Abstract
Approximately 120,000 young women are diagnosed with cancer every year in the USA. Many will have treatment that can reduce their fertility, although few will learn this fact before their treatment commences. This presents a tremendous quality of life issue post-treatment, as evidenced in this Perspectives by a personal account from a 23 year-old woman diagnosed with breast cancer. Clinicians must increase awareness about patients’ desires for motherhood and awareness about their individual reproductive potential. We demonstrate novel evidence about the wide variability in ovarian reserve in women of similar age, using assessment by antral follicle count. We show how a unified approach between oncology and fertility teams can help patients better understand their risk of treatment-related infertility, as well as how to take effective measures to mitigate it. Finally, we present options for fertility preservation, based on the time point at which consultation occurs.
Introduction
In common parlance, a description of cancer treatment often evokes further details about hair loss, decreased appetite, illness, and vomiting. In young women with cancer (aged below 45 years at cancer diagnosis), infertility is a serious concern, although in many cases this issue is not considered by patients in relation to outcomes of chemotherapy or radiation treatment.
According to 2006 Surveillance Epidemiology and End Results (SEER ) statistics, approximately 120,000 women below the age of 50 develop cancer each year in the USA.1 Many of these young women unwittingly face reproductive compromise. 2 As recently as 2009, only 34–72% of reproductive-age women treated for cancer recall having a discussion about the effects of cancer treatment on their future fertility.3 In 2009, a survey of 249 oncologists at major academic centers in the USA reported that 82% had referred a patient to a reproductive endocrinologist at one point, yet more than half reported that they rarely did so.4 The reasons given for lack of referral were: lack of knowledge, insufficient time to discuss the issue, perceptions that patients could not delay treatment, or the perception that if patients did not raise the issue themselves they were not interested.5 In this Perspectives we will posit that these issues can be overcome with an integrated, team approach between oncologists and reproductive endocrinologists.
Patient perspective
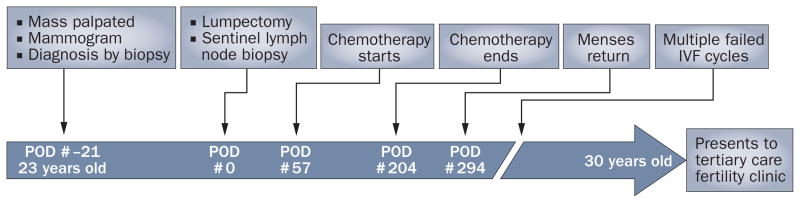
We offer a case and a patient’s letter to help exemplify the unmet need for improved fertility counseling before treatment. In 2002, a 23-year-old, gravida 0 woman presented to a medical center with a breast mass and was diagnosed with an invasive ductal carcinoma. 57 days after surgical therapy, she was treated with chemotherapy. She received four cycles of doxorubicin and cyclophosphamide, followed by four cycles of docetaxel. She then underwent local radiotherapy. The patient desired to have children, but did not have the opportunity to speak with a fertility specialist and was not offered options to preserve fertility. Regular menses returned 3 months after completion of chemotherapy. However, 7 years later, she presented to our tertiary care fertility center after several unsuccessful attempts at both natural conception and at in vitro fertilization (Figure 1).
Figure 1.
Timeline of events. Events relating to cancer detection and treatment, as well as fertility work-up and treatments in a young female patient, are chronicled. Time elapsed, relative to her date of lumpectomy, is listed at the bottom along with her age. Abbreviations: IVF, in vitro fertilization; POD, post-operative day.
This deeply personal issue warrants a greater understanding from a patient’s perspective. Consider her letter:
“I can still remember the day I was diagnosed with breast cancer. I was 23 and the doctor could barely look me in the eye before he dashed out the door. It took me about 5 years to fully acknowledge and accept the fact that I had cancer; it was a part of my life I had simply closed off. I sit here now and I feel the familiar unsettling sense of uncertainty and apprehension. Do I dare wonder whether another in vitro fertilization (IVF) cycle will work? After three unsuccessful IV F cycles, I have almost lost the luxury of hope that I will have my own biological children. Is it time to relinquish the dream of having a family of my own? The fertility clinic I first visited after cancer treatment did not specialize in cancer patients. The clinic tried to help the best they could, but in the end pointed back to the chemotherapy as the source of my infertility. It doesn’t quite feel right that this dream was taken away from me; I was not given the choice to freeze my eggs and at the time all fertility discussions were dismissed.
‘You are young.’ That seemed to be a recurring theme coming from each doctor I visited. My first doctor told me that I was too young for breast cancer given that there was none in my family history and she sent me away without further evaluation. Once I was diagnosed and I asked about fertility after cancer treatments, a doctor advised: ‘you are young, as long as you get your periods back, you’ll be fine’. My oncologist advised me that I would most likely be fertile after chemotherapy and there wasn’t much data available on fertility in young breast cancer patients— simply because there weren’t many young breast cancer patients. My periods came back but I still couldn’t get pregnant. What was wrong with me now?
Being called infertile is a cruel reminder of the time I spent with cancer treatments. To be infertile inherently would be devastating, but somehow knowing that it is stemmed from the cancer treatments is a very bitter truth. Almost as if my cancer is still mocking me, ‘I might not have taken your life but I have taken something else.’ I am truly at a loss knowing that my fertility could have been saved but wasn’t, all based on my age at the time. Perhaps it may not been saved completely, but any attempt made then would be better than what I am left with now. I still have dreams of a family of my own. That is why I am here today visiting with yet another set of doctors. Will it be worth it? I don’t know. At least at the end of it all, I can say that we tried everything that we could, this is the decision that my husband and I believed in.”
Fertility preservation: the problem
As our patient describes, despite her young age at diagnosis and resumption of normal menses after treatment, normal fertility was not restored. Thus, in this Perspectives, we propose a more nuanced clinical approach to considerations about fertility preservation for patients. To improve our understanding of such considerations, we recommend the incorporation and development of new translational research–from an improved understanding of basic ovarian biology to increased awareness of the psychosocial effects of treatment-related infertility.
In the past, when assessing the gonadotoxicity of cancer treatments, most studies used the presence or absence of normal menses as their primary outcome.6 This approach has limitations in terms of improving our understanding of fertility outcomes post-treatment. Albright and colleagues first described primary ovarian insufficiency (also known as premature ovarian failure) in 1942;7 since then an increased understanding has been gained regarding partial ovarian injury. Primary ovarian insufficiency can be overt (menses are absent or irregular) or occult (the ovary is damaged but menses remain regular).8 In our patient, menses resumed, but ovarian injury still manifested as infertility. Young cancer patients may benefit greatly from an improved understanding of the continuum of ovarian damage and, furthermore, from a more-targeted approach from clinicians and researchers regarding fertility after cancer treatment.
Recent studies have shown the loss of fertility—the loss of a potential child—has a profound impact on young women. Among young cancer survivors who are childless at diagnosis, approximately 75% desire children after treatment.9 In one study of survivors from gynecological malignancies, one-third of the patients identified with the statement that “part of ones self had died along with the idea of giving birth”.10 Furthermore, a recent qualitative study demonstrated that a lack of fertility preservation services was associated with distress in women with cancer.11 To optimize treatment in young women, evaluation and treatment of cancer must include an assessment of their reproductive potential and a discussion regarding the impact of treatment on ovarian function.
Fertility work-up
Referral to a reproductive endocrinologist should be offered to any woman younger than 45 years who desires future children and for whom any of the following is recommended: systemic chemotherapy, radiation to the abdomen or pelvis, oophorectomy, and/or pharmacologic delay in fecundity (for example, treatment with tamoxifen). A fertility specialist may be able to estimate the future fertility potential of an individual woman on the basis of the following factors: age, family history, gynecologic history, cancer treatment type and duration, and several diagnostic modalities. The combination of this information may facilitate the medical team’s understanding of not only a patient’s risk of infertility—independent of her cancer diagnosis—but also her relative chance of significant ovarian compromise after cancer treatment.
Our patient received counseling about future fertility solely on the basis of her age. Generally, the incidence of chemotherapy-related amenorrhea increases as one gets older, which reflects the importance of age.12 A plausible mechanism for this observation is the fact that egg numbers (ovarian reserve) tend to reduce with increasing age, resulting in lower egg quantity and potentially reduced egg quality. Although age is likely the most important predictor of chemotherapy-related infertility, our patient’s case highlights the need for additional prognostic tools.
The development of prognostic tools for the assessment of ovarian reserve improves our ability to counsel women undergoing fertility treatment and may also serve to improve counseling in the situation of a cancer diagnosis. Antral follicle count (AFC) and serum anti-Müllerian hormone (AMH) are currently considered the most reliable noninvasive methods for determining the ovarian reserve. This assumption is based on the correlation between these markers with the number of primordial follicles in the ovary, as well as parallel patterns of decline with age.13–15
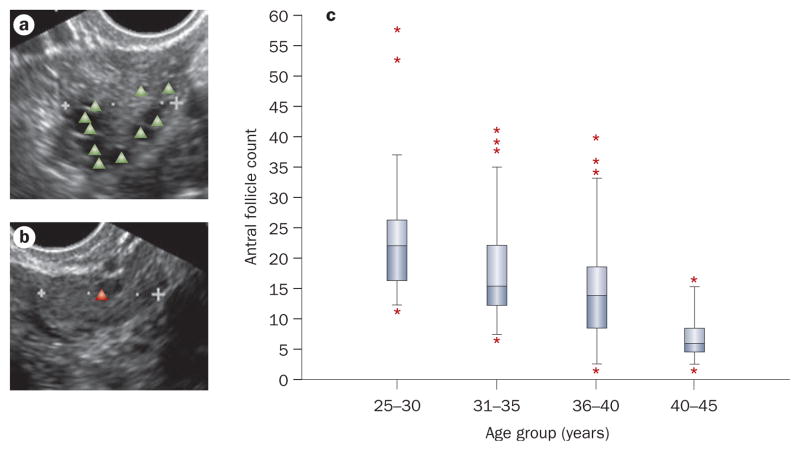
New evidence from the Ovarian Aging study (NIH R01 HD044876) suggests that there is considerable variation in ovarian reserve in women of similar age (Figure 2).14 For instance, the AFC in women aged 25–30 years ranges from 12 to 37. Some of the variation in ovarian reserve seen in these women may be accounted for by genetics. 16,17 We posit that variability in ovarian reserve may help account for the observed variation in rates of ovarian insufficiency in age-matched individuals who have undergone cancer treatment. AFC can decline notably in premenopausal women who become amenorrheic after treatment with cyclophosphamide regimens. 18 In addition, women with low levels of AMH pretreatment tend to have higher rates of chemotherapy-related amenorrhea than those with high pretreatment AMH.19 Finally, these indices have been shown to identify other subgroups most likely to experience fertility issues and/or chemotherapy-related amenorrhea.20–22
Figure 2.
Antral follicle count. Transvaginal ultrasound images reflect a | high and b | low antral follicle counts. The triangles indicate antral follicles. c | The variability in antral follicle count in 252 healthy Caucasian women stratified by age group. Boxes represent 25th and 75th percentiles. Error bars represent standard deviation; stars represent statistical outliers.
Absolute prediction of fertility outcome is difficult for the following reasons: the unknown risks to fertility of some treatment regimens, the potential for adjustments in cancer therapy, and differences in variability in assessment of reproductive potential. However, the correlation between ovarian reserve and reproductive capacity may allow us to individualize our assessment, and may aid the prediction of an individual’s post-treatment reproductive compromise. This information may also help inform patients about their chances for success with the available fertility preservation options. A woman who presents with a diminished follicle number before cancer treatment should be informed that her reproductive potential may be low or that her window of reproductive opportunity may be foreshortened–even in the absence of cancer treatment–and that fertility preservation attempts may have limited success.23,24
Reproductive endocrinologists may be able to use AFC, along with serum markers and clinical history, to make a comprehensive assessment of reproductive potential and better determine women that are suitable candidates for fertility preservation before their cancer treatment starts. Such an assessment by a reproductive endocrinologist might mean that a patient like our own, who missed an opportunity to preserve her fertility 7 years ago, could now receive better counseling.
Today, fertility centers will open emergency slots to evaluate patients with cancer as soon as possible, giving patients with cancer the most opportunity should they choose to cryopreserve oocytes or embryos. Patients may ultimately decide not to pursue these options, but the sooner they are seen, the more options they will have. This protocol allows for an active choice for treatment or not versus the historical passive exclusion of treatment.
Ovarian stimulation
The team approach to counseling can be extended further to a team approach to treatment of young cancer patients. Ovarian stimulation with standard fertility medications increases oocyte yield compared with the natural menstrual cycle. From an oncologist’s perspective, concerns might arise as to whether hormone stimulation at the start of some fertility preservation protocols (along with potential treatment delays) may worsen prognosis. For example, use of aromatase inhibitors in patients with endometrial carcinoma has been associated with lower estradiol levels than that seen in standard ovarian stimulation cycles.25 This treatment approach has also been studied in fertility preservation for young patients with breast cancer and is unlikely to result in significant increases in recurrence risk.26 Furthermore, a review of 2,600 patients with early-stage breast cancer indicates that onset of adjuvant chemotherapy could be delayed up to 12 weeks after definitive surgery without significant decreases in relapse-free survival.27
Ovarian stimulation takes about 2 weeks and usually starts with the onset of menses. Generally, cancer treatment need not be delayed for more than 2–6 weeks, depending on whether a patient is in the follicular or luteal phase of the menstrual cycle. Gonadotropin releasing hormone (GnRH) antagonists could be used to more quickly prepare the patient for ovarian stimulation. 28 In patients with breast cancer, oocyte retrieval with standard fertility medications can be completed in an average of 33 days from first fertility evaluation. Indeed, ovarian stimulation has been integrated into cancer care without delaying the onset of chemotherapy.29 In our patient’s case, 78 days elapsed between diagnostic biopsy and the start of chemotherapy–a window that would have comfortably allowed the opportunity to preserve fertility.
Preserving hope
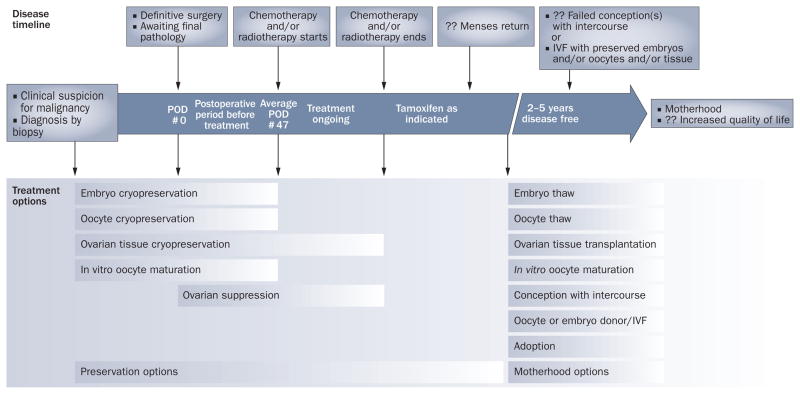
Family and happiness will mean many different things to different patients. Some may choose advanced reproductive technologies while others, of course, may not. However, we emphasize the importance of allowing patients to make their own decisions about family in the presence of the best available clinical data, and, as close to the time of diagnosis as possible, when the most options are available (Figure 3).
Figure 3.
Options for fertility preservation and for conception. A timeline of cancer and motherhood events is shown at the top of the flow chart. Patients meeting referral criteria should see a reproductive endocrinologist as soon after diagnostic biopsy as possible. Boxes demarcate viable time windows for established and experimental fertility preservation and motherhood options are shown below the timeline. Options for fertility preservation before cancer treatment commences include embryo, oocyte, and ovarian tissue cryopreservation, as well as ovarian suppression therapy. Once treatment has commenced, the only options may involve ovarian tissue cryopreservation or ovarian suppression. 2–5 years after treatment, patients may attempt natural conception, use preserved tissues, attempt IV F, use donor eggs and/or embryos, surrogacy, or choose adoption. Abbreviations: IVF, in vitro fertilization; POD, post-operative day.
An attempt to preserve a patient’s fertility before treatment offers the best chance of a successful pregnancy after treatment. Established techniques, such as embryo cryopreservation, or experimental techniques, such as oocyte cryopreservation, ovarian tissue cryopreservation, or ovarian suppression therapy can be used before commencing cancer treatment.
AS CO currently recommends the use of embryo cryopreservation whenever possible. 30 Survival rates per thawed embryo range from 35% to 90% and implantation rates of these embryos range from 8% to 30%.29 Embryo cryopreservation requires a sperm source, whereas oocyte cryopreservation does not, offering a very useful alternative to women who are single at diagnosis. Live birth rates after thawing cryopreserved oocytes were once as low as 2–5% per thawed oocyte, but new freezing techniques have dramatically improved the live birth rate of cryopreserved oocytes to approximately 40% per cycle started, near that of cryopreserved embryos.31–33
Currently available experimental alternatives for fertility preservation include tissue cryopreservation and in vitro maturation (IVM). Ovarian tissue cryopreservation involves a surgical procedure to remove all or part of the ovary, potentially offering an option for prepubertal girls and for women who require emergent treatment and cannot harvest eggs at the time of diagnosis. When thawed, this tissue provides the possibility of re-implantation or perhaps–in the future–complete in vitro growth. In addition to use before treatment, ovarian tissue cryopreservation may be one of the only available procedural options for fertility preservation once treatment commences.34 IVM involves egg harvest before treatment from antral follicles 4–12 mm in diameter, in the absence of stimulation, and subsequent maturation of oocytes in vitro. IVM also offers a viable option for prepurbertal girls and women facing emergent treatment. The procedure can be performed at any time in the menstrual cycle. Implantation rates of embryos from IVM are less than half that of embryos from oocytes matured in vivo.35
The use of ovarian suppression therapy remains controversial. In 2009 a randomized study of 78 patients with cancer reported decreased rates of amenorrhea and normalization of serum markers of ovarian function. 36 This study is interesting, although it is limited by short follow up (8 months after last chemotherapy) and lack of fertility as an outcome. Additionally, the results are considered controversial because of small sample size and higher than expected adverse outcomes in the control group.37 Another small randomized trial investigating the effect of GnRH agonist use together with an escalated chemotherapy regimen known as BEA COPP was stopped early because all patients in both study arms had very low AMH levels at 12 months of follow up.38 This study was limited by lack of a proper control group, by absence of fertility as an outcome, and by concerns that the treatment regimen (escalated BEA COPP) may be particularly gonadotoxic. Until larger randomized trials with longer follow up are conducted and a greater focus on both fertility and recurrence rates are included in trials, we view the use of GnRH agonists as a possible yet experimental option. We do not recommend GnRH agonists as replacement for more proven methods of fertility preservation, and contend that it should be considered only for patients enrolled under experimental protocol.
Following cancer treatment, and after a period of 2–5 years of disease-free survival, natural conception can be attempted. If fertility preservation was not performed, ovarian stimulation and oocyte harvest for embryo or oocyte cryopreservation can be attempted, but this is associated with diminished success.39 If natural conception is unsuccessful, and fertility preservation was performed before treatment, cryopreserved oocytes and/or embryos may be thawed and transferred to the patient. Additionally, cryopreserved tissues can also be transplanted back to the patient. To date, transpositions of cryopreserved tissue have led to approximately 10 births worldwide.40 In patients who do not respond to the abovementioned options, or who electively choose not to undergo fertility preservation before cancer treatment, may also consider options such as oocyte and/or embryo donation or adoption. Patients who either had a hysterectomy or who underwent pelvic radiation may wish to consider surrogacy.
Conclusions and future directions
As cancer treatment strategies advance, we must keep an increasingly attentive eye on how we are affecting our patients’ quality of life after cancer treatment. We must work together and plan with foresight, rather than working separately, in hindsight, to mend missed opportunities. Increased awareness of the risk of treatment-related infertility is paramount. This awareness must start with both fertility and oncology practitioners. As evidenced by our patient’s perspective, advances in the field of fertility preservation have the potential to make a tremendous impact on an individual patient’s life. With the development of newer and more-effective fertility counseling and treatments, we must continue to work as a team to provide our patients better access to these technologies.
Acknowledgments
This project was supported by NIH/NICHD and NIH/NIA Grant Number R01 HD044876 and by NIH/NCRR UCSF-CTSI Grant Number UL1 RR 024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Mr. Letourneau’s work was supported by a grant from the UCSF PACCTR Medical Student Research Fellowship. We would like to thank our patient for her generous and heartfelt submission. Without her work this manuscript would not have been possible.
Footnotes
Competing Interests
The authors declare no competing interests.
Author contributions
M. E. Melisko provided a substantial contribution to discussions of the content and to review and/or editing of the manuscript before submission. M. I. Cedar researched the data for the article and provided a substantial contribution to discussions of the content. J. M. Letourneau and M. P. Rosen contributed equally to research, discussion of content and writing the article and to review and/or editing of the manuscript before submission.
References
- 1.NCI Fast Stats. Statistics stratified by age. Surveillance Epidemiology and End Results (SEER) 2006 [online], http://seer.cancer.gov/faststats/selections.php.
- 2.Loprinzi CL, Wolf SL, Barton DL, Laack NN. Symptom management in premenopausal patients with breast cancer. Lancet Oncol. 2008;9:993–1001. doi: 10.1016/S1470-2045(08)70256-0. [DOI] [PubMed] [Google Scholar]
- 3.Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update. 2009;15:587–597. doi: 10.1093/humupd/dmp015. [DOI] [PubMed] [Google Scholar]
- 4.Forman EJ, Anders CK, Behera MA. A nationwide survey of oncologists regarding treatment-related infertility and fertility preservation in female cancer patients. Fertil Steril. doi: 10.1016/j.fertnstert.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Quinn GP, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27:5952–5957. doi: 10.1200/JCO.2009.23.0250. [DOI] [PubMed] [Google Scholar]
- 6.Sonmezer M, Oktay K. Fertility preservation in female cancer patients. Hum Reprod Update. 2004;10:251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 7.Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature. Am J Med Sci. 1942;204:625–648. [Google Scholar]
- 8.Nelson LM. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer. 2009;53:281–284. doi: 10.1002/pbc.22001. [DOI] [PubMed] [Google Scholar]
- 10.Carter J, et al. Gynecologic cancer treatment and the impact of cancer-related infertility. Gynecol Oncol. 2005;97:90–95. doi: 10.1016/j.ygyno.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Patel A, et al. Reproductive health assessment for women with cancer: a pilot study. Am J Obstet Gynecol. 2009;201:191e1–191e4. doi: 10.1016/j.ajog.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 13.Hansen KR, et al. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- 14.Rosen M, et al. Antral follicle count: absence of significant midlife decline. Fertil Steril. doi: 10.1016/j.fertnstert.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheffer GJ, et al. Antral follicle counts by transvaginal ultrasoundography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845–851. doi: 10.1016/s0015-0282(99)00396-9. [DOI] [PubMed] [Google Scholar]
- 16.Rosen MP, et al. Is antral follicle count a genetic trait? Menopause. 2009;17:109–113. doi: 10.1097/gme.0b013e3181b48a88. [DOI] [PubMed] [Google Scholar]
- 17.Schuh-Huerta SM, et al. Genetic determinants of ovarian aging assessed by antral follicle count. Fertil Steril. 2008;90:S265–S266. [Google Scholar]
- 18.Lutchman Singh K, et al. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer. 2007;96:1808–1816. doi: 10.1038/sj.bjc.6603814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anders C, et al. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Invest. 2008;26:286–295. doi: 10.1080/07357900701829777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen EC, Muller J, Rechnitzer C, Schmiegelow K, Andersen AN. Diminished ovarian reserve in female childhood cancer survivors with regular menstrual cycles and basal FSH <10 I U/l. Hum Reprod. 2003;18:417–422. doi: 10.1093/humrep/deg073. [DOI] [PubMed] [Google Scholar]
- 21.Lie Fong S, et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Mullerian hormone. Hum Reprod. 2009;24:982–990. doi: 10.1093/humrep/den487. [DOI] [PubMed] [Google Scholar]
- 22.Su HI, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116:592–599. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibreel A, Maheshwari A, Bhattacharya S, Johnson NP. Ultrasound tests of ovarian reserve; a systematic review of accuracy in predicting fertility outcomes. Hum Fertil (Camb) 2009;12:95–106. doi: 10.1080/14647270902896256. [DOI] [PubMed] [Google Scholar]
- 24.de Boer EJ, et al. A low number of retrieved oocytes at in vitro fertilization is predictive of early menopause. Fertil Steril. 2002;77:978–985. doi: 10.1016/s0015-0282(02)02972-2. [DOI] [PubMed] [Google Scholar]
- 25.Azim A, Oktay K. Letrozole for ovulation induction and fertility preservation by embryo cryopreservation in young women with endometrial carcinoma. Fertil Steril. 2007;88:657–664. doi: 10.1016/j.fertnstert.2006.12.068. [DOI] [PubMed] [Google Scholar]
- 26.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 27.Lohrisch C, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006;24:4888–4894. doi: 10.1200/JCO.2005.01.6089. [DOI] [PubMed] [Google Scholar]
- 28.von Wolff M, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril. 2009;92:1360–1365. doi: 10.1016/j.fertnstert.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Madrigrano A, Westphal L, Wapnir I. Egg retrieval with cryopreservation does not delay breast cancer treatment. Am J Surg. 2007;194:477–481. doi: 10.1016/j.amjsurg.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 31.Georgescu ES, Goldberg JM, du Plessis SS, Agarwal A. Present and future fertility preservation strategies for female cancer patients. Obstet Gynecol Surv. 2008;63:725–732. doi: 10.1097/OGX.0b013e318186aaea. [DOI] [PubMed] [Google Scholar]
- 32.Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86:70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Cao Y, Chian R. Fertility preservation with immature and in vitro matured oocytes. Sem Reprod Med. 27:456–464. doi: 10.1055/s-0029-1241055. [DOI] [PubMed] [Google Scholar]
- 34.Meirow D, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 35.Reinblatt SL, Buckett W. In vitro maturation for patients with polycystic ovary syndrome. Semin Reprod Med. 2008;26:121–126. doi: 10.1055/s-2007-992932. [DOI] [PubMed] [Google Scholar]
- 36.Badaway A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91:694–697. doi: 10.1016/j.fertnstert.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 37.Oktay K, Sönmezer M. Questioning GnRH analogs for gonadal protection in cancer patients. Fertil Steril. 2009;92:e32. doi: 10.1016/j.fertnstert.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Behringer K, et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin Lymphoma. Final results of a phase II clinical trial from the German Hodgkin Study Group. Ann Oncol. doi: 10.1093/annonc/mdq066. [DOI] [PubMed] [Google Scholar]
- 39.Ginsburg ES, et al. In vitro fertilization for cancer patients and survivors. Fertil Steril. 2001;75:705–710. doi: 10.1016/s0015-0282(00)01802-1. [DOI] [PubMed] [Google Scholar]
- 40.von Wolff M, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy—a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45:1547–1553. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]