Abstract
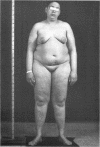
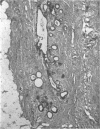
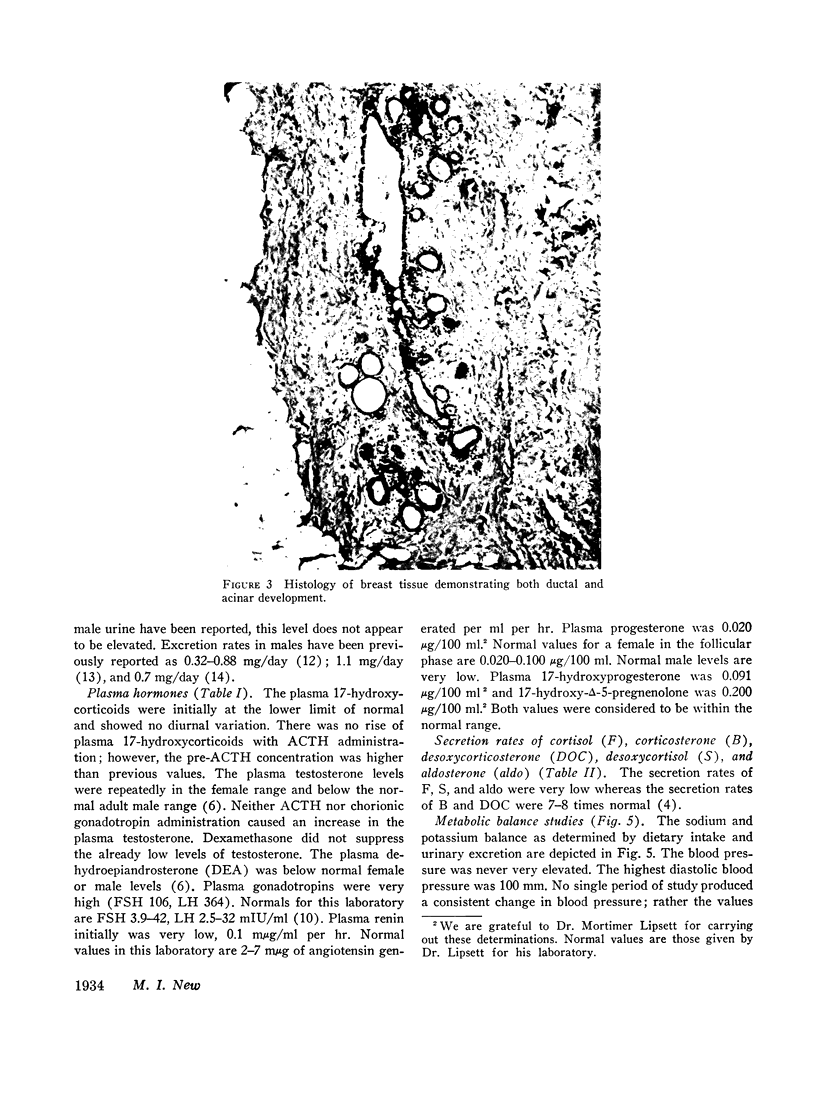
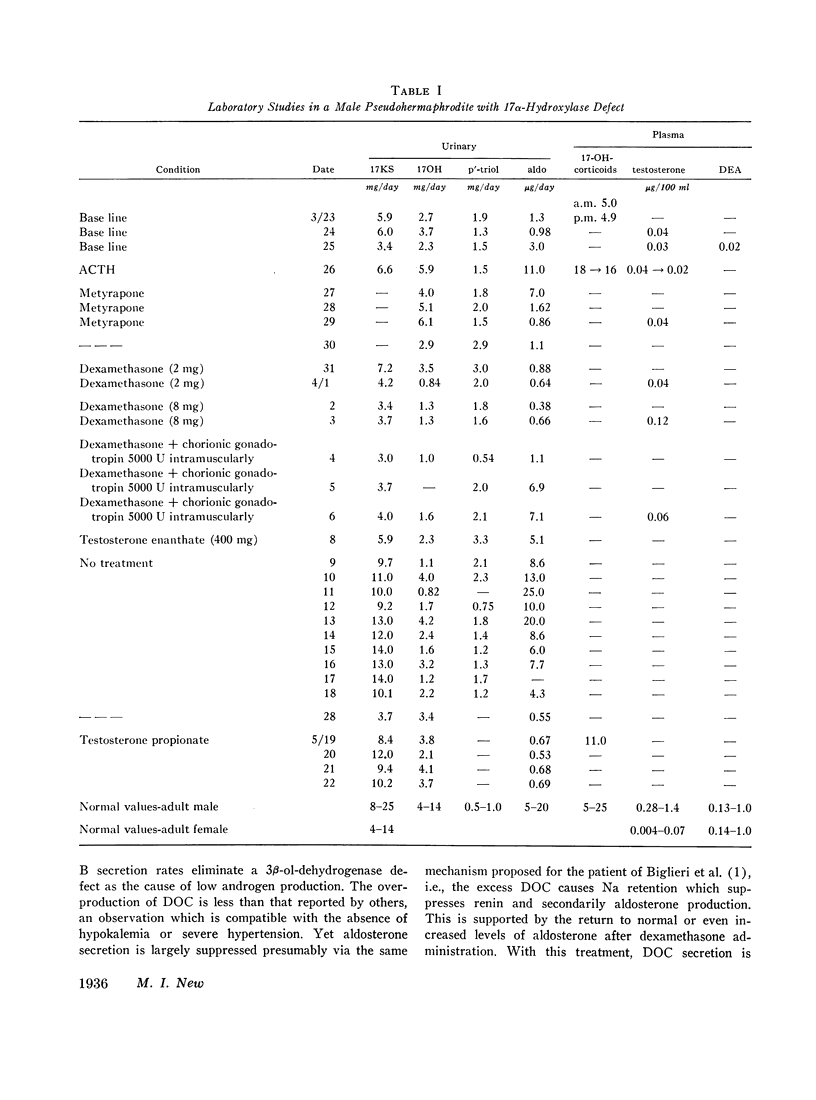
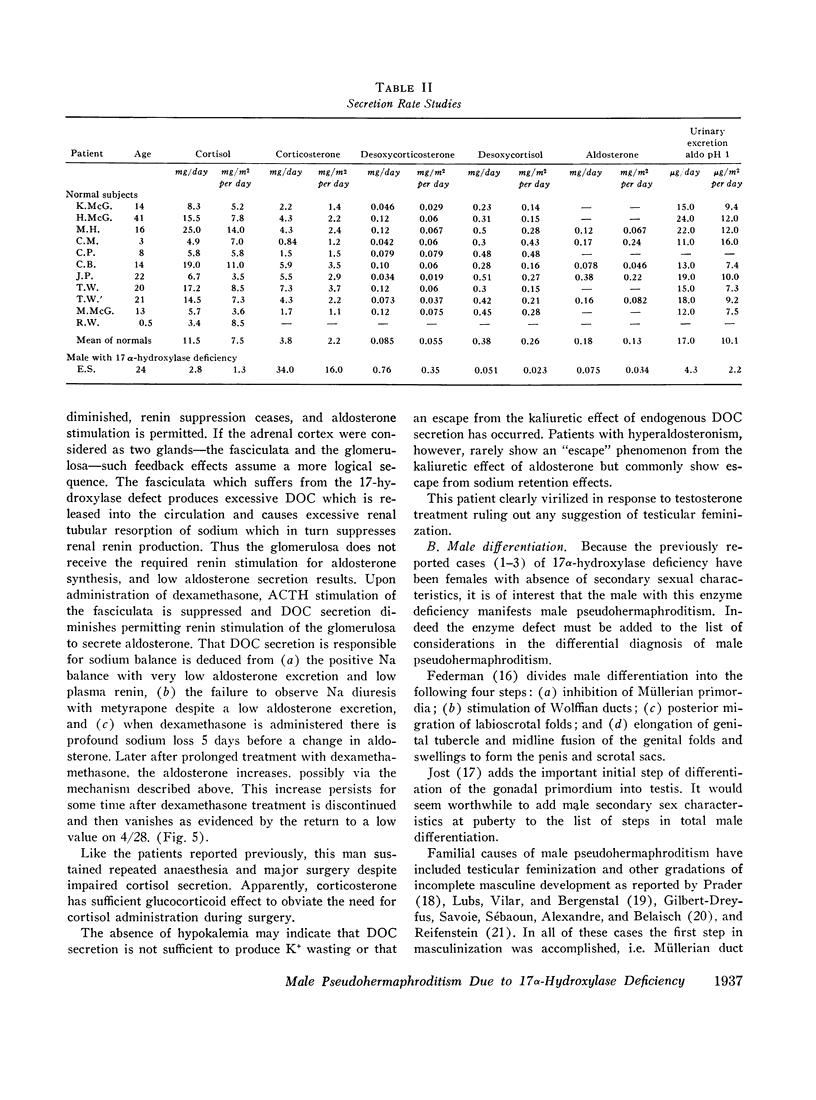
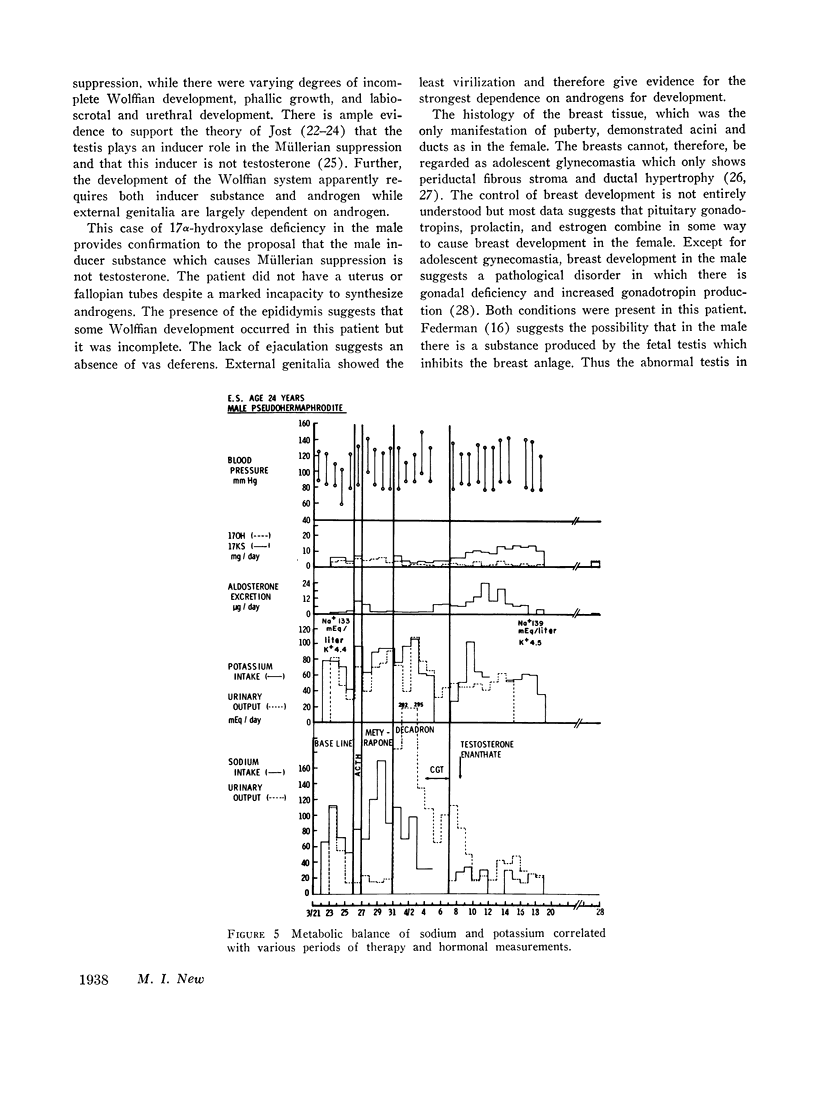
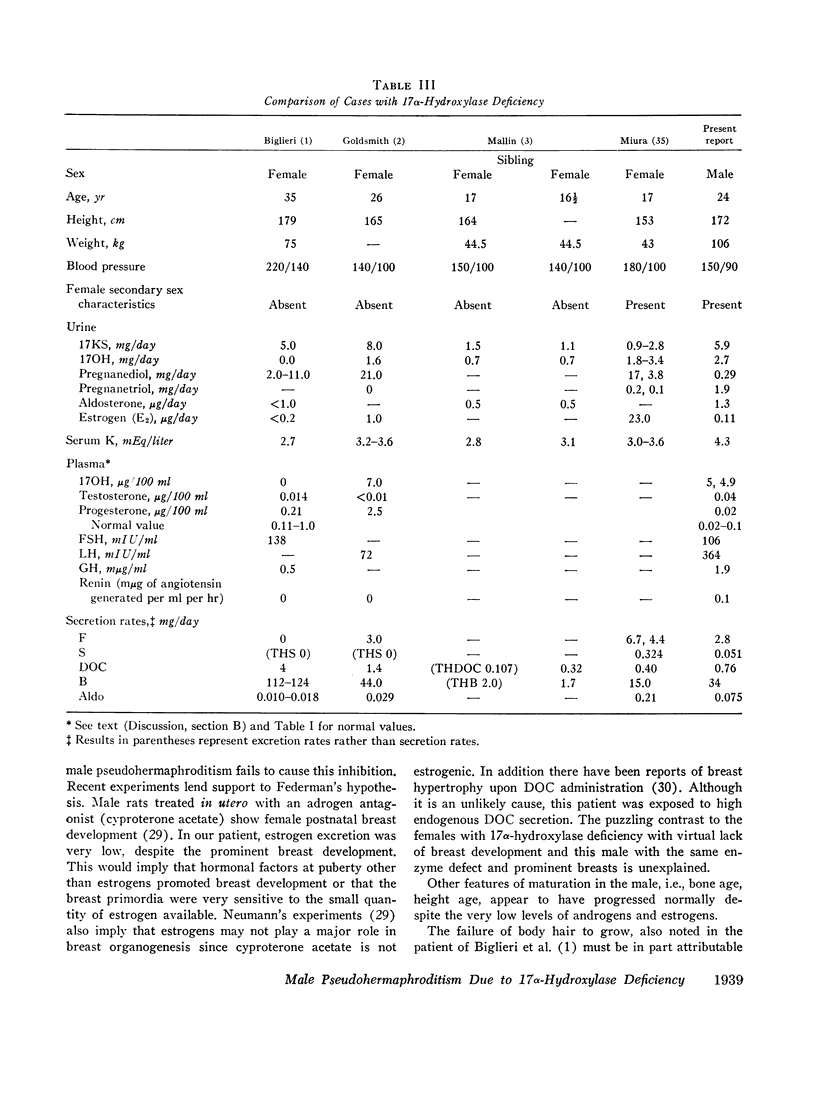
This is the first report of a male with 17α-hydroxylase deficiency resulting in male pseudohermaphroditism, ambiguous external genitalia, absence of male secondary sexual characteristics, and gynecomastia at puberty. Diagnosis was based on extensive studies of steroid metabolism including the following: low urinary excretion of 17-ketosteroids and 17-hydroxycorticoids which did not increase after ACTH; no response of very low plasma testosterone and dehydroepiandrosterone to adrenocorticotropin (ACTH) or chorionic gonadotropin; and low urinary aldosterone and plasma renin which increased after dexamethasone. Secretion rates of 17-hydroxylated steroids, cortisol (F) and 11-desoxycortisol (S), were very low while desoxycorticosterone (DOC) and corticosterone (B) secretion rates were increased sevenfold. Results expressed as milligrams per meter squared per day were as follows: F, 1.3; S, 0.023; DOC, 0.35; and B, 16 (mean normal values were F, 7.5; S, 0.26; DOC, 0.055, and B, 2.2). Plasma gonadotropins were markedly increased (FSH, 106; LH, 364 mIU/ml). Testicular biopsies revealed interstitial-cell hyperplasia and early spermatogenesis. Karyotype was 46/XY. Pedigree showed no other affected member. At laparotomy ovaries, uterus, and fallopian tubes were absent, vas deferens was incomplete, and prostate was present. External genitalia consisted of small phallus, bifid scrotum, third-degree hypospadias, and small vagina. At puberty there was no growth of body hair or phallic enlargement. Biopsy of marked gynecomastia showed both ducts and acini. Testosterone administration produced virilization. Sexual ambiguity demonstrates strong dependence of external genitalia on androgens for male differentiation. Suppression of Müllerian structures occurred despite female levels of testosterone indicating this step in male differentiation is not testosterone dependent. Pubertal breast development in this male supports the concept of femaleness during ontogeny unless counteracted by male factors. Diagnosis of other adrenocortical enzymatic deficiencies is excluded by the steroidal studies. The clinical response to testosterone excludes testicular feminization. Deficiency of 17-hydroxylation must be added to the cause of male pseudohermaphroditism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELING C. G. Purification of urinary conjugated oestrogens by gel filtration. Nature. 1961 Oct 28;192:326–327. doi: 10.1038/192326a0. [DOI] [PubMed] [Google Scholar]
- BONGIOVANNI A. M. The adrenogenital syndrome with deficiency of 3 beta-hydroxysteroid dehydrogenase. J Clin Invest. 1962 Nov;41:2086–2092. doi: 10.1172/JCI104666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN J. B., BULBROOK R. D., GREENWOOD F. C. An additional purification step for a method for estimating oestriol, oestrone and oestradiol-17 beta in human urine. J Endocrinol. 1957 Nov;16(1):49–56. doi: 10.1677/joe.0.0160049. [DOI] [PubMed] [Google Scholar]
- Biglieri E. G., Herron M. A., Brust N. 17-hydroxylation deficiency in man. J Clin Invest. 1966 Dec;45(12):1946–1954. doi: 10.1172/JCI105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy H. M., Peterson R. E. Measurement of testosterone and 17-ketosteroids in plasma by the double isotope dilution derivative technique. J Clin Endocrinol Metab. 1968 Jul;28(7):949–977. doi: 10.1210/jcem-28-7-949. [DOI] [PubMed] [Google Scholar]
- Goldsmith O., Solomon D. H., Horton R. Hypogonadism and mineralocorticoid excess. The 17-hydroxylase deficiency syndrome. N Engl J Med. 1967 Sep 28;277(13):673–677. doi: 10.1056/NEJM196709282771302. [DOI] [PubMed] [Google Scholar]
- JOST A. Hormonal factors in the development of the fetus. Cold Spring Harb Symp Quant Biol. 1954;19:167–181. doi: 10.1101/sqb.1954.019.01.023. [DOI] [PubMed] [Google Scholar]
- KLOPPER A., MICHIE E. A., BROWN J. B. A method for the determination of urinary pregnanediol. J Endocrinol. 1955 May;12(3):209–219. doi: 10.1677/joe.0.0120209. [DOI] [PubMed] [Google Scholar]
- KLOPPER A., STRONG J. A., COOK L. R. The excretion of pregnanediol and adrenocortical activity. J Endocrinol. 1957 Jun;15(2):180–189. doi: 10.1677/joe.0.0150180. [DOI] [PubMed] [Google Scholar]
- LUBS H. A., Jr, VILAR O., BERGENSTAL D. M. Familial male pseudohermaphrodism with labial testes and partial feminization: endocrine studies and genetic aspects. J Clin Endocrinol Metab. 1959 Sep;19:1110–1120. doi: 10.1210/jcem-19-9-1110. [DOI] [PubMed] [Google Scholar]
- Mallin S. R. Congenital adrenal hyperplasia secondary to 17-hydroxylase deficiency. Two sisters with amenorrhea, hypokalemia, hypertension, and cystic ovaries. Ann Intern Med. 1969 Jan;70(1):69–75. doi: 10.7326/0003-4819-70-1-69. [DOI] [PubMed] [Google Scholar]
- Merkatz I. R., New M. I., Peterson R. E., Seaman M. P. Prenatal diagnosis of adrenogenital syndrome by amniocentesis. J Pediatr. 1969 Dec;75(6):977–982. doi: 10.1016/s0022-3476(69)80334-3. [DOI] [PubMed] [Google Scholar]
- Miura K., Yoshinaga K., Goto K., Katsushima I., Maebashi M., Demura H., Iino M., Demura R., Torikai T. A case of glucocorticoid-responsive hyperaldosteronism. J Clin Endocrinol Metab. 1968 Dec;28(12):1807–1815. doi: 10.1210/jcem-28-12-1807. [DOI] [PubMed] [Google Scholar]
- Neumann F., Berswordt-Wallrabe R. Effects of the androgen antagonist cyproterone acetate on the testicular structure, spermatogenesis and accessory sexual glands of testosterone-treated adult hypophysectomized rats. J Endocrinol. 1966 Aug;35(4):363–371. doi: 10.1677/joe.0.0350363. [DOI] [PubMed] [Google Scholar]
- Neumann F., Elger W. [The effect of the anti-androgen 1,2-alpha-methylene-6-chloro-delta-4,6-pregnadiene-17-alpha-ol-3,20-dione-17-alpha-acetate (cyproterone acetate) on the development of the mammary glands of male foetal rats]. J Endocrinol. 1966 Dec;36(4):347–352. doi: 10.1677/joe.0.0360347. [DOI] [PubMed] [Google Scholar]
- New M. I., Miller B., Peterson R. E. Aldosterone excretion in normal children and in children with adrenal hyperplasia. J Clin Invest. 1966 Mar;45(3):412–428. doi: 10.1172/JCI105356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New M. I., Peterson R. E. A new form of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1967 Feb;27(2):300–305. doi: 10.1210/jcem-27-2-300. [DOI] [PubMed] [Google Scholar]
- New M. I., Seaman M. P., Peterson R. E. A method for the simultaneous determination of the secretion rates of cortisol, 11-desoxycortisol, corticosterone, 11-desoxycorticosterone and aldosterone. J Clin Endocrinol Metab. 1969 Apr;29(4):514–522. doi: 10.1210/jcem-29-4-514. [DOI] [PubMed] [Google Scholar]
- PRADER A., ANDERS G. J. [On the genitics of congenital lipoid hyperplasia of the adrenals]. Helv Paediatr Acta. 1962;17:285–289. [PubMed] [Google Scholar]
- PRADER A., GURTNER H. P. Das Syndrom des pseudohermaphroiditismus masculinus bei kongenitaler Nebennierenriden-Hyperplasie ohne Androgenüberproduktion (adrenaler Pseudohermaphroditismus masculinuS). Helv Paediatr Acta. 1955 Aug;10(4):397–412. [PubMed] [Google Scholar]
- PRADER A. Gonadendysgenesie und testikuläre Feminisierung. Schweiz Med Wochenschr. 1957 Mar 23;87(12):278–285. [PubMed] [Google Scholar]
- ROMANOFF L. P., MORRIS C. W., WELCH P., GRACE M. P., PINCUS G. Metabolism of progesterone-4-C14 in young and elderly men. J Clin Endocrinol Metab. 1963 Mar;23:286–292. doi: 10.1210/jcem-23-3-286. [DOI] [PubMed] [Google Scholar]
- Saxena B. B., Demura H., Gandy H. M., Peterson R. E. Radioimmunoassay of human follicle stimulating and luteinizing hormones in plasma. J Clin Endocrinol Metab. 1968 Apr;28(4):519–534. doi: 10.1210/jcem-28-4-519. [DOI] [PubMed] [Google Scholar]