Abstract
Despite significant advances, the radiotherapy and chemotherapy protocols marginally improve the overall survival of patients with glioblastoma. Lipoplatin™, and Lipoxal™, the liposomal formulations of cisplatin and oxaliplatin respectively, were tested on the F98 glioma cells for their ability to improve the cell uptake and increase the synergic effect when combined with ionizing radiation. The cytotoxicity and synergic effect of platinum compounds were assessed by colony formation assay, while the cellular uptake was measured by Inductively Coupled Plasma Mass Spectrometer (ICP-MS). After 4 h exposure with platinum compounds, cells were irradiated (1.5 to 6.6 Gy) with a 60Co source. The liposomal formulations were compared to their liposome-free analogs and to carboplatin. The concomitant treatment of F98 cells with carboplatin and radiation produced the highest radiosensitizing effect (30-fold increase). Among the platinum compounds tested, Lipoplatin™ produced the most promising results. This liposomal formulation of cisplatin improved the cell uptake by 3-fold, and its radiosensitizing potential was enhanced by 14-fold. Although Lipoxal™ can potentially reduce the adverse effect of oxaliplatin, a synergic effect with radiation was measured only when incubated at a concentration higher than its IC50. Conversely, concomitant treatment with cisplatin did not result in a synergic effect, as in fact a radioprotective effect was measured on the F98 cells. In conclusion, among the five platinum compounds tested, carboplatin and Lipoplatin™ showed the best radiosensitizing effect. Lipoplatin™ seems the most promising since it led to the best cellular incorporation and has already been reported to be less neurotoxic than other platinum compounds.
Introduction
Glioblastoma multiforme (GBM) is the most aggressive brain neoplasm, taking the lives of patients within a median of 12 to 14 months after diagnosis. Standard treatment typically consists of surgical resection of the tumor followed by radiation and chemotherapy. Even with complete macroscopic resection of the tumor, recurrence is the rule due to diffuse infiltrative growth of the brain parenchyma, at a distance from the main tumor nodule [1]. Radiation therapy remains the most effective single-treatment modality, resulting in at least transient disease control in most patients. Chemotherapy also lead to a slight survival benefit of GBM patients [1]. However, despite decades of research, malignant gliomas remains resistant to radiation and chemotherapeutic drugs, contributing to the poor prognosis associated with these tumors [2]. One potential means to improve the efficacy of radiation is by coupling it with a radiosensitizer.
Numerous platinum (Pt) analogs were evaluated in preclinical and clinical studies but so far only cisplatin, oxaliplatin and carboplatin have been approved for clinical use. The heavy metal compounds exert their anti-neoplastic effect by binding to DNA [3]. In numerous cell lines, combining radiotherapy and platinum compounds enhances cell killing, possibly by enhancing the production of DNA single and double-strand breaks [4]. To explain the radiosensitizing properties of platinum compounds, our group has demonstrated that the efficiency of low energy electrons produced by ionizing radiation to induce DNA strand breaks is significantly increased in presence of cisplatin [5].
Cisplatin combined with external beam radiotherapy resulted in only marginal benefits in the survival of GBM patients [6]. The clinical use of cisplatin has been impeded by severe adverse reactions including renal toxicity, gastrointestinal toxicity, peripheral neuropathy, asthenia, and ototoxicity. Theses adverse effects cause by cisplatin frequently hinder the use of higher doses to maximize its antineoplastic effects [7–10]. Concomitant radiotherapy with oxaliplatin was investigated for use against some cancers, such as oesophageal squamous cell carcinoma or adenocarcinoma [11]. However, due to neurotoxicity, no such endeavour was undertaken for the treatment of GBM with oxaliplatin in concomitance with radiotherapy.
To prevent the side effects caused by cisplatin and oxaliplatin and optimize the oncologic outcome, a potential approach consists in incorporating the platinum agent in a liposome and combines it with radiation to obtain a synergistic effect. Potential application of liposomal drugs to GBM patients might also benefit from a more avid crossing of the blood-brain barrier by liposomal formulations because of their lipid nature achieving higher concentrations of drug in the brain lesions. Lipoplatin™ and Lipoxal™, the liposomal formulations of cisplatin and oxaliplatin, respectively, were developed in order to reduce the systemic toxicity of these platinum compounds, while simultaneously improving the delivery of the drug to the primary tumor as well as to metastases by enhancing the circulation time in body fluids and tissues. Preclinical studies have shown that Lipoplatin™ reduced nephrotoxicity in rats, compared to cisplatin [12, 13]. Lipoplatin™ and Lipoxal™ are distributed into tissues and tend to concentrate preferentially at tumor sites apparently via extravasation through the leaky tumor vasculature.
The radiosensitizing ability of carboplatin was also investigated in our study since it was proposed as the most promising platinum compound. In studies of patients with locally advanced head and neck cancer, significantly longer survival rates were seen in the patients receiving concurrent radiotherapy and carboplatin, and the only major toxicity was manageable myelosuppression [14, 15]. However, the benefit for GBM patients treated with carboplatin and radiotherapy remains modest [16, 17]. In this study, the in vitro concomitant treatment of rat glioma F98 cells with Lipoplatin™ or Lipoxal™ and radiation was investigated. Their cellular accumulation, cytotoxicity and synergic effect with radiation were compared to the liposome-free cisplatin and oxaliplatin, and also to carboplatin.
Materials and Methods
Chemicals
Cisplatin, carboplatin and oxaliplatin were purchased from Sigma-Aldrich (Oakville, Ontario, Canada). Lipoplatin™ and Lipoxal™, the liposomal formulation of cisplatin and oxaliplatin, were generously provided by Regulon (Greece). All platinum solutions were prepared immediately before use in FBS-free Minimum Essential Medium Eagle (MEM) (Sigma, Oakville, Canada).
Cell line and culture conditions
The rat F98 cell line was chosen because it represents a good model of human glioblastoma [18, 19]. The F98 cell line was obtained from ATCC and tested negative for the MAP assay by Charles River Laboratories (Wilmington, MA, USA). Cells were grown in monolayer using MEM supplemented with 10% foetal bovine serum (FBS) (Gibco, Canada), 26.2 mM of sodium bicarbonate, 2 mM L-glutamine and a mix of penicillin (100UI/ml) and streptomycin (100μg/ml). Cells were incubated at 37°C in a humidified environment with 5% CO2 and propagated upon confluence, every 3 days.
Cellular uptake of platinum compounds
Cells were allowed to proliferate upon a confluence near 70% and incubated with one of the platinum compound (10 μM) for 4 h. It is to note that the concentrations that we used in our study are similar as the concentration found in plasma in clinical use [20]. The equimolarity of the platinum compounds free and loaded in liposomes was assessed using an Inductively Coupled Plasma Mass Spectrometer (ICP-MS) (ELAN DRC-II, PerkinElmer). Cells were washed twice with PBS, harvested, counted and reported as cells/ml. Cells were treated with 23% of nitric acid, 8% H2O2 and autoclaved for 1h. The resulting solutions were then injected in the ICP-MS to quantify the platinum accumulated in the cells. Data were expressed as nmol of platinum per 2 × 106 cells. Cells not incubated with platinum were used as control.
Clonogenic assay and synergistic effect determination
Efficiency of the concomitant treatment of platinum compounds with radiation was determined by calculating the combination index (CI) based on results obtained with a clonogenic assay. F98 cells were plated at a density of 1000 cells per 10 cm Petri dish. After 18 h incubation, culture medium was removed, cells were washed twice with PBS and platinum solutions or control solutions (platinum-free MEM) were added and incubated for 4 h. Thereafter the incubation medium was removed, cells were washed twice, and 10% FBS MEM was added. Colonies were counted 8 to 10 days after retrieval of the incubation medium. For the concomitance assays with radiation, cells were irradiated immediately after removal of the culture media containing the platinum compounds. Incubation then proceeded for 8 to 10 days prior to the counting of resulting colonies. Colonies containing more than 50 cells were scored. Experiments were performed a minimum of six times for each treatment modality.
The relative colony formation inhibition (surviving fraction) was plotted. Median-Effect or the concentrations of platinum compound required to inhibit 50% of colony formation (IC50) were plotted. CI as well as linear correlation coefficient (r) were calculated using CALCUSYN software from BIOSOFT (Ferguson, MO) developed by Chou [21]. Briefly, the CI were calculated using the equation:
where the denominator, (Ptx) is the concentration of platinum compound that inhibits colonies formation at x%, and (IRx) corresponds to the radiation dose which results in the same x% of colonies formation inhibition. In the numerator, (Pt) + (IR) “in combination” also inhibit the colonies formation at the equivalent x%. If the sum of these two fractions is equal to 1, additive effect is indicated. If the CI value is smaller than 1, synergism in indicated, and if the CI value is greater than 1, antagonism is suggested.
Data Analysis
Differences were analyzed with the Student t-test. p-values < 0.05 were considered significant.
Results
Cellular uptake of platinum compounds
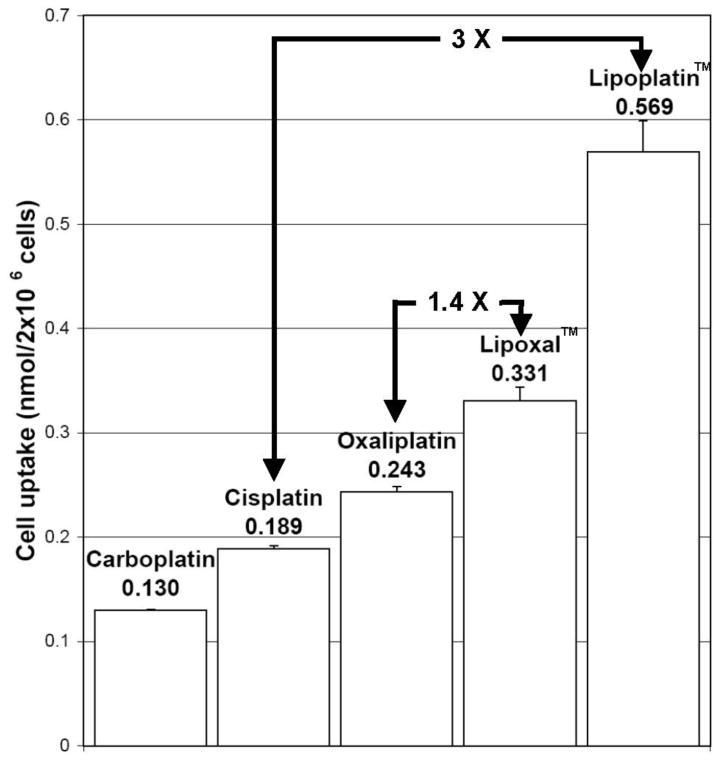
Among the liposome-free platinum compounds tested, the highest uptake in F98 cells was obtained with oxaliplatin, followed by cisplatin and carboplatin (Fig. 1). Incorporation of cisplatin in liposome (Lipoplatin™) or oxaliplatin (Lipoxal™) significantly increased the uptake in F98 cells. Incubation with Lipoplatin™ resulted in cellular uptake of 0.569 nmol/2 × 106 cells, while an accumulation of 0.331 nmol/2 × 106 cells was measured with Lipoxal™. This resulted to an overall 3 and 1.4-fold increase in cell uptake compared to their liposome-free analogs (Fig. 1).
Fig. 1.
Cellular uptake in F98 cells treated for 4h with 10μM of the platinum compounds.
Cytotoxicity induced by the platinum compounds
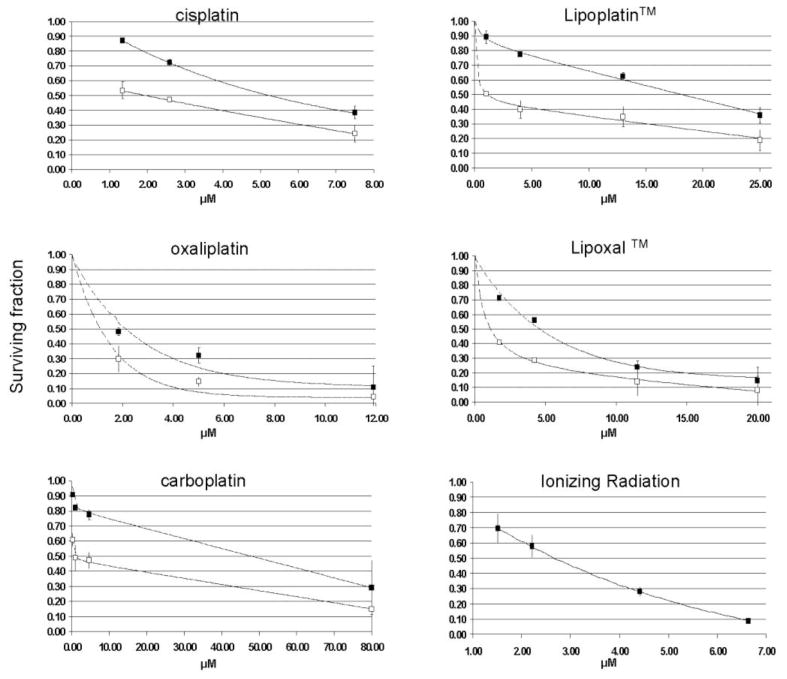
The concentration of platinum compounds required to inhibit 50% of colony formation (IC50) was measured. These IC50 values are required to determine the CI when the F98 cells are treated with the platinum compounds in concomitance with radiation. The IC50 values for the five platinum compounds are reported in Fig. 2 and 3.
Fig. 2.
Surviving fraction of F98 cells following treatment with platinum alone or radiation alone (black squares). Combinations of platinum and 2.21 Gy are represented by white squares.
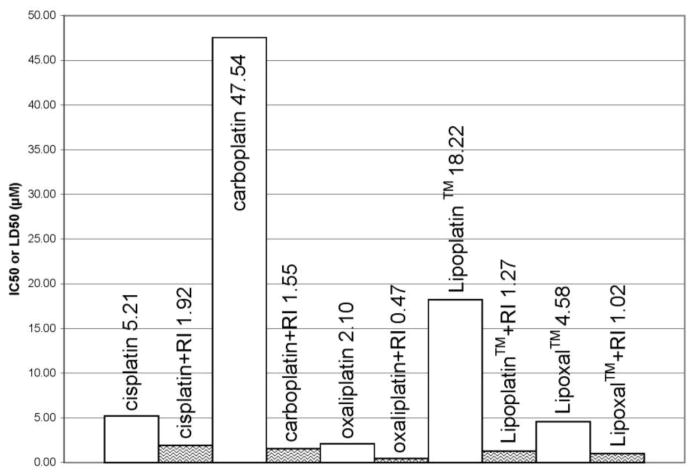
Fig. 3.
IC50 and LD50 measured for the F98 cells after treatment with the platinum compounds and radiation. Empty columns represent the dose needed to obtain the IC50 with platinum alone. Waved columns are the LD50 for the platinum drugs when combined with 2.21 Gy of gamma rays. Both IC50 and LD50 correspond to the platinum compounds concentration needed to inhibit by 50% of colony formation. RI = radiation.
The least cytotoxic drug for the F98 cells was carboplatin, which showed an IC50 between 9–20 times higher than cisplatin and oxaliplatin. Loading these latter drugs in liposome resulted in a protective effect. The liposomal formulations of cisplatin and oxaliplatin demonstrated reduced cytotoxicity by 3.1 and 2.3-fold compared to their liposome-free analogs. The overall order of cytotoxicity for the platinum compounds for the F98 cells was: oxaliplatin >Lipoxal™ >cisplatin >Lipoplatin™ ≫carboplatin.
Concomitance effect of platinum compounds with radiation
The concomitance effect was measured on F98 cells incubated at different concentrations of platinum drugs and then irradiated at the dose corresponding to the LD50. This latter value was obtained on platinum-free F98 cells and corresponds to the radiation dose which resulted in a reduction of 50% of the colonies formation. Using a colony formation assay, the LD50 for the irradiated F98 cells was 2.2 ± 0.4 Gy (Fig. 2).
When the platinum compounds were combined with a radiation dose of 2.2 Gy, the most efficient association (lowest combined IC50) to treat the F98 cells was obtained with the concomitant treatment of oxaliplatin and radiation. The overall order of efficiency for the concomitant treatment of the tested platinum compounds was as follows: oxaliplatin > Lipoxal™ > Lipoplatin™ > carboplatin > cisplatin.
It is noteworthy that the largest improvement after radiation treatment was observed with carboplatin. Although carboplatin was the least efficient platinum compound when used as a single treatment, the concomitant treatment with radiation resulted in the largest improvement with a combined IC50 of 1.55 μM compared to 47.5 μM when used alone (a 30-fold improvement). The association of Lipoplatin™ with radiation also led to an important concomitance effect with a combined IC50 14-fold lower than measured when treating with Lipoplatin™ alone. Regarding the other platinum compounds, the concomitant treatment increased the efficiency in F98 cells kill by 2.7 - 4.5 times. The order of improvement after concomitance treatment with radiation was as follows: carboplatin > Lipoplatin™ > Lipoxal™ ≈ oxaliplatin > cisplatin.
To determine whether the concomitant treatment obtained with these platinum compounds led to a synergistic effect, the CI was calculated according to the method developed by Chou et al. (2006). A CI value lower than 1 indicates that the drug tested acts as a radiosensitizer (synergic effect), whereas a CI value higher than 1 indicates that a radioprotective effect results from the addition of the drug. We also sought to determine whether the synergic effect would be reached at platinum compound concentrations lower, equal or higher than their respective IC50.
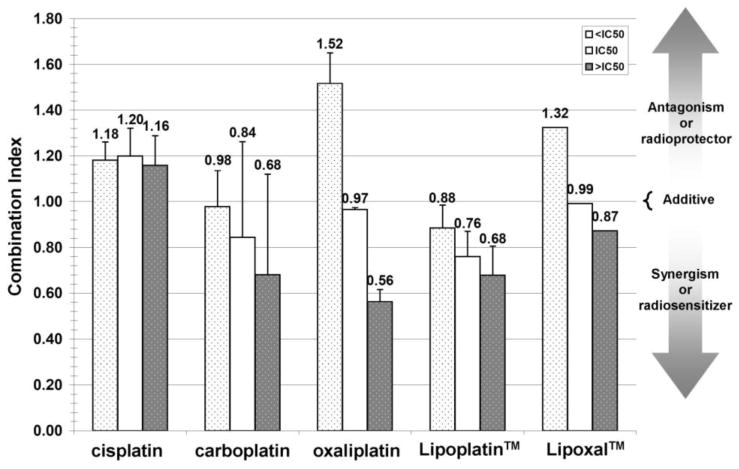
Lipoplatin™ was the most promising platinum compound to amplify the cytotoxic effect produced by radiation. A synergistic effect was observed at all the IC50 values tested while the best concomitance effect was obtained with a Lipoplatin™ concentration higher than its IC50. Carboplatin, particularly at concentration higher than the IC50, also amplified the effect of radiation, but to a lower extent than Lipoplatin™.
Conversely, a radioprotective effect was observed with cisplatin, notwithstanding the drug concentration tested. Regarding Lipoxal™ and its liposome-free analog oxaliplatin, incubation at a concentration lower than their respective IC50 in association with radiation resulted in a radioprotective effect. To reach a radiosensitizing (synergistic) effect, their concentrations had to be increased to a level higher than their IC50 values (Fig. 4).
Fig. 4.
Combination Index (CI) of F98 cell line treated with platinum plus ionizing radiation at 2.21 Gy. The CI is represented for platinum concentration lower (< IC50), equal (= IC50) and higher (> IC50) for each specific platinum. IC50 are those obtain with platinum alone (white columns in Fig. 3). When CI <1, =1 or >1, synergism, additive effect or antagonism is indicated respectively.
Discussion
So far, the therapeutic potential of platinum compounds as radiosensitizers to treat malignant tumors has been limited by the adverse effects observed on normal tissues, which hindered the use of higher doses to maximize their antineoplastic effects. Previous studies have shown that the liposomal formulation of cisplatin and oxaliplatin, (Lipoplatin™ and Lipoxal™) can reduce the adverse effects and increase the tumor uptake [8]. Our group demonstrated that a significant increase of single and double strand breaks of DNA is measured when DNA is irradiated in the presence of cisplatin [5]. These results support the fact that a sufficient number of platinum atoms must be accumulated in the cancer cell to reach a radiosensitizing effect. The present study was undertaken to determine whether loading of cisplatin and oxaliplatin into liposomes would increase their capacity to accumulate in the F98 cells and enhance their radiosensitizing properties.
Evaluating the relative cell uptake of liposomal-free platinum compounds in 8 cancer cell lines, several authors reported similar results than what was measured in our study with the F98 cells [22, 23]. The lowest cell uptake was obtained after incubation with carboplatin, while an equivalent or slightly higher accumulation was reported with oxaliplatin compared to cisplatin. After loading the drug in liposomes, an important increase in cell uptake was measured with Lipoplatin™ and Lipoxal™. It was proposed that the ability of liposome to bypass the P-glycoprotein pumps responsible for multidrug resistance can promote a higher uptake of chemotherapeutic agents in resistant cancer cells [8, 24]. Since these multidrug resistance transporters were detected in the F98 cell line [25], this could explain the higher drug uptake reached with Lipoplatin™ and Lipoxal™ compared to their liposome-free analogs.
The ability of Lipoplatin™ to enhance the accumulation of cisplatin in cancer cells was also observed in clinical studies [9]. Direct measurement of the platinum levels in specimens from the excised tumors and normal tissues showed that the total platinum levels were on average 10–200 times higher in hepatocellular adenocarcinoma, gastric cancer or colon cancer samples compared to the adjacent normal tissue specimens. The tumor targeting was likely due to the preferential accumulation caused by extravasation through the leaky tumor vasculature, and to a higher avidity for Lipoplatin™ by the cell membrane of tumor cells compared to normal cells [8].
Surprisingly, in our clonogenic assay, Lipoplatin™ and Lipoxal™ resulted in less cytotoxic effect on the F98 cells than their liposome-free analogs. The relative level of cancer cell toxicity induced by cisplatin, oxaliplatin and carboplatin measured on the F98 cells was similar to the results reported by other groups on human glioma cell lines and other cancer cell lines. The most efficient platinum compound, oxaliplatin, generally also reached the highest cell uptake [22, 23, 26]. Conversely, the liposomal formulations Lipoxal™ and Lipoplatin™ enhance the accumulation in the cancer cells but result in a lower cytotoxicity. These results suggest that carrying the cisplatin and oxaliplatin into liposome could lead to a different distribution of these drugs in the cancer cells. More so, this peculiar effect could be related to the inability of the liposomal membrane to let the platinum compound loose in the cell cytoplasm, and thus inhibit its interaction with DNA.
An ideal radiosensitizer should induce limited adverse effects when administrated, but yet amplify cell killing in irradiated cancer cells. Although carboplatin showed a lower level of cytotoxicity for cancer cells than cisplatin and oxaliplatin, it might represent a promising radiosensitizer against GBM since it is not neurotoxic. In our study, the concomitant treatment of F98 cells with carboplatin and radiation has produced a greater improvement in radiation cytotoxicity than cisplatin and oxaliplatin, supporting its clinical evaluation as a radiosensitizer to treat GBM. However, among the platinum compounds tested in our study, Lipoplatin™ remains the most promising in our opinion. This liposomal formulation of cisplatin has the ability to prevent the adverse neurotoxic effects observed with the liposome-free cisplatin [8]. The cell uptake of the liposomal form was improved by 3-fold, and its radiosensitizing potential was improved by 14-fold compared to 2.7-fold for liposome-free cisplatin. Furthermore, a synergistic effect could still be observed when the drug was incubated at lower or higher concentration than its IC50. Although Lipoxal™ can also potentially reduce the adverse effect of oxaliplatin, a synergistic effect with radiation was measured only when incubated at a concentration higher than its IC50, rendering the use of this drug impractical in the clinical situation.
In this study, five platinum formulations were tested to determine their synergistic effect with radiation in F98 glioma cell line. Even if combination of cisplatin with radiation significantly enhanced the proliferation control on F98 cell line, in term of synergism cisplatin was shown to act mostly as a weak radioprotective agent. Whereas oxaliplatin and Lipoxal™ radiosensitize F98 cells only at concentration higher than their IC50 values, carboplatin and Lipoplatin™ depicted the best radiosensitizing effect even at low concentration. Moreover, Lipoplatin™ showed the best cellular incorporation and has already been reported to be less neurotoxic than other platinum compounds. This study on the screening of platinum compounds supports their evaluation in animal models bearing GBM tumor with the ultimate goal of devising a phase I clinical study.
Acknowledgments
This research project was funded by the Canadian Institutes of Health Research, grant # MOP 81356. BP and LS are members of the FRSQ-funded Centre de recherche clinique Étienne-LeBel. Special thanks to REGULON, Inc. for the free supply of Lipoplatin™ and Lipoxal™.
References
- 1.Sathornsumetee S, Rich JN. Designer therapies for glioblastoma multiforme. Ann N Y Acad Sci. 2008;1142:108–132. doi: 10.1196/annals.1444.009. [DOI] [PubMed] [Google Scholar]
- 2.Chakravarti A, Palanichamy K. Overcoming therapeutic resistance in malignant gliomas: current practices and future directions. Cancer Treat Res. 2008;139:173–189. [PubMed] [Google Scholar]
- 3.Boulikas T. Molecular mechanisms of cisplatin and its liposomally encapsulated form, Lipoplatin. Lipoplatin as a chemotherapy and antiangiogenesis drug. Cancer ther. 2007;5:349–376. [Google Scholar]
- 4.Yang L, Douple EB, O’Hara JA, et al. Enhanced radiation-induced cell killing by carboplatin in cells of repair-proficient and repair-deficient cell lines. Radiat Res. 1995;144:230–236. [PubMed] [Google Scholar]
- 5.Zheng Y, Hunting DJ, Ayotte P, et al. Role of secondary low-energy electrons in the concomitant chemoradiation therapy of cancer. Phys Rev Lett. 2008;100:198101. doi: 10.1103/PhysRevLett.100.198101. [DOI] [PubMed] [Google Scholar]
- 6.Glass J, Silverman CL, Axelrod R, et al. Fractionated stereotactic radiotherapy with cis-platinum radiosensitization in the treatment of recurrent, progressive, or persistent malignant astrocytoma. Am J Clin Oncol. 1997;20:226–229. doi: 10.1097/00000421-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Fortin D, Desjardins A, Benko A, et al. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in malignant brain tumors: the Sherbrooke experience. Cancer. 2005;103:2606–2615. doi: 10.1002/cncr.21112. [DOI] [PubMed] [Google Scholar]
- 8.Boulikas T. Molecular mechanisms of cisplatin and its liposomally encapsulated form, Lipoplatin ™. Lipoplatin ™ as a chemotherapy and antiangiogenesis drug. Cancer Ther. 2007;5:349–376. [Google Scholar]
- 9.Boulikas T, Stathopoulos GP, Volakakis N, et al. Systemic Lipoplatin infusion results in preferential tumor uptake in human studies. Anticancer Res. 2005;25:3031–3039. [PubMed] [Google Scholar]
- 10.Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. (Review) Oncol Rep. 2003;10:1663–1682. [PubMed] [Google Scholar]
- 11.Conroy T, Viret F, Francois E, et al. Phase I trial of oxaliplatin with fluorouracil folinic acid and concurrent radiotherapy for oesophageal cancer. Br J Cancer. 2008;99:1395–1401. doi: 10.1038/sj.bjc.6604708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulikas T. Low toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenografts. Oncol Rep. 2004;12:3–12. [PubMed] [Google Scholar]
- 13.Stathopoulos GP, Boulikas T, Kourvetaris A, et al. Liposomal oxaliplatin in the treatment of advanced cancer: a phase I study. Anticancer Res. 2006;26:1489–1493. [PubMed] [Google Scholar]
- 14.Jeremic B, Milicic B, Dagovic A, et al. Radiation therapy with or without concurrent low-dose daily chemotherapy in locally advanced, nonmetastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2004;22:3540–3548. doi: 10.1200/JCO.2004.10.076. [DOI] [PubMed] [Google Scholar]
- 15.Jeremic B, Shibamoto Y, Stanisavljevic B, et al. Radiation therapy alone or with concurrent low-dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: a prospective randomized trial. Radiother Oncol. 1997;43:29–37. doi: 10.1016/s0167-8140(97)00048-0. [DOI] [PubMed] [Google Scholar]
- 16.Peterson K, Harsh G, 4th, Fisher PG, et al. Daily low-dose carboplatin as a radiation sensitizer for newly diagnosed malignant glioma. J Neurooncol. 2001;53:27–32. doi: 10.1023/a:1011891209900. [DOI] [PubMed] [Google Scholar]
- 17.Packer RJ, Krailo M, Mehta M, et al. A Phase I study of concurrent RMP-7 and carboplatin with radiation therapy for children with newly diagnosed brainstem gliomas. Cancer. 2005;104:1968–1974. doi: 10.1002/cncr.21403. [DOI] [PubMed] [Google Scholar]
- 18.Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- 19.Mathieu D, Lecomte R, Tsanaclis AM, et al. Standardization and detailed characterization of the syngeneic Fischer/F98 glioma model. Can J Neurol Sci. 2007;34:296–306. doi: 10.1017/s0317167100006715. [DOI] [PubMed] [Google Scholar]
- 20.Stathopoulos GP, Boulikas T, Vougiouka M, et al. Pharmacokinetics and adverse reactions of a new liposomal cisplatin (Lipoplatin): phase I study. Oncol Rep. 2005;13:589–595. [PubMed] [Google Scholar]
- 21.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 22.Ghezzi A, Aceto M, Cassino C, et al. Uptake of antitumor platinum(II)-complexes by cancer cells, assayed by inductively coupled plasma mass spectrometry (ICP-MS) J Inorg Biochem. 2004;98:73–78. doi: 10.1016/j.jinorgbio.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Kitada N, Takara K, Minegaki T, et al. Factors affecting sensitivity to antitumor platinum derivatives of human colorectal tumor cell lines. Cancer Chemother Pharmacol. 2008;62:577–584. doi: 10.1007/s00280-007-0640-3. [DOI] [PubMed] [Google Scholar]
- 24.Sadava D, Coleman A, Kane SE. Liposomal daunorubicin overcomes drug resistance in human breast, ovarian and lung carcinoma cells. J Liposome Res. 2002;12:301–309. doi: 10.1081/LPR-120016196. [DOI] [PubMed] [Google Scholar]
- 25.Lamprecht A, Benoit JP. Etoposide nanocarriers suppress glioma cell growth by intracellular drug delivery and simultaneous P-glycoprotein inhibition. J Control Release. 2006;112:208–213. doi: 10.1016/j.jconrel.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Pendyala L, Creaven PJ. In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res. 1993;53:5970–5976. [PubMed] [Google Scholar]