Abstract
OBJECTIVES
To determine the effect of two different community-based group exercise programs on functional balance, mobility, postural reflexes, and falls in older adults with stroke.
DESIGN
A randomized, clinical trial.
SETTING
Community centre.
PARTICIPANTS
Total of 61 community-dwelling older adults with chronic stroke.
INTERVENTION
Participants were randomly assigned to an Agility (n = 30) or Stretching/weight-shifting (n = 31) exercise group. Both groups exercised three times a week for 10 weeks.
MEASUREMENTS
Participants were assessed prior to, immediately after, and one-month following the intervention for Berg Balance, Timed Up and Go, step reaction time, Activities-specific Balance Confidence, and Nottingham Health Profile. Testing of standing postural reflexes and induced falls evoked by a translating platform was also performed. In addition, falls in the community were tracked for one year from the start of the interventions.
RESULTS
Although exercise led to improvements in all clinical outcome measures for both groups, the Agility group demonstrated greater improvement in step reaction time and paretic rectus femoris postural reflex onset latency compared to the Stretching/weight-shifting group. Further, induced falls on the platform were reduced in the Agility group.
CONCLUSION
Group exercise programs that include Agility or Stretching/weight shifting exercises improve postural reflexes, functional balance and mobility and may lead to a reduction of falls in older adults with chronic stroke.
Keywords: rehabilitation, cerebrovascular accident, posture, physical fitness, clinical trial
INTRODUCTION
About thirty percent of community-dwelling adults aged 65 and over fall at least once each year and falls and fall-related injuries have been shown to be independent determinants of functional decline.1,2 Stroke is considered one of the greatest risk factors for falls in older adults.3 Twenty-three to 73% of community-dwelling older adults with chronic stroke have been reported to fall over a four to six month period with approximately half falling repeatedly3,4 and there is a greater than seven-fold increase in fracture risk in this population. 5 Stroke-related impairments such as muscle weakness, impaired cognition, sensorimotor dysfunction, and balance and mobility problems presumably contribute to the large number of falls. One potential way of improving balance and mobility as well as reducing falls is through exercise interventions.
Recent studies have demonstrated that exercise can improve mobility6–8 and functional balance9,10 in older adults with chronic stroke. However, it is unclear as to what are the advantages to different types of exercise programs and the mechanisms, which underlie their improvements. Although falls and fall-related injuries in older adults with stroke are an enormous burden on both the individual and health care system, no studies have investigated the effect of an exercise intervention on falls reduction in this population.
Postural reflexes, in the form of coordinated muscle activity, are the first line of defence against an unexpected destabilizing force applied to the body (e.g. collision, slip, and trip) or from self-induced movements (e.g. reaching, transferring). Older adults with stroke have delayed paretic limb postural reflex muscle onset latencies compared to healthy older adults11 and it is unknown whether exercise can alter these latencies, which could lead to improved postural control and a reduction in falls. Such a concept would support an emerging idea of neural plasticity with exercise, particularly following brain injury in animal models.12
In addition to slow postural reflexes, older adults with stroke demonstrate slow rate of force production13,14, which may contribute to the unsuccessful recovery from an unexpected destabilizing force to the body. Further, integration of multi-sensory information (i.e. visual, vestibular, and somatosensory) is impaired following a stroke.15 Thus, we designed an Agility program, which involved fast-paced, dynamic movements and a multi-sensory integration component. The purpose of this study was to determine the effect of two different community-based group exercise programs (Agility versus Stretching/weight-shifting program) on functional balance, mobility, standing postural reflexes, and falls in older adults with chronic stroke. The Stretching/weight-shifting program utilized weight-shifting, as this would increase use of the paretic limb, which may improve motor function and reduce the risk of falling.16,17
METHODS
Participants
Participants living in the community were recruited from hospital databases, stroke groups, and advertisements. Inclusion criteria were (1) age ≥50 years, (2) single stroke, at least 12 months from onset, (3) able to walk, with or without an assistive device, for a minimum of 10 meters and have an activity tolerance of 60 minutes with rest intervals. Exclusion criteria were (1) not medically stable, (2) neurological conditions not related to stroke (e.g. Parkinson’s disease) or severe musculoskeletal conditions (e.g. recent joint replacement surgery, amputation), (3) < 22 on Mini-Mental State Exam18, and (4) Berg Balance > 52/56 (minimal balance deficit). Following university and hospital ethics approval, participants provided written informed consent prior to participation. The participant’s physician confirmed the presence of stroke and the inclusion/exclusion criteria. In addition, type, location, and onset of stroke were collected through medical records and/or physician information where available.
The American Heart Association Stroke Functional Classification was collected to provide an indication of the level of function of the participants. This scale19 consists of five levels and measures residual impairment and disability of stroke in the areas of basic (BADL) and instrumental (IADL) activities of daily living. Level one indicates independence in both BADL and IADL and level five indicates complete dependence.
Study design
Participants were screened for a six-month fall history, balance (Berg Balance), and dementia (Mini-Mental State Exam) and then randomly assigned alphanumeric codes through a random number generator. Following this procedure, participants were stratified20 for functional balance (Berg Balance) and falls (recalled over the past six months) to generate four subgroups: (1) Berg Balance < 40 and falls < 2 (n = 12), (2) Berg Balance < 40 and falls ≥ 2 (n = 9), (3) Berg Balance ≥ 40 and falls < 2 (n = 25), and (4) Berg Balance ≥ 40 and falls ≥ 2 (n = 15). Subsequently, a person independent of the study (i.e. concealed allocation) randomly assigned participants (using their codes) from each subgroup such that there were similar numbers of participants placed into the two exercise groups. Participants knew they were in one of two exercise groups but were unaware of the differences between them. Exercise instructors were not aware of the outcome measures of the study. All assessors were blinded to the group assignment, study design, and purpose.
Intervention
The two exercise programs consisted of one-hour sessions, 3x/week for 10-weeks held at a local community centre supervised by three instructors (physical therapist, kinesiologist, and recreation therapist). There were six classes (three for each exercise program) with a 1:3 instructor:participant ratio.
Each of the exercise programs began with a five-minute warm-up consisting of walking and light stretching and ended with a five-minute cool-down of light stretching. The Agility exercise program challenged dynamic balance and the tasks were progressively increased in difficulty based on set criteria and dependent on an individual’s ability. This program emphasized an agility and multi-sensory approach. Tasks included standing in various postures (e.g. tandem or feet apart, one foot stance, and weight-shifting) and walking with various challenges (e.g. different step lengths and speeds, tandem walking, figure of eight walking, stepping up and over low risers, side stepping, crossover stepping, and stepping over obstacles). Additional exercises included sit-to-stand movements, rapid knee raise while standing, and standing perturbations (i.e. instructor pushing participant in a controlled manner or participant pushing instructor to destabilize balance and elicit postural reflexes). Eyes closed conditions and foam surfaces were incorporated for many of the tasks.
The Stretching/weight-shifting exercise program focussed on slow, low-impact movements consisting of stretching and weight-shifting. Weight-shifting exercises incorporated Tai Chi-like movements and reaching tasks, which encouraged increased force to be taken through the paretic lower limb. Stretching of major muscle groups was performed while standing and while on mats on the floor. The act of getting down and up from the floor was considered an exercise in itself and practiced with the aid of the instructors.
Outcome measures
Participants were evaluated three times: before the intervention (baseline), after the intervention (post-intervention), and one-month following (retention). For each of these time periods, participants were assessed on two occasions separated by two days to minimize fatigue.
The Berg Balance Scale21 was used to assess functional balance while the Timed Up and Go Test22 assessed functional mobility. Balance confidence and health-related quality of life were measured using the Activities-specific Balance Confidence (ABC) Scale23 and Nottingham Health Profile (NHP)24, respectively. Higher scores on the ABC reflect better perceived balance confidence and lower scores on the NHP reflect better perceived health-related quality of life.
To assess standing postural reflexes, a total of 20 platform translations (8 cm displacement, 30 cm/s velocity, and 300 cm/s2 acceleration), separated by 15–30 second intervals, were induced while participants stood on two force plates (Bertec Corp.) embedded in the platform. Participants wore a harness attached to the ceiling and at least one spotter was present. Participants were told that the platform could move at any time but the onset and direction of the translation were unexpected in nature. The direction was counterbalanced across participants so that either 10 consecutive backward translations followed 10 forward translations or vice versa. The first trial from each direction was discarded from the analysis, as the first trial to a perturbation is highly variable compared to subsequent ones.25
Force plate data and surface electromyography (EMG) (Bortec) from bilateral tibialis anterior (TA), medial head of gastrocnemius (MG), rectus femoris (RF), and biceps femoris (BF) were recorded at 600 Hz for two seconds prior to platform movement and four seconds after. The TA and RF muscles were analyzed for the forward translations while MG and BF were analyzed for the backward translations due to their roles in the primary recovery response to those translation directions.26 EMG was band-passed (10–1000 Hz), full-wave rectified and low-pass filtered at 100 Hz (second-order, zero-lag, Butterworth algorithm). Muscle onset latency, representing a postural reflex, was defined as an increase in muscle activity that exceeded + 2 SD (beyond mean signal one sec prior to platform movement) for at least 30 ms and was determined by an interactive computer algorithm.
Test re-test reliability over two separate days for 10 older adults with chronic stroke produced Intraclass Correlation Coefficients (ICC) ± standard error of measurement (SEM) (ms) for the paretic TA, RF, and MG of 0.92 ± 9.2, 0.87 ± 11.2, 0.79 ± 9.9, respectively and for the non-paretic TA, RF, and MG of 0.79 ± 4.3, 0.79 ± 14.1, and 0.67 ± 4.4, respectively. No learning effect was observed as evident from non-significant F-tests over the two days.
Participants were instructed to step forward as fast as possible following an auditory cue to measure step reaction time. The first two and last two trials were with the non-paretic limb while the middle trial was with the paretic limb to reduce any standing postural bias. Only data from the non-paretic limb was recorded because pilot work found that older adults with stroke tended to step with this limb in response to platform translations. Reaction time, averaged over the four non-paretic limb trials, was defined as the time between the auditory cue and when the vertical force from the force plate reached zero (foot lift).
Participants kept a self-report monthly falls diary over one year from the start of the intervention and phone calls were placed if the monthly diary was not returned. A fall was defined as unintentionally coming to rest on the floor or another lower level but not due to seizure, stroke/myocardial infarction, or an overwhelming displacing force (e.g. earthquake).1 Falls were also determined during the platform translations at baseline, post-intervention, and retention assessments and were defined as being unable to recover balance following the perturbation and requiring the use of the harness system (i.e. caught by the rope and harness system) or the assistance of the spotter.
Statistical analysis
Based on a Berg Balance score (primary outcome measure) of 45.3 with a SD of 5.65 and desired five-point change10, a sample size of 21 persons per group would have 80% power with alpha of 0.05. Thirty persons per group were sought to account for dropouts. All participants received the Agility or Stretching/weight-shifting condition as allocated and were included in the analyses if measures of outcomes were available. Baseline descriptive variables were compared between groups using Chi-square (gender, affected limb), Mann-Whitney U (age, stroke duration, and Mini-Mental State Exam), Median (American Heart Association Stroke Functional Classification), or independent t (mass) tests. Outcome measures were tested for normality and, when applicable, either log (Berg Balance, Timed Up and Go, step reaction time) or rank (NHP) transformed for subsequent analysis. Baseline outcome measures between the exercise groups were compared using independent t-tests.
Three separate multivariate analyses of variance (MANOVA) were performed to compare the outcome measures of two exercise groups (Group: Agility versus Stretching/weight-shifting) at three assessment times (Time: baseline, post-intervention, retention) using a mixed model with group as the between-subjects factor and assessment time as the within-subjects factor.27 The first MANOVA included the clinical outcome measures: (1) Berg Balance, (2) Timed Up and Go, (3) step reaction time, (4) ABC, and (5) NHP. The second MANOVA included the paretic limb postural reflex muscle onset latencies for the: (1) TA, (2) RF, (3) MG, and (4) BF, and the third MANOVA included the muscles of the non-paretic limb. Following a significant MANOVA, a two-way (Group and Time) analysis of variance (ANOVA) was undertaken, and if applicable, Duncan’s post-hoc tests. A covariate (baseline scores) was included in the ANOVAs when baseline differences were significant for a particular variable.
The number of falls occurring over one year in the community for each participant was normalized to the number of months over which information was collected. Subsequently, the number of falls/month over the course of one year from the start of the intervention (excluding falls during the classes) for each group was compared using a Mann-Whitney U test. Additionally, the total number of fallers (individuals who fell one or more times) and a subset of the fallers (individuals who fell ≥ two times, i.e., repeat fallers) over the one year time period were compared between the two groups using Chi-square tests.
Finally, the total number of falls during the platform translations was rank transformed and entered in a two-way ANOVA to compare the two exercise groups across three assessment times (group as the between-subjects factor and assessment time as the within-subjects factor).
An alpha level of 0.05 was selected for all statistical analyses (SAS 8.2, SAS Institute Inc.).
RESULTS
Participant characteristics
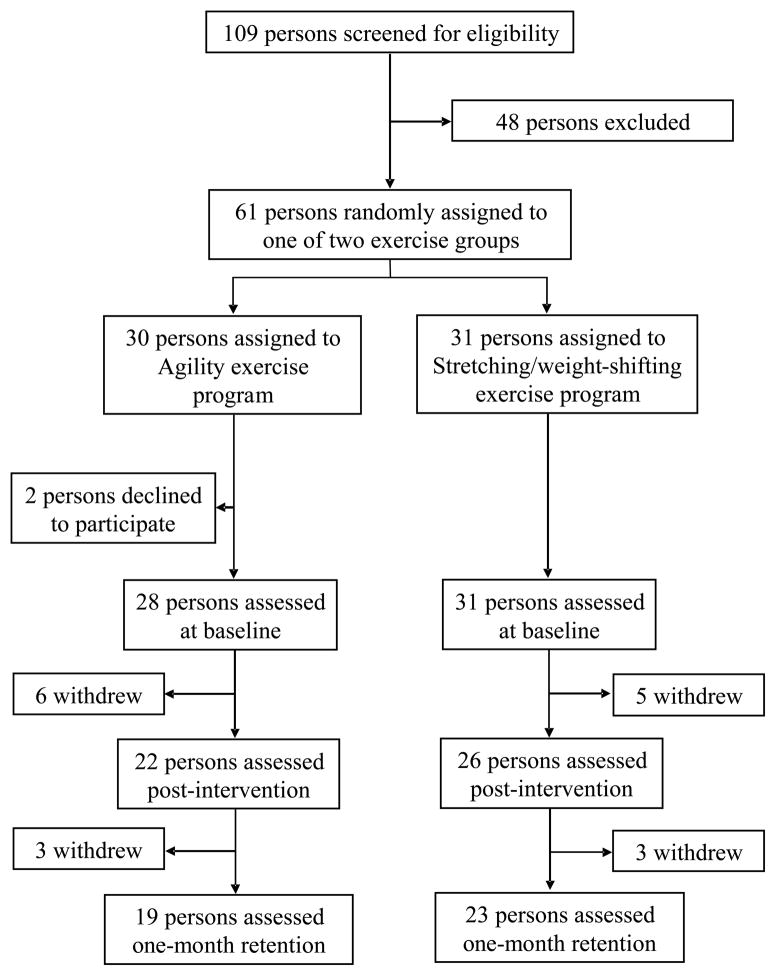
A total of 109 potential participants were identified over three months. A total of 48 participants were excluded: 35 did not meet inclusion criteria, two refused to participate, and 11 could not obtain physician approval, make the exercise class times, or were planning a conflicting extended vacation. Thus, 61 persons underwent random assignment: 31 into the Stretching/weight-shifting and 30 into the Agility program. Two individuals discontinued the study due to time commitments prior to baseline assessment. A total of 11 individuals discontinued the intervention due to time commitments (n = 2), hip fracture (n = 1, during exercise in the Agility program on a non-challenging task that was included in both programs), illness (e.g. severe flu, hospitalization) (n = 5), or personal reasons (n = 3). Six participants were lost at retention testing due to illness (n = 2), vacation (n = 3), or personal reasons (n = 1) (Figure 1). The mean (+/−SD) percent of exercise classes attended for the Stretching/weight-shifting and Agility groups were 94.4% (5.5) and 92.6% (10.4), respectively. Table 1 describes participant characteristics. There were no differences between exercise groups for baseline descriptive variables (P >.200).
Figure 1.
Trial Profile.
Table 1.
Participant Characteristics.
| Variable* | Stretching/weight- shifting (n=26) | Agility (n=22) | Dropouts (n=11) |
|---|---|---|---|
| Gender, Male/Female | 18 (69)/8 (31) | 17 (77)/5 (23) | 6 (55)/5 (45) |
| Age, yrs | 67.5 (7.2) | 68.1 (9.0) | 69.6 (10.8) |
| Mass, kg | 78.4 (15.9) | 83.5 (17.7) | 76.3 (16.3) |
| Stroke Duration, yrs | 3.8 (2.4) | 3.6 (1.8) | 4.1 (5.7) |
| Affected Side, R/L/NA | 8 (31)/18 (69)/0 (0) | 10 (45)/11 (50)/1 (5) | 3 (27)/7 (64)/1 (9) |
| AHASFC, 1 – 5 | 2.5 (2 – 3) | 2.0 (1 – 3) | 3.0 (2.5 – 3) |
| MMSE, 1 – 30 | 26 (24 – 28) | 28 (27 – 28) | 27 (26.25 – 28) |
| # of Fallers† | 15 (58) | 15 (68) | 6 (55) |
| Stroke Location | |||
| Cortical | 10 (39) | 4 (18) | 2 (18) |
| Subcortical | 8 (31) | 7 (32) | 2 (18) |
| Brainstem/cerebellum | 4 (15) | 6 (27) | 3 (27) |
| Cortical-subcortical | 0 | 0 | 1 (9) |
| Unknown | 4 (15) | 5 (23) | 3 (27) |
| Co-morbidities | |||
| Arthritis | 3 (12) | 5 (23) | 2 (18) |
| Diabetes | 9 (35) | 4 (18) | 2 (18) |
| Depression | 9 (35) | 4 (18) | 3 (27) |
| Hypertension | 20 (77) | 22 (100) | 11 (100) |
| Obesity (BMI > 30) | 9 (35) | 6 (27) | 1 (11) |
| Hyperlipidemia | 8 (31) | 12 (55) | 4 (36) |
| Assistive Devices‡ | |||
| AFO | 2 (8) | 4 (18) | 3 (27) |
| Wheelchair | 3 (12) | 2 (9) | 2 (18) |
| Four-wheel Walker | 3 (12) | 3 (14) | 2 (18) |
| Quad Cane | 2 (8) | 3 (14) | 2 (18) |
| Regular Cane | 9 (35) | 5 (23) | 5 (45) |
Values are (1) mean (+/−SD) for age, mass, (2) number (%) for gender, affected side, # of Fallers, co-morbidities, assistive devices, and (3) median (IQR) for AHASFC, MMSE.
Abbreviations: R: right; L: left; NA: not applicable; AHASFC: American Heart Association Stroke Functional Classification; MMSE: Mini-Mental State Exam; BMI: body mass index; AFO: ankle-foot orthosis;
# persons with ≥1 falls within 6 months prior to the study;
Note that some participants used multiple assistive devices.
Clinical outcome measures
There were no differences in the baseline clinical outcome measures (p-value range .089 – 1.00) except for step reaction time (P = .010), which was entered as a covariate. The MANOVA for the clinical outcome measures demonstrated an overall Group by Time interaction (Wilk’s λ = 0.75, P = .037), Time main effect (Wilk’s λ = 0.33, P < .0001), but no Group main effect (Wilk’s λ = 0.83, P = .184). Step reaction time was decreased in the Agility exercise group to a greater extent than the Stretching/weight-shifting group following the intervention (Table 2). There was also a trend (P = .077) for Group by Time effect for the Timed Up and Go test (Table 2). All clinical measures showed improvements after the intervention, which with the exception of step reaction time, were retained at follow-up (Table 2).
Table 2.
Outcome Measures.
| Measure - Mean (+/−SD)* | Stretching/weight-shifting Group | Agility Group | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n = 26) | Post-intervention (n = 26) | Retention (n = 23) | Baseline (n = 22) | Post-intervention (n = 22) | Retention (n = 19) | Time | Group X Time | |
| Clinical Measures | ||||||||
| Berg Balance, max. 56 | 44.8 (7.1) | 48.1 (5.7) | 47.5 (6.0) | 44.7 (6.5) | 49.1 (5.0) | 49.0 (5.4) | <.0001‡ | .625 |
| Timed Up & Go, sec | 18.4 (13.1) | 17.0 (10.7) | 17.5 (11.0) | 20.2 (10.8) | 16.7 (9.6) | 16.9 (10.5) | .0004‡ | .077 |
| Step Reaction Time, ms† | 590 (171) | 540 (144) | 659 (175) | 721 (170) | 608 (124) | 633 (130) | .005§ | .010 |
| ABC Scale, %, max. 100 | 58.0 (21.2) | 68.3 (19.4) | 64.8 (20.0) | 68.1 (18.6) | 74.0 (18.3) | 76.0 (17.2) | <.0001‡ | .386 |
| NHP, max. 600 | 155 (108) | 123 (133) | 133 (124) | 116 (98) | 99 (102) | 100 (98) | <.0001‡ | .360 |
| Postural Reflex Onset Latencies | ||||||||
| Paretic TA, ms | 115.7 (18.8) | 109.5 (15.9) | 115.9 (18.4) | 122.8 (19.5) | 118.1 (19.9) | 124.7 (27.8) | .054§ | .940 |
| Paretic RF, ms† | 140.3 (32.2) | 129.3 (26.6) | 138.0 (28.4) | 164.7 (33.6) | 137.2 (22.5) | 146.9 (23.1) | <.0001|| | .035 |
| Paretic MG, ms | 130.0 (33.7) | 117.7 (18.0) | 120.2 (18.3) | 131.6 (19.1) | 126.4 (21.1) | 132.1 (31.1) | .004¶ | .472 |
| Paretic BF, ms | 170.9 (37.8) | 164.6 (21.5) | 156.7 (33.6) | 185.7 (27.5) | 167.4 (13.4) | 156.6 (24.9) | .011# | .490 |
| Non-paretic TA, ms | 107.1 (13.0) | 105.1 (11.3) | 106.5 (14.5) | 109.1 (16.8) | 111.7 (20.4) | 106.7 (18.4) | .522 | .575 |
| Non-paretic RF, ms | 139.6 (33.0) | 134.5 (26.5) | 129.0 (23.4) | 148.2 (35.5) | 141.2 (24.5) | 133.1 (29.2) | .011** | .610 |
| Non-paretic MG, ms† | 109.1 (15.3) | 109.2 (20.5) | 107.6 (14.0) | 120.1 (20.5) | 113.2 (17.6) | 113.5 (18.3) | .146 | .218 |
| Non-paretic BF, ms | 149.9 (30.7) | 145.7 (22.7) | 152.7 (31.5) | 171.4 (22.2) | 162.6 (31.5) | 153.5 (33.8) | .089 | .684 |
| Falls During Platform Translations | ||||||||
| # of Falls/person† | 0.76 (2.11) | 1.20 (2.68) | 0.86 (2.63) | 1.64 (3.05) | 1.00 (2.51) | 1.06 (2.44) | .392 | .009 |
Note: The Time factor tested whether there was a difference among the pre-, post-, and retention testing over the whole sample while the Group X Time interaction tested whether the Agility group responded differently among the three time points than did the Streching/weight-shifting group.
Abbreviations: ABC = Activity-specific Balance Confidence Scale; NHP = Nottingham Health Profile, TA = tibialis anterior; RF = rectus femoris; MG = medial head of gastrocnemius; BF = biceps femoris.
Group baseline differences, P<.05
Post-intervention and retention different from baseline
Post-intervention different from baseline and retention
Three time periods all different
Post-intervention different from baseline
Retention different from baseline
Retention different from baseline and post-intervention
Muscle onset latencies
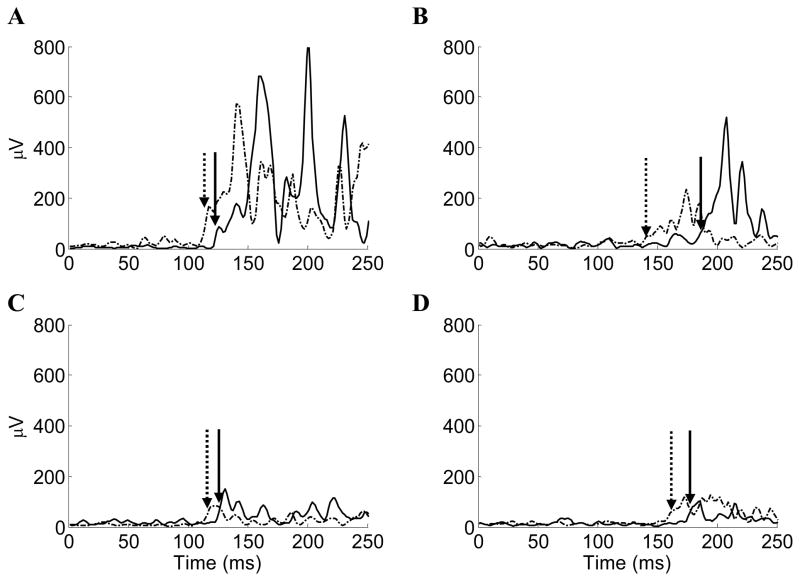
Muscle baseline measures were not different between groups (p-value range .078 – .871) except for the paretic RF and non-paretic MG (P = .026 and .040, respectively), which were entered as a covariate for their respective analyses. The MANOVA for the paretic limb onset latencies demonstrated an overall Group by Time interaction (Wilk’s λ = 0.68, P = .050), Time main effect (Wilk’s λ = 0.50, P = .0004), but no Group main effect (Wilk’s λ = 0.87, P = .312). The paretic RF onset latency was significantly faster by 27.5 ms following the Agility exercise program compared to 11 ms following the Stretching/weight-shifting program. Onset latencies improved in all paretic muscles between 4.7 and 27.5 ms (Table 2) and were not due to different recovery strategies, as muscle sequencing was similar in all test sessions. Figure 2 shows typical postural reflexes for the paretic muscles at baseline and post-intervention assessments.
Figure 2.
Typical postural reflexes evoked during platform translations at baseline (solid lines) and post-intervention (dashed lines) assessments. Sample postural reflexes of a person with stroke during forward platform translations for the (A) paretic TA and (B) paretic RF. Sample postural reflexes of a person with stroke during backward platform translations for the (C) paretic MG and (D) paretic BF. Platform translations triggered at time zero. Arrows (solid for baseline assessment and dashed for post-intervention assessment) indicate the onset latency identified by the combination of a computer algorithm and visual inspection.
The MANOVA for the non-paretic limb did not show a Group by Time interaction (Wilk’s λ = 0.84, P = .212) or Group main effect (Wilk’s λ = 0.86, P = .196), but did show a Time main effect (Wilk’s λ = 0.64, P = .0008). Of the non-paretic musculature, only the RF showed faster onset latency between the retention assessment and the baseline and post-intervention assessments (Table 2).
Falls during platform translations
The number of falls that occurred during the baseline assessment in response to platform translations was entered in the ANOVA as a covariate. There was a significant Group by Time interaction (P = .009) but no Group or Time main effects (P = .428 and P = .392, respectively) for the total number of falls experienced during the platform translations. Falls on the platform were reduced for the Agility exercise group whereas falls were increased for the Stretching/weight-shifting exercise group following the interventions (Table 2).
Prospective community-based falls
A total of 21 and 19 participants for the Stretching/weigh-shifting and Agility exercise groups, respectively, completed all 12 months of the fall diary. There were 75 falls in the Stretching/weight-shifting group (0.26 falls/month/person) compared to 25 falls in the Agility group (0.10 falls/month/person); however, this did not reach significance (P = .197). There were 16 fallers that contributed to these values for the Stretching/weight-shifting group compared to 11 fallers in the Agility group (P = .422). In addition, there were 11 repeat fallers in the Stretching/weight-shifting group compared to 7 in the Agility group (P = .454).
A sub-analysis on those who had a history of falls (15 per group with at least one fall prior to starting the intervention) revealed that less participants continued to fall in the Agility group (8/15) compared to 13/15 in the Stretching/weight-shifting group (P = .046).
DISCUSSION
Exercise leads to improvements in physical function and psychosocial measures
Older adults may have reduced functional balance and impaired mobility28, slower step reaction time29, and delayed postural reflex muscle onset latencies in response to perturbations of balance30, which is further exacerbated in those older adults with stroke. For both types of exercise programs, exercise training improved functional balance and mobility, led to faster standing paretic limb postural reflexes and step reaction time, and resulted in greater balance confidence and health-related quality of life in older adults with chronic stroke. The one-month retention of our effects is particularly important, given that mobility has been shown to deteriorate over a three-month period in older adults with chronic stroke.31
The group aspect of the programs enhanced social contact, which is important considering approximately 20% of this population suffers from depression.3 Significantly, both exercise programs demonstrated improvements in perceived health-related quality of life. This may explain, in part, the good adherence to the interventions.
Differences between the exercise interventions
The Agility exercise group demonstrated greater improvements in step reaction time and paretic RF postural reflex onset latency as well as a trend towards greater improvement in the Timed Up and Go test (representative of functional mobility). The importance of these outcome measures is reflected in the literature in that reaction time and the Timed Up and Go test have been shown to discriminate between older adult fallers and non-fallers.32,33 The task-specific nature of the Agility exercise program may have contributed to the greater improvements in step reaction time, as task-specific training is thought to drive neural plasticity.34 Task-specific interventions may be particularly important in stroke where altered motor coordination is present. For example, in stroke, muscle strengthening protocols may require complementary task-specific practice to transfer the strength gains to functional tasks.35 Specifically, the use of the RF muscle to step forward during the rapid stepping tasks in the Agility exercise classes may have facilitated the change in step reaction time.
This is the first time lower extremity postural reflex muscle onset latency has been shown to change with exercise in any population. Hu and Woollacott36 reported faster neck muscle reflex onset latency in response to platform translations following a multi-sensory exercise program in older adults. Appropriately timed postural reflexes in response to a destabilizing event presumably help prevent the occurrence of a fall; however, other factors such as the magnitude of the response might also contribute. Although participants in both the Agility and Stretching/weight-shifting groups demonstrated improvements in reflex onset latency for several muscle groups, only RF showed a Group by Time effect, with a 27 ms faster paretic RF reflex latency for the Agility group. Further, this change is as long as the monosynaptic stretch reflex and is suggestive of functional significance. The multi-sensory training in the Agility group, which enhances vestibular stimulation, may have contributed to the change in RF onset latency, as the vestibulospinal tract is believed to exert a large influence on proximal leg muscles.37 Although the improvements in postural control were demonstrated in a standing task, it is likely these changes would generalize to other dynamic tasks given the functional nature of the exercise program.
The two exercise programs had some similar components including warm-up and cool-down periods and the fact that both used weight-shifting activities. More importantly, both groups had to attend the program three times a week, which involves substantial activation in itself (transportation, walking into the centre, etc). Despite these similarities, differences between groups were found in step reaction time, rectus femoris muscle onset latency, number of falls induced by platform translations, and a trend for the Timed Up and Go test.
The neurophysiological and functional changes in this study probably have a contribution from neuronal circuitry remodelling. Exercise may promote brain plasticity through mechanisms such as increased expression of brain-derived neurotrophic factor.38 Additionally, exposure to enriched rehabilitative training has demonstrated that increased dendritic arborisation accompanies increased motor performance in rats.39 In humans, forced-use of the paretic limb in older adults with chronic stroke has shown cortical reorganization and improved motor performance.40 In addition, repetitive exercise can alter spinal circuitry as observed in spinal cats undergoing step training.41
The most noteworthy group difference was that the total number of falls experienced during platform translations was reduced to a greater extent in the Agility compared to the Stretching/weight-shifting group following the interventions. Although the stretching/weight-shifting group had a three-fold increase in the number of falls registered over the one-year period from the start of the intervention compared to the Agility group, this did not reach statistical significance. However, our sub-analysis showed the Agility exercise program reduced by half the number of fallers in the year following the intervention for individuals who had a prior history of falling in the community. There was a large amount of group variability in the falls in response to the platform translations and the prospective falls recorded over the year. Because of the variability in the fall measures and our small sample size, our results should be interpreted with caution. Our study was powered for functional balance measures and not falls. In addition, only 83 % of exercising participants completed the 12-month prospective fall diaries. Based on our fall data, a sample size of 292 participants per exercise group would be required to detect differences for the number of fallers between the two interventions.42
Our study had several limitations. Although our results can be generalized to community-dwelling ambulatory chronic stroke patients, many of whom regain walking ability43, our results may not generalize to stroke patients who are not ambulatory, or who reside in a nursing home. Overall, our participants are representative of a sample at risk for falls as the mean baseline Berg Balance score of our participants was 45, which has been reported as a threshold value for fall-risk.21 It is important to note that our study compared individuals randomized to two different types of exercise programs, but did not include a non-exercising control group. In addition, since our participants responded to a recruitment advertisement regarding exercise, it is possible that they were more interested and motivated to pursue activities, which may benefit their health than those who did not respond.
Implications and conclusions
Our results suggest that exercise programs in older adults with chronic stroke should include dynamic balance training, with emphasis on multi-sensory and agility tasks. Agility exercises have been effective in exercise studies aimed at reducing fall risk and improving balance in older women with osteoporosis.44 The reduction in falls in the Agility exercise program is encouraging, although larger studies are required to confirm these findings. Getting to the floor for mat exercises in the Stretching/weight-shifting group was a major challenge, and several people reported that it was the first time they had attempted this task since their injury. Thus, incorporating this task into the Agility exercise program would be beneficial. The instructors reported that the Stretching/weight-shifting exercise program was easier to administer than the Agility program because the latter required closer supervision to ensure that the tasks were appropriately graded to provide a challenge to the participant, yet not result in a fall. Future studies could consider the use of hip protectors in conjunction with exercise in this patient population.
The community-based group exercise interventions were effective in reducing fall-risk factors in this older adult group with chronic stroke including functional balance, mobility, and standing postural reflexes. These programs increase regular physical activity for older adults with chronic conditions and could offset the secondary complications which often occur following sedentary lifestyle.
Acknowledgments
Funding: This study was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) (MOP-57862), salary support to JJE from CIHR (MSH-63617) and the Michael Smith Foundation for Health Research (MSFHR), and trainee support to DSM from MSFHR and the Natural Sciences and Engineering Research Council of Canada.
The research team wishes to thank the participants, exercise instructors, and testers.
References
- 1.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 1998;53:M112–M119. doi: 10.1093/gerona/53a.2.m112. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen L, Engstad T, Jacobsen BK. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke. 2002;33:542–547. doi: 10.1161/hs0202.102375. [DOI] [PubMed] [Google Scholar]
- 4.Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ. 1995;311:83–86. doi: 10.1136/bmj.311.6997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanis J, Oden A, Johnell O. Acute and long-term increases in fracture risk after hospitalization for stroke. Stroke. 2001;32:702–706. doi: 10.1161/01.str.32.3.702. [DOI] [PubMed] [Google Scholar]
- 6.Ada L, Dean CM, Hall JM, et al. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo-controlled, randomized trial. Arch Phys Med Rehabil. 2003;84:1486–1491. doi: 10.1016/s0003-9993(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 7.Dean CM, Richards CL, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: a randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000;81:409–417. doi: 10.1053/mr.2000.3839. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira-Salmela LF, Olney SJ, Nadeau S, et al. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Arch Phys Med Rehabil. 1999;80:1211–1218. doi: 10.1016/s0003-9993(99)90018-7. [DOI] [PubMed] [Google Scholar]
- 9.Tangeman PT, Banaitis DA, Williams AK. Rehabilitation of chronic stroke patients: changes in functional performance. Arch Phys Med Rehabil. 1990;71:876–880. [PubMed] [Google Scholar]
- 10.Eng JJ, Chu KS, Kim CM, et al. A community-based group exercise program for persons with chronic stroke. Med Sci Sports Exerc. 2003;35:1271–1278. doi: 10.1249/01.MSS.0000079079.58477.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Fabio RP, Badke MB, Duncan P. Adapting human postural reflexes following localized cerebrovascular lesion: analysis of bilateral long latency responses. Brain Res. 1986;363:257–264. doi: 10.1016/0006-8993(86)91010-3. [DOI] [PubMed] [Google Scholar]
- 12.Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24:1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- 13.Bohannon RW, Walsh S. Nature, reliability, and predictive value of muscle performance measures in patients with hemiparesis following stroke. Arch Phys Med Rehabil. 1992;73:721–725. [PubMed] [Google Scholar]
- 14.Tsuji I, Nakamura R. The altered time course of tension development during the initiation of fast movement in hemiplegic patients. Tohoku J Exp Med. 1987;151:137–143. doi: 10.1620/tjem.151.137. [DOI] [PubMed] [Google Scholar]
- 15.Bonan IV, Colle FM, Guichard JP, et al. Reliance on visual information after stroke. Part I: balance on dynamic posturography. Arch Phys Med Rehabil. 2004;85:268–273. doi: 10.1016/j.apmr.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Cheng P-T, Wu S-H, Liaw M-Y, et al. Symmetrical body-weight distribution training in stroke patients and its effect on fall prevention. Arch Phys Med Rehabil. 2001;82:1650–1654. doi: 10.1053/apmr.2001.26256. [DOI] [PubMed] [Google Scholar]
- 17.Sackley CM. The relationships between weight-bearing asymmetry after stroke, motor function and activities of daily living. Physiother Theory Pract. 1990;6:179–185. [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Kelly-Hayes M, Robertson JT, Broderick JP, et al. The American Heart Association Stroke Outcome Classification: executive summary. Circulation. 1998;97:2475–2478. doi: 10.1161/01.cir.97.24.2474. [DOI] [PubMed] [Google Scholar]
- 20.Tate DG, Findley T, Dijkers M, et al. Randomized clinical trials in medical rehabilitation research. Am J Phys Med Rehabil. 1999;78:486–499. doi: 10.1097/00002060-199909000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Berg KO, Wood-Dauphinee SL, Williams JI, et al. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83 (Suppl 2):S7–S11. [PubMed] [Google Scholar]
- 22.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 23.Powell L, Myers AM. The activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 24.Visser MC, Koudstaal PJ, Erdman RAM, et al. Measuring quality of life in patients with myocardial infarction or stroke: a feasibility study of four questionnaires in The Netherlands. J Epidemiol Community Health. 1995;49:513–517. doi: 10.1136/jech.49.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marigold DS, Patla AE. Strategies for dynamic stability during locomotion on a slippery surface: effects of prior experience and knowledge. J Neurophysiol. 2002;88:339–353. doi: 10.1152/jn.00691.2001. [DOI] [PubMed] [Google Scholar]
- 26.Horak FB, Nashner LM. Central programming of postural movements: adaptations to altered support-surface configurations. J Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 27.Domholdt E. Physical therapy research: principles and applications. 2. Philadelphia: W.B. Saunders; 2000. [Google Scholar]
- 28.Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, Berg balance scale, timed up & go test, and gait speeds. Phys Ther. 2002;82:128–137. doi: 10.1093/ptj/82.2.128. [DOI] [PubMed] [Google Scholar]
- 29.Rogers MW, Johnson ME, Martinez KM, et al. Step training improves the speed of voluntary step initiation in aging. J Gerontol A Biol Sci Med Sci. 2003;58A:46–51. doi: 10.1093/gerona/58.1.m46. [DOI] [PubMed] [Google Scholar]
- 30.Lin S-I, Woollacott MH. Postural muscle responses following changing balance threats in young, stable older, and unstable older adults. J Mot Behav. 2002;34:37–44. doi: 10.1080/00222890209601929. [DOI] [PubMed] [Google Scholar]
- 31.Wade DT, Collen FM, Robb GF, et al. Physiotherapy intervention late after stroke and mobility. BMJ. 1992;304:609–613. doi: 10.1136/bmj.304.6827.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord SR, Ward JA, Williams P, et al. Physiological factors associated with falls in older community-dwelling women. J Am Geriatr Soc. 1994;42:1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 33.Gunter KB, White KN, Hayes WC, et al. Functional mobility discriminates nonfallers from one-time and frequent fallers. J Gerontol A Biol Sci Med Sci. 2000;55A:M672–M676. doi: 10.1093/gerona/55.11.m672. [DOI] [PubMed] [Google Scholar]
- 34.Sherpherd RB. Exercise and training to optimize functional motor performance in stroke: driving neural reorganization? Neural Plasticity. 2001;8:121–129. doi: 10.1155/NP.2001.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim CM, Eng JJ, MacIntyre DL, et al. Effects of isokinetic strength training on walking in persons with stroke: a double-blind controlled pilot study. J Stroke Cerebrovasc Dis. 2001;10:265–273. doi: 10.1053/jscd.2001.123775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu M-H, Woollacott MH. Multisensory training of standing balance in older adults: II. Kinematic and electromyographic postural responses. J Gerontol A Biol Sci Med Sci. 1994;49:M62–M71. doi: 10.1093/geronj/49.2.m62. [DOI] [PubMed] [Google Scholar]
- 37.Allum JHJ, Honegger F, Acuna H. Differential control of leg and trunk muscle activity by vestibulo-spinal and proprioceptive signals during human balance corrections. Acta Otolaryngol. 1995;115:124–129. doi: 10.3109/00016489509139273. [DOI] [PubMed] [Google Scholar]
- 38.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 39.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liepert J, Bauder H, Miltner WHR, et al. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 41.Edgerton VR, Tillakaratne NJK, Bigbee AJ, et al. Plasticity of the spinal neural circuitry after injury. Ann Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- 42.Rosner BA. Fundamentals of Biostatistics. 5. Pacific Grove: Duxbury Press; 2000. [Google Scholar]
- 43.Jorgensen HS, Nakayama H, Raaschou HO, et al. Stroke: Neurologic and functionalrecovery. The Copenhagen Stroke Study. Phys Med Rehabil Clin N Am. 1999;10:887–906. [PubMed] [Google Scholar]
- 44.Liu-Ambrose T, Khan KM, Eng JJ, et al. Resistance and agility training reduce fall risk in women aged 75 to 85 with low bone mass: a 6-month randomized, controlled trial. J Am Geriatr Soc. 2004;52:657–665. doi: 10.1111/j.1532-5415.2004.52200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]