Abstract
Polyamines are essential for cell proliferation. Their production is dysregulated in many cancers and polyamine depletion leads to tumor regression in mouse models of SCC. The purpose of this study was to determine the maximally tolerated dose of the polyamine transport inhibitor, MQT 1426, when combined with the ornithine decarboxylase inhibitor, DFMO, and to determine whether this therapy results in reduction in tumor polyamine levels. Thirteen cats with oral SCC received both drugs orally and serial tumor biopsies were obtained for polyamine measurement. Cats were monitored for response to therapy and toxicity. A maximum tolerated dose of MQT 1426 when combined with DFMO was determined. Dose limiting toxicity was vestibular in nature, but was fully reversible. Spermidine and total polyamine levels decreased significantly in tissues, 2 cats experienced objective tumor regression, and 6 cats had stable disease. These results suggest that further study of polyamine depletion therapies is warranted.
The polyamines putrescine, spermidine, and spermine are required for cell proliferation and growth. They are produced as a result of myc oncogene signaling and play a role in epigenetic gene regulation through DNA stabilization and RNA processing. Polyamine metabolism is known to be dysregulated in many cancers and neoplastic cells require higher levels of polyamines than normal tissues for growth and replication.1 Polyamines are obtained by cells through de novo synthesis as well as through transport from the extra-cellular fluid.2 Ornithine decarboxylase (ODC) is the main enzyme involved in polyamine production within the cell and is overexpressed in many cancers, including breast and colon cancers and squamous cell carcinoma (SCC). This enzyme has been extensively evaluated as a target for cancer treatment and prevention during the last decade.3
2-difluoromethylornithine (DFMO) is an irreversible inhibitor of ODC that has been studied for its role as a possible cancer therapeutic or chemopreventative agent. Studies testing the effect of DFMO on cancer cell lines and in murine tumor models have shown promising results, but clinical trials in humans using DFMO to treat established tumors have met with limited success.4,5,6 One mechanism of cellular resistance to DFMO is through polyamine uptake from the extracellular fluid through a membrane transport system. 3 Compounds are now being synthesized to inhibit this polyamine transporter and MQT 1426 is an example.7,8 In a study using a murine model of SCC and treatment with a combination of DFMO and MQT 1426, 13 of 18 tumors resolved completely and 2 additional tumors responded partially (>50%).8 These data suggest that effective treatment of SCC will require prevention of both polyamine uptake and de novo synthesis.
Head and neck cancer represents 3-5% of cancers in the United States and is the 6th most commonly diagnosed cancer worldwide. It usually arises in adults secondary to prolonged exposure to tobacco, alcohol, and/or other carcinogens.9 Additionally, the incidence is increasing in the population under 40 years of age and epidemiologic studies suggest this is due to human papillomavirus infections.10 The vast majority of head and neck cancers are SCC and they are both locally invasive and have a high metastatic rate. Treatment consisting of surgery and chemoirradiation is effective as front-line intervention, but those patients with recurrent or treatment refractory tumors have few effective therapeutic options.9
Research is ongoing to identify new therapeutic targets for patients with treatment refractory SCC of the head and neck. Studies in vitro and in vivo using inbred immunodeficient mouse models can often show misleadingly positive results. Consequently, recent attention has been given to the advantages of research using spontaneous models of cancer, particularly in pet animals.11 SCC represents at least 70% of tumors of the feline mouth.12 Similar to the human disease, feline oral SCC is extremely locally aggressive exhibiting extension into bone causing pain, ptylism, and inability to eat and, without treatment, usually leads to death or euthanasia within 4-8 weeks of diagnosis.13 The majority of cats present with tumors too large to be amenable to surgical resection and studies have shown that radiation therapy and chemotherapy are largely ineffective at prolonging life beyond 3-4 months.12,14 Because of the demonstrated similarities in biologic behavior between feline oral SCC and treatment refractory head and neck SCC in humans, the domestic cat may be a more predictive model of response to novel cancer therapies than mouse models.
The primary purpose of this study was to determine the maximum tolerated dose of MQT 1426 when paired with a fixed dose of DFMO and to determine whether this combination therapy results in a reduction in tumor polyamine levels in vivo. A secondary purpose was to assess the potential efficacy of this dual therapy in head and neck SCC using the pet cat as a relevant spontaneous, immune-competent large animal tumor model.
Materials and Methods
Study design
The owners of pet cats presenting to the Veterinary Medical Teaching Hospital (VMTH) at the University of California, Davis with a histologic diagnosis of oral SCC were offered entry into the study, which was approved by the institution's Animal Care and Use Committee. Prior to enrollment, cats were evaluated through physical exam, complete blood count, serum biochemistry, thoracic radiographs and, when indicated, regional lymph node aspirates. Cats were excluded from the study if they had concurrent disease expected to limit life to less than 4 months. Concurrent anti-neoplastic therapy was not allowed, but concurrent use of non-steroidal anti-inflammatory drugs, antibiotics, and opioid analgesics was allowed.
Dose escalation
Cats were initially enrolled in cohorts of 3 based on planned dose escalation of one of the two study drugs (MQT 1426). DFMO and MQT 1426 were formulated into a single fish flavored, water-based liquid for oral administration. The dose of DFMO was fixed at 100 mg/kg/day (divided twice daily) based on efficacy data in murine models and a previous study in cats where this dose was determined to be safe (Lewis J, manuscript submitted, 2010). The dose of MQT 1426 was escalated in 3 cat cohorts starting at 50 mg/kg/day (divided twice daily) and escalated by 25 mg/kg/day increments until dose-limiting toxicity was reached and maximum tolerated dose (MTD) was determined. Once the MTD was determined, all further cats were enrolled at this dose level.
Tissue collection and polyamine measurement
Cats were briefly anesthetized on day 0 and 2-3 4 mm punch biopsies were collected from tumor tissue for baseline polyamine levels. At the end of the 2 month study period or at early study withdrawal, cats were again briefly anesthetized and 2-3 4mm punch biopsies were collected for polyamine level measurement. Tissues were extracted with 0.2 N perchloric acid and polyamines were then derivatized with dansyl chloride and, following extraction with methanol, analyzed using high performance liquid chromatography equipped with fluorescence detection as previously described.15
Response and toxicity evaluation
Cats returned to the VMTH every 2 weeks for a total study period of 2 months to evaluate response to therapy and treatment-related toxicity. Between hospital visits, pet owners were asked to monitor and report, in writing, the activity level, appetite, vomiting, diarrhea, and changes in hearing, behavior and skin appearance. A complete blood count was run at each of the 4 rechecks and a biochemistry screen was run at the one and two month rechecks or at the last visit if the cat was removed from the study due to progressive disease or toxicity. Toxicities were graded according to the published Veterinary Co-operative Oncology Group common terminology criteria for adverse events (VCOG-CTCAE).16 Dose limiting toxicity was defined as any grade 3 or greater toxicity. Pre-treatment tumor volume was estimated with three orthogonal caliper measurements. Response to therapy was determined at each recheck visit by measurement of the longest tumor diameter using published Response Evaluation Criteria in Solid Tumors (RECIST).17 By these criteria, complete response (CR) was defined as disappearance of all tumors confirmed at 4 weeks, partial response (PR) was defined as a 30% decrease in the sum of the longest tumor diameters confirmed at 4 weeks, stable disease (SD) was defined as meeting neither PR nor progressive disease (PD) criteria, and PD was defined as a 20% increase in the sum of the longest tumor diameters.
Statistics
Paired pre- and post-treatment tissue putrescine, spermidine, spermine and total polyamine levels were compared using the Wilcoxan signed rank test. Changes in individual and total polyamine levels were compared between treatment cohorts using the Kruskal-Wallis test. Changes in individual and total polyamine levels were compared between responders (PR and SD) versus non-responders (PD) using the Mann-Whitney U test. Statistical significance was set at a p value of .05.
Results
Patient characteristics
Thirteen cats with head and neck SCC were enrolled. Mean age was 11.7 years. Eight were castrated males and 5 were spayed females. Eleven cats were mixed breed, 1 was a Persian and one was a Burmese. The mean body weight at diagnosis was 3.7 kg. Five cats had SCC involving maxillary bone, 4 involving tongue, 2 involving mandibular bone, and 2 involving buccal soft tissues at the time of diagnosis. Median tumor volume was 5.4 cm3 (range, 0.3 cm3 -17.2 cm3). There was no evidence of lymph node or lung metastasis in any cat at the time of diagnosis. Nine cats received treatment with meloxicam during the study period.
Dose escalation and toxicity (Table 1)
Table 1.
Summary of toxicity type and frequency by treatment cohort.
| MQT 1426 Dose Level | Number of Cats | Toxicity | |||
|---|---|---|---|---|---|
| Vestibular | Alopecia | Vomiting | Anorexia | ||
| 50 mg/kg/day | 3 | 1 grade 2 | 1 grade 2 | ||
| 75 mg/kg/day* | 7 | 1 grade 2 | 1 grade 2 | ||
| 100 mg/kg/day | 3 | 1 grade 2 2 grade 3 |
1 grade 1 1 grade 2 |
1 grade 1 | 2 grade 2 |
Maximum tolerated dose when paired with 100mg/kg/day DFMO and divided BID
No hematologic or clinicopathologic toxicity occurred. Reversible vestibular toxicity was seen in 5 cats including 3 grade 2 and 2 grade 3 toxicities. These cats acutely developed generalized ataxia, most obvious in the pelvic limbs. Symptoms began as early as 2 days and as late as 80 days after starting oral therapy with DFMO and MQT 1426. The first cat to develop vestibular symptoms underwent a complete neurologic exam and Brain Auditory Evoked Response (BAER) testing. BAER results indicated a bilaterally symmetrical central vestibular dysfunction. Polyamine depletion therapy was discontinued in this cat and it was neurologically normal within 48 hours. A post-treatment tumor biopsy was not collected from this cat.
Both grade 3 vestibular toxicities occurred in cats on the 3rd dose level. Based on this finding, the maximum tolerated dose of MQT 1426 was determined to be 75 mg/kg/day when divided twice daily and combined with 100 mg/kg/day DFMO (dose level 2).
Two cats experienced grade 2 anorexia and 1 cat had grade 1 vomiting, all occurring during episodes of vestibular toxicity. Four cats experienced hair loss including 1 grade 1 alopecia and 3 grade 2 alopecia (Table 1).
Polyamine tissue levels
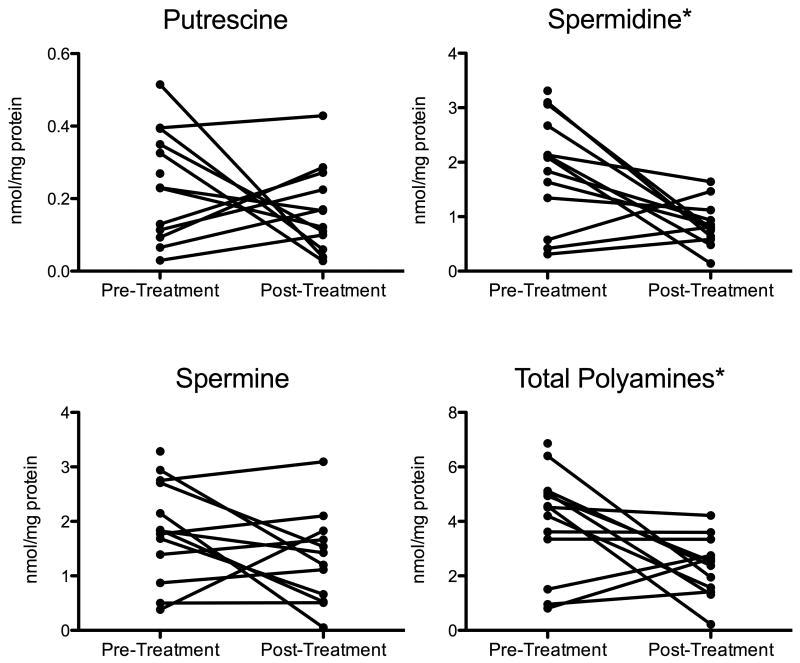
The mean±SD pre-treatment tumor polyamine level for all cats was 3.99±1.92 nmol/mg protein. Mean pre-treatment putrescine, spermidine, and spermine levels were 0.24±0.15, 1.89±1.02, and 1.85±0.92 nmol/mg protein, respectively. Statistically significant decreases in total tissue polyamines (p=.03) and spermidine (p=.01) were found in post-treatment tumor samples, but not in putrescine or spermine levels (Figure 1). There were no significant differences found between changes in individual or total polyamine levels depending on dose cohort or response to treatment.
Figure 1.
Line graph plotting pre-and post-treatment tumor tissue putrescine, spermidine, spermine, and total polyamine levels in each of the 13 cats. Post-treatment measurements were not available for one cat. Statistically significant differences (noted by asterisk) between pre- and post-treatment spermidine and total polyamine tissue levels were found.
Tumor response
Two PRs were documented, 6 cats had SD, and 4 had PD. One cat's response was not evaluable because therapy was discontinued after 2 days due to vestibular toxicity. The median overall progression free interval was 50 days (range, 13-83 days). In the 6 cats with SD, the median progression free interval was 57.5 days (range, 41-83 days). Three cats classified as SD experienced reduction in tumor diameter less than 30%. The cats experiencing PR both had buccal soft tissue tumors with starting tumor volumes of 5.4 and 17.2 cm3. PR lasted 76 and 80 days, but progressive disease occurred in both cats after temporary discontinuation of polyamine depletion therapy due to vestibular toxicity. After resolution of symptoms, both cats were able to restart therapy and the dose was slowly escalated to their original dose. Second less complete and less durable tumor responses (52 and 86 days) were documented in both cats after restarting therapy. These cats remained on therapy until death and survived to 108 and 398 days. Concurrent treatment with meloxicam occurred in 1 of 2 cats with PR, 4 of 6 with SD, and 4 of 5 with PD.
Discussion
This study identified the maximum tolerated dose of the polyamine transport inhibitor MQT 1426 when combined with a fixed dose of DFMO when administered orally twice daily to pet cats with spontaneous oral SCC. Overall, the combination of DFMO and MQT 1426 was well tolerated with no hematologic or clinicopathologic toxicities observed. The dose limiting toxicity in this study was vestibular in nature, a toxicity not documented in human clinical trials with DFMO at doses similar to those used in this study. The vestibular signs were quickly reversible with all cats recovering within 24-48 hours of discontinuing polyamine depletion therapy. Two cats experiencing vestibular toxicity were able to restart the drug combination with a gradual escalation to the previous dose and without return of vestibular symptoms.
Polyamines have been identified as modulators of N-methyl-D-aspartate (NMDA) excitatory amino acid receptors, which mediate vestibular synaptic plasticity.18,19 Vestibular symptoms have not been documented in human clinical trials of polyamine depletion therapies, but severe vestibular dysfunction occurs in transgenic mouse models that have a complete lack of polyamines when treated with DFMO.20 In addition, DFMO prevents vestibular compensation that occurs after surgical labyrinthectomy in several species.18 It is possible that systemic polyamine depletion achieved with the combination therapy in this study was more significant than in previous studies of DFMO alone, resulting in vestibular signs. Additionally, it is possible that cats are more sensitive to vestibular dysfunction caused by polyamine depletion than are other species.
Another possible mechanism for the vestibular toxicity is damage to vestibular hair cells. DFMO has long been known to damage cochlear hair cells leading to reversible hearing loss in all species.21 In human clinical trials using doses of DFMO comparable to those used in this study, hearing loss is common and may be incomplete or fluctuate over time.22 Although owners of cats were asked to monitor for signs of hearing loss, symptoms of deafness were not noted. It is likely, however, that the cats did experience hearing loss that was not detected by the owners due to their sedentary indoor lifestyle. BAER testing performed in the first cat to develop vestibular toxicity did confirm hearing loss and results were consistent with cochlear hair cell dysfunction. Further study is needed to determine the exact mechanism of the vestibular toxicity seen in this trial and to determine if an alternative dosing scheme could minimize the symptoms.
The results of this study also suggest that combination therapy with DFMO and the polyamine transport inhibitor MQT 1426 can result in significant reductions in polyamine levels within tumor tissues. We measured statistically significant reductions in total polyamine levels and spermidine levels in tumor tissue after therapy. These results support the findings of a previous study using these agents in a murine model of SCC where depletion of spermidine, putrescine, and total polyamines occurred with this combination therapy.8 Pre-treatment total and individual polyamine levels were highly variable between cats, suggesting significant heterogeneity in polyamine metabolism between tumors. Likewise, changes in polyamine levels between cats were highly variable and did not correlate statistically with dose or tumor response, though sample size was small. This may reflect the variability expected in a spontaneous tumor model and further study with a larger cohort is necessary to determine whether pre-treatment polyamine levels or ODC expression could predict response to polyamine depletion therapy. Additionally, there is evidence that other factors not controlled for in this study, including increasing age and dietary factors, can affect tissue polyamine levels, at least in mice.23 Despite these limitations, the results of this study suggest that the combination of DFMO and MQT 1426 may reduce tumor polyamine levels and support further study of this therapy.
The results of this study also suggest that dual agent polyamine depletion therapy can result in objective tumor responses in spontaneously occurring SCC. Overall, the anti-tumor effect of the ODC inhibitor, DFMO, on human malignancies has been disappointing. DFMO has been studied in cats with oral SCC as a single agent and significant reductions in tissue polyamines and objective tumor responses did not occur (Lewis J, manuscript in preparation, 2011). It appears that the addition of a polyamine transport inhibitor may allow for more complete depletion of polyamines in tumor cells by also preventing uptake of polyamines from the extracellular space.
Two cats had significant reductions in tumor size and prolonged survivals and additional cats had more minor responses or temporary disease stabilization. It is worth noting that both cats that experienced partial responses also had tumor progression when vestibular toxicity necessitated therapy discontinuation. Additionally, responses occurred again in both cats when therapy was re-started. These second responses were less complete and durable, perhaps due to cautious dosing of the drug combination to avoid vestibular symptoms. These findings suggest a direct correlation between tumor response and polyamine depletion therapy.
The tumor responses to this therapeutic strategy seen in this study were less robust and durable than those seen published studies of murine models of SCC treated with the same therapy. Naturally occurring oral SCC in cats represents a heterogeneous tumor population that is more similar to spontaneous human cancers, making the disease in cats an excellent model for human head and neck SCC. It is expected that responses to experimental cancer therapies will be less significant in spontaneous, heterogeneous tumors than in experimental murine models. Additionally, it should be noted that the etiology of the previously tested murine SCC is known to be driven by an ODC transgene.8 The etiology of spontaneous feline (and human) oral SCC may be less dependent on overexpression of ODC or other mechanisms of polyamine dysregulation, which may also explain the less robust responses to polyamine depletion therapy.
The drug dosing scheme used in the present study was different from that used in previously published murine studies. This may have contributed to the less significant reductions in tissue polyamine measurements and tumor responses seen in the feline study. Previous studies mixed DFMO into the drinking water of subjects and the polyamine transport inhibitor was administered twice daily intraperitoneally. The authors of the present study chose a pulse-dosing scheme (orally twice daily) for both drugs that would be most likely to be adhered to by pet owners. Pulse dosing of these drugs may have resulted in depletion of tissue polyamines for only short periods of time and reduced the overall efficacy of the treatment protocol, but further study is necessary. Additionally, the time between oral dosing and post-treatment tissue biopsies was not standardized in this study, which may have affected the results of polyamine measurements in tissues.
Concurrent treatment with medications such as non-steroidal anti-inflammatories (NSAIDs), opioid pain medications, and antibiotics were allowed in this study to help maintain quality of life in study subjects. Although objective tumor regression of feline oral SCC due to NSAIDs has not been reported in the literature, it should be noted that NSAIDs could possibly reduce intratumoral inflammation enough to reduce tumor measurements. In addition, NSAIDs such as aspirin and indomethacin have been shown to decrease polyamine levels in colon cancer cell lines, but the effect of NSAIDs on polyamines in naturally-occurring SCC tumors is unknown.24,25 Because the majority of cats in this study received meloxicam during the study period, it is impossible to completely eliminate the possibility that this drug may have confounded study results. Many cats received meloxicam therapy prior to study enrollment, which should minimize the confounding effect.
Further investigation into the role of DFMO and polyamine transport inhibitors as therapy for the various forms of feline and human SCC is indicated. Additionally, there may be a role for polyamine depletion therapy in other tumors such as bladder, breast or prostate cancer.1 Newer, more potent polyamine transport inhibitors and drugs with other mechanisms of polyamine depletion are becoming available and may serve as additional agents for future investigations.7
In conclusion, the combination of DFMO and MQT 1426 was safe and effective in reducing total tissue polyamine levels. Additionally, objective tumor responses were observed. Further study of SCC and other tumor types is necessary to determine an optimal dosing scheme to minimize vestibular toxicity and maximize tumor responses.
Acknowledgments
We thank Dr. Michael Kent for his assistance in statistical analysis. This study was supported by grants from the Center for Comparative Animal Health, School of Veterinary Medicine, University of California, Davis, the National Institutes of Health grant #CA094107; and the Toni Wiebe Memorial Fund.
Footnotes
This research was presented, in part, at the Veterinary Cancer Society Annual Meeting, 2008.
Disclosure of possible conflict of interest: At the time this research was done, Mark Burns was employed by MediQuest Therapeutics, Inc. and MQT 1426 was originally produced during his association with this company. He is now owner of Aminex Therapeutics and holds the patent for MQT 1426 and its analogs.
Contributor Information
Katherine A. Skorupski, Department of Surgical and Radiological Sciences, University of California, Davis, CA.
Thomas G. O'Brien, Lankenau Institute for Medical Research, Wynnewood, PA
Teri Guerrero, Department of Surgical and Radiological Sciences, University of California, Davis, CA.
Carlos O. Rodriguez, Jr., Department of Surgical and Radiological Sciences, University of California, Davis, CA.
Mark R. Burns, MediQuest Therapeutics, Inc., Bothell, WA
References
- 1.Casero RA, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6:373–90. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 2.Thomas T, Thomas TJ. Polyamine metabolism and cancer. J Cell Mol Med. 2003;7:113–26. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shantz LM, Levin VA. Regulation of ornithine decarboxylase during oncogenic transformation: mechanisms and therapeutic potential. Amino Acids. 2007;33:213–23. doi: 10.1007/s00726-007-0531-2. [DOI] [PubMed] [Google Scholar]
- 4.O'Shaughnessy JA, Demers LM, Jones SE, Arseneau J, Khandelwal P, George T, Gersh R, Mauger D, Manni A. Alpha-difluoromethylornithine as a treatment for metastatic breast cancer patients. Clin Cancer Res. 1999;5:3438–44. [PubMed] [Google Scholar]
- 5.Mitchell MF, Tortolero-Luna G, Lee JJ, Hittelman WN, Lotan R, Wharton JT, Hong WK, Nishioka K. Phase I dose de-escalation trial of alpha-difluoromethylornithine in patients with grade 3 cervical intraepithelial neoplasia. Clin Cancer Res. 1998;4:303–10. [PubMed] [Google Scholar]
- 6.Levin VA, Uhm JH, Jaeckle KA, Choucair A, Flynn PJ, Yung WKA, Prados MD, Bruner JM, Chang SM, Kyritsis AP, Gleason MJ, Hess KR. Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosourea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme. Clin Cancer Res. 2000;6:3878–84. [PubMed] [Google Scholar]
- 7.Burns MR, Graminski GF, Weeks RS, Chen Y, O'Brien TG. Lipophilic lysine-spermine conjugates are potent polyamine transport inhibitors for use in combination with a polyamine biosynthesis inhibitor. J Med Chem. 2009;52:1983–93. doi: 10.1021/jm801580w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Weeks RS, Burns MR, Boorman DW, Klein-Szanto A, O'Brien TG. Combination therapy with 2-difluoromethylornithine and a polyamine transport inhibitor against murine squamous cell carcinoma. Int J Cancer. 2006;118:2344–49. doi: 10.1002/ijc.21621. [DOI] [PubMed] [Google Scholar]
- 9.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and –unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 11.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–56. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 12.Liptak JM, Withrow SJ. Cancer of the gastrointestinal tract. In: Withrow SJ, Vail DM, editors. Withrow & MacEwen's Small animal clinical oncology. Fourth. St. Louis: Saunders Elsevier; 2007. pp. 455–510. [Google Scholar]
- 13.Hayes AM, Adams VJ, Scase TJ, Murphy S. Survival of 54 cats with oral squamous cell carcinoma in United Kingdom general practice. J Small Anim Pract. 2007;48:394–9. doi: 10.1111/j.1748-5827.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 14.Fidel JL, Sellon RK, Houston RK, Wheeler BA. A nine-day accelerated radiation protocol for feline squamous-cell carcinoma. Vet Radiol Ultrasound. 2007;48:482–5. doi: 10.1111/j.1740-8261.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 15.Koza RA, Megosh LC, Palmieri M, O'Brien TG. Constitutively elevated levels of ornithine and polyamines in mouse epidermal papillomas. Carcinogenesis. 1991;12:1619–25. doi: 10.1093/carcin/12.9.1619. [DOI] [PubMed] [Google Scholar]
- 16.Vail DM. Veterinary Co-operative Oncology Group-Common criteria for adverse events (VCOG-CTCAE) following chemotherapy or biologic antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol. 2004;2:194–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Coker NJ, Henley CM. Polyamines increase in the brain stem and cerebellum following labyrinthectomy. Am J Otol. 1997;18:214–22. [PubMed] [Google Scholar]
- 19.Williams K. Interactions of polyamines with ion channels. Biochem J. 1997;325:289–97. doi: 10.1042/bj3250289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Levic S, Gratton MA, Doyle KJ, Yamoah EN, Pegg AE. Spermine synthase deficiency leads to deafness and a profound sensitivity to alpha-difluoromethylornithine. J Biol Chem. 2009;284:930–937. doi: 10.1074/jbc.M807758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MC, Tinling S, Doyle KJ. Difluoromethylornithine-induced reversible hearing loss across a wide frequency range. Laryngoscope. 2004;114:1113–7. doi: 10.1097/00005537-200406000-00029. [DOI] [PubMed] [Google Scholar]
- 22.Pasic TR, Heisey D, Love RR. Alpha-difluoromethylornithine ototoxicity. Chemoprevention clinical trial results. Arch Otolaryngol Head Neck Surg. 1997;123:1281–6. doi: 10.1001/archotol.1997.01900120031004. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 2006;139:81–90. doi: 10.1093/jb/mvj003. [DOI] [PubMed] [Google Scholar]
- 24.Hughes A, Smith NI, Wallace HM. Polyamines reverse non-steroidal anti-inflammatory drug-induced toxicity in human colorectal cancer cells. Biochem J. 2003;374:481–8. doi: 10.1042/BJ20030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babbar N, Gerner EW, Casero RA., Jr Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem J. 2006;394:317–24. doi: 10.1042/BJ20051298. [DOI] [PMC free article] [PubMed] [Google Scholar]