Abstract
Antepartum bed-rest treatment is based on assumptions that it is both effective and safe for mother and fetus. However, research indicates, that bed-rest treatment is ineffective for preventing preterm birth and fetal growth restriction, and for increasing gestational age at birth and infant birthweight. Studies of women treated with pregnancy bed-rest identify numerous side effects, including muscle atrophy, bone loss, weight loss, decreased infant birthweight in singleton gestations and gestational age at birth, and psychosocial problems. Studies conducted by aerospace scientists who have used bed rest as a model for the study of weightlessness in space using nonpregnant individuals report similar results. Antepartum bed-rest treatment should be discontinued until evidence of effectiveness is found.
Keywords: activity restriction, bed rest, effectiveness, high-risk pregnancy, pregnancy, pregnancy complications, preterm birth, safety, side effects
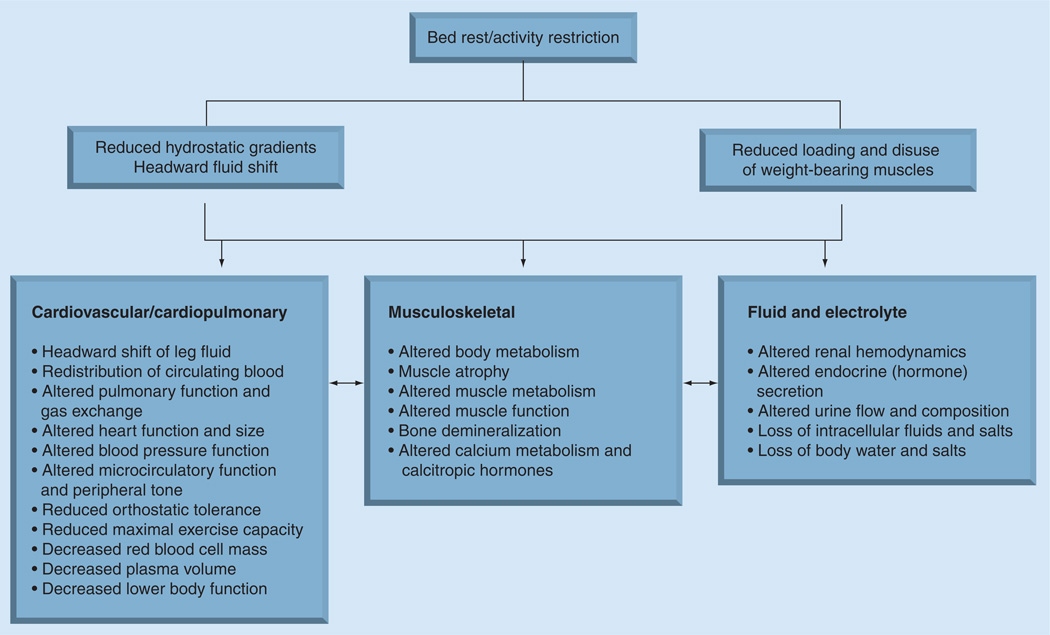
Antepartum bed rest is currently used in an attempt to prevent preterm birth by approximately 71–87% of USA obstetricians and, to a lesser extent, in Canadian physicians and midwives to prevent preterm birth, despite questions regarding its effectiveness and safety [1–4]. The use of bed rest as a treatment in healthcare dates to the early 1900s [2]. However, during World War II, the value of bed rest began to be questioned as physicians noted that injured soldiers who were immobilized developed muscle weakness and excessive calcium excretion, while those who returned to battle early out of necessity recovered more quickly [5]. Concomitantly, in the 1940s aerospace scientists began to use bed rest as a model to investigate the anticipated effects of weightlessness and space flight. Studies primarily investigated males and, only occasionally, were women included. In numerous investigations, aerospace scientists discovered that not only was muscle atrophy and calcium excretion a side effect of bed rest, but that bed rest also produced an array of other physiological and psychosocial side effects as the body attempted to adapt to the prone state. Figure 1 summarizes the purported causes of physiological change during bed rest and some of the physiological adaptations to bed rest [5,6]. Every major organ system is rapidly affected by reduced hydrostatic gradients, and reduced loading and disuse of weight-bearing tissues during bed rest [5–7]. Some resulting physiological changes include, but are not limited to, redistribution of circulating blood, altered renal hemodynamics, fluid and electrolyte loss, decreased plasma volume, muscle atrophy, bone demineralization, and altered body metabolism, glucose metabolism, vestibular function, sensory and balance information, and circadian rhythms. A more detailed summary of the side effects of bed rest is described by both the Sandler and Vernikos and the Fortney, Schneider and Greenleaf groups [5,6]. Scientists also discovered that adaptation to the prone state begins within hours and the side effects are differential, depending on the body system; for example, muscle loss rapidly occurs but atrophy is greater in the gastrocnemius than in the biceps brachii, while altered circadian rhythms and glucose metabolism occur more slowly [5,6,8]. Side effects also vary with the length and severity of activity restriction [6]. As this extensive research became known, clinical care for postoperative and cardiovascular patients began to change, and ambulation was incorporated into care for many conditions. Knowledge regarding the adverse side effects of bed rest, however, has not been applied to the treatment of women with pregnancy complications, and the use of antepartum activity restriction continues [1,4].
Figure 1. Physiologic effects of bed rest.
Adapted from [7].
Definition of bed rest/activity restriction
Antepartum bed rest is also at times referred to as activity restriction. The two terms are often used interchangeably, and thus lead to some confusion. Of note, aerospace studies use the term bed rest or ‘muscle disuse’. In our research, we defined antepartum bed rest as confinement to bed in hospital with activity limited to toileting and found that during this time women were in bed for approximately 22 h per day. Fox and colleagues defined bed rest as no more than 1–2 h out of bed per day [1]. Both Sprague et al. [4] and Sciscione [9] used categorical definitions of bed rest/activity restriction. Recently Sciscione stated that the term activity restriction should be used, and use of other terms abandoned, but did not provide a clear rationale for doing so [9]. In truth, obstetric practice related to bed rest/activity restriction varies across the USA, from complete confinement in bed either in the hospital or at home, to resting in a reclining position on a bed or couch or chair a few times a day [3]. Some practitioners may not confine women to bed rest but restrict sexual activity, lifting of children or other behaviors, while allowing women to remain ambulatory. Of importance, if ambulation occurs, the side effects of bed rest are reduced in proportion to the amount of ambulation allowed. Thus, for the purposes of this article we use the terms activity restriction and bed rest interchangeably, as restriction of ambulation as indicated by aerospace research produces extensive physiologic adaptations and adverse side effects [5,6]. Further, all antepartum studies reviewed used extensive restriction of ambulation and confined women to one particular site, although the term bed rest was not always clearly defined.
Evidence for effectiveness of bed-rest treatment
Antepartum bed rest is used to prevent preterm labor, and as a treatment for pregnancy-related complications such as preterm rupture of membranes, placenta previa, incompetent cervix, fetal growth retardation, preeclampsia and multiple gestation [10]. There are two assumptions behind antepartum bed-rest treatment; that bed rest is effective in preventing preterm birth, and that bed rest is safe for mothers and their fetuses/infants [10]. The first assumption of effectiveness began to be questioned in the 1980s. Randomized controlled trials were initiated and assessed birth outcomes such as neonatal morbidity and mortality, gestational age (GA) at birth, and infant birthweight among singleton and multiple gestations [10]. Outcomes for women treated with hospital bed rest were compared with those treated in an outpatient clinic and allowed to ambulate, but who were hospitalized if severe complications developed. With a few exceptions, studies found no group differences in maternal–fetal outcomes [10,11]. Furthermore, studies that compared outcomes of twin pregnancies among a hospitalized bed-rest group with an ambulatory group found that there was a higher rate of preterm birth and greater morbidity and mortality in the hospital group [12,13]. Subsequent studies and meta-analyses confirm that there are no group differences in fetal growth restriction, GA at birth and infant birthweight [14–18]. Thus, a Cochrane database meta-analysis has concluded that antepartum bed rest is a treatment whose effectiveness has not yet been demonstrated. There is no evidence to support the use of activity restriction during pregnancy at home or in hospital, and practitioners should not assume efficacy for bed-rest treatment until evidence is produced [9,10,18,19].
Research regarding leisure-time physical activity during pregnancy provides additional insight into the effects of activity restriction. Regular leisure physical activity appears to protect against prematurity, low birthweight, gestational diabetes and preeclampsia [20–24]. In a prospective study, leisure-time activity before pregnancy was unrelated to preterm birth [25]. Vigorous leisure-time activity was somewhat associated with a reduced risk of preterm birth during the first trimester and even more so during the second trimester. In a large Scandinavian study (n = 5749), sedentary pregnant women were compared with those who participated in more than one type of leisure sports activity [22]. Active women had a significantly reduced risk of preterm birth. Women who engaged in light physical activity (walking) has a 24% reduced risk of preterm delivery and women who engaged in moderate to heavy activity (sports such as tennis, swimming or weekly running, to competitive sports several times a week) had a 66% reduced risk. The greater the intensity of the activity, the greater the reduced risk of preterm birth. Weissgerber and colleagues proposed four mechanisms that explain the protective effects of exercise during pregnancy: enhanced placental growth and vascularity, reduced oxidative stress, reduced inflammation, and correction of disease-related endothelial dysfunction [24]; while others propose that cardiovascular adaptations during moderate regular exercise improve physiologic outcomes of pregnancy [23]. Exercise may also reduce pregnancy complications linked with maternal obesity [24]. Therefore, physical activity, especially during the second trimester, may not only be a healthy behavior but may also have a protective effect against disease.
Physiological side effects of antepartum bed-rest treatment
The second assumption behind antepartum bed-rest treatment, that it is safe for mothers and their fetuses/infants, has no data providing evidence to support it [10]. Prior to the last two decades, research into the potential side effects of bed rest among pregnant women was nearly nonexistent. Aerospace scientists provided extensive data regarding the bodily adaptation to bed rest, but these investigations included only a few women, none of whom were pregnant. Therefore, because the physiology of pregnancy is vastly different from the nonpregnant state, new research was needed in order to determine whether young, healthy pregnant women experience adverse effects of bed rest.
Studies into the side effects of bed rest have since identified several adverse physiological and psychosocial side effects of pregnancy-associated bed rest [10]. Altered gastrocnemius muscle metabolism is rapidly induced during activity restriction [26–27]. In two studies, Maloni and colleagues used a dual-wavelength hemoglobin/myoglobin spectrophotometer and portable ergometer to assess muscle reoxygenation times after plantar flex exercises in women who had been prescribed pregnancy bed rest from hospital admission through 6 weeks postpartum [11,26]. Across a mean of approximately 4 weeks of bed rest (range: 5–70 days), the muscle reoxygenation time needed to recover from plantar flexion exercise significantly increased across bed rest and significantly decreased across 6 weeks postpartum. Women on complete bed rest; that is, never allowed out of bed, had greater antepartum increases in muscle reoxygenation time than those on partial bed rest – that is, out of bed for toileting – suggesting a dose effect. Women also reported symptoms associated with antepartum muscle loss, including muscle deconditioning and weakness; in addition to pain and discomfort, particularly in the back and hips. With resumption of postpartum ambulation, women reported symptoms of weight-bearing muscle soreness and deconditioning [26–28]. These results are consistent with research in nonpregnant subjects. Muscle deterioration begins with approximately 6 h of bed rest [5,6]. Muscle loss is differential, with greater loss among the weight-bearing muscles of the gastrocnemius medialis and vastus lateralis and across longer periods of bed rest [8,29,30].
There are also indications of maternal bone loss during antepartum activity restriction. Three studies of maternal bone loss during pregnancy bed rest have been conducted, each assessing different bone components and each using different methods. In a study of trabecular bone loss in the radius and ulna among 181 women using dual x-ray absorptiometry, women treated with bed rest had an adjusted mean loss of 4.6% compared with 1.5% for ambulatory women [31]. Women on prolonged bed rest had sixfold higher odds of bone loss. In a study of bone turnover during pregnancy, activity restriction increased bone turnover markers [32]. Bone resorption rapidly increased as bed rest was initiated. Last, in a recent pilot study of bone loss in the calcaneus assessed by quantitative ultrasound, the speed of sound and broadband attenuation scores were not significantly different after 7 days of bed rest between an ambulatory and bed-rest group [33]. However, bone stiffness index scores, were significantly different between the groups, suggesting a greater relative risk for fracture in the women treated with activity restriction. Again, these results are consistent with results in nonpregnant subjects [6,29,30,34]. In a recent study assessing bone turnover in women hospitalized and treated with bed rest for anorexia nervosa, activity restriction was associated with suppressed bone formation and resorption and an imbalance of bone turnover [35].
Concomitant with muscle and bone loss, evidence indicates that pregnant women treated with activity restriction lose weight. Maloni and colleagues conducted three studies of maternal weight change across antepartum activity restriction in both singleton and multiple gestations [11,28,36]. In a pilot study, women with singleton or multiple gestations either lost weight during activity or did not gain weight during an average of 29 days of bed rest. Weight loss occurred rapidly after admission. Some women continued to lose weight across time while others began to gain, but their total weight gain to date was outside the lower limits of normal for the GA of pregnancy. Healthy pregnant women in the control group, however, gained weight. In a longitudinal study of 141 women with a singleton gestation, the weekly rate of weight change was significantly lower than the Institute of Medicine recommendations for pregnancy weight gain by BMI [36]. Last, in a study of 31 women with multiple gestations, maternal weight gain was appropriate prior to hospital admission for bed-rest treatment [28]. Again, however, once bed rest was initiated, the weekly weight gain was significantly less than the Institute of Medicine recommendations for multiple gestations [28]. A total of 86% of the women with triplet gestations and 65% of those with twin gestations either lost weight during bed rest or did not gain any weight during nearly 24 days of bed rest. In nonpregnant individuals treated with bed rest, weight loss is due to loss of fluids, muscle, bone and appetite. Bed rest also alters carbohydrate metabolism [5,6,37].
Of concern, maternal weight gain is directly related to infant birthweight, and infant birthweight is a predictor of neonatal morbidity and mortality [38,39]. Maternal weight gain is especially important in women with multiple gestations as both twins and triplets are, on average, born 3–6 weeks earlier, respectively, than their singleton counterparts, and one out of eight twins and one out of three triplets are born at less than 32 weeks gestation [40]. Therefore, infant birthweight was also assessed. In a bed-rest pilot study, hospitalized women on complete bed rest gained less weight and had infants who were born at an earlier GA and weighed significantly less than women on partial bed rest (allowed out of bed for toileting) [11]. In a large study of mothers of singleton infants, infant birthweights were matched with the US mean for each infant’s gestation age, race and gender. Infants born to mothers treated with bed rest weighed significantly less (p = 0.001) than their matched controls [36]. A total of 75% of the infants had a birthweight below the national mean. Of interest, however, is that only 12 infants were small for GA. There appears to be a shift away from large for GA infants. In a study of multiple gestations, while women lost weight, the infant birthweights were appropriate for GA [28]. National standards for a multiple pregnancy, however, do not allow for comparisons by GA and gender only, and triplet infant weights could only be compared with US standards for GA. Therefore, further study is needed using infant birthweight standards that are sensitive not only to weight by GA, but also race and gender.
Intuitively it would seem that the incidence of thrombosis during bed rest would be high; however, research among previously treated women with activity restriction for pregnancy complications is unclear. One retrospective chart review found an increased prevalence of 15.6 per 1000 among women treated with bed rest compared with 0.8 among women not treated with bed rest [41]. In a prospective study, however, bed rest was not a risk factor for deep-vein thrombosis and pulmonary embolism [42]. In our studies, however, we excluded women with nonpregnancy-related complications, including those admitted with DVT or pulmonary embolism. We have never noted subsequent development of antepartum thrombosis [11,27,28]; additional research is needed.
Psychosocial side effects of antepartum bed-rest treatment
A host of psychosocial side effects are associated with bed-rest treatment; the most common being antepartum depressive symptoms (Box 1). In total, six studies that have used standardized instruments support evidence that antepartum depression symptoms are high among women treated with bed rest [11,18,43–46]. Depression, anxiety and hostility, a group of depressive symptoms commonly referred to as dysphoria, appear to be highest upon hospital admission and are not likely caused by bed-rest treatment, but rather the result of some other factor, perhaps knowing that the status of the pregnancy is in jeopardy. In longitudinal studies, however, antepartum depressive symptoms gradually decrease from hospital admission as positive affect develops with the increasing GA of the pregnancy [11,28,45,46]. Across 4 weeks of bed rest, lowest scores were obtained at delivery and depressive symptoms decreased across the first six postpartum weeks for some women, but remained somewhat elevated, particularly for women whose infants had complications [45].
Box 1. Physiological and psychosocial side effects of antepartum bed rest.
Physiological
Psychosocial
Lack of control [47]
Maternal stress is also high during either home or hospital bed rest and emanates from a variety of sources [47–50]. Confinement to bed rest is a type of sensory deprivation where kinesthetic stimulation is severely limited and, as a result, other sensory stimulation is also reduced [10]. A type of environmental ‘sameness’ develops when one is confined to a bed or room for long periods. Another primary source of stress is ‘altered temporality’: a type of waiting and accompanying elongation of time that develops during bed rest when women become very focused on trying to avoid preterm birth and are waiting for the time to pass until the fetus can be safely born [47]. Women are well aware that each new day brings increased chances for infant survival. Completion of another day of fetal growth is paramount in their mind. When women spend long, isolated, fright-filled hours in bed, time is perceived as slowing down, in seconds and minutes, rather than hours or days [47–50]. Women also feel out of control of what is happening with their bodies [47]. Women report feeling imprisoned, as activity is physically possible but is restricted by prescription [47,51]. Being separated from the family while hospitalized, and worrying about family matters and their care, concern for negative emotions, and health status are also sources of stress [10,11,28,47,51]. Studies confirm that the experience of bed rest is stressful for the entire family [52–55]. Concern for family disruption, financial difficulties, care for children at home and the partner’s assumption of maternal responsibilities, in addition to worry about maternal and fetal outcome, combine into a traumatic experience for the mother, her partner and children. These experiences are similar when bed rest is prescribed at home, but women on bed rest at home worry about the proximity of healthcare providers should an emergency arise [47].
Expert commentary
A few early studies reported that bed rest delayed preterm birth or improved fetal outcome, but were methodologically flawed [56]. Current evidence indicates that bed-rest treatment is not effective; however, there are gaps in research and there is an urgent need for additional randomized controlled trials of bed-rest treatment to definitively determine its effectiveness for each diagnosis. Such studies are difficult to conduct, however, as they must be longitudinal, are labor intensive, and include women with one diagnosis uncomplicated by other diseases. Since the severity of adverse effects appears to be directly proportional to the severity of restriction, a consistent and precise definition of bed rest or activity restriction that uses the ratio, rather than categorical, level of measurement for the length of bed rest is needed [9,11]. A description of the setting in which restriction takes place is also needed so that generalizations can be made across studies.
Using maternal activity logs or pedometers facilitate more precise ratio level measurement of activity. Furthermore, a practice management protocol for managing high-risk pregnancy is necessary for consistency and control of internal validity of the study, as currently, management of women with a high-risk pregnancy varies within and between healthcare providers [3,4,57]. Multisite trials may be necessary to enroll women with the same diagnosis and have sufficiently large sample size based on a power analysis. To extend the science, the ideal design should include an experimental group of women with the same diagnosis who are permitted to be ambulatory but who will be admitted if emergent complications occur, but discharged to activity once their status stabilizes; that is, an intensively monitored ambulatory outpatient group. Outcome variables of main interest should focus on assessments of maternal and fetal morbidity and mortality, particularly precise assessment of maternal weight change during bed rest, and infant GA at birth assessed by one or two consistent methods, as well as infant birthweight, and other measures of physical status. Secondary outcomes could include length of hospital stay and cost of treatment, as well as evidence of other documented side effects of bed rest.
There is also a need for additional studies on the side effects of bed rest. Investigations need to focus on the side effects that have long-term implications for maternal and fetal health, such as, cardiovascular and musculoskeletal deconditioning. Decreased aerobic capacity and muscle strength rapidly resulting from inactivity interact to produce deconditioning [8]. Lower postpartum maternal activity resulting from deconditioning could set into motion a downward spiral of inactivity leading to further deconditioning and disability. In particular, studies assessing bone loss are needed. During bed rest in nonpregnant individuals, bone loss is highest in the trabecular bone [6,58–61]. Of concern, bone loss associated with bed rest may interfere with obtaining peak bone mass because it occurs during the first 25 years of life. Individuals with the highest peak bone mass have the greatest protective advantage against bone loss associated with aging and illness [62–65]. Those who do not reach optimal peak bone mass are at risk of developing osteoporosis [62]. If the pregnant woman is older, bed-rest treatment occurs as natural bone loss with aging begins. Age-related bone loss may be temporarily accelerated by bed rest, resulting in a greater decline in mass and an earlier arrival of fracture threshold. Since trabecular bone loss in the spine and hip may not be fully recoverable, failure to fully recover bone loss may compromise lifelong maternal skeletal integrity [6,59] and increase the risk for osteoporosis and susceptibility to fractures, falls and resultant morbidity and mortality [62,63].
Additional data are needed regarding the impact of maternal weight loss upon fetal birthweight. Research about maternal weight change and altered body and glucose metabolism during a high-risk pregnancy treated with bed rest is needed, as infant birthweight is a major predictor of infant morbidity and mortality, both of which increase as GA and infant birthweight decrease [62]. Last, additional research is needed about postpartum recovery. Women, particularly those on long-term bed rest, are debilitated in the postpartum, yet they are discharged from the hospital after birth at the same time as healthy postpartum women. In our pilot study of post-bed-rest maternal endurance and lower leg muscle strength at 2–3 days postpartum, maternal performance corresponded to standards for the tenth percentile of 70-year-old women [66]. Therefore, after bed rest, women are discharged in a physically deconditioned state and are at risk for subsequent injury or illness. Fainting and falls were reported in 10–11% of our sample before 6 weeks [27]. Early identification of the extent of deconditioning and subsequent physical therapy assessment can lead to an individualized post-bed-rest planned program of postpartum rehabilitation.
Limitations to the study of the side effects of bed rest have been extensively detailed elsewhere [10]. It is possible, for example, that maternal weight loss is a side effect of having a high-risk pregnancy, rather than of bed rest. The ideal comparison bed-rest group is women with high-risk pregnancies who are not prescribed activity restriction. Predicting those women, however, who will have a high-risk pregnancy prior to initiation of bed-rest treatment remains elusive, as the causes of preterm birth are not well understood and a large percent of preterm births occur spontaneously without any previous indication [62]. Thus, the variables of bed-rest treatment and high-risk pregnancy are confounded [10]. In addition, research into the side effects of bed rest is more complex than research in nonpregnant bed-rest subjects. Instruments used to measure physiological variables such as muscle atrophy, cardiovascular deconditioning, and hip and spine bone mineral density may not be safe or well tolerated in pregnancy.
Despite limitations in studying the effectiveness and side effects of bed rest, there is sufficient evidence to suggest that bed rest should not be used until proven to be effective [14–18]. There is also sufficient evidence to indicate that bed-rest treatment is not benign. Rather, bed rest produces multiple adverse side effects, some of which have major implications for long-term maternal–infant health. Furthermore, the body of evidence regarding antepartum bed rest side effects is strongly supported by similar research conducted in nonpregnant individuals [6,8,29,60]. Therefore, it would appear that healthcare providers should engage in prevention and discontinue the use of bed rest until evidence indicates that it is effective.
Five-year view
The Cochrane collaboration first challenged the effectiveness of antepartum bed-rest treatment in 1989 by stating that “There is currently no evidence to support such recommendations” [67]. Additional research since that time continues to support the Cochrane recommendation. Furthermore, it is now clear that antepartum bed rest is associated with adverse side effects of concern for immediate and long-term maternal–fetal health. Despite these new data, prescription of bed rest in the USA, Canada and other countries continues [1–4,10]. In 1998, Maloni and colleagues reported that 88–93% of obstetricians and maternal–fetal medicine specialists would prescribe bed rest [3]. In 2009, Fox and colleagues reported that between 71 and 87% of maternal–fetal medicine specialists prescribed bed rest for women with both cervical dilation and arrested preterm labor or women with preterm premature rupture of membranes, while between 5 and 11% would not [1]. In a recent Canadian study, despite the belief of the majority that bed rest was not likely to be effective, a much lower percent of obstetricians (35%), family practitioners (42.7%) and midwives (21.4%) prescribed bed rest in hospital, but these numbers increased dramatically when prescribing bed rest at home [4]. These data are consistent with our observations that while it may appear that the use of bed rest is declining somewhat, there has been a shift in the site of bed rest from hospital to home. Explanations for continued use despite existing evidence are weak or lacking. Prescription of bed rest appears to be based upon the belief that rest does little harm and that maternal activity is related to physical forces that stimulate uterine contractions, cervical effacement and dilation [1,4,10]. Some practitioners rely on their clinical expertise and conclude that since they have had good outcomes, bed-rest treatment is effective, when it is unknown whether the same outcome would have occurred without bed-rest treatment. Others state that they use bed rest because there are limited effective interventions to prevent preterm birth, and that abstaining from using interventions in high-risk pregnancy is difficult since neonatal outcomes are often serious [4,10]. Prescription of bed rest may continue because fellow colleagues also prescribe it [4,10]. Still, other practitioners believe that bed rest is of little benefit but report continued use [1,4,10]. Continued prescription of antepartum bed rest may also be due to lack of access to evidence, or decisional conflict [4]. Research documents that most obstetric healthcare providers are unaware of the adverse effects of bed rest or they believe that effects are of minor consequence [1,3,4,10,18]. Therefore, continued use appears to be due to a lack of knowledge or attention to research regarding effectiveness and the adverse side effects, or a discounting of the evidence [4,10]. One possible reason could be because obstetric providers primarily read medical research while the majority of evidence regarding the side effects of bed rest in nonpregnant and pregnant individuals is produced by other related disciplines.
Perhaps once knowledge of the side effects of bed rest becomes widely known and concerns for patient safety are realized, as well as the lack of evidence for effectiveness of bed rest, the use of this treatment will decline. The science of behavioral change is not well understood. Some obstetric practices appear to change rapidly upon publication of research such as change in the management of breech birth, while other practices, such as routine episiotomy, cesarean section and electronic fetal monitoring, have not changed despite evidence. Behavioral change often lags behind science [57]. Fox and colleagues concluded that they cannot explain why bed rest continues to be used despite obstetricians’ belief that it is not effective, and suggested that bed rest is an ingrained practice [1]. Given the length of time that evidence for effectiveness and safety of bed rest has existed, only a few factors may stimulate change in practice: requirement for obtained informed consent for bed-rest treatment with a full patient explanation of its unknown efficacy and side effects [4]; patient legal action related to adverse antepartum or postpartum outcomes; institution of some type of incentive, such as cessation of insurance reimbursement for bed-rest care, or professional direction to implement evidence-based protocols for management of pregnancy complications. For example, The Society of Obstetricians and Gynaecologists of Canada has created a national protocol to guide treatment of women with hypertensive disorders of pregnancy [68]. Patient demand may also institute change as, in the past, women’s demands have stimulated change in obstetrics; for example, being awake for childbirth, having partners in delivery, and having Lamaze educational preparation for labor and other education classes. If not, it is likely that women will continue to struggle with the untreated side effects of bed rest during pregnancy and the postpartum, and wonder why they do not recover like other childbearing women.
Key issues.
The assumption that antepartum bed-rest treatment is effective in preventing preterm birth, preventing fetal growth restriction and increasing gestational age of birth and infant birthweight is not supported by research.
The assumption that antepartum bed-rest treatment is safe – that is, without major adverse effects for mother and or infant – is not supported by research.
Aerospace scientists have discovered that during bed rest, every major organ system begins to adapt to the prone state and an array of physiological and psychological side effects rapidly occur.
The purported causes of side effects of bed rest are the reduction of hydrostatic gradients, reduced loading and disuse of weight-bearing tissues.
Adaptation to the prone state begins within hours and the side effects of bed rest are differential depending upon the body system and vary with the length and degree of activity restriction.
The major adverse side effects of antepartum bed-rest treatment include muscle atrophy, bone loss, maternal weight loss and decreased infant birthweight in singleton gestations, and psychosocial problems including depression, anxiety, stress, family disruption and financial burden.
Cochrane database meta-analyses conclude that the practice of prescribing antepartum bed rest to prevent preterm birth should be discontinued until evidence is produced that it is effective.
Research in nonpregnant individuals has shown that trabecular bone loss in the spine and hip during bed rest may not be fully recoverable. Therefore, failure to fully recover bone loss during antepartum bed rest may compromise lifelong maternal skeletal integrity and increase risk for susceptibility to fractures, falls, osteoporosis, and resultant morbidity and mortality.
Antepartum bed rest should not continue to be prescribed because its effectiveness has not been demonstrated and it produces adverse side effects for both mother and infant.
Acknowledgements
Judith A Maloni would like to thank Mathew McManus, Editor, Case Western Reserve University, Frances Payne Bolton School of Nursing, for his editorial comments related to this manuscript.
Footnotes
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Fox NS, Gelber SE, Kalish RB, Chasen ST. The recommendation for bed rest in the setting of arrested preterm labor and premature rupture of membranes. Am. J. Obstet. Gynecol. 2009;200(2):165.e1–165.e6. doi: 10.1016/j.ajog.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Sprague AE. The evolution of bed rest as a clinical intervention. J. Obstet. Gynecol. Neonatal Nurs. 2004;33(5):542–549. doi: 10.1177/0884217504268523. [DOI] [PubMed] [Google Scholar]
- 3.Maloni JA, Cohen AW, Kane JH. Prescription of activity restriction to treat high-risk pregnancies. J. Womens Health. 1998;7(3):351–358. doi: 10.1089/jwh.1998.7.351. [DOI] [PubMed] [Google Scholar]
- 4.Sprague AE, O’Brien B, Newburn-Cook C, Heaman M, Nimrod C. Bed rest and activity restriction for women at risk for preterm birth: a survey of Canadian prenatal care providers. J. Obstet. Gynaecol. Can. 2008;30(4):317–326. doi: 10.1016/S1701-2163(16)32800-6. [DOI] [PubMed] [Google Scholar]
- 5.Sandler H, Vernikos J. Inactivity: Physiological Effects. Orlando, USA: Academic Press; 1986. [Google Scholar]
- 6.Fortney SM, Schneider VS, Greenleaf JE. The physiology of bed rest. In: Fregley MJ, Blatteis CM, editors. Handbook of Physiology. NY, USA: Oxford University Press; 1989. [Google Scholar]
- 7.Lujan BF, White RJ, Barber H. Human Physiology in Space: A Curriculum Supplement for Secondary Schools. Washington, DC, USA: National Aeronautics and Space Administration; 1994. [Google Scholar]
- 8.de Boer MD, Seynnes OR, di Prampero PE, et al. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur. J. Appl. Physiol. 2008;104(2):401–407. doi: 10.1007/s00421-008-0703-0. [DOI] [PubMed] [Google Scholar]
- 9.Sciscione AC. Maternal activity restriction and the prevention of preterm birth. Am. J. Obstet. Gynecol. 2010;202(3):232.e1–232.e5. doi: 10.1016/j.ajog.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Maloni JA. Antepartum bed rest for pregnancy complications: efficacy and safety for preventing preterm birth. Biol. Res. Nurs. 2010;12(2):106–124. doi: 10.1177/1099800410375978. [DOI] [PubMed] [Google Scholar]
- 11.Maloni JA, Chance B, Zhang C, Cohen AW, Betts D, Gange SJ. Physical and psychosocial side effects of antepartum hospital bed rest. Nurs Res. 1993;42(4):197–203. [PubMed] [Google Scholar]
- 12.MacLennan AH, Green RC, O’Shea R, Brookes C, Morris D. Routine hospital admission in twin pregnancy between 26 and 30 weeks’ gestation. Lancet. 1990;335(8684):267–269. doi: 10.1016/0140-6736(90)90079-k. [DOI] [PubMed] [Google Scholar]
- 13.Saunders MC, Dick JS, Brown IM, McPherson K, Chalmers I. The effects of hospital admission for bed rest on the duration of twin pregnancy: a randomised trial. Lancet. 1985;2(8459):793–795. doi: 10.1016/s0140-6736(85)90792-5. [DOI] [PubMed] [Google Scholar]
- 14.Crowther CA, Han S. Hospitalisation and bed rest for multiple pregnancy. Cochrane Database Syst. Rev. 2010;7 doi: 10.1002/14651858.CD000110.pub2. CD000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott JP, Miller HS, Coleman S, et al. A randomized multicenter study to determine the efficacy of activity restriction for preterm labor management in patients testing negative for fetal fibronectin. J. Perinatol. 2005;25(10):626–630. doi: 10.1038/sj.jp.7211359. [DOI] [PubMed] [Google Scholar]
- 16.Meher S, Abalos E, Carroli G. Bed rest with or without hospitalization for hypertension during pregnancy. Cochrane Database of Syst. Rev. 2010;4 doi: 10.1002/14651858.CD003514.pub2. CD003514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Say L, Gulmezoglu AM, Hofmeyr GJ. Bed rest in hospital for suspect impaired fetal growth. Cochrane Database of Syst Rev. 2010;2 doi: 10.1002/14651858.CD000034. CD000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosa C, Althabe F, Belizan J, Bergel E. Bed rest in singleton pregnancies for preventing preterm birth. Cochrane Database Syst. Rev. 2004;1 doi: 10.1002/14651858.CD003581.pub2. CD003581. [DOI] [PubMed] [Google Scholar]
- 19.Smith V, Devane D, Begley CM, Clarke M, Higgins S. A systematic review and quality assessment of systematic reviews of randomised trials of interventions for preventing and treating preterm birth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;142(1):3–11. doi: 10.1016/j.ejogrb.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Domingues MR, Matijasevich A, Barros AJ. Physical activity and preterm birth: a literature review. Sports Med. 2009;39(11):961–975. doi: 10.2165/11317900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Hegaard HK, Pedersen BK, Nielsen BB, Damm P. Leisure time physical activity during pregnancy and impact on gestational diabetes mellitus, preeclampsia, preterm delivery and birth weight: a review. Acta. Obstet. Gynecol. Scand. 2007;86(11):1290–1296. doi: 10.1080/00016340701647341. [DOI] [PubMed] [Google Scholar]
- 22.Hegaard HK, Hedegaard M, Damm P, Ottesen B, Petersson K, Henriksen TB. Leisure time physical activity is associated with a reduced risk of preterm delivery. Am. J. Obstet. Gynecol. 2008;198(2):180 e1–180 e5. doi: 10.1016/j.ajog.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Melzer K, Schutz Y, Boulvain M, Kayser B. Physical activity and pregnancy: cardiovascular adaptations, recommendations and pregnancy outcomes. Sports Med. 2010;40(6):493–507. doi: 10.2165/11532290-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Weissgerber TL, Wolfe LA, Davies GA, Mottola MF. Exercise in the prevention and treatment of maternal–fetal disease: a review of the literature. Appl. Physiol. Nutr. Metab. 2006;31(6):661–674. doi: 10.1139/h06-060. [DOI] [PubMed] [Google Scholar]
- 25.Evenson KR, Siega-Riz AM, Savitz DA, Leiferman JA, Thorp JM., Jr Vigorous leisure activity and pregnancy outcome. Epidemiology. 2002;13(6):653–659. doi: 10.1097/00001648-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Maloni JA, Schneider BS. Inactivity: symptoms associated with gastrocnemius muscle disuse during pregnancy. AACN Clin. Issues. 2002;13(2):248–262. doi: 10.1097/00044067-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Maloni JA, Park S. Postpartum symptoms after antepartum bed rest. J. Obstet. Gynecol. Neonatal Nurs. 2005;34(2):163–171. doi: 10.1177/0884217504274416. [DOI] [PubMed] [Google Scholar]
- 28.Maloni JA, Margevicius SP, Damato EG. Multiple gestation: side effects of antepartum bed rest. Biol. Res. Nurs. 2006;8(2):115–128. doi: 10.1177/1099800406291455. [DOI] [PubMed] [Google Scholar]
- 29.Rittweger J, Felsenberg D. Recovery of muscle atrophy and bone loss from 90 days bed rest: results from a one-year follow-up. Bone. 2009;44(2):214–224. doi: 10.1016/j.bone.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 30.Rittweger J, Beller G, Armbrecht G, et al. Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise. Bone. 2010;46(1):137–147. doi: 10.1016/j.bone.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 31.Promislow JH, Hertz-Picciotto I, Schramm M, Watt-Morse M, Anderson JJ. Bed rest and other determinants of bone loss during pregnancy. Am. J. Obstet. Gynecol. 2004;191(4):1077–1083. doi: 10.1016/j.ajog.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 32.Kaji T, Yasui T, Suto M, et al. Effect of bed rest during pregnancy on bone turnover markers in pregnant and postpartum women. Bone. 2007;40(4):1088–1094. doi: 10.1016/j.bone.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Brandao KL, Mottola MF, Gratton R, Maloni JA. Bone status in activity restricted pregnant women assessed using calcaneal quantitative ultrasound. Biol. Res. Nurs. 2011 doi: 10.1177/1099800411423807. (In press). [DOI] [PubMed] [Google Scholar]
- 34.Armbrecht G, Belavy DL, Gast U, et al. Resistive vibration exercise attenuates bone and muscle atrophy in 56 days of bed rest: biochemical markers of bone metabolism. Osteoporos. Int. 2004;21(4):597–607. doi: 10.1007/s00198-009-0985-z. [DOI] [PubMed] [Google Scholar]
- 35.DiVasta AD, Feldman HA, Quach AE, Balestrino M, Gordon CM. The effect of bed rest on bone turnover in young women hospitalized for anorexia nervosa: a pilot study. J. Clin. Endocrinol. Metab. 2009;94(5):1650–1655. doi: 10.1210/jc.2008-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maloni JA, Alexander GR, Schluchter MD, Shah DM, Park S. Antepartum bed rest: maternal weight change and infant birth weight. Biol. Res. Nurs. 2004;5(3):177–186. doi: 10.1177/1099800403260307. [DOI] [PubMed] [Google Scholar]
- 37.Krebs JM, Schneider VS, Evans H, Kuo MC, LeBlanc AD. Energy absorption, lean body mass, and total body fat changes during 5 weeks of continuous bed rest. Aviat. Space Environ. Med. 1990;61(4):314–318. [PubMed] [Google Scholar]
- 38.Behrman RE, Butler AS, editors. Preterm birth: Causes, Consequences, and Prevention. Washington, DC, USA: National Academies Press; 2007. Institute of Medicine committee on understanding premature birth and assuring healthy outcomes. Behavioral and psychosocial contributors to preterm birth. [PubMed] [Google Scholar]
- 39.Requejo JH, Merialdi M. The global impact of preterm birth. In: Berghella V, editor. Preterm Birth: Prevention and Management. Chichester, West Sussex, UK: Wiley Blackwell; 2010. [Google Scholar]
- 40.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2004. National Vital Statistics Reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics. Natl Vital. Stat. Rep. 2009;57(7):1–104. [Google Scholar]
- 41.Kovacevich GJ, Gaich SA, Lavin JP, et al. The prevalence of thromboembolic events among women with extended bed rest prescribed as part of the treatment for premature labor or preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 2000;182(5):1089–1092. doi: 10.1067/mob.2000.105405. [DOI] [PubMed] [Google Scholar]
- 42.Danilenko-Dixon DR, Heit JA, Silverstein MD, et al. Risk factors for deep vein thrombosis and pulmonary embolism during pregnancy or post partum: a population-based, case–control study. Am. J. Obstet. Gynecol. 2001;184(2):104–110. doi: 10.1067/mob.2001.107919. [DOI] [PubMed] [Google Scholar]
- 43.Heaman M. Stressful life events, social support, and mood disturbance in hospitalized and non-hospitalized women with pregnancy-induced hypertension. Can. J. Nurs. Res. 1992;24(1):23–37. [PubMed] [Google Scholar]
- 44.Mercer RT, Ferketich SL. Stress and social support as predictors of anxiety and depression during pregnancy. ANS Adv. Nurs. Sci. 1988;10(2):26–39. doi: 10.1097/00012272-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Maloni JA, Kane JH, Suen LJ, Wang KK. Dysphoria among high-risk pregnant hospitalized women on bed rest: a longitudinal study. Nurs Res. 2002;51(2):92–99. doi: 10.1097/00006199-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Maloni JA, Park S, Anthony MK, Musil CM. Measurement of antepartum depressive symptoms during high-risk pregnancy. Res. Nurs. Health. 2005;28(1):16–26. doi: 10.1002/nur.20051. [DOI] [PubMed] [Google Scholar]
- 47.Gupton A, Heaman M, Ashcroft T. Bed rest from the perspective of the high-risk pregnant woman. J. Obstet. Gynecol. Neonatal Nurs. 1997;26(4):423–430. doi: 10.1111/j.1552-6909.1997.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 48.Schroeder CA. Women’s experience of bed rest in high-risk pregnancy. Image J. Nurs. Sch. 1996;28(3):253–258. doi: 10.1111/j.1547-5069.1996.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 49.Thornburg P. ‘Waiting’ as experienced by women hospitalized during the antepartum period. MCN Am. J. Matern. Child Nurs. 2002;27(4):245–248. doi: 10.1097/00005721-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Stainton MC, Lohan M, Woodhart L. Women’s experiences of being in high-risk antenatal care: day stay and hospital stay. responses to two models of antepartum high-risk care: day stay and hospital stay. Australian Midwifery. 2005;18(4):16–20. doi: 10.1016/j.wombi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Heaman M, Gupton A. Perceptions of bed rest by women with high-risk pregnancies: a comparison between home and hospital. Birth. 1998;25(4):252–258. doi: 10.1046/j.1523-536x.1998.00252.x. [DOI] [PubMed] [Google Scholar]
- 52.Maloni JA, Ponder MB. Fathers’ experience of their partners’ antepartum bed rest. Image J. Nurs. Sch. 1997;29(2):183–188. doi: 10.1111/j.1547-5069.1997.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 53.Maloni JA, Brezinski-Tomasi JE, Johnson LA. Antepartum bed rest: effect upon the family. J. Obstet. Gynecol. Neonatal Nurs. 2001;30(2):165–173. doi: 10.1111/j.1552-6909.2001.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 54.May KA. Impact of maternal activity restriction for preterm labor on the expectant father. J. Obstet. Gynecol. Neonatal Nurs. 1994;23(3):246–251. doi: 10.1111/j.1552-6909.1994.tb01876.x. [DOI] [PubMed] [Google Scholar]
- 55.May KA. Impact of prescribed activity restriction during pregnancy on women and families. Health Care Women Int. 2001;22(1–2):29–47. doi: 10.1080/073993301300003063. [DOI] [PubMed] [Google Scholar]
- 56.Maloni JA, Kasper CE. Physical and psychosocial effects of antepartum hospital bedrest: a review of the literature. Image J. Nurs. Sch. 1991;23(3):187–192. doi: 10.1111/j.1547-5069.1991.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 57.Ramsey PS, Nuthalapaty FS, Lu G, Ramin S, Nuthalapaty ES, Ramin KD. Contemporary management of preterm premature rupture of membranes (PPROM): a survey of maternal-fetal medicine providers. Am. J. Obstet. Gynecol. 2004;191(4):1497–1502. doi: 10.1016/j.ajog.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Giangregorio L, Blimkie CJ. Skeletal adaptations to alterations in weight-bearing activity: a comparison of models of disuse osteoporosis. Sports Med. 2002;32(7):459–476. doi: 10.2165/00007256-200232070-00005. [DOI] [PubMed] [Google Scholar]
- 59.Ohshima H. Secondary osteoporosis UPDATE. Bone loss due to bed rest and human space flight study. Clin. Calcium. 2010;20(5):709–716. [PubMed] [Google Scholar]
- 60.Spector ER, Smith SM, Sibonga JD. Skeletal effects of long-duration head-down bed rest. Aviat. Space Environ. Med. 2009;80 Suppl. 5:A23–A28. doi: 10.3357/asem.br02.2009. [DOI] [PubMed] [Google Scholar]
- 61.Zwart SR, Hargens AR, Lee SM, et al. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone. 2007;40(2):529–537. doi: 10.1016/j.bone.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Institute of Health Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 63.Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J. Bone Miner. Res. 2000;15(3):557–563. doi: 10.1359/jbmr.2000.15.3.557. [DOI] [PubMed] [Google Scholar]
- 64.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med. Sci. Sports Exerc. 1997;29:197–206. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Sowers MF, Scholl T, Harris L, Jannausch M. Bone loss in adolescent and adult pregnant women. Obstet. Gynecol. 2000;96(2):189–193. doi: 10.1016/s0029-7844(00)00903-0. [DOI] [PubMed] [Google Scholar]
- 66.Rikli RE, Jones CJ. Senior Fitness Test Manual. IL, USA: Human Kinetics; 2001. [Google Scholar]
- 67.Crowther D, Chalmers I. Bed rest and hospitalization during pregnancy. In: Chalmers I, Enkin M, Keirse M, editors. Effective Care in Pregnancy and Childbirth. NY, USA: Oxford University Press; 1989. [Google Scholar]
- 68.Society of Obstetricians and Gynaecologists of Canada. Diagnosis, management, and evaluation of hypertensive disorders of pregnancy. J. Obstet. Gynaecol. Can. 2008;30(3):S1–S48. doi: 10.1016/S1701-2163(16)32992-9. [DOI] [PubMed] [Google Scholar]