Management Self- | 0.51*** | Management Self- | 0.34*** | Timed-up and Go | −0.25*** |
| Left Grip Strength | 0.43*** | Left Grip Strength | 0.22** | ||
| Left Leg strength | 0.53*** | Left Leg strength | 0.31*** | ||
| NIA Balance Test | 0.58*** | NIA Balance Test | 0.42*** | ||
| Number of medications | −0.39*** | Number of medications | −0.19* | ||
| Timed Up and Go | −0.65*** | Timed Up and Go | −0.50*** |
Significant at P ≤ .001;
significant at P ≤ .01;
significant at P ≤ .05.
ABC= Activities of Balance Confidence score; CES-D= Centre for Epidemiological Studies-Depression; ISEL= Instrumental Support Evaluation List; NIA=National Institute for Aging balance score; PASIPD= Physical Activity Scale for Individuals with Physical Disabilities.
Table 4.
Detailed forward multiple regression models for PASIPD, Pedometer and the Six Minute Walk Test.
| Dependent Variable | Independent Variables in Final Model | Adjusted r2 | Standardized Beta | Significance Level |
|---|---|---|---|---|
| PASIPD€ | Timed Up and Go | 0.04 | −0.21 | P=.007 |
|
| ||||
| Pedometer Mean Steps | Timed Up and Go | 0.27 | −0.35 | P=.000 |
| Body Mass Index | −0.24 | |||
| Age | −0.23 | |||
|
| ||||
| Six Minute Walk Test | Timed Up and Go | 0.58 | −0.27 | P=.000 |
| Leg Strength | 0.16 | |||
| Number of medications | −0.17 | |||
| Management Self-efficacy | 0.19 | |||
| NIA Balance | 0.21 | |||
| Body Mass Index | −0.16 | |||
| Grip Strength | 0.12 | |||
Management Self-efficacy= Stanford Chronic Disease Management Self-efficacy Scale; NIA Balance=National Institutes of Aging Balance Test; PASIPD=Physical Activity Scale for Individuals with Physical Disabilities.
Variables excluded: CES-depression scale, NIA balance test, normalized left leg strength, number of medications, and Stanford Chronic Disease Management Self-efficacy.
Significant at P ≤ .001;
significant at P ≤ .01;
significant at P ≤ .05.
Table 5.
Characteristics of participants for six-minute walk test > 400 meters and >7500 steps/day using a pedometer.
| Variable | Pedometer (steps/day) (N=188) | Six Minute Walk Test (meters) (N=163) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| <7500 steps/day (N/%) | >7500 Steps/Day (N/%) | Univariate Odds Ratio | <400 meters (N/%) | ≥400 meters (N/%) | Univariate Odds Ratio | |
| Chronic Conditions | ||||||
| Cardiovascular | 95 (51%) | 41 (22%) | 1.33 (0.68–2.62) | 49 (30%) | 69 (42%) | 1.57 (0.76–3.26) |
| Arthritis | 67(36%) | 28 (15%) | 1.25 (0.68–2.32) | 39 (24%) | 40 (25%) | 2.44 (1.28–4.66) |
| Back problems | 49 (26%) | 15 (8%) | 1.86 (0.94–3.69) | 27 (17%) | 29 (18%) | 1.84 (0.95–3.55) |
| Cataracts | 78 (41%) | 27 (14%) | 1.91 (1.03–3.55) | 38 (23%) | 52 (32%) | 1.40 (0.74–2.66) |
| Diabetes mellitus | 27(14%) | 10 (5%) | 1.34 (0.60–2.98) | 16 (10%) | 13 (8%) | 2.28 (1.01–5.14) |
| COPD | 10 (5%) | 2(1%) | 2.46 (0.52–11.58) | 5 (3%) | 5 (3%) | 1.64 (0.46–5.90) |
| Stroke | 14 (7%) | 4 (2%) | 1.72 (0.54–5.46) | 6 (4%) | 9 (6%) | 1.06 (0.36–3.15) |
|
|
||||||
| Impairments | ||||||
| ABC | 41 (22%) | 4 (2%) | 6.55 (2.22–19.32) | 26 (16%) | 7 (4%) | 9.94 (3.95–25.01) |
| BMI | 31 (16%) | 5 (3%) | 3.59 (1.32–9.77) | 17 (10%) | 12 (7%) | 2.83 (1.24–6.45) |
| CES-Depression | 40 (21%) | 5 (3%) | 5.00 (1.86–13.44) | 21 (13%) | 18 (11%) | 2.52 (1.20–5.27) |
| Management Self-efficacy | 41 (22%) | 3 (2%) | 9.27 (2.74–29.39) | 27 (17%) | 11 (7%) | 6.97 (3.09–15.69) |
| FEV/FVC % | 25 (13%) | 14 (7%) | 0.82 (0.38–1.77) | 13 (8%) | 24 (15%) | 1.08 (0.49–2.41) |
| Grip Strength | 29 (15%) | 8 (4%) | 2.04 (0.86–4.79) | 20 (12%) | 13 (8%) | 3.43 (1.55–7.59) |
| NIA Balance Score | 32 (17%) | 6 (3%) | 3.14 (1.23–8.00) | 19 (12%) | 9 (6%) | 4.52 (1.89–10.84) |
| Timed Up and Go | 14 (7%) | 0 | 0.89 (0.83–0.95) | 4 (2%) | 2 (1%) | 3.38 (0.60–19.03) |
| Walking aid | 12 (6%) | 1 (1%) | 6.21 (0.79–48.93) | 8 (5%) | 0 | 0.873 (0.79–0.96) |
ABC=Activities of Balance Confidence Scale; BMI=Body Mass Index CES-Depression= Centre for Epidemiological Studies – Depression Scale; COPD=Chronic Obstructive Lung Disease; FEV/FVC %= ratio of forced expiratory volume/forced vital capacity; Management Self-efficacy= Stanford Chronic Disease Management Self-efficacy; NIA= National Institutes of Aging balance score.
DISCUSSION
This study provides novel data highlighting the disparity between physical capacity and physical activity participation in a cohort of people with different chronic diseases. Secondly, these results support the hypothesis that the common impairments across diseases increase risk for inactivity more than the presence of the disease itself.
Our performance measure is consistent with other investigations of older adults, highlighting the importance of physical domains (25); the 6MWT is a measure of endurance but also considered a means to quantify function in older adults (7). Although 60% of participants were able to complete more than 400 meters in the 6MWT (19), only a third of these participants were considered active as measured by the pedometer and a third were achieving the recommended 1000 kcal/week of activity (15). That is, despite the fact that our participants were community dwelling and had sufficient ability, not all achieve recommended physical activity levels. This is an important consideration because if individuals with chronic disease are not utilizing their current abilities to engage in physical activity, they are at risk for developing a reduced tolerance to activity, further sedentary lifestyle and compromised health.
Interestingly, we found that the self-efficacy was an important determinant and was significant lower in the group who had the capacity but did not meet the recommended activity level. Self-efficacy, based on the work by Bandura (2), describes the relation between the beliefs in one’s ability and achievement. More recently, the self-efficacy model has been used in health research, in particular to assess a confidence to change negative health behaviours such as smoking and inactivity. Self-efficacy scales are also used to determine one’s balance confidence for a number of environmental situations (Activities of Balance Confidence Scale) (20). Self-efficacy explores an individual’s motivation to start and maintain healthy behaviours despite obvious setbacks. According to Bandura, health promotion activities is dependent on multiple domains (2) and as the majority of our measures were performance based, this may explain why were only able to account for some of the variance in reported physical activity. As only 50% of people who start an exercise program maintain it at 6 months (31) it is therefore prudent to educate individuals on the benefits of regular activity, assist in the development of specific exercise motivators and goals, assess self-efficacy to sustain a program despite setbacks— including discussing contingency plans in the event of a setback; and of course understanding individual impairments and abilities to minimise risk of injury.
These results were able to explain a larger variance for the pedometer record compared with the PASIPD. This may occur for several reasons; first the PASIPD is a self-report measure that requires the respondent to first remember their actions in the previous seven days and then make decisions about the effort level – thus creating potential reporting bias. Despite the known limitations, they remain an important tool for capturing activity amount and patterns.
There are several limitations noted in this study. First, this was a cross-sectional observational investigation; therefore interpreting any causality of the results needs to be viewed cautiously. With a cross-sectional design we recognize that there may be potential limitations (such as anxiety or lack of experience) to our performance measures which could influence our results. Third, some of the questionnaires used in this study relied on self-report measurements and are therefore subject to recall and respondent bias. We also acknowledge that we only captured the self-reported presence of a disease where the participant sought medical attention. It is possible that participants had mild stages of more common diseases (states such as cardiovascular disease and arthritis) and it was not included in this study. These results are only generalisible to people with mild to moderate disease severity and this is likely a reflection of a sample that are ambulatory, living in the community and were able to visit their community pharmacist. We acknowledge that we were not able to test 18% of people using the 6MWT; however, we believe that those who did complete the 6MWT were still representative of the community.
In conclusion, this novel investigation highlighted an important distinction between an individual’s functional capacity and their participation in physical activity and that impairments/limitations increase the risk of inactivity more than the presence of the chronic disease itself. As only one-third of older adults achieve recommended physical activity levels, there is the potential for older adults to engage in more physical activity. Further, researchers and clinicians can do more to develop and implement interventions that target the uptake and maintenance of positive health behaviours for older adults who have the potential to engage in more physical activity. Ultimately, this study reinforces that physical activity is a complex entity that requires understanding of many domains and not simply one’s physical capacity.
Figure 1.
Scatterplot with quadrants highlighting physical capacity (Six Minute Walk Test) vs. physical performance (pedometer steps) for 156 participants aged 65 years and older living with two or more chronic diseases.
Acknowledgments
We thank all the participants who volunteered to be part of the study. We appreciate the contribution of Chihya Hung, Amira Tawashy, Nicole Elfring, Karin McFarlane, Lee Boyer, Alex Korotchenko, Jennifer Cumal and Miho Asano. We gratefully acknowledge the support of the Canadian Institutes for Health Research (CIHR) Team Development Grant. We also thank CIHR (MOP-57862 to JJE) and Michael Smith Foundation for Health Research (MCA, JJE, WCM) for Investigator support. Drs. Ashe, Eng and Miller were involved in all aspects of the research study and manuscript preparation. Dr. Soon was involved in the study concept and design as well as the participant recruitment.
References
- 1.American Thoracic Society. ATS Guidelines for the Six Minute Walk Test. 2002. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 2.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 3.Bootsma-van der Wiel A, Gussekloo J, De Craen AJ, Van Exel E, Bloem BR, Westendorp RG. Common chronic diseases and general impairments as determinants of walking disability in the oldest-old population. J Am Geriatr Soc. 2002;50:1405–1410. doi: 10.1046/j.1532-5415.2002.50363.x. [DOI] [PubMed] [Google Scholar]
- 4.Calverley PMA, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362:1053–61. doi: 10.1016/s0140-6736(03)14416-9. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N.J: L. Erlbaum Associates; 1988. pp. 8–14. [Google Scholar]
- 6.Enright PL. The six-minute walk test. Respir Care. 2003;48:783–785. [PubMed] [Google Scholar]
- 7.Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 8.Fiatarone Singh MA. Exercise in the oldest old: some new insights and unanswered questions. J Am Geriatr Soc. 2002;50:2089–2091. doi: 10.1046/j.1532-5415.2002.50626.x. [DOI] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 11.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 12.Heath GW, Fentem PH. Physical activity among persons with disabilities--a public health perspective. Exerc Sport Sci Rev. 1997;25:195–234. [PubMed] [Google Scholar]
- 13.Heitzman CA, Kaplan RM. Assessment of methods for measuring social support. Health Psychol. 1988;7:75–109. doi: 10.1037//0278-6133.7.1.75. [DOI] [PubMed] [Google Scholar]
- 14.Kujala UM. Evidence for exercise therapy in the treatment of chronic disease based on at least three randomized controlled trials--summary of published systematic reviews. Scand J Med Sci Sports. 2004;14:339–345. doi: 10.1111/j.1600-0838.2004.413.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones DA, Ainsworth BE, Croft JB, Macera CA, Lloyd EE, Yusuf HR. Moderate leisure-time physical activity: who is meeting the public health recommendations? A national cross-sectional study. Arch Fam Med. 1998;7:285–289. doi: 10.1001/archfami.7.3.285. [DOI] [PubMed] [Google Scholar]
- 16.Liu-Ambrose T, Khan KM, Eng JJ, Lord SR, McKay HA. Balance confidence improves with resistance or agility training. Increase is not correlated with objective changes in fall risk and physical abilities. Gerontology. 2004;50:373–382. doi: 10.1159/000080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorig K, Stewart A, Ritter P, González V, Laurent D, Lynch J. Outcome Measures for Health Education and other Health Care Interventions. Thousand Oaks CA: Sage Publications; 1996. pp. 24–25.pp. 41–45. [Google Scholar]
- 18.Luna-Heredia E, Martin-Pena G, Ruiz-Galiana J. Handgrip dynamometry in healthy adults. Clin Nutr. 2005;24:250–258. doi: 10.1016/j.clnu.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 20.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 21.Rimmer JH, Braddock D. Health promotion for people with physical, cognitive and sensory disabilities: an emerging national priority. Am J Health Promot. 2002;16:220–4. doi: 10.4278/0890-1171-16.4.220. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59:1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 23.Shephard RJ. Training and the respiratory system--therapy for asthma and other obstructive lung diseases? Ann Clin Res. 1982;14(Suppl 34):86–96. [PubMed] [Google Scholar]
- 24.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- 25.Tiedemann A, Sherrington C, Lord SR. Physiological and psychological predictors of walking speed in older community-dwelling people. Gerontology. 2005;51:390–395. doi: 10.1159/000088703. [DOI] [PubMed] [Google Scholar]
- 26.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 27.Tsuyuki RT, Johnson JA, Teo KK, et al. A randomized trial of the effect of community pharmacist intervention on cholesterol risk management: the Study of Cardiovascular Risk Intervention by Pharmacists (SCRIP) Arch Intern Med. 2002;162:1149–1155. doi: 10.1001/archinte.162.10.1149. [DOI] [PubMed] [Google Scholar]
- 28.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Tudor-Locke C, Burkett L, Reis JP, et al. How many days of pedometer monitoring predict weekly physical activity? Prev Med. 2005;40:293–298. doi: 10.1016/j.ypmed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil. 2002;83:193–200. doi: 10.1053/apmr.2002.27467. [DOI] [PubMed] [Google Scholar]
- 31.White JL, Ransdell LB, Vener J, Flohr JA. Factors related to physical activity adherence in women: review and suggestions for future research. Women Health. 2005;41:123–148. doi: 10.1300/J013v41n04_07. [DOI] [PubMed] [Google Scholar]
- 32.Whitney JC, Lord SR, Close JC. Streamlining assessment and intervention in a falls clinic using the Timed Up and Go Test and Physiological Profile Assessments. Age Ageing. 2005;34:567–571. doi: 10.1093/ageing/afi178. [DOI] [PubMed] [Google Scholar]