Abstract
Background
Dilated cardiomyopathy (DCM), characterized by dilatation and dysfunction of the left ventricle, is an important cause of heart failure. Many mutations in various genes, including cytoskeletal protein genes and contractile protein genes, have been identified in DCM patients, but the mechanisms of how such mutations lead to DCM remain unknown.
Methods and Results
We established the mouse model of DCM by expressing a mutated cardiac α-actin gene, which has been reported in patients with DCM, in the heart (mActin-Tg). mActin-Tg mice showed gradual dilatation and dysfunction of the left ventricle, resulting in death by heart failure. The number of apoptotic cardiomyocytes and protein levels of p53 were increased in the hearts of mActin-Tg mice. Overexpression of Bcl-2 or downregulation of p53 decreased the number of apoptotic cardiomyocytes and improved cardiac function. This mouse model showed a decrease in myofilament calcium sensitivity and activation of calcium/calmodulin-dependent kinase IIδ (CaMKIIδ). The inhibition of CaMKIIδ prevented the increase in p53 and apoptotic cardiomyocytes and ameliorated cardiac function.
Conclusion
CaMKIIδ plays a critical role in the development of heart failure in part by accumulation of p53 and induction of cardiomyocyte apoptosis in the DCM mouse model.
Keywords: apoptosis, CaMKII, cardiomyopathy, heart failure, genes, p53
Heart failure is an important cause of morbidity and mortality in many industrial countries, and dilated cardiomyopathy (DCM) is one of its major causes.1 Although treatments for heart failure have been progressed well in both pharmacological and nonpharmacological aspects, mortality of DCM patients remains high, and the only treatment for DCM patients with severe symptoms is heart transplantation. Because the number of hearts for transplantation is limited, the development of novel therapies for DCM has been awaited.
DCM, characterized by dilatation and impaired contraction of the left ventricle, is a multifactorial disease that includes both hereditary and acquired forms. The acquired forms of DCM are caused by various factors.2 Twenty percent to 35% of patients have hereditary forms,1 and advances in molecular genetic studies during the last decade have revealed many mutations of various genes in DCM patients.3–5
Several hypotheses have been reported on the mechanisms of how gene mutations lead to DCM phenotypes. Mutations in genes encoding cytoskeletal proteins such as desmin and muscle LIM protein might disturb the interaction between the sarcomere and Z disk, resulting in impaired force transmission from the sarcomere to the surrounding syncytium.4,6 On the other hand, mutations in genes encoding contractile proteins such as α-tropomyosin and cardiac troponin T have been reported to induce the decrease in myofilament calcium (Ca2+) sensitivity.7 An increase in apoptotic cardiomyocytes and/or destruction of membrane structure by calpain activation have been reported to play a critical role in mutant gene–induced cardiac dysfunction.8–10 However, the precise mechanisms remain largely unknown as a result, at least in part, of a lack of good animal models of DCM.
Several animal models of DCM have been reported.11–13 The mdx mouse is a model of Duchenne muscular dystrophy, which has mutations in the dystrophin gene.11 Unlike humans, mdx mice rarely show cardiac abnormality, which has limited the utility of mdx mice as a model to examine the pathogenesis of DCM. Although Golden Retriever–based muscular dystrophy dogs show DCM phenotypes,12 the muscular dystrophy dogs are very difficult to maintain and handle. Although BIO 14.6 hamsters lacking δ-sarcoglycan are a good model of DCM,13 it is difficult to apply genetic approaches to the hamster. To elucidate the molecular mechanisms of how gene mutations cause DCM, appropriate animal models, particularly mouse models, are necessary. We established here a mouse model of DCM by expressing a mutated cardiac α-actin gene (mActin-Tg), which has been reported in patients with DCM, in the heart.5 mActin-Tg mice showed gradual dilatation and dysfunction of the left ventricle, resulting in death by heart failure. These phenotypes of mActin-Tg mice were quite similar to those of human DCM. In this study, we examined the underlying mechanisms of how this gene mutation leads to DCM using the new mouse model of DCM.
Methods
Detailed experimental methods are described in the online-only Data Supplement.
Mice
We generated transgenic mice (mActin-Tg) that expressed a mutated cardiac α-actin (R312H) with an HA tag in the heart. This mutation has been reported in patients with DCM.5 Generation of transgenic mice with cardiac-restricted overexpression of human Bcl-2, AC3-I, or nuclear factor of activated T cell (NFAT)–luciferase has been described previously.14–16 Heterozygous p53-deficient mice were purchased from The Jackson Laboratory (Bar Harbor, Me).17 Wild-type littermates served as controls for all studies. KN-93 (10 μmol · kg−1 ·/d−1) was used to inhibit activation of Ca2+/calmodulin-dependent kinase II (CaMKII). Echocardiography was performed on conscious mice.
Histology
For detection of apoptotic cardiomyocytes, we performed terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) staining, along with immunostaining for dystrophin.
Western Blot Analysis
Whole-cell lysates were resolved by SDS-PAGE. Western blot analyses were performed with some antibodies. The intensities of Western blot bands were measured with NIH ImageJ software (National Institutes of Health, Bethesda, Md).
Luciferase Assay
Left ventricles were homogenized in luciferase assay buffer as described previously.15
Force Measurements
A small fiber was dissected from the skinned left ventricular papillary muscle, and isometric force was measured as described previously.7
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction Analysis
Quantitative real-time polymerase chain reaction was performed with the LightCycler with the Taqman Universal Probe Library and Light Cycler Master. Relative levels of gene expressions were normalized to the mouse GAPDH expression with the ΔΔCt method.18
Statistical Analysis
Data are shown as mean±SEM. Multiple-group comparison was performed by 1-way ANOVA followed by the Bonferroni procedure for comparison of means. The F test was used to assess equal variances before comparison between 2 groups. Then, comparisons between 2 groups were performed with the Student t test (when P>0.05 in the F test) and the Welch t test (when P<0.05 in the F test). Survival rates were analyzed with the log-rank test. Values of P<0.05 were considered statistically significant.
Results
DCM Model Mouse
Because there are few useful DCM mouse models, we first generated transgenic mice that expressed a cardiac α-actin R312H mutant with an HA tag under the control of α-myosin heavy chain promoter (mActin-Tg). We obtained 3 independent founders of the transgenic mice (lines 301, 307, and 311). The protein levels of the cardiac α-actin R312H mutant were 1.6-fold in line 301, 3.3-fold in line 307, and 2.2-fold in line 311 compared with those of endogenous cardiac α-actin (Figure IA in the online-only Data Supplement). To confirm the expression of the transgene in cardiomyocytes, we performed immunohistological analyses with antibodies against HA and actinin. The mutated cardiac α-actin protein was colocalized with actinin, suggesting that the cardiac α-actin R312H mutant is incorporated into myofilaments (Figure IB in the online-only Data Supplement). Cardiac systolic function was decreased in mActin-Tg mice at 10 months of age, and the reduction was well correlated with protein levels of the cardiac α-actin R312H mutant (Figure IC in the online-only Data Supplement). To further investigate whether cardiac expression of the cardiac α-actin R312H mutant led to heart failure, we examined another transgenic mouse that expressed cardiac α-actin A331P mutant with an HA tag in the heart. This mutant has been reported to cause hypertrophic cardiomyopathy in human.19 We obtained 2 independent founders of the transgenic mice that expressed almost the same levels of the cardiac α-actin A331P mutant protein. Although the protein levels of the mutant in the A331P mutant transgenic mice were almost same as those of the R312H mutant in line 307, which had the highest expression (Figure II in the online-only Data Supplement), echocardiography revealed that there were no significant differences in cardiac systolic function, wall thickness, and left ventricular dimension between cardiac α-actin A331P mutant transgenic mice and their wild-type littermates (Table I in the online-only Data Supplement). Although it is not known at present why the expression of cardiac α-actin A331P mutant did not induce hypertrophic cardiomyopathy, these results suggest that cardiac dysfunction of mActin-Tg mice is due to cardiac expression of the cardiac α-actin R312H mutant in the heart, not to high-level expression of the cardiac α-actin protein with the tag (lines 307 and 311).
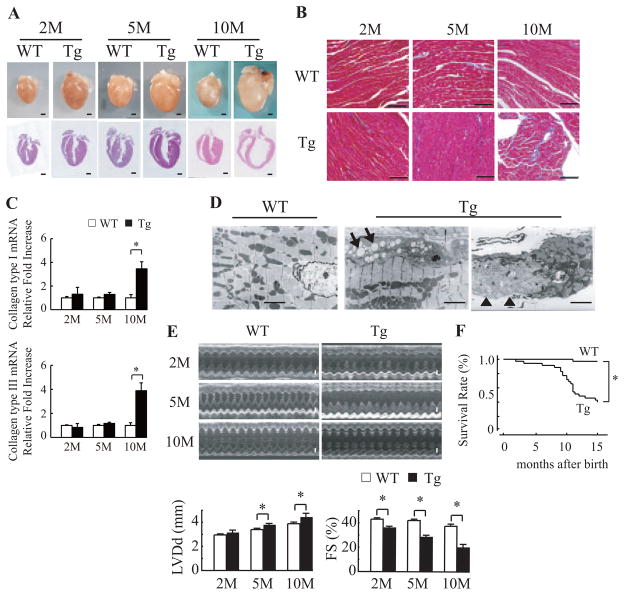
We used line 307, which expressed the cardiac α-actin R312H mutant at the highest levels, for further studies. The hearts in mActin-Tg mice were larger than those of wild-type littermates (Figure 1A), and heart weight and the ratio of heart weight to body weight were much increased in mActin-Tg mice (Table II in the online-only Data Supplement). Marked cardiac fibrosis was observed in mActin-Tg mice at 10 months of age, with increased expression of collagen types I and III (Figure 1B and 1C). Electron microscopic analyses showed that there were degenerated cardiomyocytes with an increase in vacuolar formation and lysis of myofibrils in mActin-Tg mice (Figure 1D). Echocardiography revealed that left ventricular dimension was gradually increased and that fractional shortening was reduced in mActin-Tg mice compared with wild-type littermates (Table II in the online-only Data Supplement and Figure 1E). The expression levels of ANP and SERCA2a were gradually increased and decreased in mActin-Tg mice, respectively (Figure III in the online-only Data Supplement). There was no significant difference in blood pressure, but heart rate was increased in mActin-Tg mice (Table II in the online-only Data Supplement), suggesting that the sympathetic nervous system is activated. Surface ECG monitoring showed low amplitude of the R wave in mActin-Tg mice (Table II in the online-only Data Supplement), which is often observed in human DCM patients. Many mActin-Tg mice died by 35 weeks of age (Figure 1F). Although telemetric ECG recording did not show life-threatening arrhythmia in mActin-Tg mice (data not shown), spontaneous Ca2+ sparks and Ca2+ waves were significantly increased in the cardiomyocytes of mActin-Tg mice (Table III in the online-only Data Supplement), suggesting that not only cardiac pump failure but also arrhythmia could be the cause of death. These phenotypes of mActin-Tg mice were quite similar to those of human DCM.
Figure 1.
Mutated cardiac α-actin R312H transgenic mice. A, Gross morphology (top) and sections (bottom) of wild-type littermates (WT) or mActin-Tg (Tg) hearts at 2, 5, and 10 months (M) of age. Scale bar=1 mm. B, Masson trichrome staining. Scale bar=100 μm. C, Relative levels of collagen types I and III in hearts were normalized to GAPDH expression. *P<0.05 vs WT mice. n=4 in each group. D, Electron microscopic analyses. Cytoplasmic vacuolization (arrow) and lysis of myofibrils (arrowhead) were detected in the hearts of Tg mice. Scale bar=10 μm. E, Echocardiographic analysis. Scale bar=1 mm. LVDd indicates left ventricular end-diastolic dimension; FS, fractional shortening. *P<0.05. F, Kaplan-Meier survival curve. *P<0.05 vs WT mice. WT, n=32; Tg, n=37.
Apoptotic Cardiomyocytes Are Increased in mActin-Tg Hearts
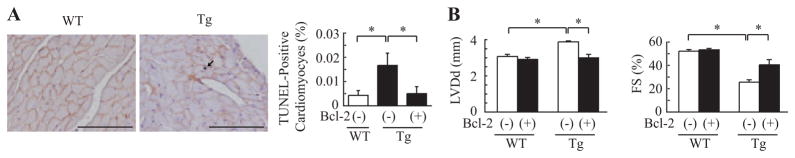
It has been reported that apoptosis of cardiomyocytes is observed in hearts of human DCM10 and that cardiomyocyte death might cause cardiac dysfunction.20 We thus examined apoptosis of cardiomyocytes by TUNEL labeling in left ventricular sections of wild-type littermates and mActin-Tg mice at 5 months of age. The number of TUNEL/dystrophin double-positive cardiomyocytes was significantly larger in mActin-Tg mice compared with wild-type littermates (Figure 2A). To examine whether the increase in apoptotic cardiomyocytes causes cardiac dysfunction in mActin-Tg mice, we generated double-transgenic mice by crossing mActin-Tg mice and the transgenic mice, which overexpress the antiapoptotic protein Bcl-2 in cardiomyocytes [mActin(+)/Bcl-2(+)-DTg].14 The number of apoptotic cardiomyocytes in mActin(+)/Bcl-2(+)-DTg mice was significantly less compared with mActin-Tg mice (Figure 2A). Echocardiography revealed that the left ventricular dimension was smaller and fractional shortening was better in mActin(+)/Bcl-2(+)-DTg mice than in mActin-Tg mice at 5 months of age (Figure 2B), suggesting that the increase in apoptotic cardiomyocytes causes cardiac dysfunction in the DCM mouse model.
Figure 2.
Increase in Bcl-2 preserves cardiac function in mActin-Tg mice. A, Double immunostaining for TUNEL (black) and dystrophin (red) of the heart (left). The graph indicates quantitative analyses of TUNEL-positive cardiomyocytes. Scale bar=100 μm. n=4 in each group. *P<0.05. B, Echocardiographic analyses at 5 months of age. *P<0.05. WT/Bcl-2(−), n=5; WT/Bcl-2(+), n=10; Tg/Bcl-2(−), n=10; Tg/Bcl-2(+), n=5. WT indicates wild-type littermates; Tg, mActin-Tg mice; LVDd, left ventricular end-diastolic dimension; and FS, fractional shortening.
p53 Is Involved in Cardiomyocyte Apoptosis in mActin-Tg Mice
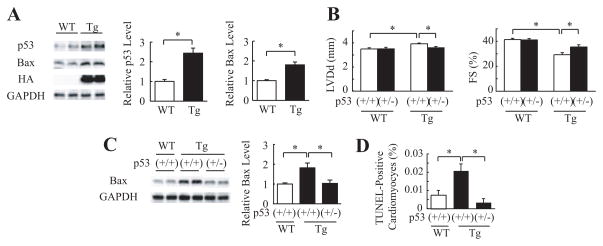
To clarify the mechanisms of how the cardiac α-actin R312H mutant induces apoptosis of cardiomyocytes, we examined expression levels of apoptosis-related proteins by Western blot analyses. The protein levels of p53 and Bax were higher in mActin-Tg mice compared with wild-type littermates (Figure 3A). Several key proapoptotic genes have been reported to be positively regulated by p53,21 and increased expression of p53 induces left ventricular dilatation and dysfunction in several types of mice.22,23 To determine the role of p53 in gene mutation–induced DCM, we crossed mActin-Tg mice and heterozygous p53-deficient mice [p53(+/−)]. Because many of homozygous p53-deficient mice [p53(−/−)] died of tumors before 5 months of age,17 we used heterozygous p53-deficient mice [p53(+/−)] for this study. Echocardiography revealed that left ventricular dimension was smaller and fractional shortening was better in mActin-Tg/p53(+/−) mice than in mActin-Tg/p53(+/+) mice at 5 months of age (Figure 3B). Loss of a single p53 allele attenuated the increase of Bax (Figure 3C) and reduced the number of apoptotic cardiomyocytes in mActin-Tg mice (Figure 3D). These results suggest that p53-induced cardiomyocyte apoptosis induces dilatation and dysfunction of the left ventricle in the DCM mouse model.
Figure 3.
Inhibition of p53 preserves cardiac function in mActin-Tg mice. A, Western blot analyses in the hearts of wild-type littermates (WT) or mActin-Tg (Tg) mice at 5 months of age. The graph indicates relative protein levels of p53 (n=8 in each group) or Bax (n=10 in each group). *P<0.05. B, Echocardiographic analyses at 5 months of age. WT/p53(+/+), n=12; WT/p53(+/−), n=10; Tg/p53(+/+), n=19; Tg/p53(+/−), n=14. *P<0.05. C, Western blot analyses in the hearts. The graph indicates relative protein levels of Bax. n=6 in each group. *P<0.05. D, Quantitative analyses of TUNEL-positive cardiomyocytes. n=5 in each group. *P<0.05.
Myofilament Calcium Sensitivity Is Decreased and Calcium-Dependent Enzymes Are Activated in mActin-Tg Mice
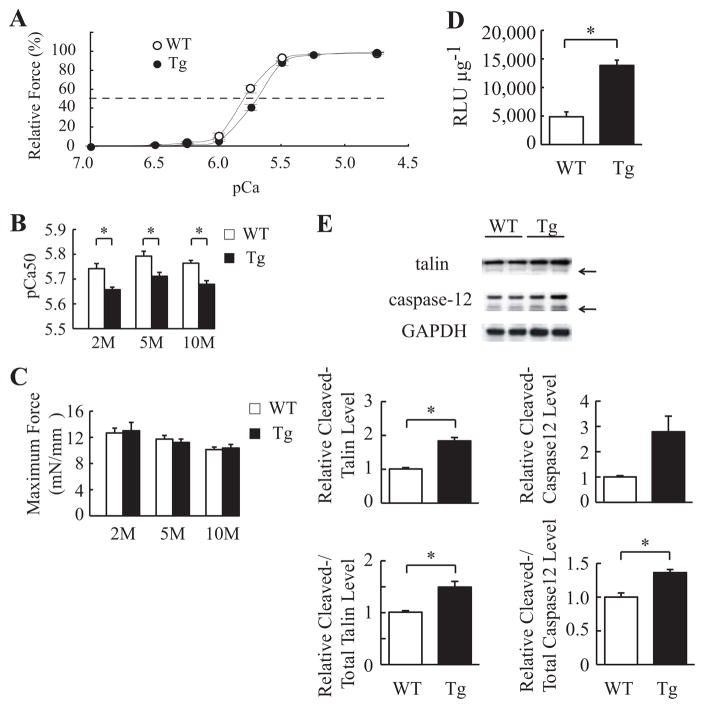
Many gene mutations associated with DCM have been reported to induce the decrease of myofilament Ca2+ sensitivity.7 We examined myofilament Ca2+ sensitivity in mActin-Tg mice. The force-pCa relationship was shifted rightward in mActin-Tg mice compared with wild-type littermates (Figure 4A). The pCa value at half-maximal force generation (pCa50, an index of Ca2+ sensitivity) was significantly lower in mActin-Tg mice (Figure 4B), suggesting that skinned cardiac muscle fibers prepared from mActin-Tg mice show a decrease in Ca2+ sensitivity of force generation. The degree was the same between 2 and 10 months of age (Figure 4B), suggesting that the reduction in Ca2+ sensitivity is not a result of cardiac dysfunction. Despite the reduced Ca2+ sensitivity, there was no significant difference in maximum force-generating capabilities between wild-type littermates and mActin-Tg mice (Figure 4C). The decrease in myofilament Ca2+ sensitivity is expected to influence intracellular Ca2+ handling in cardiomyocytes of mActin-Tg mice. To clarify whether intracellular Ca2+ levels in cardiomyocytes are changed in mActin-Tg mice, we examined the activity of Ca2+-dependent enzymes such as calcineurin and calpain. We generated double-transgenic mice by crossing mActin-Tg mice and the transgenic mice carrying a luciferase reporter driven by a cluster of NFAT binding sites, which is activated by calcineurin-dependent NFAT proteins.15 The NFAT–luciferase reporter activity was higher in mActin-Tg mice than in wild-type littermates at 5 months of age (Table IV in the online-only Data Supplement and Figure 4D). Furthermore, the ratio of the calpain-induced cleaved forms of talin and caspase-12 to total proteins was significantly increased in mActin-Tg mice compared with wild-type littermates (Figure 4E). We next examined Ca2+ transients in cardiomyocytes using fluo-3AM (Figure IVA in the online-only Data Supplement). Although the time to peak amplitude of Ca2+ was significantly slower in mActin-Tg mice than in wild-type littermates (Figure IVB in the online-only Data Supplement), there was no significant difference in peak amplitude between wild-type littermates and mActin-Tg mice at 2 and 10 months of age (Figure IVC in the online-only Data Supplement). The expression levels of SERCA2a, but not Na+/Ca2+ exchanger, were decreased in mActin-Tg mice (Figure III in the online-only Data Supplement).
Figure 4.
Myofilament Ca2+ sensitivity is decreased and Ca2+-dependent enzymes are activated in mActin-Tg mice (Tg). A, Force-pCa relationship in skinned cardiac muscle fiber at 5 months of age. The broken line indicates pCa50. Wild-type (WT; n=11) and Tg (n=10) fibers were prepared from 3 isolated hearts. B, Ca2+ sensitivity (pCa50) of force-pCa relationships in skinned cardiac muscle fibers at 2, 5, and 10 months (M) of age. *P<0.05. C, Maximum force-generating capabilities. Fibers (n=9 to 11) were prepared from 3 isolated hearts of each group. D, The NFAT–luciferase reporter activity (RLU μg−1) in the hearts at 5 months of age. n=4 in each group. *P<0.05. E, Western blot analyses in the hearts. Arrows indicate the calpain cleaved forms of talin and caspase-12. The graph indicates relative protein levels of cleaved talin or caspase-12 and ratio of cleaved forms to total proteins. n=4 in each group. *P<0.05.
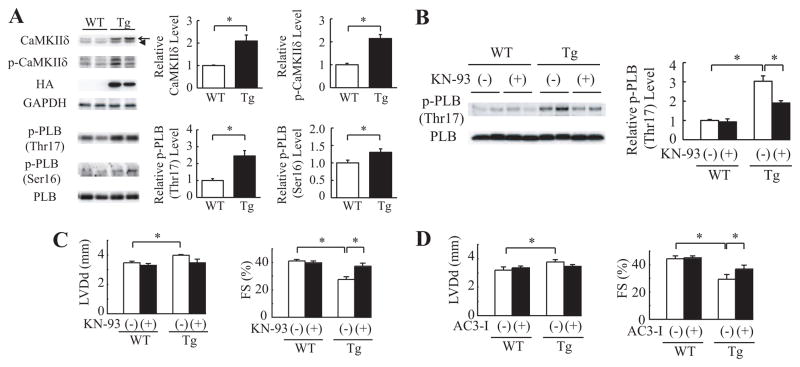
CaMKIIδ Is Activated in mActin-Tg Mice
It has been reported that among Ca2+-dependent proteins, expression of CaMKIIδ is increased in human DCM hearts24 and that overexpression of CaMKIIδ induces heart failure in mice.25,26 We thus examined the expression and phosphorylation of CaMKIIδ and phosphorylation of its target protein, phospholamban (Thr17). The protein levels of total (both CaMKIIδB and CaMKIIδC) and phosphorylated CaMKIIδ and of phosphorylated phospholamban (Thr17) were increased in mActin-Tg mice compared with wild-type litter-mates (Figure 5A and Figure VA in the online-only Data Supplement), suggesting that CaMKIIδ is activated in mActin-Tg mice. The protein levels of phosphorylated phospholamban (Ser16), which is activated by protein kinase A, were also increased in mActin-Tg mice (Figure 5A).
Figure 5.
CaMKIIδ is activated in mActin-Tg mice. A, Western blot analyses in the hearts of wild-type littermates (WT) or mActin-Tg (Tg) mice at 5 months of age. The graph indicates relative protein levels of total and phosphorylated CaMKIIδ (p- CaMKIIδ) or phosphorylat-ed phospholamban (p-PLB). Arrow and arrowhead indicate CaMKIIδB and CaMKIIδC, respectively. n=6 in each group. *P<0.05. B, Western blot analyses in the hearts at 5 months of age. The graph indicates relative protein levels of p-PLB (Thr17). n=4 in each group. *P<0.05. C and D, Echocardiographic analyses at 5 months of age. WT/KN-93(−), n=11; WT/KN-93(+), n=7; Tg/KN-93(−), n=8; Tg/KN-93(+), n=6; WT/AC3-I(−), n=8; WT/AC3-I(+), n=18; Tg/AC3-I(−), n=10; Tg/AC3-I(+), n=14. KN indicates KN-93; LVDd, left ventricular end-diastolic dimension; and FS, fractional shortening. *P<0.05.
Because it has been reported that the sympathetic nervous system is activated in failing hearts and that β-adrenergic receptor signal activates CaMKIIδ,27 we treated mActin-Tg mice with the β-blocker bisoprolol to clarify the relationship between β-adrenergic receptor signal and activation of CaMKIIδ. The treatment with bisoprolol ameliorated cardiac dysfunction of mActin-Tg mice, and there was no significant difference in cardiac function between wild-type littermates and mActin Tg mice with bisoprolol treatment (Figure VB in the online-only Data Supplement). Furthermore, the increase in CaMKIIδ levels in mActin-Tg mice was prevented by bisoprolol treatment (Figure VC in the online-only Data Supplement), suggesting that the activation of CaMKIIδ in mActin-Tg mice might be due to activation of β-adrenergic receptor signaling.
To test whether activation of CaMKIIδ induces cardiac dysfunction, we first treated mActin-Tg mice with KN-93, a CaMKII inhibitor. Levels of both phosphorylated phospho-lamban (Thr17) and phospholamban (Ser16) were decreased by KN-93 treatment in mActin-Tg mice (Figure 5B and Figure VD in the online-only Data Supplement). Echocardiography revealed that KN-93 treatment prevented left ventricular dilatation and preserved cardiac function in mActin-Tg mice (Figure 5C). On the other hand, KN-92, an inactive derivative of KN-93, did not show any effects (Figure VE in the online-only Data Supplement). To confirm the role of CaMKIIδ in mActin-Tg mice, we crossed mActin-Tg mice and AC3-I mice, which expressed the CaMKII-inhibitory peptide AC3-I in the heart [mActin(+)/AC3-I(+)-DTg].16 Echocardiography revealed that fractional shortening was better in mActin(+)/AC3-I(−)-DTg mice than in mActin(+)/AC3-I(−)-Tg mice (Figure 5D), suggesting that the activation of CaMKIIδ in the DCM mouse model induces left ventricular dilatation and contractile dysfunction.
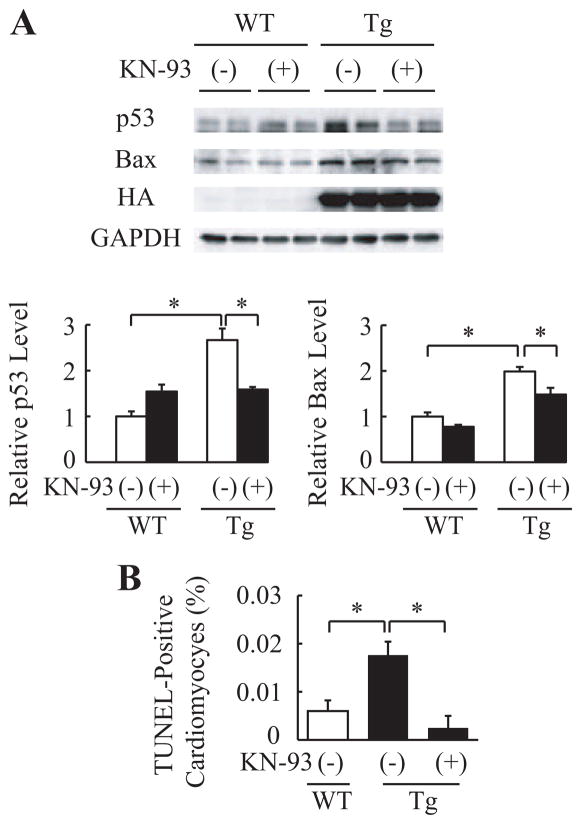
We next examined the relation between CaMKIIδ activation and p53. The increase in p53 was attenuated by treatment with KN-93 or overexpression of AC3-I (Figure 6A and Figure VIA in the online-only Data Supplement). Furthermore, KN-93 treatment inhibited the increase in Bax expression and TUNEL-positive cardiomyocytes (Figure 6A and 6B). It has been reported that CaMKIIδC, but not CaMKIIδB, induces cardiomyocyte death.27–29 To clarify the mechanism of how CaMKIIδ increases protein levels of p53 and which CaMKIIδ, δB or δC, plays an important role in apoptosis of cardiomyocytes, we transfected constitutively active forms of CaMKIIδ (caCaMKIIδ) into cardiomyocytes. Only caCaMKIIδC, not caCaMKIIδB, increased protein levels of p53 (Figure VIB in the online-only Data Supplement). Furthermore, p53 protein levels in caCaMKIIδC-transfected cardiomyocytes did not increase with MG132 treatment compared with MOCK-treated cardiomyocytes (Figure VIC in the online-only Data Supplement). These results suggest that activation of CaMKIIδC increases apoptotic cardiomyocytes at least in part via stabilization of p53 in the DCM mouse model.
Figure 6.
CaMKIIδ regulates expression of p53 in cardiomyocytes. A, Western blot analyses in the hearts of wild-type littermates (WT) or mActin-Tg (Tg) mice. The graph indicates relative protein levels of p53 or Bax. n=4 in each group. *P<0.05. B, Quantitative analyses of TUNEL-positive cardiomyocytes. n=5 in each group. *P<0.05.
Discussion
In the present study, we established a novel mouse model of DCM to clarify the mechanisms of how mutant genes lead to DCM (Table II in the online-only Data Supplement and Figure 1). The mice expressing cardiac α-actin R312H mutant in the heart, which has been reported to cause DCM in humans,5 showed dilatation and dysfunction of left ventricle with an increase in ANP messenger RNA levels, which is consistent with human heart failure (Figure 1A and 1E and Table II and Figure III in the online-only Data Supplement). Higher heart rate and hyperphosphorylated phospholamban (Ser16) (Table II in the online-only Data Supplement and Figure 5A) suggest the activation of the sympathetic nervous system to compensate for reduced cardiac systolic function, resulting in an increase in spontaneous Ca2+ sparks and Ca2+ waves (Table III in the online-only Data Supplement). Myofilament Ca2+ sensitivity was decreased in mActin-Tg mice even at 2 months of age (Figure 4B), when cardiac phenotypes such as left ventricular dilatation and cardiac fibrosis were not recognized (Table II in the online-only Data Supplement and Figure 1). These results suggest that the decrease in myofilament Ca2+ sensitivity is a primary cause of, not a secondary result from, cardiac dysfunction. Because these phenotypes were quite similar to those of human DCM, mActin-Tg mice are useful for examining the underlying mechanisms of how gene mutations lead to DCM.
There was no significant difference in the peak amplitude of Ca2+ transients between wild-type littermates and mActin-Tg mice (Figure IVC in the online-only Data Supplement), suggesting that global Ca2+ levels underlying each contractile cycle do not differ between the 2 groups. It has been reported that the peak amplitude of Ca2+ transients, which is associated with decreased Ca2+ sensitivity and systolic dysfunction, is higher in another mouse model of DCM, 7 suggesting that Ca2+ transients are augmented to compensate for decreased myofilament Ca2+ sensitivity in this model. In mActin-Tg mice, despite the preserved Ca2+ transients (Figure IVC in the online-only Data Supplement), global cardiac function was gradually impaired (Table II in the online-only Data Supplement). Local Ca2+ concentration has been reported to be important for the activation of Ca2+-dependent enzymes such as calcineurin, calpain, and CaMKII in cardiomyocytes.30 The activation of these molecules in mActin-Tg mice (Figures 4D, 4E, and 5A) might be attributed to an increase in local Ca2+ levels. It still remains to be determined whether local Ca2+ levels are really increased and, if so, how the decrease in Ca2+ sensitivity increases local Ca2+ levels.
Recent reports have shown that CaMKIIδ plays a crucial role in cardiovascular diseases.16,31 The transgenic mice that overexpressed CaMKIIδ showed heart failure with systolic dysfunction and left ventricular dilatation.25,26 In this study, CaMKIIδ was activated in the hearts of mActin-Tg mice (Figure 5A), and inhibition of CaMKIIδ by KN-93 or AC3-I ameliorated cardiac dysfunction in mActin-Tg mice (Figure 5C and 5D), suggesting that CaMKIIδ also plays an important role in gene mutation–induced cardiac dysfunction.
It has been reported that apoptosis of cardiomyocytes is observed in hearts of human DCM10 and that cardiomyocyte death could cause cardiac dysfunction.20 However, it remains unclear whether apoptosis of cardiomyocytes causes cardiac dysfunction and how cardiomyocyte apoptosis is induced in hearts of DCM. In this study, there were more apoptotic cardiomyocytes in mActin-Tg mice (Figure 2A), and cardiac function was improved by protecting cardiomyocytes from apoptosis through overexpression of Bcl-2 (Figure 2B). These results suggest that cardiomyocyte apoptosis plays a crucial role in the development of DCM. Several key proapoptotic and antiapoptotic genes have been reported to be positively or negatively regulated by p53, and increased expression of p53 induces left ventricular dilatation and dysfunction in mice deficient in MDM4, an E3 ligase for p53.23 Furthermore, we have recently demonstrated that p53 is critically involved in pressure overload–induced cardiac dysfunction.22 The protein levels of p53 were increased in mActin-Tg mice (Figure 3A), and loss of a single p53 allele reduced the number of apoptotic cardiomyocytes (Figure 3D) and improved cardiac function (Figure 3B). These results suggest that p53 is critically involved in induction of cardiomyocyte apoptosis, resulting in left ventricular dysfunction in the mouse model of DCM.
The present study indicates that p53 might be a therapeutic target for DCM. In this study, CaMKIIδ was activated in the hearts of mActin-Tg mice (Figure 5A), and the inhibition of CaMKIIδ attenuated the increase in p53 protein levels (Figure 6A and Figure VIA in the online-only Data Supplement), suggesting that CaMKIIδ regulates protein levels of p53 in the DCM model mice. Although it remains to be determined how CaMKIIδ regulates protein levels of p53, inhibition of CaMKIIδ may become a new therapeutic strategy for DCM patients by reducing p53 protein levels in the heart.
Limitations
This study has a couple limitations. First, we cannot completely rule out the nonspecific effects of overexpression of cardiac α-actin gene with tag because of a lack of transgenic mice that overexpress wild-type cardiac α-actin gene. However, we think the cardiac dysfunction observed in mActin-Tg was due to cardiac expressions of the cardiac α-actin R312H mutant in the heart, not to high-level expressions of the cardiac α-actin protein with tag because of the following reasons: We obtained 3 independent founders of the transgenic mice, and the reduction in cardiac function was well correlated with protein levels of the cardiac α-actin R312H mutant (Figure I in the online-only Data Supplement). Another transgenic mouse that expressed cardiac α-actin A331P mutant with an HA tag in the heart did not show cardiac dysfunction (Table I in the online-only Data Supplement), although the protein levels of the mutant in the A331P mutant transgenic mice were almost same as those of the R312H mutant in line 307, which had the highest expression (Figure II in the online-only Data Supplement). Second, we found that CaMKIIδC increases p53 protein levels mainly by its stabilization, but the underlying mechanisms remain to be determined.
Supplementary Material
CLINICAL PERSPECTIVE.
Heart failure is an important cause of morbidity and mortality in many industrial countries, and dilated cardiomyopathy (DCM) is one of its major causes. Molecular genetic studies over the last 2 decades have revealed many mutations of various genes in DCM patients, but the precise mechanisms of how such mutations lead to DCM remain largely unknown partly because of a lack of good animal models of DCM. Here, we established the mouse model of DCM by expressing a mutated cardiac α-actin gene, which has been reported in patients with DCM, in the heart. The transgenic mice showed gradual dilatation and dysfunction of the left ventricle, resulting in death by heart failure. These phenotypes of the transgenic mice were quite similar to those of human DCM. The number of apoptotic cardiomyocytes and protein levels of p53 were increased in the hearts of the DCM mice. Overexpression of Bcl-2, an antiapoptotic factor, or downregulation of p53 decreased the number of apoptotic cardiomyocytes and improved cardiac function. The DCM mice showed activation of CaMKIIδ. The inhibition of CaMKIIδ prevented the increase in p53 and apoptotic cardiomyocytes and ameliorated cardiac function. These results suggest that CaMKIIδ plays a critical role in the development of heart failure in part by accumulation of p53 and induction of cardiomyocyte apoptosis in the DCM mouse model. The inhibition of CaMKIIδ may become a new therapeutic strategy for DCM patients.
Acknowledgments
We thank J. Robbins (Children’s Hospital Research Foundation, Cincinnati, Ohio) for a fragment of α-myosin heavy chain gene promoter, J.D. Molkentin (Children’s Hospital Research Foundation, Cincinnati, Ohio) for NFAT–luciferase reporter transgenic mice, M.D. Schneider (Imperial College, London, UK) for Bcl-2 transgenic mice, and E.N. Olson (UT Southwestern Medical Center, Dallas, Tex) for constitutively active forms of CaMKIIδ. We thank E. Fujita, R. Kobayashi, Y. Ishiyama, M. Ikeda, I. Sakamoto, A. Furuyama, and Y. Ohtsuki for technical support, as well as M. Iiyama, K. Matsumoto, Y. Ishikawa, and Y. Yasukawa for animal care.
Sources of Funding
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (to Dr Komuro) and a Grant-in-Aid for Scientific Research (C) (20590857 to Dr Oka) from the Ministry of Education, Culture, Sports, Science and Technology; the Japan Heart Foundation/Novartis Grant for Research Award on Molecular and Cellular Cardiology; Japan Foundation for Applied Enzymology; Suzuken Memorial Foundation; and Mitsubishi Pharma Research Foundation (to Dr Toko). This work was supported by National Institutes of Health grants R01 HL079031, R01 HL070250, and R01 HL096652 and by the Fondation Leducq Award to the Alliance for Calmodulin Kinase Signaling in Heart Disease (to Dr Anderson).
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.109.935296/DC1.
Disclosures
Dr Anderson is named on patents claiming to treat heart failure by CaMKII inhibition and is a cofounder of Allosteros. The other authors report no conflicts.
References
- 1.Michels VV, Moll PP, Miller FA, Tajik AJ, Chu JS, Driscoll DJ, Burnett JC, Rodeheffer RJ, Chesebro JH, Tazelaar HD. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med. 1992;326:77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet. 2005;6:185–216. doi: 10.1146/annurev.genom.6.080604.162132. [DOI] [PubMed] [Google Scholar]
- 4.Knoll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–955. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- 5.Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT. Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science. 1998;280:750–752. doi: 10.1126/science.280.5364.750. [DOI] [PubMed] [Google Scholar]
- 6.Towbin JA, Bowles NE. Genetic abnormalities responsible for dilated cardiomyopathy. Curr Cardiol Rep. 2000;2:475–480. doi: 10.1007/s11886-000-0063-9. [DOI] [PubMed] [Google Scholar]
- 7.Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, Lu QW, Wang YY, Zhan DY, Mochizuki M, Kita S, Miwa Y, Takahashi-Yanaga F, Iwamoto T, Ohtsuki I, Sasaguri T. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185–194. doi: 10.1161/CIRCRESAHA.106.146670. [DOI] [PubMed] [Google Scholar]
- 8.Kawada T, Masui F, Tezuka A, Ebisawa T, Kumagai H, Nakazawa M, Toyo-Oka T. A novel scheme of dystrophin disruption for the progression of advanced heart failure. Biochim Biophys Acta. 2005;1751:73–81. doi: 10.1016/j.bbapap.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Kyoi S, Otani H, Matsuhisa S, Akita Y, Tatsumi K, Enoki C, Fujiwara H, Imamura H, Kamihata H, Iwasaka T. Opposing effect of p38 MAP kinase and JNK inhibitors on the development of heart failure in the cardiomyopathic hamster. Cardiovasc Res. 2006;69:888–898. doi: 10.1016/j.cardiores.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 11.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper BJ, Winand NJ, Stedman H, Valentine BA, Hoffman EP, Kunkel LM, Scott MO, Fischbeck KH, Kornegay JN, Avery RJ, Williams JR, Schmickel RD, Sylvester JE. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988;334:154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- 13.Nigro V, Okazaki Y, Belsito A, Piluso G, Matsuda Y, Politano L, Nigro G, Ventura C, Abbondanza C, Molinari AM, Acampora D, Nishimura M, Hayashizaki Y, Puca GA. Identification of the Syrian hamster cardiomyopathy gene. Hum Mol Genet. 1997;6:601–607. doi: 10.1093/hmg/6.4.601. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Nakae S, Terry RD, Mokhtari GK, Gunawan F, Balsam LB, Kaneda H, Kofidis T, Tsao PS, Robbins RC. Cardiomyocyte-specific Bcl-2 overexpression attenuates ischemia-reperfusion injury, immune response during acute rejection, and graft coronary artery disease. Blood. 2004;104:3789–3796. doi: 10.1182/blood-2004-02-0666. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 17.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 18.VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619–626. doi: 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- 19.Olson TM, Doan TP, Kishimoto NY, Whitby FG, Ackerman MJ, Fananapazir L. Inherited and de novo mutations in the cardiac actin gene cause hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2000;32:1687–1694. doi: 10.1006/jmcc.2000.1204. [DOI] [PubMed] [Google Scholar]
- 20.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan KM, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol. 2001;13:332–337. doi: 10.1016/s0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 22.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 23.Xiong S, Van Pelt CS, Elizondo-Fraire AC, Fernandez-Garcia B, Lozano G. Loss of Mdm4 results in p53-dependent dilated cardiomyopathy. Circulation. 2007;115:2925–2930. doi: 10.1161/CIRCULATIONAHA.107.689901. [DOI] [PubMed] [Google Scholar]
- 24.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T, Johnson EN, Gu Y, Morissette MR, Sah VP, Gigena MS, Belke DD, Dillmann WH, Rogers TB, Schulman H, Ross J, Jr, Brown JH. The cardiac-specific nuclear delta(B) isoform of Ca2+/calmodulin-dependent protein kinase II induces hypertrophy and dilated cardiomyopathy associated with increased protein phosphatase 2A activity. J Biol Chem. 2002;277:1261–1267. doi: 10.1074/jbc.M108525200. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 27.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng W, Zhang Y, Zheng M, Cheng H, Zhu W, Cao CM, Xiao RP. Cardioprotection by CaMKII-deltaB is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circ Res. 2010;106:102–110. doi: 10.1161/CIRCRESAHA.109.210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu W, Woo AY, Yang D, Cheng H, Crow MT, Xiao RP. Activation of CaMKIIdeltaC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J Biol Chem. 2007;282:10833–10839. doi: 10.1074/jbc.M611507200. [DOI] [PubMed] [Google Scholar]
- 30.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller Brown J. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.