Abstract
OBJECTIVE
— Sedentary lifestyle and a western diet promote subacute-chronic inflammation, obesity, and subsequently dysglycemia. The aim of the current study was to evaluate the efficacy of the anti-inflammatory drug salsalate to improve glycemia by reducing systemic inflammation in obese adults at risk for the development of type 2 diabetes.
RESEARCH DESIGN AND METHODS
— In a double-masked, placebo controlled trial, we evaluated 20 obese nondiabetic adults at baseline and after 1 month of salsalate or placebo.
RESULTS
— Compared with placebo, salsalate reduced fasting glucose 13% (P < 0.002), glycemic response after an oral glucose challenge 20% (P < 0.004), and glycated albumin 17% (P < 0.0003). Although insulin levels were unchanged, fasting and oral glucose tolerance test C-peptide levels decreased in the salsalate-treated subjects compared with placebo (P < 0.03), consistent with improved insulin sensitivity and a known effect of salicylates to inhibit insulin clearance. Adiponectin increased 57% after salsalate compared with placebo (P < 0.003). Additionally, within the group of salsalate-treated subjects, circulating levels of C-reactive protein were reduced by 34% (P < 0.05).
CONCLUSIONS
— This proof-of-principle study demonstrates that salsalate reduces glycemia and may improve inflammatory cardiovascular risk indexes in overweight individuals. These data support the hypothesis that subacute-chronic inflammation contributes to the pathogenesis of obesity-related dysglycemia and that targeting inflammation may provide a therapeutic route for diabetes prevention.
Obesity, occurring at epidemic rates worldwide, is a major risk factor for diabetes and cardiovascular disease. Thus, there is an urgent need for effective interventions to prevent diabetes in obese populations. The importance of lifestyle modification in obesity and diabetes is well recognized. However, disappointing long-term results of these treatments have led to increased interest in pharmaceutical intervention. Obesity and high-fat western diets activate inflammatory processes, which promote development of insulin resistance (1,2). Thus, targeting the inflammatory pathway may be a novel pharmacologic intervention for diabetes prevention and treatment.
Salicylates are among the most commonly used nonsteroidal anti-inflammatory drugs. The benefits of salicylates for treatment of diabetes have long been recognized (3,4). High doses of the salicylate aspirin (4–7 g/day) improve fasting and postprandial hyperglycemia in patients with diabetes (5–7). In recent studies, the hypoglycemic actions of salicylates have been reinvestigated, and the molecular target was identified to be the IκB kinase complex β (IKKβ)/nuclear factor κB (NF-κB) pathway (8,9), a central integrator of proinflammatory signals (2). The therapeutic potential of high-dose aspirin is limited by bleeding risk. Salsalate, a dimer of salicylic acid, has an established safety profile after decades of use for rheumatic pain. As a nonacetylated salicylate, salsalate is an equipotent inhibitor of NF-κB but has a lower bleeding risk than aspirin (10,11).
To our knowledge, this is the first study to assess metabolic changes with administration of salicylates to obese individuals without diabetes. We hypothesized that salicylates administered for 1 month would improve glycemia in obese young adults.
RESEARCH DESIGN AND METHODS
The Joslin Diabetes Center institutional review board approved the double-masked, placebo-controlled study. Written informed consent was obtained. Subjects were <30 years and obese, with BMI ≥30 kg/m2. Participants were excluded for recent blood donation, change in weight >5% in the preceding 6 months, use of medication known to alter glucose metabolism, acute febrile illness, biochemical evidence of renal or hepatic dysfunction, aspirin allergy, history of gastritis or gastrointestinal bleeding, or diabetes. Women were excluded for pregnancy, lactation, or lack of contraception use.
Participants were instructed to consume a high-carbohydrate diet (250–300 g/day) and abstain from strenuous exercise for 3 days before evaluations and not to alter dietary or exercise habits during the study. Blood pressure was measured twice (DINAMAP PRO-100; General Electric Healthcare) with the patient supine for 10 min. Fasting lipids and cytokines were measured, and oral glucose tolerance tests (OGTTs) were performed with glucose, insulin, and C-peptide levels measured before and 30, 60, 90, and 120 min after a 75-g glucose load. All subjects were nondiabetic on the basis of American Diabetes Association guidelines (12). Insulin resistance was determined using homeostasis model assessment of insulin resistance (HOMA-IR) for insulin and HOMA-IR calculated using C-peptide (HOMA-IRC-peptide), as described by the modified formula HOMA-IRC-peptide = (fasting C-peptide × fasting glucose)/22.5 (13).
Subjects were randomly assigned by a research pharmacist to receive salsalate, 4.0 g/day (Caraco Pharmaceutical Laboratories, Detroit, MI) divided in two doses, or identical placebo for 4 weeks. Participants and study personnel were blinded to the treatment assignment. The starting dose was selected on the basis of tolerability data in patients with arthritis (14). A dose-reduction plan was specified a priori with stepped reductions of 500 mg for symptoms related to salicylate use, such as tinnitus or headache. Compliance was evaluated by salicylate levels.
Twenty-seven subjects were enrolled. One subject became ineligible because of blood donation after screening. Three subjects withdrew consent because of personal conflicts and were considered noninformative; two had been randomly assigned to placebo and one to salsalate. Three subjects were withdrawn because of rash.
Assays
Glucose was measured by glucose oxidation, cholesterol and HDL were measured by a cholesterol esterase assay, triglycerides were measured via hydrolysis to glycerol and free fatty acids (FFAs) (Beckman Synchron CX3 Delta and CX9; Beckman Coulter, Brea, CA), and glycohemoglobin was measured by high-performance liquid chromatography (Tosho 2.2; Tosoh Bioscience, San Francisco, CA). Immunoassays were performed in duplicate using commercial assays including radioimmunoassays for insulin and C-peptide (Diagnostic Systems Laboratories, Webster, TX) and adiponectin (Linco Research, St. Charles, MO) and enzyme-linked immunoabsorbent assays for FFAs (Wako Chemicals, Richmond, VA), and interleukin-6 and vascular cell adhesion molecule-1 (R&D Systems, Minneapolis, MN). C-reactive protein (CRP) was analyzed by immunoturbidometry (Wako Chemicals). Salicylate levels were assessed commercially by colorimetric assay (Quest Laboratories, Cambridge, MA). Glycated albumin was evaluated by a Hitachi 911 lipid and protein analyzer and kits from AsahiKasei (Tokyo, Japan). Glycated albumin was calculated by determining the colorimetric measurement of total albumin (a), partial enzymatic digestion of albumin at glycated sites (b), reassessment of albumin (c), and a − c.
Statistical analysis
The primary end point was change from baseline in glycemic measures between salsalate and placebo groups. Secondary outcomes were also assessed as change from baseline within group. Data are presented as mean ± SEM. Unpaired (salsalate versus placebo) and paired (before versus after treatment) Student’s t tests were performed. Treatment effects were determined by calculating percent change for each participant. Repeated measures and area under the curve analyses were performed to compare response to OGTT before and after treatment. Completer analysis was prespecified. Multiple regression analysis was performed to evaluate the contribution of covariates to the primary end points. Results were considered significant with two-tailed P < 0.05.
RESULTS
Twenty participants completed the protocol. Subject baseline characteristics were similar (Table 1) in both groups. All participants had central obesity (15) and normal fasting glucose values. Three subjects (two in the placebo group and one in the treatment group) had baseline 120-min OGTT glucose values consistent with impaired glucose tolerance. No subject had diabetes at baseline.
Table 1.
Baseline subject characteristics
| Salsalate therapy | Placebo | |
|---|---|---|
| Sex (male/female) | 1/8 | 2/9 |
| Ethnicity (W/H/B/other) | 5/3/1 | 5/1/4/1 |
| Age (years) | 23.5 ± 1.1 | 24.1 ± 1.0 |
| BMI (kg/m2) | 36.3 ± 2.2 | 38.9 ± 2.5 |
| Waist circumference (cm) | 106 ± 5 | 116 ± 6 |
| Current smoking | 3 of 9 | 2 of 11 |
| Cholesterol (mmol/l) | 4.0 ± 0.3 | 4.6 ± 0.2 |
| Triglycerides (mmol/l) | 1.0 ± 0.1 | 1.1 ± 0.2 |
| Systolic blood pressure | 121 ± 4 | 123 ± 4 |
| Diastolic blood pressure | 67 ± 3 | 71 ± 3 |
| Fasting glucose (mmol/l) | 5.0 ± 0.2 | 4.8 ± 0.1 |
| 120-min glucose (mmol/l) | 6.7 ± 0.4 | 6.8 ± 0.4 |
Data are means ± SEM. There were no significant differences in the baseline characteristics between the salsalate and placebo groups. W, white, non-Hispanic; H, Hispanic; B, African-American.
In salsalate-treated participants, mean serum salicylate levels were in the therapeutic range established in rheumatology practice (0.7–2.2 mmol/l): 1.35 ± 0.18 mmol/l at 2 weeks and 1.23 ± 0.25 mmol/l at 4 weeks. Salicylate levels were undetectable in placebo-treated participants. There were no significant changes in weight, systolic or diastolic blood pressure, or standard lipid profiles in either group (Table 2).
Table 2.
Glycemic, inflammatory, lipid, and body composition parameters
| Salsalate
|
Placebo
|
P value for comparison of change between groups | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 month | % change from baseline | Baseline | 1 month | % change from baseline | ||
| Glucose metabolism | |||||||
| Glucose (mmol/l) | 5.0 ± 0.2 | 4.6 ± 0.1* | −8 | 4.8 ± 0.1 | 5.1 ± 0.1* | +5 | <0.002 |
| Glycated albumin (%) | 12.4 ± 0.4 | 10.3 ± 0.3* | −17 | 12.5 ± 0.3 | 12.4 ± 0.4 | −1 | <0.0003 |
| Glucose AUC (mmol · min−1 · l−1) | 905 ± 42 | 776 ± 50 | −14 | 801 ± 40 | 840 ± 42 | +6 | <0.004 |
| C-peptide (nmol/l) | 1.6 ± 0.2 | 1.1 ± 0.2* | −24 | 1.5 ± 0.2 | 1.5 ± 0.2 | +5 | <0.01 |
| Insulin (pmol/l) | 112 ± 21 | 124 ± 18 | +27 | 97 ± 12 | 98 ± 10 | +6 | 0.5 |
| HOMA IR C-peptide | 1.1 ± 0.2 | 0.7 ± 0.1* | −29 | 1.0 ± 0.1 | 1.0 ± 0.1 | +10 | <0.004 |
| Inflammatory parameters | |||||||
| Adiponectin (mg/l) | 10.6 ± 1.7 | 16.2 ± 2.7* | +56 | 10.8 ± 1.5 | 10.5 ± 1.5 | −1 | <0.003 |
| FFA (mEq/l) | 0.46 ± 0.08 | 0.25 ± 0.05 | −14† | 0.42 ± 0.06 | 0.37 ± 0.08 | −14 | 0.9 |
| CRP (nmol/l) | 48.2 ± 10.5 | 30.2 ± 7.4* | −34 | 45.4 ± 8.4 | 42.7 ± 9.6 | −8 | 0.2 |
| Lipid parameters | |||||||
| LDL (mmol/l) | 2.9 ± 0.3 | 2.8 ± 0.2 | −5 | 3.3 ± 0.2 | 3.2 ± 0.2 | −4 | 0.8 |
| Triglycerides (mmol/l) | 1.0 ± 0.1 | 0.8 ± 0.1 | −12 | 1.1 ± 0.2 | 1.3 ± 0.2 | +15 | 0.08 |
| BMI (kg/m2) | 36.3 ± 2.2 | 36.5 ± 2.2 | +0.5 | 38.9 ± 2.5 | 38.8 ± 2.5 | −0.3 | 0.5 |
Data are means ± SEM.
P < 0.05 for within-group analysis.
There was one outlier in the treatment group. With the exclusion of this subject, the change in FFA concentrations is a significant reduction of 46% (P = 0.02).
Glucose metabolism
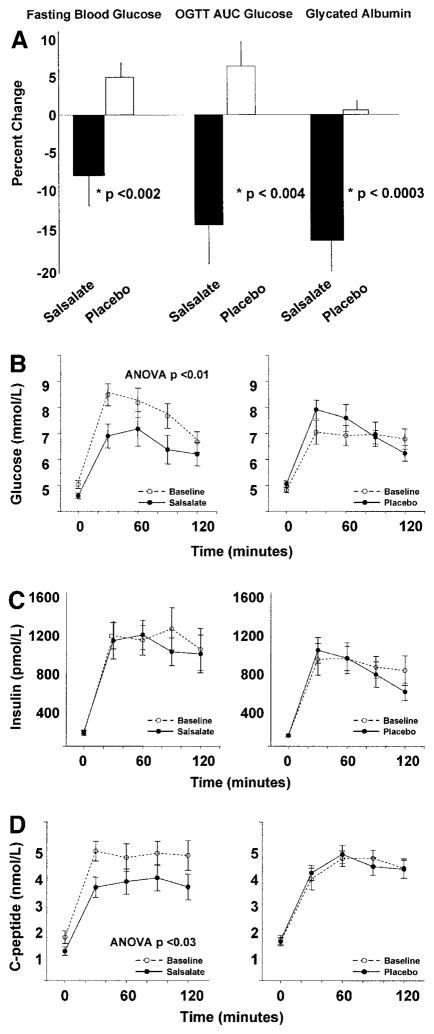
Fasting glucose decreased 13% in the salsalate group compared with the placebo group after 1 month (−0.4 ± 0.2 vs. +0.2 ± 0.1 mmol/l, respectively, P < 0.002) (Fig. 1A, left). The glucose area under the curve after a 75-g OGTT was also significantly reduced in salsalate compared with placebo-treated subjects (−130 ± 53 vs. +38 ± 15 mmol · min−1 · l−1, P < 0.004) (Fig. 1A, center). Likewise, within groups the glycemic response to an OGTT improved after salsalate treatment (repeated-measures analysis P < 0.01) but not placebo (Fig. 1B). Consistently, we found a 17% reduction in the percentage of glycated albumin between salsalate and placebo (−2.2 ± 0.5 vs. −0.1 ± 0.1%, P < 0.0003) (Fig. 1A, right). Therefore, glycemia, assessed by fasting glucose postglucose load and glycated albumin, was significantly reduced after salsalate compared with placebo. No hypoglycemia was noted in either group.
Figure 1.
Changes in glycemia, insulin, and C-peptide. A: Fasting glucose, AUC after an OGTT, and glycated albumin were reduced in salsalate-treated subjects compared with the placebo group. B: The salsalate-treated group (left panel) showed improvements in glycemia after an OGTT compared with the placebo group (right panel). Mean and SEM data are shown before and 30, 60, 90, and 120 min after 75 g oral glucose. ○, baseline data. C and D: Data for insulin (C) and C-peptide (D) are shown before and 30, 60, 90, and 120 min after a 75-g oral glucose load for both the salsalate-treated (left panels) and placebo (right panels) groups. AUC, area under the curve.
Fasting insulin and levels during an OGTT remained unchanged after salsalate or placebo (Fig. 1C). However, the reduction in fasting C-peptide comparing salsalate and placebo-treated groups was significant (−0.53 ± 0.14 vs. +0.06 ± 0.1 nmol/l, respectively, P < 0.01). The percent difference in C-peptide area under the curve after an OGTT between groups was also significant (−19 ± 6 vs. +2 ± 6%, P < 0.03) (Fig. 1D).
As salicylates have previously been demonstrated to reduce insulin clearance (7), we assessed insulin sensitivity by HOMA IRC-peptide, which was significantly lowered by 29 ± 10% after salsalate and increased 10 ± 7% after placebo (between groups, P < 0.004). However, HOMA-IR using insulin was not significantly changed in either group (4.3 ± 0.9 vs. 4.2 ± 0.6 and 3.5 ± 0.5 vs. 3.7 ± 0.4, before vs. after salsalate and placebo, respectively), with no difference in the change between groups (P = 0.7). These findings are consistent with reduced insulin clearance and improved insulin sensitivity contributing to the improved glycemia.
Cytokines and adipokines
In secondary analyses, cytokines and adipokines were assessed to evaluate the anti-inflammatory mechanism for improvement in glycemia. Of importance, adiponectin increased significantly comparing salsalate-treated and placebo groups (+5.6 ± 1.8 vs. −0.3 ± 0.4 mg/l, respectively, P < 0.003) (Fig. 2a). In change from baseline within-group analysis, CRP concentrations decreased 34% (−18.0 ± 7.7 nmol/l, P < 0.05) after salsalate, with a nonsignificant decrease after placebo (−2.8 ± 7.2 nmol/l, P = 0.7), and fasting nonesterified FFAs showed a tendency to decline by 14% after salsalate (−0.20 ± 0.10 mEq/l, P = 0.07) with no change after placebo (−0.06 ± 0.05 mEq/l, P = 0.6) (Fig. 2B), although the between-group comparisons did not reach statistical significance. Additional inflammatory markers interleukin-6 and soluble vascular cell adhesion molecule-1 did not change significantly after salsalate therapy (data not shown). Using multiple regression analysis, salsalate assignment independently predicted the change in fasting blood glucose (P < 0.009), response to glucose load (P = 0.05), and glycated albumin (P < 0.003) when adjusting for the change in adiponectin, FFAs, and CRP.
Figure 2.
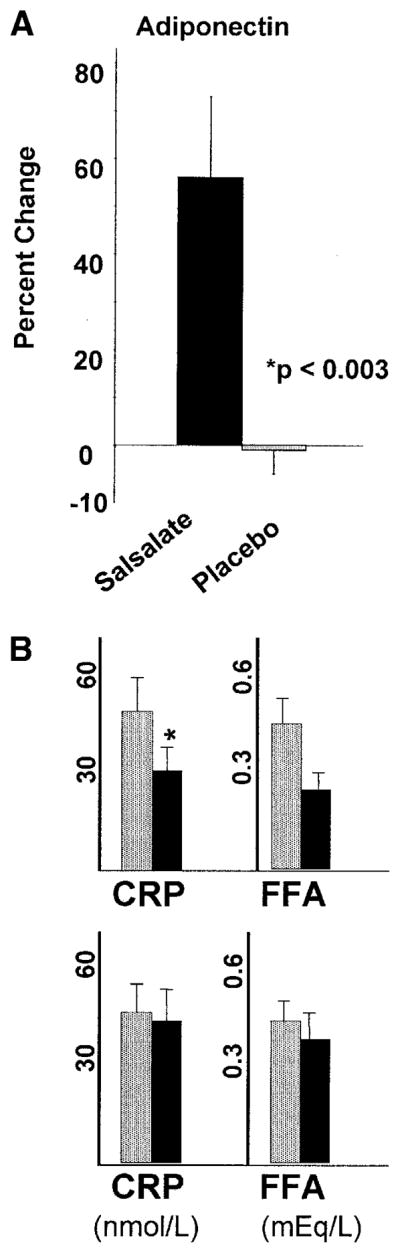
Changes in inflammatory markers and mediators. A: Adiponectin increased significantly in the salsalate-treated group compared with the placebo group. B: CRP and FFA were lower after salsalate (upper panel) but unchanged after placebo (lower panel) by within-group analysis (*P < 0.05).
 , baseline; ■, after therapy.
, baseline; ■, after therapy.
Safety and tolerability
A priori, the protocol specified dose reduction for signs of salicylism so the maximum tolerable dose was evaluated for efficacy. Three participants required dose reduction attributable to complaints of tinnitus, headache, or dizziness. Two of the three participants were receiving active medication; one tolerated 3.5 g/day, one tolerated 3.0 g/day, and both completed the trial without symptoms.
There were no noted changes in laboratory analyses of renal function, electrolytes, or anion gap. Three participants who received active therapy were withdrawn for rash. No respiratory distress was noted. There was no statistically significant change in mean alanine aminotransferase (ALT) or aspartate aminotransferase (AST) in the treatment or placebo groups (P > 0.1). However, one participant had an isolated rise in ALT to less than twice the upper range of normal, and a second participant had a similarly mild elevation in ALT and AST. Both resolved spontaneously.
CONCLUSIONS
Obesity is an established risk factor for development of type 2 diabetes and is associated with a chronic inflammatory process (16,17). Higher fasting glucose levels within the normoglycemic range independently predict risk for diabetes (18). In this proof-of-principle study, we evaluated whether the widely prescribed anti-inflammatory agent salsalate could have an impact on glycemia in obese young adults. In a randomized, double-masked, placebo-controlled study, we demonstrate reductions in glycemia, both fasting and after an OGTT, and reduced glycated albumin after 1 month of salsalate. As evidence of the anti-inflammatory effects of this therapy, we demonstrated increased adiponectin and a trend toward reductions in CRP and FFA levels, which may also be important for reducing risks of diabetes and cardiovascular disease. This is the first human trial to demonstrate that salsalate can produce significant improvements in glycemia and adiponectin in obese individuals. Although our study was limited in sample size and duration, the data are consistent with the hypothesis that targeting inflammation may provide a potential treatment for dysglycemia and a method for diabetes prevention.
Both sodium salicylate and acetylsalicylic acid (aspirin) have been shown to improve glycemia in humans with type 2 diabetes (5,7,19). Previous human studies have lasted for 1–2 weeks. The current study is the first to suggest that effects may be sustained over longer durations. On rare occasions, salicylates have been noted to cause hypoglycemia. Notably, there was no symptomatic or measured hypoglycemia in our study group. Additionally, our data suggest that improved insulin sensitivity may contribute to reduced glucose levels. Evaluation of insulin resistance was limited by the fact that insulin clearance has been shown to be reduced by salicylates, as demonstrated during a hyperinsulinemiceuglycemic clamp before and after 2 weeks of high-dose aspirin (7). Although we did not directly assess insulin clearance in this cohort, altered insulin clearance would confound interpretation of insulin sensitivity based on circulating insulin levels. No effect of salicylates on C-peptide clearance has been demonstrated. Thus, the finding of an improvement in insulin sensitivity was based on interpretation of HOMA-IRC-peptide, as described previously (13), and was supported by increased adiponectin.
Mechanism(s) by which salicylates lower glucose have historically been poorly understood and attributed to prostaglandin suppression (20). However, aspirin doses required for both anti-inflammatory and glucose-lowering effects exceed levels necessary for inhibition of cyclooxygenase enzymes and prostaglandin synthesis. Nonacetylated salicylates, such as salsalate, lack an acetyl group and do not effectively inhibit cyclooxygenase enzymes (21) and thus are unlikely to act via this proposed mechanism. Recently, high doses of salicylates have been recognized to inhibit NF-κB activity (9), possibly through inhibition of IKKβ (22). The direct role of the IKKβ/NF-κB pathway in development of diet-induced obesity and insulin resistance has been validated in animal models (23,24). In addition, insulin resistance in suppressor of cytokine signaling 3 knockout mice is improved by sodium salicylate, suggesting an inflammatory mechanism of action (25). Although we did not directly assess NF-κB or suppressor of cytokine signaling 3 activity, we hypothesize that the mechanism by which salsalate improves glycemia is via the established effect of salicylates on the IKKβ/NF-κB signaling pathways.
Other mechanisms could contribute to the altered metabolic parameters demonstrated. Adiponectin is an adipocyte-derived cytokine, which has insulin sensitizing, anti-inflammatory, and anti-atherosclerotic properties (26,27), and is decreased in obese populations (28). Increased adiponectin could affect glucose homeostasis. Likewise, although modest, the reduction in levels of FFAs could also contribute to the improvements in glycemic parameters and insulin sensitivity through the decreased activation of inflammatory mediators (29). Multiple regression analyses suggest that observed changes in the measured circulating inflammatory cytokines do not fully account for the glycemic-lowering effects of salsalate. Although no weight change was noted in our current study, the impact of alterations in food intake or energy expenditure precipitated by salsalate therapy cannot be excluded. Future mechanistic studies are required to confirm the hypothesis that salsalate improves glycemia by affecting the IKKβ/NF-κB signaling pathways in humans.
Although efficacious for treatment of patients with diabetes, the adverse safety profile of high doses of aspirin, particularly the risk of bleeding, limits clinical therapeutic utility. Nonacetylated salicylates, such as salsalate, do not inhibit platelets and have reduced risk of bleeding (10,11). Additionally, salsalate is not soluble in the acidic gastric environment with a resultant lack of gastrointestinal side effects (30). Salsalate has been administered to humans for decades and has an established long-term safety profile. Therefore, the choice of salsalate, a salicylate dimer that is an equipotent inhibitor of NF-κB activity without adverse effects on platelet aggregation and bleeding risk, is a logical choice for study.
Side effects reported in the current study were tinnitus, headache, rash, and transient elevations in ALT and AST. Tinnitus, an expected side effect, was reported in two patients receiving active therapy and one receiving placebo. Symptoms resolved with dose adjustment as anticipated. Rash occurred at a rate higher than reported by registration trials (31). The reason for the increased rate of this side effect is not apparent in this group without a history of allergy to nonsteroidal anti-inflammatory drugs. There was no evidence of respiratory distress or anaphylaxis in our participants. The transient, mild ALT and AST elevation is a rare reported side effect, and attribution to the medication is unclear. Obesity itself is associated with mild elevations in serum ALT and AST and with hepatic steatosis and/or inflammation (32). Recent estimates suggest that >33% of obese individuals may have hepatic steatosis (33). Therefore, mild elevations in hepatic enzymes may be consistent with expected findings in the obese population. Salsalate has been widely prescribed for more than a half century, and no specific concerns in overweight individuals have been noted. However, safety in obese subjects needs to be monitored and established in larger, long-term studies.
This study is limited by the relatively small sample size and short-term duration. However, the excellent efficacy to lower glycemia and improve adiponectin in obese individuals in this proof-of-principle trial does support potential use of anti-inflammatory modulators for prevention of insulin resistance and diabetes. Metformin (34,35), rosiglitazone (36), and troglitazone (37) have previously been shown to reduce inflammation in obesity through the inhibition of NF-κB activity or related cytokines, which may contribute to their impact on diabetes prevention and treatment. The reduction in fasting glucose in this study is consistent with or greater in magnitude than the change demonstrated in the larger, longer term diabetes prevention trials with metformin (38), rosiglitazone (39), or troglitazone (40). Whether these effects will be sustained with continued administration of salsalate needs to be determined.
In summary, our data demonstrate that targeting inflammation using salsalate improves glycemia, insulin resistance, and inflammatory profiles in obese individuals at risk for development of diabetes. This proof-of-principle study suggests a physiologically relevant approach to improving the inflammatory milieu associated with obesity. More extensive studies are needed to evaluate the use of anti-inflammatory strategies in general and salsalate specifically as preventative and therapeutic interventions in obesity and diabetes.
Acknowledgments
This work was supported by the National Institutes of Health (Grants K23-DK02795 to A.B.G., P50-HL083813 to A.B.G. and S.E.S., P30-DK36836, and General Clinical Research Center Grant M01-RR001032), a Lilly Fellowship Grant, and Clinical Investigator Training Program, Harvard-MIT Division of Health Sciences and Technology-Beth Israel Deaconess Medical Center, with support from Pfizer and Merck (A.F.).
We thank the patients and staff of the Joslin Diabetes Center General Clinical Reasearch Center, Dr. Laura Coombs, George Washington Biostatistical Center, and Drs. Ernie Schaefer and Bela Asztalos, New England Medical Center.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CRP
C-reactive protein
- FFA
free fatty acid
- HOMA-IR
homeostasis model assessment of insulin resistance
- HOMA-IRC-peptide
homeostasis model assessment of insulin resistance calculated using C-peptide
- IKKβ
IκB kinase complex β
- NF-κB
nuclear factor-κB
- OGTT
oral glucose tolerance test
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebstein W. Zur therapie des diabetes mellitus, insbesondere über die anwendeng der salicylauren natron bei demselben. Berl Klin Wochenschr. 1876;13:337–340. [Google Scholar]
- 4.Williamson R. On the treatment of glycosuria and diabetes mellitus with sodium salicylate. Br Med J. 1901;1:760–762. doi: 10.1136/bmj.1.2100.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid J, Macdougall AI, Andrews MM. Aspirin and diabetes mellitus. Br Med J. 1957;33:1071–1074. doi: 10.1136/bmj.2.5053.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilgore S. The influence of salicylate on hyperglycemia. Diabetes. 1960;9:392–393. [Google Scholar]
- 7.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 9.Kopp E, Ghosh S. Inhibition of NF-κB by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 10.Estes D, Kaplan K. Lack of platelet effect with the aspirin analog, salsalate. Arthritis Rheum. 1980;23:1303–1307. doi: 10.1002/art.1780231113. [DOI] [PubMed] [Google Scholar]
- 11.Mielants H, Veys EM, Verbruggen G, Schelstraete Comparison of serum salicylate levels and gastro-intestinal blood loss between salsalate (Disalcid)and other forms of salicylates. Scand J Rheumatol. 1981;10:169–173. doi: 10.3109/03009748109095292. [DOI] [PubMed] [Google Scholar]
- 12.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28:S37–S42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 13.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 14.April P, Abeles M, Baraf H, Cohen S, Curran N, Doucette M, Ekholm B, Goldlust B, Knee CM, Lee E, et al. Does the acetyl group of aspirin contribute to the antiinflammatory efficacy of salicylic acid in the treatment of rheumatoid arthritis? Semin Arthritis Rheum. 1990;19:20–28. [PubMed] [Google Scholar]
- 15.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Thorand B, Lowel H, Schneider A, Kolb H, Meisinger C, Frohlich M, Koenig W. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998. Arch Intern Med. 2003;163:93–99. doi: 10.1001/archinte.163.1.93. [DOI] [PubMed] [Google Scholar]
- 17.Duncan BB, Schmidt MI. The epidemiology of low-grade chronic systemic inflammation and type 2 diabetes. Diabetes Technol Ther. 2006;8:7–17. doi: 10.1089/dia.2006.8.7. [DOI] [PubMed] [Google Scholar]
- 18.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353:1454–1462. doi: 10.1056/NEJMoa050080. [DOI] [PubMed] [Google Scholar]
- 19.Gilgore SG, Rupp JJ. Response of blood glucose to intravenous salicylate. Metabolism. 1961;10:419–421. [PubMed] [Google Scholar]
- 20.Baron SH. Salicylates as hypoglycemic agents. Diabetes Care. 1982;5:64–71. doi: 10.2337/diacare.5.1.64. [DOI] [PubMed] [Google Scholar]
- 21.Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol. 1995;2:637–643. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- 22.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 23.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-β links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 25.Torisu T, Sato N, Yoshiga D, Kobayashi T, Yoshioka T, Mori H, Iida M, Yoshimura A. The dual function of hepatic SOCS3 in insulin resistance in vivo. Genes Cells. 2007;12:143–154. doi: 10.1111/j.1365-2443.2007.01044.x. [DOI] [PubMed] [Google Scholar]
- 26.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 28.You T, Yang R, Lyles MF, Gong D, Nicklas BJ. Abdominal adipose tissue cytokine gene expression: relationship to obesity and metabolic risk factors. Am J Physiol. 2005;288:E741–E747. doi: 10.1152/ajpendo.00419.2004. [DOI] [PubMed] [Google Scholar]
- 29.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 30.Roth S, Bennett R, Caldron P, Hartman R, Mitchell C, Doucette M, Ekholm B, Gold-lust B, Lee E, Wilson R. Reduced risk of NSAID gastropathy (GI mucosal toxicity) with nonacetylated salicylate (salsalate): an endoscopic study. Semin Arthritis Rheum. 1990;19:11–19. [PubMed] [Google Scholar]
- 31.Disalcid package insert. Northridge, CA: 3M Pharmaceuticals; 1998. [Google Scholar]
- 32.Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;126:137–145. doi: 10.7326/0003-4819-126-2-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 33.Choi JW. Association between elevated serum hepatic enzyme activity and total body fat in obese humans. Ann Clin Lab Sci. 2003;33:257–264. [PubMed] [Google Scholar]
- 34.Dandona P, Aljada A, Ghanim H, Mohanty P, Tripathy C, Hofmeyer D, Chaudhuri A. Increased plasma concentration of macrophage migration inhibitory factor (MIF) and MIF mRNA in mononuclear cells in the obese and the suppressive action of metformin. J Clin Endocrinol Metab. 2004;89:5043–5047. doi: 10.1210/jc.2004-0436. [DOI] [PubMed] [Google Scholar]
- 35.Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schonbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor-κB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–617. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- 36.Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al-Haddad W, Dhindsa S, Dandona P. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–2735. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- 37.Aljada A, Garg R, Ghanim H, Mohanty P, Hamouda W, Assian E, Dandona P. Nuclear factor-κB suppressive and inhibitor-κB stimulatory effects of troglitazone in obese patients with type 2 diabetes: evidence of an antiinflammatory action? J Clin Endocrinol Metab. 2001;86:3250–3256. doi: 10.1210/jcem.86.7.7564. [DOI] [PubMed] [Google Scholar]
- 38.Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, Andres R, Saudek C, Edelstein SL, Arakaki R, Murphy MB, Shamoon H. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 40.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]