Abstract
Perioperative myocardial infarction is a common and potentially fatal complication after noncardiac surgery, particular among patients with cardiovascular risk factors. β-blockers have been considered a mainstay in prevention and treatment of perioperative myocardial infarction, yet recent evidence suggests that β-blockers may have an unfavorable risk profile in this setting and the use has become controversial. What seems conspicuously absent from the current discussion is the appreciation of how much interindividual genetic variation influences the clinical response to β-blocker therapy. Genetic variation in the adrenergic signaling pathway is common, and has a major impact on adrenergic receptor function and β-blocker efficacy in other cardiovascular diseases such as heart failure and hypertension. Genetic variation in the cytochrome P450 2D6 enzyme, which is responsible for the metabolism of most β-blockers, is also important and can lead to poor metabolizing of β-blockers (potential toxicity) or their ultra-rapid degradation (decreased efficacy). Here, we review the molecular, cellular and physiologic consequences of polymorphisms in the adrenergic signaling pathway and CYP2D6 gene, and show that these are likely relevant factors influencing efficacy, safety and toxicity of β-blocker therapy in prevention and treatment of perioperative myocardial infarction.
The use of β-blockers has been considered the standard of care to reduce myocardial ischemia and infarction both during, and after, noncardiac surgery and has been recommended by international practice guidelines.1,2 However, since the publication of the POISE study in 2008,3 a large clinical trial with more than 8,000 patients that showed an increased risk of death and stroke among patients randomized to receive metoprolol in these settings, the use of β-blockers to reduce perioperative cardiac risk has become controversial. While the debate is still ongoing and the controversy far from being settled4, one crucial aspect regarding the efficacy and safety of β-blockers in the perioperative period has not been explored: the influence of genetic factors.
Ample evidence exists that the individual response to β-blockers in other clinical settings is substantially influenced by genetic variation in adrenergic signaling5–8 and drug metabolism pathways, most notably in the cytochrome P450 2D6 (CYP2D6) enzyme9–12 which is responsible for the metabolism of most β-blockers.13 Here we provide a concise overview of the genetic variability within these pathways relevant to β-blocker responses. Furthermore, we discuss potential links between genetic factors and the risk for the adverse outcomes during β-blocker treatment, such as hypotension and stroke, observed in recent clinical trials in the perioperative period.3,14–18
Perioperative myocardial infarction – a hidden epidemic
Perioperative myocardial infarction (MI) is a common and serious complication after surgery, often referred to as a “hidden epidemic”.19,20 A recent study among 85,000 inpatient surgeries showed an overall incidence rate of 0.5% for perioperative MI, which was associated with a 30–40% mortality rate.21 The risk for perioperative MI is at least on an order of magnitude higher among patients with preexisting coronary artery disease undergoing major noncardiac surgery (reported risk: 5–6%).14,22–24 Taking into account that worldwide an estimated 230 million surgeries are performed annually, more than 1 million patients are expected to suffer from perioperative MI or cardiac death each year.
The pathophysiology of perioperative MI
Several important differences exist between perioperative MI and acute MI in a nonoperative setting.20 Perioperative myocardial ischemia and infarction are often “silent”, with minimal classical clinical symptoms of an acute MI such as chest pain or dyspnea. 23,25–27
Two major causes of perioperative MI can be distinguished.28,29 The first cause involves the destabilization of a vulnerable atherosclerotic plaque, followed by acute coronary artery thrombosis, and subsequent myocardial ischemia and infarction.30 Causes for plaque destabilization in the perioperative period are manifold, but the most apparent are surgical stress resulting in hypertension, tachycardia and increased catecholamine levels, and hypercoagulability caused by surgical trauma. This pathophysiological process is identical to an acute coronary syndrome.31 While potentially fatal, the minority of perioperative MIs, however, are caused by an acute coronary syndrome.
The second, more common, mechanism for perioperative MI is an imbalance between myocardial oxygen demand and supply. In the setting of stable coronary artery disease with fixed atherosclerotic lesions, events resulting in higher oxygen demands in the myocardium that cannot be met by the limited blood flow can lead to myocardial ischemia and infarction. Common reasons for high myocardial oxygen demand and/or reduced oxygen supply in the perioperative period include tachycardia, acute hemorrhage, hypotension, hypoxemia, hypertension (increased myocardial wall stress), fever, and sepsis syndrome. In addition, endothelial dysfunction also plays a crucial role.32,33 Clinically, this type of perioperative MI resembles a non-ST segment elevation MI and is typically associated with a smaller increase in cardiac biomarkers than an acute coronary syndrome.23–25,34–41
Common to both mechanisms is the activation of the sympathetic nervous system, which increases heart rate and cardiac oxygen consumption by catecholamine-mediated activation of β-adrenergic receptors (βAR) on the myocardium. A logical intervention to potentially prevent myocardial ischemia in the perioperative period is the administration of βAR antagonists (β-blockers) to decrease myocardial energy expenditure.
β-blockers in the prevention of perioperative MI
Because of results showing a significant reduction in mortality among patients with acute coronary syndrome who were treated with β-blockers,42,43 clinicians concerned about perioperative myocardial infarction reasoned that β-blockers may provide the same benefits to surgical patients. Mangano et al. showed in the 1990’s that prophylactic atenolol reduced perioperative myocardial ischemia by 50% and the incidence of cardiac deaths after noncardiac surgery by 10% in patients with or at risk for coronary artery disease.44,45 When bisoprolol was given to high-risk patients who had a positive dobutamine stress echocardiography 30 days before surgery in the unblinded DECREASE I trial, a similar observation was made: nonfatal MI and cardiac death rates were significantly lower in patients who received bisoprolol compared to placebo.46 These consistent findings along with evidence from the efficacy of β-blockers in acute coronary syndrome led to a strong recommendation (class I and IIa) by the American College of Cardiology and the American Heart Association in their 2002 ACC/AHA guideline for perioperative evaluation for noncardiac surgery.47 However, a systematic review published in 2005 which included a meta-analysis of all randomized controlled trials that evaluated β-blockers in noncardiac surgery raised new issues. This analysis showed that β-blockers were likely efficacious in lowering the rate of perioperative MI and cardiac death, but were also associated with a substantial risk for adverse cardiovascular side effects such as hypotension and bradycardia requiring treatment, suggesting that there was risk of hypoperfusion of other organ systems.48 Two large randomized controlled trials were published in 2006, in which patients undergoing major noncardiac surgery who received either metoprolol or placebo on the day of or one day before surgery had no difference in postoperative cardiovascular morbidity or overall mortality.15,17 The authors from both trials concluded that β-blockers were not effective in reducing the rate of postoperative cardiac events. Then, in 2008 the largest perioperative β-blocker study so far, the POISE trial, was published.3 The POISE study randomized more than 8,300 β-blocker-naïve patients to either extended-release metoprolol or placebo on the morning of and for 30 days after surgery and used a composite of nonfatal MI, cardiovascular death and nonfatal cardiac arrest as primary outcome. The results indicated that metoprolol usage was associated with reduced risk for MI and the composite endpoint (hazard ratio of 0.84 and 0.73, respectively), but also was associated with significantly higher risks for death and stroke (hazard ratio 1.33 and 2.17, respectively) compared to placebo. A subsequent meta-analysis including 33 trials with more than 12,300 patients, which was largely driven by the POISE data, found similar results and concluded that evidence did not support the use of β-blockers to prevent perioperative cardiovascular events in noncardiac surgery.49 In the light of these data and the fact that patients who were chronically titrated to β-blockers had reportedly better outcomes (which is supported by two very recent studies50,51), the 2007 ACC/AHA guidelines2 were again updated in 2009 to reflect the new evidence and now recommend the use of β-blockers titrated to heart rate and blood pressure1.
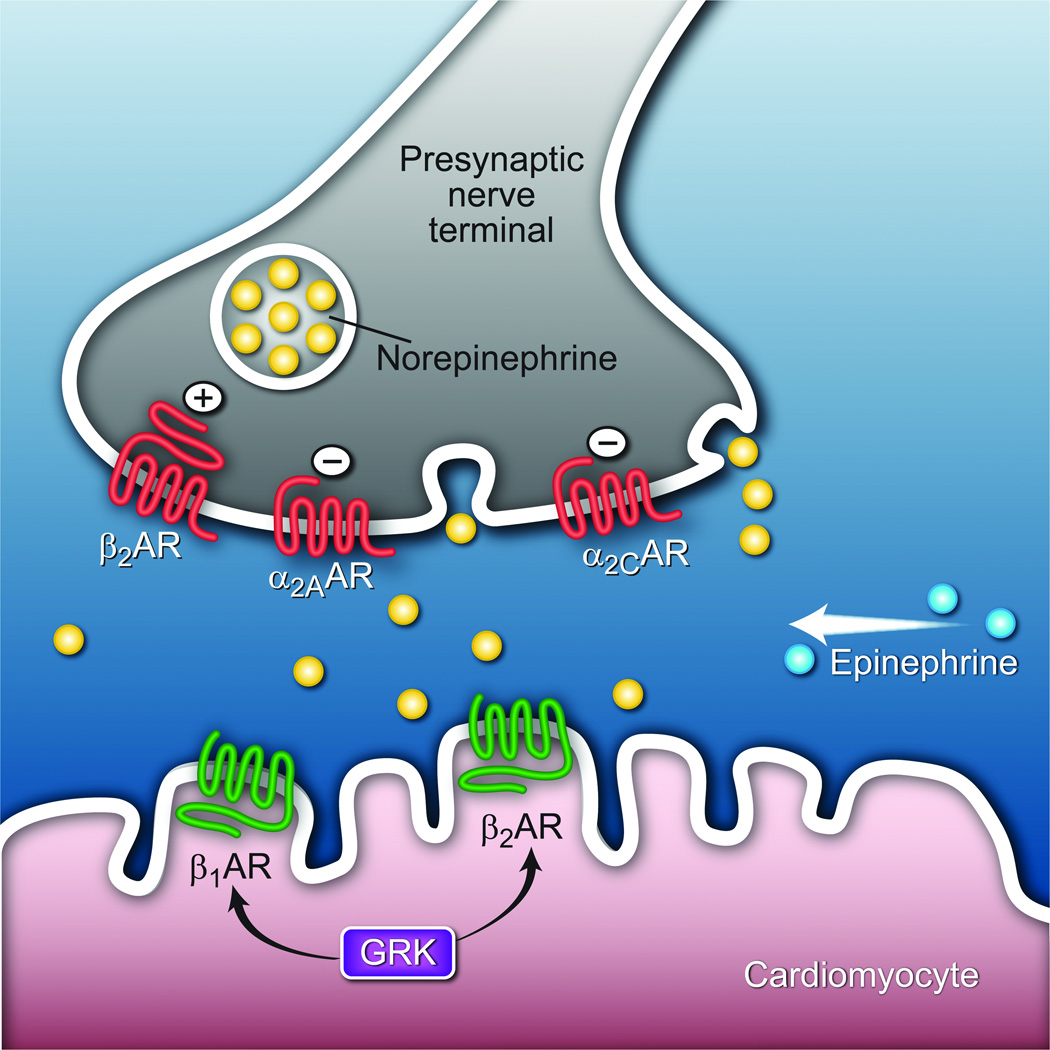
β-blockers are most often used in the treatment of hypertension, angina and myocardial infarction, and more recently, congestive heart failure. By antagonizing the action of norepinephrine and epinephrine on β1- or β2-adrenergic receptors (AR) expressed on cardiac myocytes, these agents can fully, or partially, block these actions of the sympathetic nervous system. The source of cardiac norepinephrine is from its release from the prejunctional cleft of sympathetic neurons innervating the heart (fig. 1). The release of norepinephrine is, in part, mediated by a negative feedback loop by α2AAR and α2CAR expressed on the prejunctional cleft: increased norepinephrine in the cleft binds these α2AR subtypes, which depress subsequent release.52 This mechanism is one of several that are thought to regulate neurotransmitter release and potentially mitigate against “over-stimulation.” There is also evidence that prejunctional β2AR can regulate release in a positive feedback loop,53 although norepinephrine has a low affinity for the β2AR subtype. The source for epinephrine is systemic in nature, arising from the adrenal gland. In the “basal-state” sympathetic nervous activity at the heart is modest at best (as indicated by a small decrease in resting heart rate from a single dose of atenolol in normal subjects). However, when activated by the aforementioned perioperative conditions such as pain and surgical stress, sympathetic nervous activity can be marked, with β-blocker administration causing a substantial reduction in heart rate, cardiac contractility, and myocardial oxygen consumption.
Figure 1.
Cardiac adrenergic receptors and polymorphisms relevant to β-blocker responsiveness. Shown are the presynaptic α2A- and α2CARs that when activated by epinephrine or norepinephrine decrease norepinephrine release from the presynaptic nerve terminal. Presynaptic β2AR activation increases norepinephrine release: on the cardiomyocyte, catecholamine activated β1AR and β2AR increase inotropy and chronotropy, and can under signal dampening due to receptor phosphorylation by GRKs (G-protein coupled receptor kinase).
Recent evidence, however, suggests that there is substantial inter-individual difference in how patients respond to β-blockers: some patients experience strong side effects such as excessive hypotension and bradycardia whereas others experience no measurable response. Several lines of evidence suggest that the individual genetic background is responsible for these observed response differences.
Impact of genetic polymorphisms on β-blocker response
The most common form of genetic variation is the exchange of a single base pair in the DNA strand: this is referred to as single nucleotide polymorphism (SNP). The genome of a human contains approximately 3 billion (3 × 109) base pairs and each individual harbors approximately 10–20 million SNPs. Most of these SNPs are silent as they fall outside of the coding region of genes (known as exons). Of those in the coding region, some do not change the encoded amino acid, due to redundancy of the genetic code. These are termed “synonymous” SNPS, while those that result in the encoding of a different amino acid are termed “nonsynonymous” SNPs. Of note, noncoding SNPs, such as those in gene promoter regions, may also have significant effects leading to clinical phenotypes. Within the genes of interest that are discussed in this review, the ones coding for adrenergic receptors and cytochrome P450, several important nonsynonymous SNPs are known as well as their functional consequence on the receptor and enzyme function. Most commonly nonsynonymous SNPs cause a reduced function or activity of the affected protein, but on rare occasions novel protein functions may result. In the next section, we will discuss the most important SNPs and gene variants within adrenergic receptors and the β-blocker-metabolizing cytochrome P450 2D6 enzyme.
Genetic variation of the cardiac adrenergic axis
α2-adrenergic receptors
As introduced above and summarized in figure 1, there are multiple receptor pathways that might influence β-blocker response, and if genetic variations in these pathways are present, they could represent the basis for inter-individual variation in the response to perioperative β-blockers. The physiologic effect of β-blockers is dependent, in part, on the presence of a cardiac βAR agonist to antagonize. Thus polymorphisms (defined as a genetic variation with a prevalence of >5% in a population) of the α2A and the α2CAR, which partially control norepinephrine release could alter β-blocker responsiveness.54,55 Within the coding block of the intronless (consisting only of a single exon) α2AAR gene there is one nonsynonymous polymorphism. At amino acid position 251, the most common allele results in Asn (Aspargine), but a relatively rare polymorphism results in an encoded Lys (Lysine, table 1, fig. 2). In African-Americans the frequency of the α2ALys251allele is 4%, and in Caucasians it is 0.4%. In functional studies using the wild-type and Lys variant recombinantly expressed in Chinese hamster ovary cells, the α2ALys251 receptor had a ~50% increase in function.56 Thus, the minor variant represents a gain-of-function, and if present in an individual would be expected to be manifested as a decrease in norepinephrine release, compared to an individual expressing α2AAsn251, under the same stimulation. Given the rare prevalence of α2ALys251, this variant has not been studied in clinical trials. In contrast, a coding polymorphism of the α2CAR, which consists of an in-frame deletion of 12 nucleotides and results in deletion of four amino acids, occurs in ~40% of African-Americans and ~3% in Caucasians (table 1). This deletion is in the third intracellular loop of the receptor, and results in a nearly complete loss of function due to loss of receptor coupling to its cognate G-protein (Gi).57 This polymorphism, termed α2CDel322–325, has been associated with increased norepinephrine (or its transporter) in the cardiac presynaptic cleft,58 increased risk for heart failure,59 and a significantly reduced survival benefit in heart failure patients when taking the β-blocker bucindolol.54 The other presynaptic adrenergic receptor that has been thought to have some control over norepinephrine release is the β2AR, although the relevance is less certain as compared to the α2A- and α2CAR. The β2AR is also polymorphic, and will be discussed below in the context of myocardial adrenergic receptor.
Table 1.
Localization of Polymorphisms of Selected Adrenergic Receptors or Related Genes.
| Receptor/ Protein |
Gene | Reference | “Variant” | Allele Frequency | Phenotype | |
|---|---|---|---|---|---|---|
| Caucasians | African-Americans | |||||
| α2A | ADRA2A | Asn-251 | Lys-251 | 0.0040 | 0.040 | Increased Coupling |
| α2C | ADRA2C | - | Del322–325 | 0.04 | 0.43 | Heart Failure |
| β1 | ADRB1 | Ser-49 | Gly-49 | 0.15 | 0.15 | Downregulation |
| Arg-389 | Gly-389 | 0.73 | 0.58 | Decreased coupling | ||
| β2 | ADRB2 | Arg-16 | Gly-16 | 0.61 | 0.50 | Increased desensitization |
| Gln-27 | Glu-27 | 0.43 | 0.27 | Reduced desensitization | ||
| Thr-164 | Ile-164a | ~0.02 | ~0.004 | Loss-of-function | ||
| β3 | ADRB3 | Trp64 | Arg64 | 0.10 | ? | Loss-of-function |
| GRK5 | GRK5 | Gln-41 | Leu-41 | 0.01 | 0.23 | enhanced desensitization of β1AR |
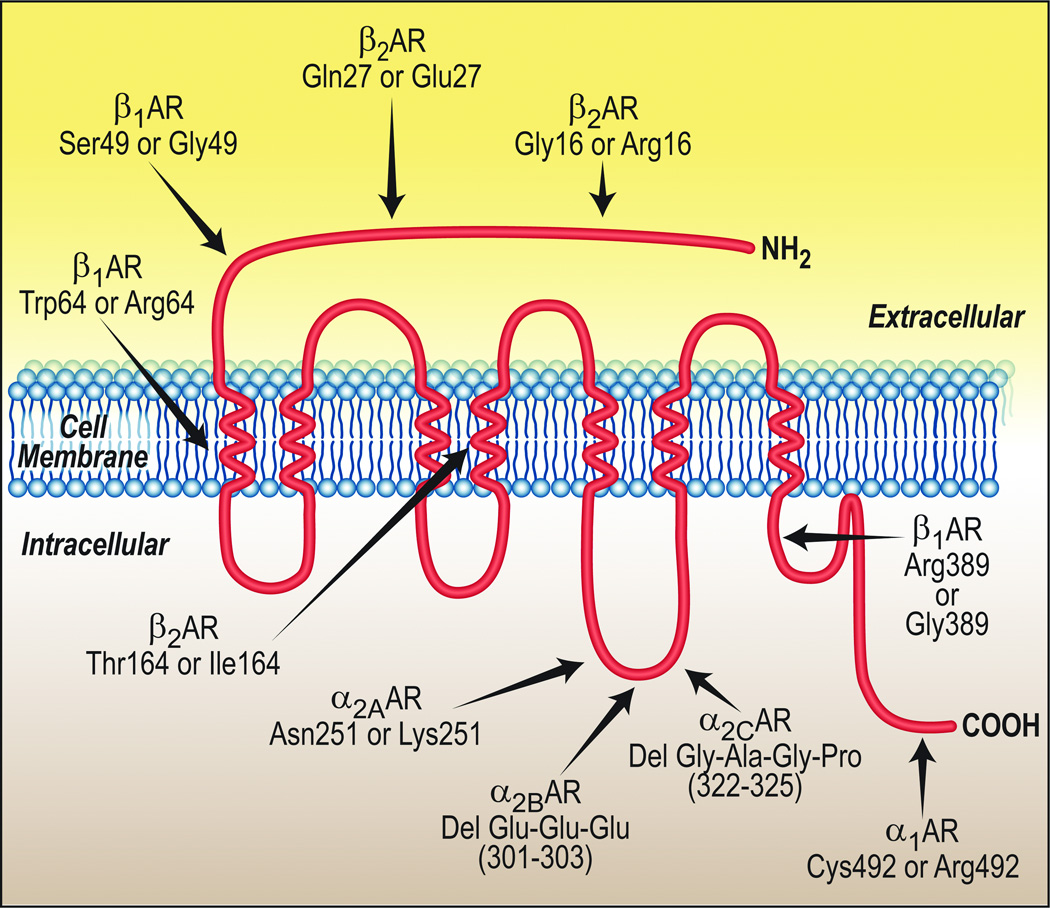
Figure 2.
Localization of the common polymorphisms of the adrenergic receptors. The schematic shows a prototypic 7-transmembrane spanning receptor. The amino acid positions within the respective receptor protein are given, while their physical positions within the prototypic receptor are approximated.
β1-adrenergic receptor polymorphisms
β1AR are the predominant βAR in the heart and mediate an increase in heart rate and contractility (fig. 1). β1AR are encoded by the ADRB1 gene which consists only of a single coding block (exon). Myocyte β1AR are a major target for epinephrine and norepinephrine. Not only does this subtype increase cardiac inotropy and chronotropy, signaling of β1AR has been shown to evoke specific proapoptosis signals.60 A common polymorphism of the β1AR is observed at amino acid position 389, where Arg or Gly can be found (table 1, fig. 2). The originally cloned β1AR had a Gly at this position, but it is now clear that in African-Americans Gly and Arg allele frequencies are approximately the same, and in Caucasians the Arg allele frequency is ~70%. In transfected cells, we found that β1Arg389 exhibited a 3-fold greater stimulation of adenylyl cyclase and cyclic adenosine monophosphate compared to β1Gly389.61 This was found to be due to an increase in the formation of the agonist-receptor-Gs complex. The gain-of-function was also observed in [35S]GTPγS binding studies, confirming that the phenotype was due to enhanced coupling of the receptor to Gαs.
Transgenic mice were then constructed62 to express β1Arg389 and –Gly389 receptor in cardiomyocytes in a targeted manner using the α-myocin heavy chain promoter. In lines with equivalent expression, we found higher basal and dobutamine-stimulated cardiac contractility in β1Arg389 mice compared to -Gly389 mice at 3 months of age.62 Interestingly, by 6 months of age Arg389 mice were unresponsive to agonist while Gly389 mice retained responsiveness and showed only a minor decline in agonist-promoted contractility. At this juncture we considered the possibility of a “phenotypic switch,” which might imply that any pharmacogenomic effect would also have a time-dependent element. However, this possibility was subsequently shown not to be the case when we examined cardiac explants. In these transgenic mice, we also observed a decrease in heart rate to acutely or chronically administered β-blocker only in the Arg389 mice, which was the first evidence that this locus might have an effect on β-blocker outcomes in a clinical setting.62 The basis for this appeared to be the greater potential for an enhanced inotropic state in hearts expressing β1Arg389, and thus a higher potential to be antagonized by a β-blocker back towards baseline. Subsequent studies in explanted human hearts confirmed the enhanced function of β1Arg389 in normal hearts, as well as hearts with end-stage failure.63 The difference in maximal contraction between Arg- and Gly389 failing human hearts was not as pronounced as was observed in normal hearts. So there was no switch in the phenotype (Arg389 was still the hyperfunctioning receptor) but rather a dampening of the phenotypic difference between the variants as heart failure progresses. These results are consistent with studies of agonist-promoted desensitization (which occurs in heart failure due to the increased catecholamines) of these two receptors expressed in model cells, which showed that Arg389 undergoes greater desensitization than Gly389.64 In clinical studies, β1Arg389 has been associated with enhanced exercise capacity in heart failure65 and an improved mortality outcome in response to β-blocker in heart failure.63 It should be noted that in this latter study the β-blocker was bucindolol, which had failed to show a group mean improvement in survival. However, with patient stratification by β1AR genotype, an improvement in survival was clearly evident for those with the β1Arg389 genotype receiving bucindolol compared to those with the same genotype receiving placebo, or the Gly389 genotype receiving bucindolol or placebo (fig. 3). These results highlight the potential for pharmacogenomic markers to bring drugs, previously thought to be ineffective, into use for selected populations based on genotype. DNA from a placebo-controlled trial with other β-blockers (such as metoprolol and carvedilol) using similar outcomes is not available, so a direct comparison of these results with different drugs has not been carried out. However, other studies without placebo arms have suggested that the findings with bucindolol in heart failure may be unique to that β-blocker.66 This may be due to the fact that bucindolol acts as an inverse agonist at β1Arg389 (but not Gly389), while metoprolol and carvedilol are neutral antagonists for both allelic forms of the receptor.63 As introduced above, there appears to be a small but independent effect of the α2CDel322–325 genotype on heart failure outcome in response to bucindolol as well.54 The mechanisms behind this effect are not altogether clear, but appear to be due to a loss of counterregulatory function during a marked decrease in norepinephrine that is found in a subset of heart failure patients treated with bucindolol. The β1Arg389 polymorphism has also been associated with a greater decrease in blood pressure during atenolol treatment of hypertension67 and improved treatment outcomes in hypertensive patients.68
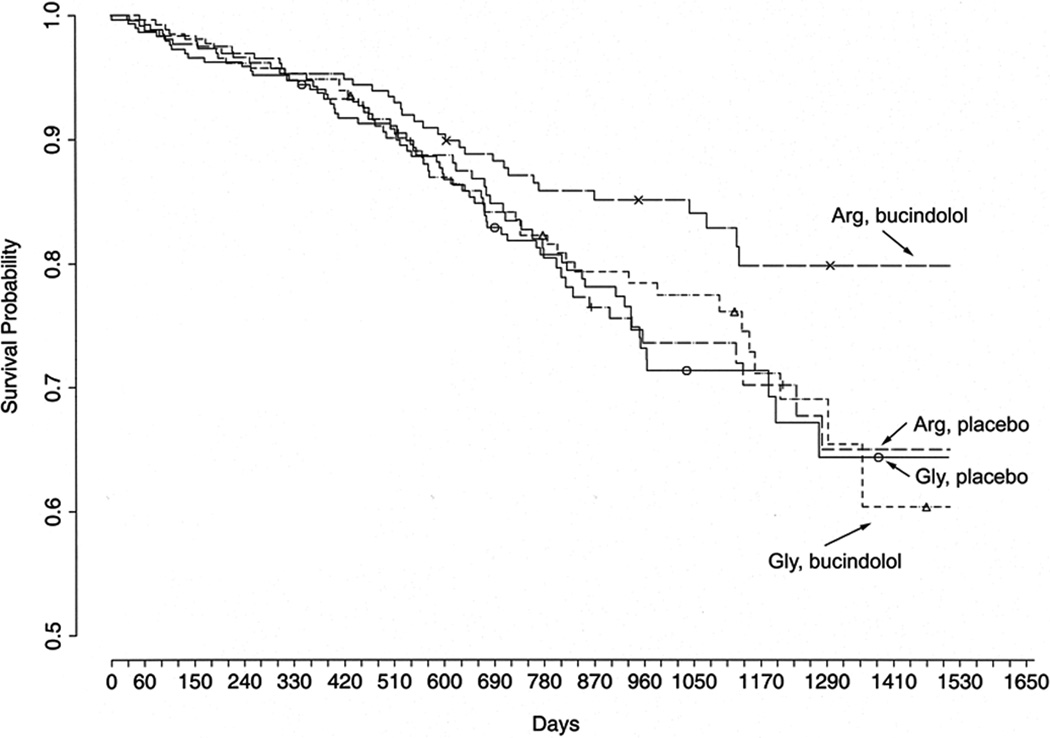
Figure 3.
Pharmacogenetics of the β-blocker bucindolol in chronic heart failure. Shown are Kaplan-Meyer curves showing survival stratified by treatment group and the β1Arg- or β1Gly389 polymorphism. Those with the β1Arg389 genotype receiving bucindolol had a 38% improvement in survival over placebo patients of the same genotype. Gly389 carriers on bucindolol showed no improvement. From Liggett SB et al., Proc Natl Acad Sci USA 2006; 103:11288-93. Reprinted with permission from the National Academy of Sciences, USA.63
A less common polymorphism of the β1AR is found in the amino terminus, where Ser is substituted by Gly. The β1-Gly49 receptor appears to undergo enhanced agonist-promoted downregulation in recombinantly expressed cell lines.69 The low allele frequency of Gly49 has generally made investigations of this polymorphism not feasible due to statistical power considerations.
β2-adrenergic receptor polymorphisms
β2AR, encoded by the ADRB2 gene, are widely expressed in virtually all cell-types. Their presence on smooth muscle mediates relaxation and thus dilation of vasculature and the airways. Their somewhat more limited expression on cardiac myocytes mediates increased inotropy and chonotropy, as well as antiapoptotic effects. The β2AR has three nonsynonymous (amino acid-changing) polymorphisms at amino acid positions 16, 27 and 164 (table 1, fig. 2). The most pronounced phenotype is the substitution of Ile for Thr at position 164, which is in the fourth transmembrane domain of the β2AR. The β2Ile164 receptor is markedly uncoupled from stimulation of adenylyl cyclase due to impaired agonist-receptor interaction.70,71 However, the variant is rare, with the heterozygous state being found in <5% of the population (a homozygous individual has never been reported). In heart failure patients, β2Ile164 is associated with a marked decrease in exercise capacity in otherwise matched patients72 and also increased mortality.73 β-blocker responsiveness has not been adequately assessed with this variant due to its low allele frequency. The other two polymorphisms at positions 16 and 27 are in the extracellular amino terminus of the β2AR, and appear to have effects on agonist-promoted downregulation of the receptor.74,75 In terms of pharmacogenetics, these two polymorphisms have been primarily studied in the context of β-agonist treatment for asthma.76 However, several studies have indicated that the polymorphism at 16 and/or 27 may have effects on heart failure survival during β-blocker therapy (including “β1-specific antagonists”).77,78 The molecular basis for these observations remains unclear.
β3-adrenergic receptor polymorphisms
Of adrenergic receptors, the β3AR is the least understood in terms of cardiovascular function.79 Initial work showed that the β3AR has an important role in regulating metabolism in adipocytes.80 Recent data, however, suggest that β3ARs mediate vasodilation when β1AR and β2AR are not functional, perhaps preventing excessive overstimulation by catecholamines.81 Other studies have demonstrated that activation of β3AR increases formation of nitric oxide and evokes a decrease in inotropy.82,83 One polymorphism has been identified in the coding region of this receptor (ADRB3 gene), at position 64 (Trp/Arg, table 1, fig. 2). One group has reported that the Arg receptor has depressed agonist-promoted coupling to cyclic adenosine monophosphate production,84 while another group has found no differences.85 Several studies have suggested relationships between β3AR alleles and cardiac or metabolic risk factors.86,87 A recent paper showed that in diabetic patients β3AR are often up-regulated while β1AR are simultaneously down-regulated88 which may result in an altered response to β-blocker therapy.
GRK5 polymorphisms
In addition to these variations in the relevant receptors, one polymorphism in the second messenger system within a G-protein-coupled receptor kinase (GRK5, fig. 1) has recently shown in vitro and in vivo evidence for relevance to β-blocker responsiveness.89 The GRKs phosphorylate multiple G-protein coupled receptors during agonist activation, which acts to partially uncouple the receptor form Gs, and is a major mechanism of desensitization. A nonsynonymous polymorphism of GRK5, where the major allele Gln at amino acid position 41 is substituted by Leu, has been found.89 It is prevalent in African-Americans but not Caucasians (table 1). In transfected cells and transgenic mice, GRK5-L41 has been shown to exhibit enhanced desensitization of β1AR.89 And in a mouse model of heart failure those mice expressing GRK5-L41 were partially protected from failure and had no additional benefit from β-blockers compared to mice expressing the Q41 allele. Similar findings were also observed in a prospective clinical trial in heart failure, where those not receiving β-blockers but with the L41 allele had survival similar to Q41 subjects on β-blocker and improved survival over Q41 subjects not receiving β-blocker. Thus GRK5-L41 acts as a “genetic β-blocker,” and when present may obfuscate the need for these agents, or, may indicate that a lower dosage is necessary to achieve the desired outcome.
A recent study of the gene encoding the α subunit of Gs (GNAS) showed that patients with a certain haplotype) had a significantly different Gαs expression, cyclic adenosine monophosphate production and cardiac performance during cardiac surgery,90 indicating that genetic variation within the adrenergic second messenger system may also play an important role for how patients respond to β-blockade.
Genetic variation of drug metabolism of β-blockers – cytochrome P450 2D6 (CYP2D6)
Genetic variation not only influences the pharmacodynamics, but also the pharmacokinetics of β-blocker treatment. Most β-blockers, such as metoprolol and propranolol, are extensively metabolized in the liver by cytochrome P450 2D6 (CYP2D6), a hepatic enzyme of the cytochrome P450 family.91 CYP2D6 is responsible for the phase I metabolism of approximately 25% of all commonly used drugs and thus one of the most important drug metabolizing enzymes.92,93 CYP2D6 is involved in the metabolism of antidepressants, antiemetics, anticancer drugs, antipsychotics, opioids (tramadol, morphine, codeine), and β-blockers.
The CYP2D6 gene is very polymorphic with close to 100 known variants.93,94 These CYP2D6 gene variants have a major impact on the CYP2D6 enzyme activity, with some variants resulting in a complete loss-of-function phenotype whereas others lead to a gain-of-function. With β-blockers, several studies have reported a clinically relevant, severalfold difference in metoprolol plasma concentrations among patients with different CYP2D6 variants.95–97 The ability of the CYP2D6 enzyme to metabolize substrates has been stratified into four classes: ultra-rapid metabolizers (UM), extensive metabolizers (EM, considered the normal phenotype), intermediate metabolizers (IM) and poor metabolizers (PM).93 Table 2 lists the most important CYP2D6 alleles and haplotypes and their respective allele frequency. The genetic basis of these CYP2D6 phenotypes has also been ascribed to copy number variation within the CYP2D6 gene. Copy number variation is defined as a variable number of DNA segments compared to the reference genome and include deletion, but also duplication or multiplication of large segments (>1 kB) of DNA. For CYP2D6, the most functional allele determines the phenotype. If an individual has at least one fully functional CYP2D6 allele, the resultant phenotype is considered wild-type or extensive metabolizer. With one or two reduced-function alleles, an intermediate metabolizer phenotype results and with two nonfunctional alleles, for example due to deletion, a PM phenotype results. On the contrary, if individuals possess more than two copies of a fully functional CYP2D6 allele (up to 13 have been described), likely after several ancestral duplication events, an UM phenotype ensues (fig. 4).94,98 It is of note that substantial ethnic differences exist in the distribution of CYP2D6 alleles: PM are more common in people from European ancestry whereas UM are more prevalent in people from North Africa and Oceania.98
Table 2.
Common and Important CYP2D6 Polymorphisms
| CYP2D6 Allele/Haplotype |
Nucleotide Change | Metabolizer Class |
Caucasians | African- Americans |
|---|---|---|---|---|
| *1 | reference | EM (wild-type) | 0.33 – 0.36 | 0.29 – 0.35 |
| *2 | 2850C>T, 4180G>C | EM | 0.22 – 0.33 | 0.18 – 0.27 |
| *3 | 2549delA | PM | 0.01 – 0.04 | 0 |
| *4 | 1846G>A | PM | 0.12 – 0.21 | 0.06 – 0.08 |
| *5 | CYP2D6 deleted | PM | 0.02 – 0.07 | 0.06 – 0.07 |
| *6 | 1707delT | PM | 0.01 | 0 |
| *9 | 2615–2617delAAG | IM | 0 – 0.02 | 0 |
| *10 | 100C>T | IM | 0.01 – 0.02 | 0.03 – 0.08 |
| *17 | 1023C>T, 2850C>T | IM | 0 | 0.15 – 0.23 |
| *29 | 1659G>A; 1661G>C; 2850C>T; 3183G>A; 4180G>C | IM | N/D | N/D |
| *41 | 2988G>A | IM | N/D | N/D |
| UM | Multiple copies | UM | 0.02 | 0.01 – 0.05 |
EM = extensive metabolizer; IM = intermediate metabolizer; PM = poor metabolizer; UM = ultrarapid metabolizer.
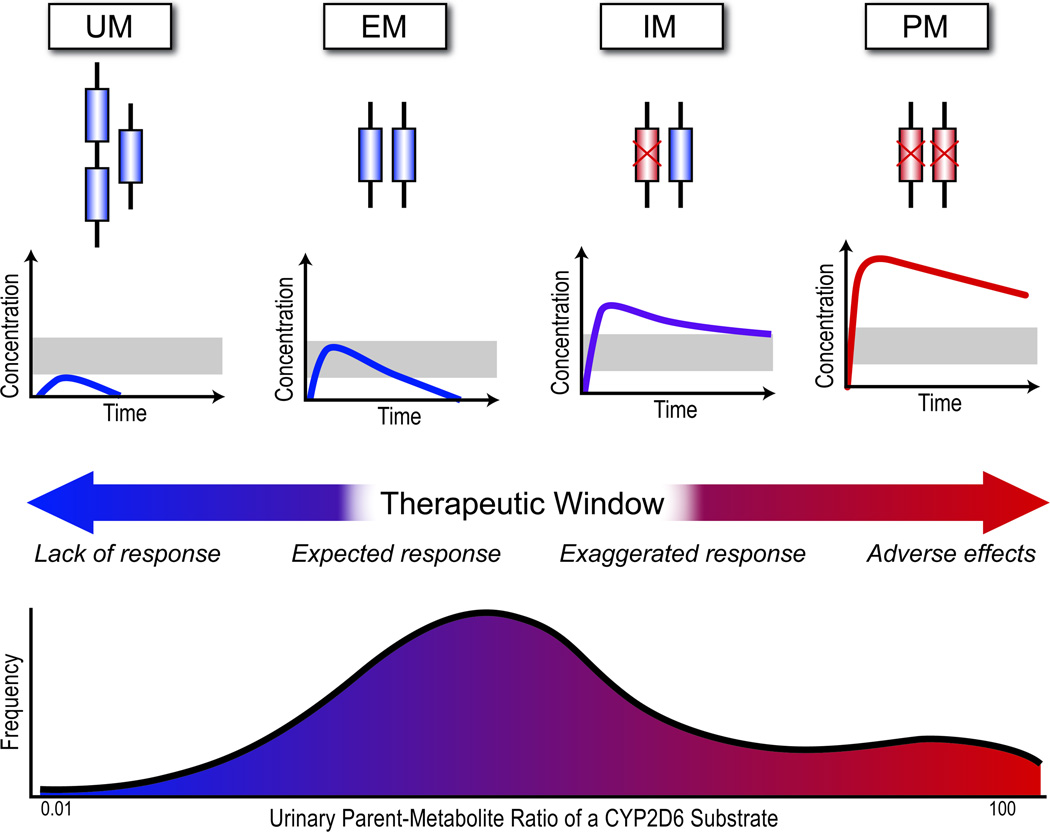
Figure 4.
Effects of CYP2D6 genotype on CYP2D6 substrate metabolism. Extensive metabolizers (EM) have one or two normal copies of the CYP2D6 gene and are considered wild-type. A normal β-blocker dose will likely result in a therapeutic drug concentration. Ultra-rapid metabolizers (UM) possess more than 2 functional copies (up to 13). A CYP2D6 substrate (e.g., metoprolol) will be rapidly metabolized and the drug effect minimal. Intermediate metabolizers (IM) have one or two hypo-functional CYP2D6 alleles which results in a reduced CYP2D6 function. Substrate metabolism is reduced and higher drug concentrations result with the possibility of an exaggerated response. Poor metabolizers (PM) have two nonfunctional copies of CYP2D6 which results in a nonfunctional enzyme. This may lead to toxic drug concentrations.
The functional consequences of copy number variants or SNPs in CYP2D6 enzyme activity are nontrivial: individuals with a PM phenotype are unable to adequately metabolize CYP2D6 substrates (e.g., drugs) and higher, potentially dangerous, plasma drug concentrations can result. Patients with an UM phenotype will rapidly metabolize the drug and plasma drug concentrations from standard dosing can be too low to be efficacious. On the other hand, if a prodrug must be metabolized into an active form by CYP2D6, an opposite picture results: PM will not reach effective drug concentrations whereas UM will develop elevated plasma levels and are at higher risk for adverse drug effects. An example for the latter is the case of a fatal morphine poisoning in a neonate that was breastfed by a mother who received codeine.99 Codeine is a prodrug that must be metabolized by CYP2D6 to morphine and because the mother was a CYP2D6 UM (and required high doses of codeine), toxic plasma concentrations of morphine resulted in the neonate.
It is of note that CYP2D6 is the only enzyme in the cytochrome P450 family that is not inducible which results in a substantially higher contribution of genetic differences to enzyme activity level and clinical phenotype. However, CYP2D6 can be inhibited by many drugs and, as a consequence, a lower enzyme activity may result than may be expected simply based on the CYP2D6 genotype. This process is called phenocopying. Two of the clinically most important inhibitors of CYP2D6 are paroxetine100, a selective serotonin reuptake inhibitor, and statins101.
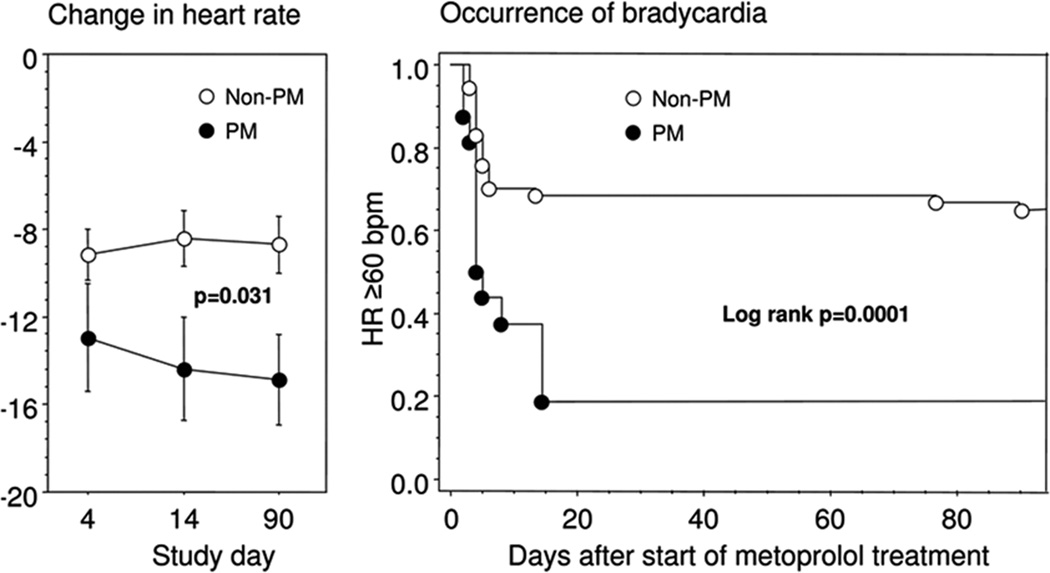
The evidence clearly supports a major role of CYP2D6 polymorphisms in the pharmacokinetics of β-blockers, but does this role also translate into relevant differences in clinical outcomes? Current evidence suggests so. Several studies independently found that patients with PM phenotype, who were treated with metoprolol, had a higher risk to develop bradycardia and lower blood pressure and had a higher incidence of adverse drug reactions.9–12 UM subjects have been reported to not achieve a therapeutic effect of standard dosing of metoprolol10 (fig. 5). It is of note, however, that routine genotyping for CYP2D6 variants is not commonly performed clinically.
Figure 5.
Clinical relevance of CYP2D6 genotype on β-blocker treatment. Poor CYP2D6 metabolizers experience a greater reduction in heart rate (a) and a higher risk of bradycardia when treated with metoprolol.
HR = heart rate; PM = poor metabolizer.
Reprinted with permission from Macmillan Publishers Ltd.: [Clinical Pharmacology & Therapeutics] Rau T et al., Impact of the CYP2D6 Genotype on the Clinical Effects of Metoprolol: A Prospective Longitudinal Study 2009; 85: 269–72.
Impact of genetic variation on the use of beta-blockers in perioperative MI
Data presented in the previous sections show clearly that gene variants have a significant impact on the individual response to β-blocker-therapy – but is there evidence to support the influence on perioperative myocardial infarction? A study published in 2005 with more than 700 patients with acute coronary syndrome who received standard β-blocker therapy in the emergency department shows a significant impact of ADRB2 genotype on survival.102
For perioperative β-blockade, three recent papers provide indirect evidence that genetic variation in CYP2D6-dependent metabolism as well as adrenergic signaling may influence outcomes. 50,103,104 Particularly the apparent decreased risk associated with atenolol, which is not metabolized by CYP2D6, compared to metoprolol which undergoes extensive CYP2D6-dependent metabolism is very interesting.
Whether, however, the conditions of perioperative MI are such that these polymorphisms have a significant impact on outcomes, remains a critical question that needs to be addressed. The idea that “one drug fits all” is being questioned in virtually all of clinical medicine. Given the apparent interindividual variation in efficacy and adverse effects of β-blockers for prevention of perioperative MI, the biologic plausibility, and the low costs of genotyping by modern methods, it seems to us that a rigorous pharmacogenomic investigation is indicated. Ultimately, this could lead to a “genetic scorecard” that would recommend when a β-blocker should not, or should, be used, and the dose, for prevention of perioperative MI. As these trials are being contemplated, we implore investigators in other current trials of perioperative MI prevention to collect blood for archival purposes such that a DNA bank can be established and subsequent pharmacogenomic hypotheses pursued.
In conclusion, we believe that there is strong evidence to suspect that polymorphisms in the adrenergic signaling pathway and CYP2D6-dependent β-blocker metabolism influence efficacy, safety and toxicity of β-blocker therapy in prevention and treatment of perioperative MI. It is to be expected that the emphasis on careful β-blocker dose titration, as recommended in the most recent ACC/AHA guidelines,1 might lessen the disparate effects of genetic polymorphisms.
Acknowledgments
This work was supported by grants 1K23GM087534 from the National Institute of General Medical Sciences (to Dr. Nagele), HL45967 and HL077101 from the National Heart, Blood and Lung Institute (to Dr. Liggett), and an institutional grant for the Washington University Institute of Clinical and Translational Sciences (UL1RR024992) from the National Institutes of Health (Bethesda, Maryland), and the American Heart Association (09CRP2240001; Dallas, Texas) (to Dr. Nagele). Dr. Nagele reports receiving research support from Roche Diagnostics (Indianapolis, Indiana).
Appendix 1: Glossary
- Allele
A version of a gene. Usually two or more versions exist.
- Codon
Sequence of three nucleotides of DNA that encodes a single amino acid.
- Exon
Nucleic acid sequence, usually within a gene, that is transcribed into messenger RNA and protein often after splicing which removes introns.
- Gene
Commonly defined as a stretch of DNA that encodes for a protein or RNA; in humans, genes often consist of multiple exons and introns and may span tens of thousands of base pairs.
- Intron
Nucleic acid sequence within a gene that is removed before the gene is transcribed into protein by splicing.
- Locus
Specific position on a chromosome
- Mutation
Rare changes in the DNA sequence; in classic genetics mutations are often associated with specific traits.
- Nonsynonymous
a DNA substitution that causes a change in the amino acid sequence of a protein.
- Polymorphism
A variation in DNA sequence as compared to a “reference” sequence (usually the more common allele). Typically, polymorphisms are so noted when the frequency is greater than 1% in a given population. A single-nucleotide polymorphism changes only a single nucleotide and is the most common form of genetic variation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work should be attributed to the Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri.
References
- 1.Fleischmann KE, Beckman JA, Buller CE, Calkins H, Fleisher LA, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Robb JF, Valentine RJ. ACCF/AHA Focused Update on Perioperative Beta Blockade. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:e169–e276. doi: 10.1161/CIRCULATIONAHA.109.192689. [DOI] [PubMed] [Google Scholar]
- 2.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2007;116:e418–e499. doi: 10.1161/CIRCULATIONAHA.109.192690. [DOI] [PubMed] [Google Scholar]
- 3.POISE Study Group. Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, Xavier D, Chrolavicius S, Greenspan L, Pogue J, Pais P, Liu L, Xu S, Málaga G, Avezum A, Chan M, Montori VM, Jacka M, Choi P. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 4.Poldermans D, Devereaux PJ. The experts debate: Perioperative beta-blockade for noncardiac surgery—proven safe or not? Cleve Clin J Med. 2009;76:S84–S92. doi: 10.3949/ccjm.76.s4.14. [DOI] [PubMed] [Google Scholar]
- 5.Dorn GW, Liggett SB. Mechanisms of pharmacogenomic effects of genetic variation within the cardiac adrenergic network in heart failure. Mol Pharmacol. 2009;76:466–480. doi: 10.1124/mol.109.056572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liggett SB. Polymorphisms of adrenergic receptors: Variations on a theme. Assay Drug Dev Technol. 2003;1:317–326. doi: 10.1089/15406580360545134. [DOI] [PubMed] [Google Scholar]
- 7.Shin J, Johnson JA. Pharmacogenetics of β-blockers. Pharmacotherapy. 2007;27:874–887. doi: 10.1592/phco.27.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small KM, McGraw DW, Liggett SB. Pharmacology and Physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol. 2003;43:381–411. doi: 10.1146/annurev.pharmtox.43.100901.135823. [DOI] [PubMed] [Google Scholar]
- 9.Bijl MJ, Visser LE, van Schaik RHN, Kors JA, Witteman JCM, Hofman A, Vulto AG, van Gelder T, Stricker B. Genetic variation in the CYP2D6 gene is associated with a lower heart rate and blood pressure in β blocker users. Clin Pharmacol Ther. 2008;85:45–50. doi: 10.1038/clpt.2008.172. [DOI] [PubMed] [Google Scholar]
- 10.Goryachkina K, Burbello A, Boldueva S, Babak S, Bergman U, Bertilsson L. CYP2D6 is a major determinant of metoprolol disposition and effects in hospitalized Russian patients treated for acute myocardial infarction. Eur J Clin Pharmacol. 2008;64:1163–1173. doi: 10.1007/s00228-008-0525-3. [DOI] [PubMed] [Google Scholar]
- 11.Rau T, Wuttke H, Michels LM, Werner U, Bergmann K, Kreft M, Fromm MF, Eschenhagen T. Impact of the CYP2D6 genotype on the clinical effects of metoprolol: A prospective longitudinal study. Clin Pharmacol Ther. 2008;85:269–272. doi: 10.1038/clpt.2008.218. [DOI] [PubMed] [Google Scholar]
- 12.Wuttke H, Rau T, Heide R, Bergmann K, Bohm M, Weil J, Werner D, Eschenhagen T. Increased frequency of cytochrome P450 2D6 poor metabolizers among patients with metoprolol-associated adverse effects. Clin Pharmacol Ther. 2002;72:429–437. doi: 10.1067/mcp.2002.127111. [DOI] [PubMed] [Google Scholar]
- 13.Lennard MS, Silas JH, Freestone S, Ramsay LE, Tucker GT, Woods HF. Oxidation phenotype--a major determinant of metoprolol metabolism and response. N Engl J Med. 1982;307:1558–1560. doi: 10.1056/NEJM198212163072505. [DOI] [PubMed] [Google Scholar]
- 14.POBBLE Trial Investigators. Perioperative β-blockade (Pobble) for patients undergoing infrarenal vascular surgery: Results of a randomized double-blind controlled trial. J Vasc Surg. 2005;41:602–609. doi: 10.1016/j.jvs.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Juul AB, Wetterslev J, Gluud C, Kofoed-Enevoldsen A, Jensen G, Callesen T, Norgaard P, Fruergaard K, Bestle M, Vedelsdal R, Miran A, Jacobsen J, Roed J, Mortensen M-B, Jorgensen L, Jorgensen J, Rovsing M-L, Petersen PL, Pott F, Haas M, Albret R, Nielsen LL, Johansson G, Stjernholm P, Molgaard Y, Foss NB, Elkjaer J, Dehlie B, Boysen K, Zaric D, Munksgaard A, Madsen JB, Oberg B, Khanykin B, Blemmer T, Yndgaard S, Perko G, Wang LP, Winkel P, Hilden J, Jensen P, Salas N DIPOM Trial Group. Effect of perioperative β blockade in patients with diabetes undergoing major non-cardiac surgery: Randomised placebo controlled, blinded multicentre trial. BMJ. 2006;332:1482. doi: 10.1136/bmj.332.7556.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace A, Layug B, Tateo I, Li J, Hollenberg M, Browner W, Miller D, Mangano DT. Prophylactic atenolol reduces postoperative myocardial ischemia. Anesthesiology. 1998;88:7–17. doi: 10.1097/00000542-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Raymer K, Butler r, Parlow J, Roberts R. The effects of perioperative β-blockade: Results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152:983–990. doi: 10.1016/j.ahj.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Zaugg M, Bestmann L, Wacker J, Lucchinetti E, Boltres A, Schulz C, Hersberger M, Kälin G, Furrer L, Hofer C, Blumenthal S, Müller A, Zollinger A, Spahn DR, Borgeat A. Adrenergic receptor genotype but not perioperative bisoprolol therapy may determine cardiovascular outcome in at-risk patients undergoing surgery with spinal block: The Swiss Beta Blocker in Spinal Anesthesia (BBSA) Study: A double-blinded, placebo-controlled, multicenter trial with 1-year follow-up. Anesthesiology. 2007;107:33–44. doi: 10.1097/01.anes.0000267530.62344.a4. [DOI] [PubMed] [Google Scholar]
- 19.Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: A review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J. 2005;173:627–634. doi: 10.1503/cmaj.050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation. 2009;119:2936–2944. doi: 10.1161/CIRCULATIONAHA.108.828228. [DOI] [PubMed] [Google Scholar]
- 21.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 22.Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology. 1998;88:572–578. doi: 10.1097/00000542-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Landesberg G, Mosseri M, Zahger D, Wolf Y, Perouansky M, Anner H, Drenger B, Hasin Y, Berlatzky Y, Weissman C. Myocardial infarction after vascular surgery: The role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37:1839–1845. doi: 10.1016/s0735-1097(01)01265-7. [DOI] [PubMed] [Google Scholar]
- 24.Le Manach Y, Perel A, Coriat P, Godet G, Bertrand M, Riou B. Early and delayed myocardial infarction after abdominal aortic surgery. Anesthesiology. 2005;102:885–891. doi: 10.1097/00000542-200505000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Landesberg G, Vesselov Y, Einav S, Goodman S, Sprung CL, Weissman C. Myocardial ischemia, cardiac troponin, and long-term survival of high-cardiac risk critically ill intensive care unit patients. Crit Care Med. 2005;33:1281–1287. doi: 10.1097/01.ccm.0000166607.22550.87. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang P, Gerstenblith G, Furman WR, Golueke PJ, Gottlieb SO. Frequency and significance of early postoperative silent myocardial ischemia in patients having peripheral vascular surgery. Am J Cardiol. 1989;64:1113–1116. doi: 10.1016/0002-9149(89)90862-x. [DOI] [PubMed] [Google Scholar]
- 27.Raby KE, Barry J, Creager MA, Cook EF, Weisberg MC, Goldman L. Detection and significance of intraoperative and postoperative myocardial ischemia in peripheral vascular surgery. JAMA. 1992;268:222–227. [PubMed] [Google Scholar]
- 28.Thygesen K, Alpert JS, White HD on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal Definition of Myocardial Infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 29.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 30.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 31.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 32.Le Manach Y, Coriat P, Collard CD, Riedel B. Statin therapy within the perioperative period. Anesthesiology. 2008;108:1141–1146. doi: 10.1097/ALN.0b013e318173ef8e. [DOI] [PubMed] [Google Scholar]
- 33.Ouattara A, Benhaoua H, Le Manach Y, Mabrouk-Zerguini N, Itani O, Osman A, Landi M, Riou B, Coriat P. Perioperative statin therapy is associated with a significant and dose-dependent reduction of adverse cardiovascular outcomes after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2009;23:633–638. doi: 10.1053/j.jvca.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Adams JE, Sicard GA, Allen BT, Bridwell KH, Lenke LG, Davila-Roman VG, Bodor GS, Ladenson JH, Jaffe AS. Diagnosis of perioperative myocardial infarction with measurement of cardiac troponin I. N Engl J Med. 1994;330:670–674. doi: 10.1056/NEJM199403103301003. [DOI] [PubMed] [Google Scholar]
- 35.Bursi F, Babuin L, Barbieri A, Politi L, Zennaro M, Grimaldi T, Rumolo A, Gargiulo M, Stella A, Modena MG, Jaffe AS. Vascular surgery patients: Perioperative and long-term risk according to the ACC/AHA guidelines, the additive role of post-operative troponin elevation. Eur Heart J. 2005;26:2448–2456. doi: 10.1093/eurheartj/ehi430. [DOI] [PubMed] [Google Scholar]
- 36.Kim LJ, Martinez EA, Faraday N, Dorman T, Fleisher LA, Perler BA, Williams GM, Chan D, Pronovost PJ. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation. 2002;106:2366–2371. doi: 10.1161/01.cir.0000036016.52396.bb. [DOI] [PubMed] [Google Scholar]
- 37.Landesberg G, Mosseri M, Shatz V, Akopnik I, Bocher M, Mayer M, Anner H, Berlatzky Y, Weissman C. Cardiac troponin after major vascular surgery: The role of perioperative ischemia, preoperative thallium scanning, and coronary revascularization. J Am Coll Cardiol. 2004;44:569–575. doi: 10.1016/j.jacc.2004.03.073. [DOI] [PubMed] [Google Scholar]
- 38.Landesberg G, Mosseri M, Wolf Y, Vesselov Y, Weissman C. Perioperative myocardial ischemia and infarction: Identification by continuous 12-lead electrocardiogram with online ST-segment monitoring. Anesthesiology. 2002;96:264–270. doi: 10.1097/00000542-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Landesberg G, Shatz V, Akopnik I, Wolf YG, Mayer M, Berlatzky Y, Weissman C, Mosseri M. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol. 2003;42:1547–1554. doi: 10.1016/j.jacc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Jimenez F, Goldman L, Sacks DB, Thomas EJ, Johnson PA, Cook EF, Lee TH. Prognostic value of cardiac troponin T after noncardiac surgery: 6-month follow-up data. J Am Coll Cardiol. 1997;29:1241–1245. doi: 10.1016/s0735-1097(97)82754-4. [DOI] [PubMed] [Google Scholar]
- 41.Martinez EA, Nass CM, Jermyn RM, Rosenbaum SH, Akhtar S, Chan DW, Malkus H, Weiss JL, Fleisher LA. Intermittent cardiac troponin-I screening is an effective means of surveillance for a perioperative myocardial infarction. J Cardiothorac Vasc Anesth. 2005;19:577–582. doi: 10.1053/j.jvca.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 42.ISIS-1. Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. First International Study of Infarct Survival Collaborative Group. Lancet. 1986;2:57–66. [PubMed] [Google Scholar]
- 43.The MIAMI Trial Research Group. Metoprolol in acute myocardial infarction (MIAMI). A randomised placebo-controlled international trial. The MIAMI Trial Research Group. Eur Heart J. 1985;6:199–226. [PubMed] [Google Scholar]
- 44.Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335:1713–1720. doi: 10.1056/NEJM199612053352301. [DOI] [PubMed] [Google Scholar]
- 45.Wallace A, Layug B, Tateo I, Li J, Hollenberg M, Browner W, Miller D, Mangano DT. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology. 1998;88:7–17. doi: 10.1097/00000542-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Poldermans D, Boersma E, Bax JJ, Thomson IR, van de Ven LLM, Blankensteijn JD, Baars HF, Yo T-I, Trocino G, Vigna C, Roelandt JRTC, van Urk H, Fioretti PM, Paelinck B. The Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study G: The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341:1789–1794. doi: 10.1056/NEJM199912093412402. [DOI] [PubMed] [Google Scholar]
- 47.Eagle KA, Berger PB, Calkins H, Chaitman BR, Ewy GA, Fleischmann KE, Fleisher LA, Froehlich JB, Gusberg RJ, Leppo JA, Ryan T, Schlant RC, Winters WL, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Jacobs AK, Hiratzka LF, Russell RO, Smith SC. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery--executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) J Am Coll Cardiol. 2002;39:542–553. doi: 10.1016/s0735-1097(01)01788-0. [DOI] [PubMed] [Google Scholar]
- 48.Devereaux PJ, Beattie WS, Choi PTL, Badner NH, Guyatt GH, Villar JC, Cina CS, Leslie K, Jacka MJ, Montori VM, Bhandari M, Avezum A, Cavalcanti AB, Giles JW, Schricker T, Yang H, Jakobsen C-J, Yusuf S. How strong is the evidence for the use of perioperative β blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ. 2005;331:313–321. doi: 10.1136/bmj.38503.623646.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative β blockers in patients having non-cardiac surgery: A meta-analysis. Lancet. 2008;372:1962–1976. doi: 10.1016/S0140-6736(08)61560-3. [DOI] [PubMed] [Google Scholar]
- 50.Ellenberger C, Tait G, Beattie WS. Chronic β blockade is associated with a better outcome after elective noncardiac surgery than acute β blockade: A single-center propensity-matched cohort study. Anesthesiology. 2011;114:817–823. doi: 10.1097/ALN.0b013e31820fca0b. [DOI] [PubMed] [Google Scholar]
- 51.Wallace AW, Au S, Cason BA. Association of the pattern of use of perioperative β-blockade and postoperative mortality. Anesthesiology. 2010;113:794–805. doi: 10.1097/ALN.0b013e3181f1c061. [DOI] [PubMed] [Google Scholar]
- 52.Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 53.Schelb V, Gobel I, Khairallah L, Zhou H, Cox SL, Trendelenburg AU, Hein L, Starke K. Postnatal development of presynaptic receptors that modulate noradrenaline release in mice. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:359–371. doi: 10.1007/s002100100455. [DOI] [PubMed] [Google Scholar]
- 54.Bristow MR, Murphy GA, Krause-Steinrauf H, Anderson JL, Carlquist JF, Thaneemit-Chen S, Krishnan V, Abraham WT, Lowes BD, Port JD, Davis GW, Lazzeroni LC, Robertson AD, Lavori PW, Liggett SB. An a2C-adrenergic receptor polymorphism alters the norepinephrine-lowering effects and therapeutic response of the β-blocker bucindolol in chronic heart failure. Circ Heart Fail. 2010;3:21–28. doi: 10.1161/CIRCHEARTFAILURE.109.885962. [DOI] [PubMed] [Google Scholar]
- 55.Small KM, Liggett SB. Identification and functional characterization of α2-adrenoceptor polymorphisms. Trends Pharmacol Sci. 2001;22:471–477. doi: 10.1016/s0165-6147(00)01758-2. [DOI] [PubMed] [Google Scholar]
- 56.Small KM, Forbes SL, Brown KM, Liggett SB. An asn to lys polymorphism in the third intracellular loop of the human alpha 2A-adrenergic receptor imparts enhanced agonist-promoted Gi coupling. J Biol Chem. 2000;275:38518–38523. doi: 10.1074/jbc.M004550200. [DOI] [PubMed] [Google Scholar]
- 57.Small KM, Forbes SL, Rahman FF, Bridges KM, Liggett SB. A four Amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem. 2000;275:23059–23064. doi: 10.1074/jbc.M000796200. [DOI] [PubMed] [Google Scholar]
- 58.Gerson MC, Wagoner LE, McGuire N, Liggett SB. Activity of the uptake-1 norepinephrine transporter as measured by I-123 MIBG in heart failure patients with a loss-of-function polymorphism of the presynaptic alpha2C-adrenergic receptor. J Nucl Cardiol. 2003;10:583–589. doi: 10.1016/j.nuclcard.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347:1135–1142. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 60.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human β1-adrenergic receptor. J Biol Chem. 1999;274:12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 62.Mialet Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, Schwartz A, Dorn GW, Liggett SB. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9:1300–1305. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 63.Liggett SB, Mialet-Perez J, Thaneemit-Chen S, Weber SA, Greene SM, Hodne D, Nelson B, Morrison J, Domanski MJ, Wagoner LE, Abraham WT, Anderson JL, Carlquist JF, Krause-Steinrauf HJ, Lazzeroni LC, Port JD, Lavori PW, Bristow MR. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A. 2006;103:11288–11293. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rathz DA, Gregory KN, Fang Y, Brown KM, Liggett SB. Hierarchy of polymorphic variation and desensitization permutations relative to β1- and β2-adrenergic receptor signaling. J Biol Chem. 2003;278:10784–10789. doi: 10.1074/jbc.M206054200. [DOI] [PubMed] [Google Scholar]
- 65.Wagoner LE, Craft LL, Zengel P, McGuire N, Rathz DA, Dorn GW, 2nd, Liggett SB. Polymorphisms of the β1-adrenergic receptor predict exercise capacity in heart failure. Am Heart J. 2002;144:840–846. doi: 10.1067/mhj.2002.125325. [DOI] [PubMed] [Google Scholar]
- 66.Sehnert AJ, Daniels SE, Elashoff M, Wingrove JA, Burrow CR, Horne B, Muhlestein JB, Donahue M, Liggett SB, Anderson JL, Kraus WE. Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol. J Am Coll Cardiol. 2008;52:644–651. doi: 10.1016/j.jacc.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 67.Johnson JA, Zineh I, Puckett BJ, McGorray SP, Yarandi HN, Pauly DF. Beta 1-adrenergic receptor polymorphisms and antihypertensive response to metoprolol. Clin Pharmacol Ther. 2003;74:44–52. doi: 10.1016/S0009-9236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 68.Pacanowski MA, Gong Y, Cooper-DeHoff RM, Schork NJ, Shriver MD, Langaee TY, Pepine CJ, Johnson JA. β-adrenergic receptor gene polymorphisms and β-blocker treatment outcomes in hypertension. Clin Pharmacol Ther. 2008;84:715–721. doi: 10.1038/clpt.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rathz DA, Brown KM, Kramer LA, Liggett SB. Amino acid 49 polymorphisms of the human beta1-adrenergic receptor affect agonist-promoted trafficking. J Cardiovasc Pharmacol. 2002;39:155–160. doi: 10.1097/00005344-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Green SA, Cole G, Jacinto M, Innis M, Liggett SB. A polymorphism of the human beta 2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J Biol Chem. 1993;268:23116–23121. [PubMed] [Google Scholar]
- 71.Turki J, Lorenz JN, Green SA, Donnelly ET, Jacinto M, Liggett SB. Myocardial signaling defects and impaired cardiac function of a human beta 2-adrenergic receptor polymorphism expressed in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:10483–10488. doi: 10.1073/pnas.93.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagoner LE, Craft LL, Singh B, Suresh DP, Zengel PW, McGuire N, Abraham WT, Chenier TC, Dorn GW, 2nd, Liggett SB. Polymorphisms of the beta(2)-adrenergic receptor determine exercise capacity in patients with heart failure. Circ Res. 2000;86:834–840. doi: 10.1161/01.res.86.8.834. [DOI] [PubMed] [Google Scholar]
- 73.Liggett SB, Wagoner LE, Craft LL, Hornung RW, Hoit BD, McIntosh TC, Walsh RA. The Ile164 beta2-adrenergic receptor polymorphism adversely affects the outcome of congestive heart failure. J Clin Invest. 1998;102:1534–1539. doi: 10.1172/JCI4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of beta 2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 1995;13:25–33. doi: 10.1165/ajrcmb.13.1.7598936. [DOI] [PubMed] [Google Scholar]
- 75.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 76.Liggett SB, Hall IP. β2-adrenergic receptor polymorphisms and asthmatic phenotypes. In: Postma DS, Weiss ST, editors. Genetics of Asthma and COPD. New York: Taylor & Francis; 2006. pp. 299–316. [Google Scholar]
- 77.de Groote P, Lamblin N, Helbecque N, Mouquet F, Mc Fadden E, Hermant X, Amouyel P, Dallongeville J, Bauters C. The impact of beta-adrenoreceptor gene polymorphisms on survival in patients with congestive heart failure. Eur J Heart Fail. 2005;7:966–973. doi: 10.1016/j.ejheart.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 78.Kaye DM, Smirk B, Williams C, Jennings G, Esler M, Holst D. β-adrenoceptor genotype influences the response to carvedilol in patients with congestive heart failure. Pharmacogenetics. 2003;13:379–382. doi: 10.1097/00008571-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Raake PW, Koch WJ, Most P. Polymorphisms present in G-protein-coupled receptor kinases and their effect on β-blocker treatment. Pharmacogenomics. 2011;12:295–297. doi: 10.2217/pgs.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Redfern G, Rodseth RN, Biccard BM. Outcomes in vascular surgical patients with isolated postoperative troponin leak: A meta-analysis. Anaesthesia. 2011;66:604–610. doi: 10.1111/j.1365-2044.2011.06763.x. [DOI] [PubMed] [Google Scholar]
- 81.Dessy C, Balligand JL. Beta3-adrenergic receptors in cardiac and vascular tissues emerging concepts and therapeutic perspectives. Adv Pharmacol. 2010;59:135–163. doi: 10.1016/S1054-3589(10)59005-7. [DOI] [PubMed] [Google Scholar]
- 82.Birenbaum A, Tesse A, Loyer X, Michelet P, Andriantsitohaina R, Heymes C, Riou B, Amour J. Involvement of β3-adrenoceptor in altered β-adrenergic response in senescent heart: Role of nitric oxide synthase 1–derived nitric oxide. Anesthesiology. 2008;109:1045–1053. doi: 10.1097/ALN.0b013e31818d7e5a. [DOI] [PubMed] [Google Scholar]
- 83.Zaugg M, Schaub MC. β3-adrenergic receptor subtype signaling in senescent heart: Nitric oxide intoxication or “endogenous” β blockade for protection? Anesthesiology. 2008;109:956–959. doi: 10.1097/ALN.0b013e31818d4942. [DOI] [PubMed] [Google Scholar]
- 84.Pietri-Rouxel F, St John Manning B, Gros J, Strosberg AD. The biochemical effect of the naturally occurring Trp64-->Arg mutation on human beta3-adrenoceptor activity. Eur J Biochem. 1997;247:1174–1179. doi: 10.1111/j.1432-1033.1997.01174.x. [DOI] [PubMed] [Google Scholar]
- 85.Candelore MR, Deng L, Tota LM, Kelly LJ, Cascieri MA, Strader CD. Pharmacological characterization of a recently described human beta 3-adrenergic receptor mutant. Endocrinology. 1996;137:2638–2641. doi: 10.1210/endo.137.6.8641219. [DOI] [PubMed] [Google Scholar]
- 86.Pacanowski MA, Zineh I, Li H, Johnson BD, Cooper-DeHoff RM, Bittner V, McNamara DM, Sharaf BL, Merz CN, Pepine CJ, Johnson JA. Adrenergic gene polymorphisms and cardiovascular risk in the NHLBI-sponsored Women's Ischemia Syndrome Evaluation. J Transl Med. 2008;6:11. doi: 10.1186/1479-5876-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Luk AO, Ma RC, So WY, Tam CH, Ng MC, Yang X, Baum L, Lam V, Tong PC, Chan JC. Independent predictive roles of eotaxin Ala23Thr, paraoxonase 2 Ser311Cys and beta-adrenergic receptor Trp64Arg polymorphisms on cardiac disease in type 2 diabetes--an 8-year prospective cohort analysis of 1297 patients. Diabet Med. 2010;27:376–383. doi: 10.1111/j.1464-5491.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- 88.Amour J, Loyer X, Le Guen M, Mabrouk N, David J-S, Camors E, Carusio N, Vivien B, Andriantsitohaina R, Heymes C, Riou B. Altered contractile response due to increased β3-adrenoceptor stimulation in diabetic cardiomyopathy: The role of nitric oxide synthase 1–derived nitric oxide. Anesthesiology. 2007;107:452–460. doi: 10.1097/01.anes.0000278909.40408.24. [DOI] [PubMed] [Google Scholar]
- 89.Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, Diwan A, Martini JS, Sparks L, Parekh RR, Spertus JA, Koch WJ, Kardia SLR, Dorn Ii GW. A GRK5 polymorphism that inhibits β-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–517. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frey UH, Adamzik M, Kottenberg-Assenmacher E, Jakob H, Manthey I, Broecker-Preuss M, Bergmann L, Heusch G, Siffert W, Peters J, Leineweber K. A novel functional haplotype in the human GNAS gene alters Gαs expression, responsiveness to β-adrenoceptor stimulation, and peri-operative cardiac performance. Eur Heart J. 2009;30:1402–1410. doi: 10.1093/eurheartj/ehn572. [DOI] [PubMed] [Google Scholar]
- 91.Gardiner SJ, Begg EJ. Pharmacogenetics, drug-metabolizing enzymes, and clinical practice. Pharmacol Rev. 2006;58:521–590. doi: 10.1124/pr.58.3.6. [DOI] [PubMed] [Google Scholar]
- 92.Owen RP, Sangkuhl K, Klein TE, Altman RB. Cytochrome P450 2D6. Pharmacogenet Genomics. 2009;19:559–562. doi: 10.1097/FPC.0b013e32832e0e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zanger U, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: Overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:23–37. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]
- 94.Daly AK, Brockmoller J, Broly F, Eichelbaum M, Evans WE, Gonzalez FJ, Huang JD, Idle JR, Ingelman-Sundberg M, Ishizaki T, Jacqz-Aigrain E, Meyer UA, Nebert DW, Steen VM, Wolf CR, Zanger UM. Nomenclature for human CYP2D6 alleles. Pharmacogenetics. 1996;6:193–201. doi: 10.1097/00008571-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 95.Ismail R, Teh LK. The relevance of CYP2D6 genetic polymorphism on chronic metoprolol therapy in cardiovascular patients. J Clin Pharm Ther. 2006;31:99–109. doi: 10.1111/j.1365-2710.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 96.Nozawa T, Taguchi M, Tahara K, Hashimoto Y, Igarashi N, Nonomura M, Kato B-I, Igawa A, Inoue H. Influence of CYP2D6 genotype on metoprolol plasma concentration and β-adrenergic inhibition during long-term treatment: A comparison with bisoprolol. J Cardiovasc Pharmacol. 2005;46:713–720. doi: 10.1097/01.fjc.0000184117.76188.68. [DOI] [PubMed] [Google Scholar]
- 97.Rau Ta, Heide Ra, Bergmann Kb, Wuttke Ha, Werner Ua, Feifel Na, Eschenhagen Ta. Effect of the CYP2D6 genotype on metoprolol metabolism persists during long-term treatment. Pharmacogenetics. 2002;12:465–472. doi: 10.1097/00008571-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: Pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116:496–526. doi: 10.1016/j.pharmthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet. 2006;368:704. doi: 10.1016/S0140-6736(06)69255-6. [DOI] [PubMed] [Google Scholar]
- 100.Crewe HK, Lennard MS, Tucker GT, Woods FR, Haddock RE. The effect of selective serotonin re-uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol. 1992;34:262–265. doi: 10.1111/j.1365-2125.1992.tb04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Transon C, Leemann T, Dayer P. In vitro comparative inhibition profiles of major human drug metabolising cytochrome P450 isozymes (CYP2C9, CYP2D6 and CYP3A4) by HMG-CoA reductase inhibitors. Eur J Clin Pharmacol. 1996;50:209–215. doi: 10.1007/s002280050094. [DOI] [PubMed] [Google Scholar]
- 102.Lanfear DE, Jones PG, Marsh S, Cresci S, McLeod HL, Spertus JA. β2-adrenergic receptor genotype and survival among patients receiving β-blocker therapy after an acute coronary syndrome. JAMA. 2005;294:1526–1533. doi: 10.1001/jama.294.12.1526. [DOI] [PubMed] [Google Scholar]
- 103.Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of β-blockers may contribute to heterogeneous results in trials of perioperative β-blockade. Anesthesiology. 2010;113:585–592. doi: 10.1097/ALN.0b013e3181e73eea. [DOI] [PubMed] [Google Scholar]
- 104.Wallace AW, Au S, Cason BA. Perioperative β-blockade: Atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology. 2011;114:824–836. doi: 10.1097/ALN.0b013e3182110e83. [DOI] [PubMed] [Google Scholar]