Abstract
The extracellular levels of the neurotransmitter glycine in the brain are tightly regulated by the glycine transporter 1 (GlyT1) and the clearance rate for glycine depends on its rate of transport and the levels of cell surface GlyT1. Over the years, it has been shown that PKC tightly regulates the activity of several neurotransmitter transporters. In the present work, by stably expressing three N-terminus GlyT1 isoforms in porcine aortic endothelial cells and assaying for [32P]-orthophosphate metabolic labeling, we demonstrated that the isoforms GlyT1a, GlyT1b, and GlyT1c were constitutively phosphorylated, and that phosphorylation was dramatically enhanced, in a time dependent fashion, after PKC activation by phorbol ester. The phosphorylation was PKC-dependent, since pre-incubation of the cells with bisindolylmaleimide I, a selective PKC inhibitor, abolished the phorbol ester-induced phosphorylation. Blotting with specific anti-phospho-tyrosine antibodies did not yield any signal that could correspond to GlyT1 tyrosine phosphorylation, suggesting that the phosphorylation occurs at serine and/or threonine residues. In addition, a 23-40% -inhibition on Vmax was obtained by incubation with phorbol ester without a significant change on the apparent Km value. Furthermore, pre-incubation of the cells with the selective PKCα/β inhibitor Gö6976 abolished the downregulation effect of phorbol ester on uptake and phosphorylation, whereas the selective PKCβ inhibitors (PKCβ inhibitor or LY333531) prevented the phosphorylation without affecting glycine uptake, defining a specific role of classical PKC on GlyT1 uptake and phosphorylation. Taken together, these data suggest that phosphorylation that conventional PKCα/β regulates the uptake of glycine, whereas PKCβ is responsible for GlyT1 phosphorylation.
Keywords: phosphorylation, glycine, transporter, PKC
1. Introduction
The neurotransmitter glycine plays an important role at inhibitory synapses in several regions of the central nervous system, where it regulates a variety of sensory and motor information (Zafra et al., 1997; Gomeza et al., 2003a). In addition, along with glutamate, it is an obligatory co-agonist at excitatory synapses containing the N-methyl-d-aspartate (NMDA) receptors (Nong et al., 2003; Gabernet et al., 2005). At both types of synapses, the extracellular concentration of glycine is tightly modulated by the action of the plasma membrane glycine transporter 1 (GlyT1), which is responsible for the rapid re-uptake of glycine back into the presynaptic terminals and surrounding glia cells through a Na+/Cl--dependent symport mechanism. In addition to GlyT1, the strictly neuronal glycine transporter 2 (GlyT2), encoded by a different gene, participates in regulating glycine availability at glycinergic synapses. This high affinity glycine transport is dependent on the Na+ concentration gradient generated by the plasma membrane Na+/K+-ATPase (Johnston and Iversen, 1971; Aragon et al., 1987). The essential role of GlyT1 and GlyT2 in neurotransmission was revealed by the presence of severe motor and respiratory deficits followed by neonatal mortality in the knockout mice deficient in GlyT1 or GlyT2 (Gomeza et al., 2003a; Gomeza et al., 2003b). The GlyTs belongs to the Na+/Cl--dependent solute carrier SLC6 family of transporters that includes those for GABA, serotonin, dopamine and norepinephrine. Structurally, all members of the family share a similar topology that consist of 12 transmembrane domains, intracellular amino and carboxy-termini, and a large extracellular loop that contains multiple N-glycosylation sites (Olivares et al., 1997; Gether et al., 2006). This topology is supported by the crystal structure of the leucine transporter from Aquifex aeolicus, a bacterial related transporter (Yamashita et al., 2005). The GlyT1 has been shown to exist in five different functional splice variants expressed throughout the brain. Three of these variants differ in the length of the amino-terminal tail, with GlyT1a representing the shortest isoform followed by GlyT1b and GlyT1c. Although the three N-terminal splice variants do not show major differences in their kinetic properties, they display different expression patterns in the central nervous system and peripheral tissues (Kim et al., 1994; Borowsky and Hoffman, 1998; Hanley et al., 2000).
Sequence analysis of most members of the SLC6 family of transporters, including GlyT1, predicts the presence of multiple consensus sites for phosphorylation by several protein kinases. Not surprising, a large number of studies indicate that activation of protein kinase C (PKC) by 4-α-phorbol 12-myristate 13-acetate (PMA) leads to increased phosphorylation of the transporters for dopamine (DAT), norepinephrine (NET) and serotonin (SERT) in brain synaptosomes and cultured model cells expressing recombinant transporter, however it has not been demonstrated for GlyT1or GlyT2 (Sato et al., 1995; Ramamoorthy et al., 1998; Foster et al., 2002; Lin et al., 2003; Jayanthi et al., 2004). The role of transporter phosphorylation is still unclear or unknown for GlyTs; however, recent findings on DAT suggest that phosphorylation is implicated in the PKC-dependent reversal of neurotransmitter transport rather than endocytosis (Kantor and Gnegy, 1998; Cowell et al., 2000; Granas et al., 2003; Khoshbouei et al., 2004; Fog et al., 2006). While reverse transport of glycine has been shown by using whole-cell patch-clamp in stably expressing GlyT1b CHO cells and synaptosomes containing GlyT2, whether PKC-dependent phosphorylation plays a role in glycine efflux is something that remains to be determined (Roux and Supplisson, 2000; Aubrey et al., 2005; Luccini and Raiteri, 2007).
The PKC's belong to the serine/threonine kinase family implicated in the regulation of a broad number of pathways including membrane trafficking. This kinase family includes at least ten members and it appears that each isoenzyme could have a particular role in the cell. Traditionally, the PKC family is divided into three groups: conventional, novel and atypical PKCs. The basis for this classification is found in the PKC's N-terminal end that is responsible for regulating kinase activity and cofactor binding (Newton, 2010). The conventional PKCs include the α, βI, βII, and γ isoenzymes, and they require diacylglycerol (DAG) or phosphatidylserine (PS) and Ca2+ for their activation. The novel PKCs include ε, δ, θ, and η/L, and they are independent of Ca2+. Activation of both, conventional and novel PKCs can be achieved by binding of DAG or the analog PMA. The atypical PKCs include the isoenzymes ζ, ι/λ, and they require only PS or phosphatidylinositides, but not Ca2+ or DAG/PMA for activation. Although it is well established that PKC is involved in the regulation of most members of the SLC6 family including GlyT1 and GlyT2, whether PKC directly phosphorylates transporter remains to be determined, given that other kinases have also been shown to be able to phosphorylate transporter in vitro, such as the Ca2+/calmodulin-dependent kinase α (CaMKIIα) (Fog et al., 2006; Ramamoorthy et al., 2011). In spite of the great amount of information in the literature regarding transporter regulation, the hallmark in the field has been to clarify the relationship between transporter phosphorylation, reduction of transport velocity, ubiquitination and endocytosis. In the present study, we demonstrate that the three GlyT1 isoforms (a, b and c) are phosphorylated in a time- and PKC-dependent fashion. Moreover, by using selective inhibitors for different conventional PKCs, we provide strong experimental evidence to demonstrate that GlyT1 phosphorylation is dependent on the activation of PKCβ in PAE whereas PKCα/β are important for regulating transporter activity. These findings altogether provide evidence that conventional PKCα/β play a regulatory role on controlling several of the GlyT1 properties.
2. Experimental Procedures
2.1. Chemicals and antibodies
[3H]-glycine and 32P-orthophosphate were purchased from Perkin Elmer Life Scientific (Boston, MA). Antibody to α-actin, Flag-M2 agarose beads, PMA, N-ethylmaleimide, KN-92, sodium fluoride, phosphatase inhibitor cocktail, sodium orthovanadate, phenylmethanesulfonyl fluoride, aprotinin and leupeptine were from Sigma (St. Louis, MO). The PKA inhibitor KT5720 was kindly provided by Dr. Robert Kirken's laboratory (University of Texas at El Paso). Bisindolylmaleimide I (BIM), HDBBE (2,2′,3,3′,4,4′-Hexahydroxy-1,1′-biphenyl-6,6′-dimethanol Dimethyl Ether), rottlerin, KN-93, Gö6976, Gö6983, LY333531 and PKCβ inhibitor (3-(1-(3-Imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione) were from EMD Biochemicals (San Diego, CA). HRP-labeled secondary antibodies were from Promega (Madison, WI). Rabbit polyclonal antibodies to GlyT1 were kindly donated by Drs. Detlev Boison and Dietmar Benke (University of Zurich). Monoclonal mouse to PKCβI (E3) and the polyclonal PKCβII (C–16) and PKCβII (C–18) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal mouse antibodies to PKCα was from Abcam Inc. (Cambridge, MA), PKCβ and EEA1 (cat# 123-324) were from BD Bioscience (San Jose CA). Monoclonal antibody specific to multiple phosphotyrosine (pY) was from Invitrogen (Carlsbad, CA).
2.2. Plasmid constructs
The cDNA encoding for the mouse glycine transporter 1a (GlyT1a) was purchased from the American Type Culture Collection (ATCC), and the human isoforms GlyT1b and GlyT1c were kindly donated by Bruno Giros (INSERM, France). The three isoforms were tagged with Flag and 10X-His epitopes at their N-termini (FH-GlyT1) as previously described (Miranda et al., 2005). The resulting constructs were cloned into pCDNA 3.1 and the DNA sequence was verified by automatic deoxynucleotide sequencing.
2.3. Cell culture and transfections
Porcine aortic endothelial (PAE) cells were kindly provided by Dr. A. Sorkin (University of Colorado at Denver) and grown at 37°C and 5% CO2 in Ham's F12 medium containing 10% fetal bovine serum (FBS) and antibiotics. PAE cells were grown to 50–80% confluence and transfected with appropriate plasmids using Effectene, according to the manufacturer's recommendations (Qiagen, Hilden, Germany). PAE cells stably expressing FH-GlyT1a, b or c were selected by growing them in the presence of G418 (400 μg/ml). C6 glioma cell were grown in Ham's F-12 Kaighn's modified medium containing 1.25% FBS and 7.5% of horse serum under the same conditions described above.
2.4. Metabolic labeling and GlyT1 purification
Confluent PAE cells expressing GlyT1a, b or c isoforms were incubated in a DMEM-phosphate free media supplemented with 5% dialyzed-FBS for 6 h following the addition of 100-125 μCi of [32P]-orthophosphoric acid/ml. After 2 h incubation, GlyT1 phosphorylation was stimulated by 1 μM PMA for different time intervals (120, 60, 30 and 15 minutes) or inhibited with 1 μM of BIM for 30 min before addition of PMA. As a negative control, parental cells transfected with pCDNA3.1 were incubated with PMA or DMSO. After incubation, cells were washed twice with Ca2+- and Mg2+-free cold phosphate-buffered saline (CMF-PBS) and lysed in a ice-cold-buffer containing 25 mM HEPES, pH 8.0, 100 mM NaCl, 15 mM imidazole, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, 10 mM N-ethymaleimide, 10 mM sodium fluoride, 1X phosphatase inhibitor cocktail, 1 mM sodium orthovanadate, 1 mM phenylmethanesulfonyl fluoride and 10 μg/ml aprotinin and 10 μg/ml leupeptine. Lysates were cleared by centrifugation at 14,000g and soluble GlyT1 was purified by a double affinity chromatography. Briefly, cleared lysates were incubated with Ni-NTA affinity agarose beads for 1 hour and eluted with imidazole. The eluted protein was incubated further with anti-flag affinity agarose beads for 4 h and eluted with 100 mM glycine, pH 3.5 (Miranda et al., 2005). The fractions containing GlyT1 were subjected to 8.0% SDS-PAGE and transferred to a nitrocellulose membrane as previously described. Finally, the nitrocellulose membranes containing 32P-GlyT1 were subjected to autoradiography followed by Western blot using a rabbit polyclonal antibody to GlyT1. Signal intensities were quantitated with ImageJ (NIH).
2.5. Glycine uptake
PAE cells stably expressing the GlyT1 were incubated for 30 min with specific inhibitors of PKA, CaMKII, novel PKCδ, or conventional PKCα/β1, PKCβ, or PKCα/γ; before the addition of 1 μM PMA. After treatment, glycine uptake was measured as previously described with some modifications (Sato et al., 1995). Briefly, confluent cells were washed with 0.25 ml of reaction media containing: 10 mM HEPES pH 7.4, 135 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgSO4 and 10 mM glucose. Uptake was initiated by the addition of reaction media containing 4 μCi [3H]glycine/ml and 200 μM cold glycine for 10 minutes at 37°C. After incubation, cells were washed twice with reaction buffer, and [3H]glycine was extracted with 0.2 N of NaOH. Finally, glycine uptake was determined by scintillation spectroscopy, and protein concentration was determined as described by Bradford (Bradford, 1976). The specific glycine uptake is represented as the difference between the total glycine uptake from GlyT1 expressing cells and glycine uptake by the parental PAE cells transfected with pCDNA 3.1.
2.6. Immunofluorescence and microscopy
The cells grown on glass coverslips were treated with DMSO or 1μM PMA for 60 min at 37°C. After treatment, the cells were washed with CMF-PBS, fixed with freshly prepared 4% paraformaldehyde for 15 min at room temperature and mildly permeabilized using a 3-min incubation in CMF-PBS containing 0.1% Triton X-100 and 0.5% bovine serum albumin at room temperature. The cells were then incubated in CMF-PBS containing 0.5% bovine serum albumin at room temperature for 1-2 h with primary rabbit GlyT1 and mouse EEA1 antibodies, and subsequently incubated for 60 min with secondary antibodies labeled with CY3 or Alexa-488 (Jackson Laboratories, West-Glove, PA). Both primary and secondary antibody solutions were precleared by centrifugation at 100,000 × g for 20 min. After staining, the coverslips were mounted in Mowiol (Calbiochem). Images were acquired with a Zeiss laser scanning confocal microscope (LSM700), controlled with the ZEN 2009 software (Carl Zeiss, New York, NY). Alexa-488 and Cy3 fluorophores were excited with 488 nm and 555 nm lasers respectively, and high resolution optical section images were acquired and processed with ZEN2009. The final arrangement of all images was performed using Photoshop software.
2.7. Statistical analysis
Statistical and kinetic analysis was performed using SigmaPlot® 10 software. Unless otherwise stated, glycine uptake values are represented as the mean of at least three independent experiments, each performed in triplicate +/- average SE, with statistical significance of p<0.05 determined by t- student's paired t test. Signal intensities from western blotting were plotted as the mean +/- SE of at least two independent experiments.
3. Results
3.1. Protein Kinase C-dependent endocytosis and reduction of glycine uptake
It is well documented that the activity of several neurotransmitter transporters is reduced after PKC activation, and this reduction is accompanied by transporter endocytosis into different types of endosomes (Sorkina et al., 2003). To analyze the effect of PKC activation on GlyT1 function, PAE cells stably expressing the GlyT1a, b or c isoforms tagged with Flag and His at the N-terminus (FH-GlyT1) were used in this study (Fig. 1A). It was decided to introduce these short epitope tags at the N-terminus given that tagging this region of the protein in related transporters does not affect the functional properties such as uptake and trafficking. Stable expression in PAE cells and GlyT1 immunostaining followed by confocal microscopy showed that all FH-GlyT1 isoforms trafficked through the biosynthetic pathway and reached the plasma membrane, particularly accumulated in ruffle-like structures (Fig. 1B, see inset). Not surprising, activation of PKC in cells expressing GlyT1a or GlyT1b by 60 min incubation with PMA resulted in redistribution of GlyT1 molecules from the plasma membrane and accumulatation into many type of endosomes, the majority being early endosomes carrying the endosomal antigen 1 (EEA1, Fig. 1). Similar behavior was found for GlyT1c (not shown). In addition, the functional properties of the FH-tagged transporters were assayed by measuring the glycine dependence. As summarized in Table 1, incubation of FH-GlyT1/PAE cells with DMSO resulted in Vmax ranging from 37-41 nmol/min/mg and a Km value of 112-176 μM, values that were consistently similar to those described for non-tagged transporter (Kim et al., 1994; Olivares et al., 1994). In agreement with previous findings obtained by expressing GlyTs in different model cell types, when FH-GlyTs/PAE cells were incubated with phorbol ester a 23-40% reduction in the Vmax (23-31nmol/min/mg) was observed without a significant change in the apparent Km values (Table 1). These results together demonstrate that Flag-, His-tagged-GlyT1 isoforms trafficked to the plasma membrane and are fully functional in PAE cells, and that the addition of the N-terminal epitope tags did not affect the response to PMA and retained similar properties to those described in other expression systems.
Figure 1. Localization of GlyT1 isoforms in PAE cells.
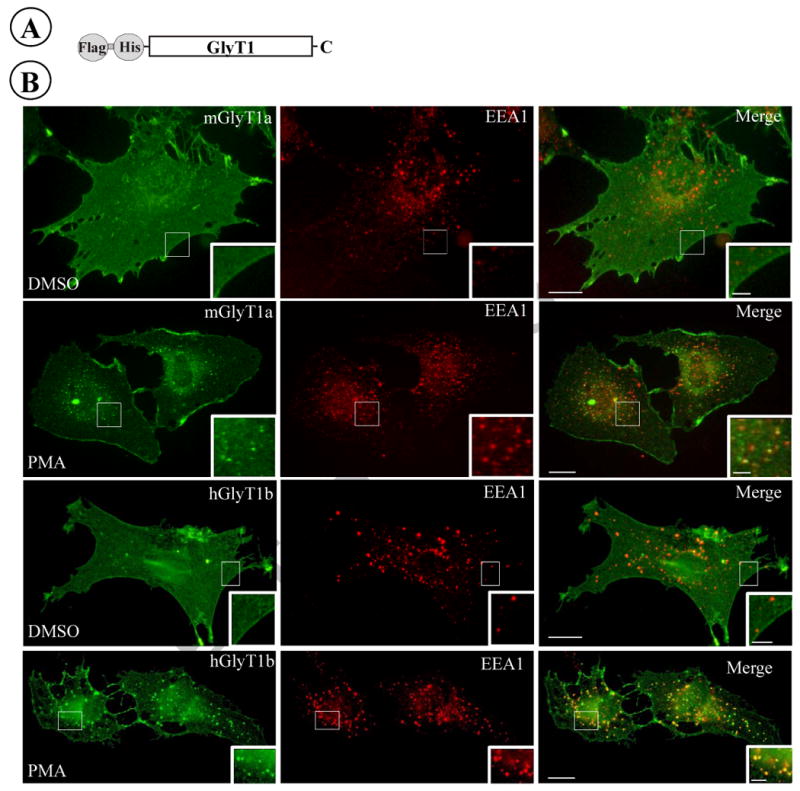
(A) Schematic representation of the N-terminal Flag, His-tagged GlyT1. (B) PAE cells stably expressing FH-GlyT1a or FH-GlyT1b were incubated with DMSO or 1 μM PMA for 60 min at 37°C, fixed with paraformaldehyde, and stained with GlyT1 and EEA1 antibodies followed by incubation with Alexa-488 and Cy3 conjugated secondary antibodies respectively. A representative optical section of single cells is shown from 15-25 cell images representing the cell population; scale bar, 10 μm. Insets represent higher magnification of the area labeled by the rectangle; scale bar, 2.5 μm.
Table I. Kinetic properties of GlyT1 isoforms.
| Transporter | Treatment | Vmax (nmol/min/mg) | Km (μM) |
|---|---|---|---|
| FH-GlyT1a | DMSO | 40 ± 3 | 176 ± 34 |
| PMA | 31 ± 3* | 170 ± 40 | |
| FH-GlyT1b | DMSO | 37 ± 3 | 112 ± 28 |
| PMA | 23 ± 2** | 72 ± 20 | |
| FH-GlyT1c | DMSO | 41 ± 3 | 174 ± 28 |
| PMA | 30 ± 0.9*** | 116 ± 8.8 |
PAE cells stably expressing GlyT1a, b or c isoforms were incubated with DMSO or 1 μM PMA for 1 h, followed by glycine uptake. The assay was performed in the presence of increasing concentrations of cold glycine 10-400 μM and 4 μCi/ml of [3H]glycine at 37°C. Kinetic parameters were calculated using the Michaelis-Menten equation and Sigma Plot 10. Values represent the mean of at least 3 determinations ± SE.
p=0.005,
p=0.008,
p=0.022 compared to the cells treated with vehicle (DMSO).
3.2 PKC activation enhances GlyT1 phosphorylation
Another PKC-dependent event described for DAT, SERT and NET is phosphorylation ((Vaughan et al., 1997; Ramamoorthy et al., 1998; Foster et al., 2002; Jayanthi et al., 2004) The GlyT1 sequence contains multiple canonical serine/threonine phosphorylation sites for PKC, PKA and CaMKII, all located in cytosolic regions of the protein. Although it has been suggested that GlyT1b is phosphorylated in response to PKC activation, there is as yet no experimental evidence to suggest that this post-translational modification takes place in any of the GlyT1 isoforms (Sato et al., 1995). To determine whether GlyT1isoforms were phosphorylated in response to PKC activation, we performed metabolic labeling assays of the FH-GlyT1/PAE cells with [32P]-orthophosphate, followed by stimulation with PMA over different periods of time (0-120 min). After PKC activation by PMA, the GlyT1 was purified by double affinity chromatography and further subjected to SDS-PAGE and autoradiography. As shown in Fig. 2A, B and C, when the transporter was purified from non-stimulated cells a faint, sometimes undetectable, radiolabeled band of 75 kDa was observed, consistent with the molecular weight for GlyT1. By contrast, treatment of the cells with 1 μM PMA induced a time-dependent increase in phosphorylated GlyT1, with more than 2-3 fold increase in the phosphorylation signal after 15 min and reaching a maximum level (3-5 fold increase) at 30-60 min of incubation with PMA, followed by a small reduction in phosphorylation after 120 min. Interestingly, intensity measurement of the phosphorylated band normalized by the total transporter suggest that transporter phosphorylation is transient, with peaks at 30-60 min followed by slow dephosphorylation. The PKC dependence was assayed by incubation of the cells with BIM, a potent and specific inhibitor of conventional, novel and some atypical PKCs. A similar pattern of phosphorylation was obtained for GlyT1 isoforms b and c (Fig. 2B and C). In control experiments, the 75 kDa band was absent in the precipitates obtained from parental PAE cells that were labeled with 32P and no immunoreactive band was detected with GlyT1 antibodies demonstrating the specificity of the radiolabeled band.
Figure 2. Activation of PKC triggers phosphorylation of GlyT1.
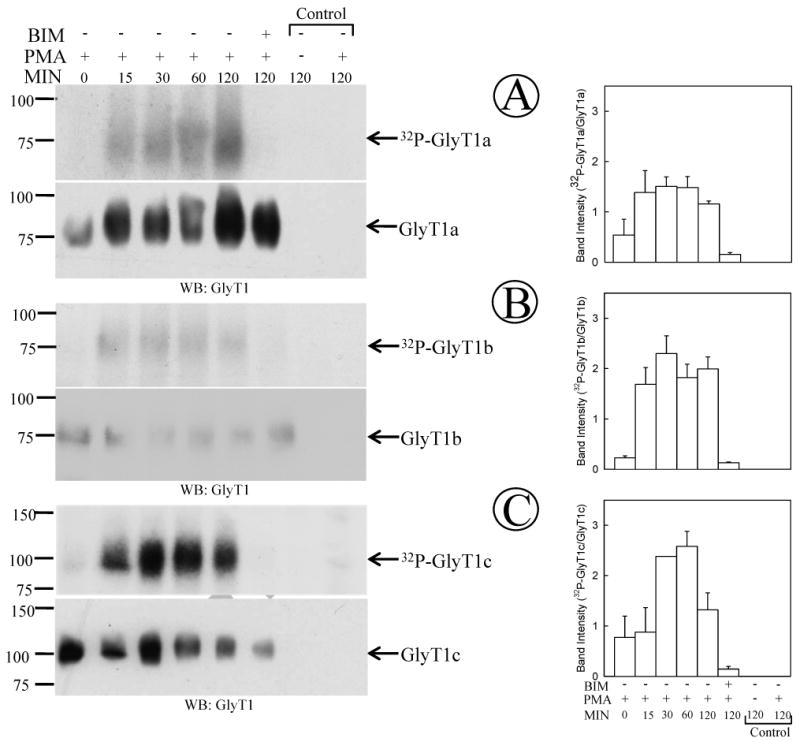
PAE cells expressing GlyT1a, b or c isoforms (pannels A, B and C respectively) were labeled with 125 μCi 32P-orthophosphate/ml as described in “Experimental Procedures” followed by incubation with 1 μM PMA for 0-120 min, or 1 μM BIM followed by the addition of 1 μM PMA. Labeled GlyT1 was purified by tandem affinity chromatography and analyzed by autoradiography and Western blotting with GlyT1 antibodies. The right panel shows the quantification of phosphorylated GlyT1 normalized by total GlyT1 (mean ± SE, n=2-3). (A) GlyT1a, (B) GlyT1b, and (C) GlyT1c. As controls experiments, purification from parental cells is presented.
3.3 Tyrosine residues are not phosphorylation sites on GlyT1
Although serine/threonine phosphorylation is well documented for DAT, SERT and NET, whether tyrosine or serine/threonine residues are responsible for GlyT1 phosphorylation remains unexplored. The GlyT1a sequence contains several tyrosine residues located in the cytoplasmic side, one tyrosine in the third (Y312) and two in the fifth (Y476 and Y483) intracellular loop, and two additional in the C-terminus (Y554 and Y593). To rule out the potential contribution of tyrosine residues on GlyT1 phosphorylation, we incubated FH-GlyT1a or GlyT1b/PAE cells with PMA for 60 min, followed by transporter purification under the same condition used in the metabolic labeling experiments, and the purified protein was subjected to Western blotting with specific anti-phosphotyrosine antibodies. As a control, we used the epidermal growth factor (EGF) receptor, known to be phosphorylated at tyrosine residues, and which was immunoprecipitated from cells previously stimulated with or without 20 ng/ml of EGF for 2 min. As shown in Fig. 3A, blotting with the specific anti-phophotyrosine antibody pY2 produced a readily detectable signal for the EGF-induced tyrosine phosphorylated receptor whereas no signal at all was detected in the lanes corresponding to GlyT1a and GlyT1b. The blots were further incubated with EGF receptor and GlyT1 antibodies, which resulted in the appearance of bands corresponding to the receptor and transporter at their expected molecular weights (Fig. 3B and 3C). The same findings were obtained for GlyT1c isoform (not shown). Essentially, these results suggest that, similar to other members of the SLC6 family, serine/threonine residues found in GlyT1 rather than tyrosines are PKC-dependent phosphorylation sites. In addition, phosphorylated peptides containing Ser/Thr residues have been confirmed by mass spectrometry from a GlyT1a and GlyT1b preparation obtained from PMA-treated cells (unpublished data), supporting this findings. Whether PKC directly phosphorylates GlyT1 or activates an additional kinase that could target the transporter are questions that still remain to be determined.
Figure 3. Blotting for tyrosine phosphorylation of EGF receptor and GlyT1.
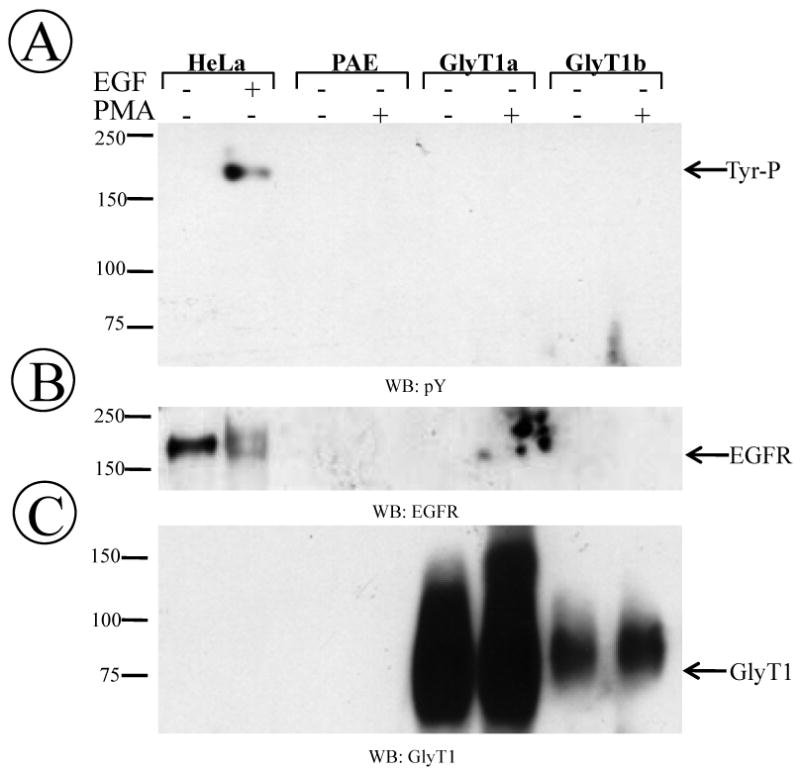
(A) FH-GlyT1/PAE cells were incubated with DMSO or PMA for 60 min followed by purification of the transporter. In control samples, HeLa cells were incubated with 20 ng of EGF for 2 min followed by immunoprecipiation of EGF receptor. Purified transporter and immunoprecipitates were subjected to Western blotting with phosphotyrosine (pY). (B) blotting with EGFR or (C) GlyT1 antibodies. A representative blot from one of three different experiments is shown.
3.4. Inhibition of conventional PKCα/β abolished the PKC-dependent reduction in [3H]glycine uptake
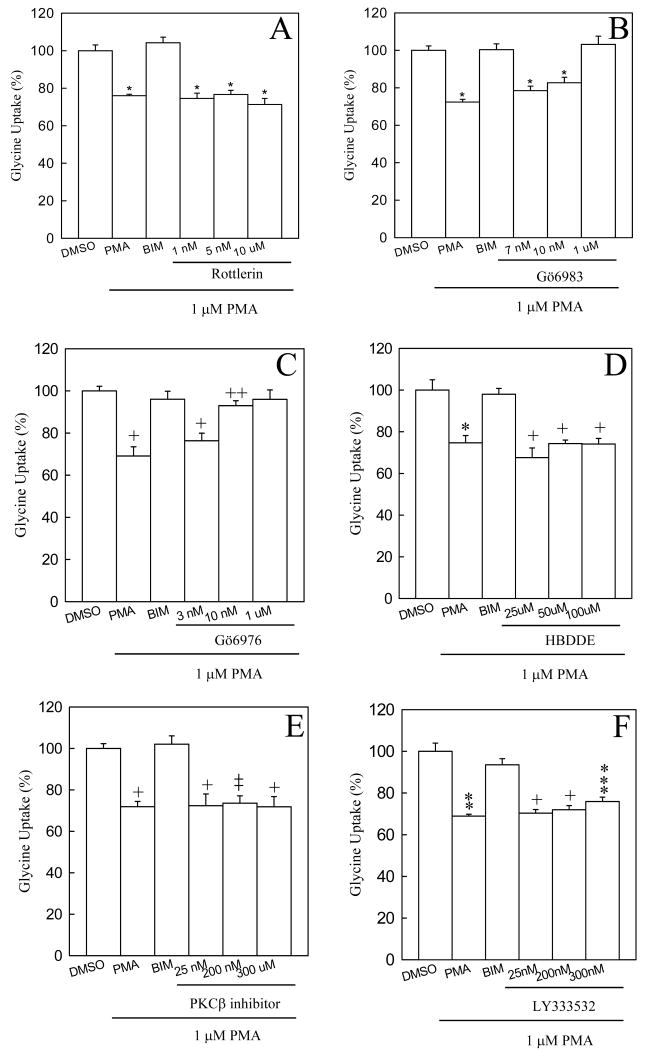
A number of studies have reported that the classical PKCβII isoform physically associates with DAT and regulates trafficking and amphetamine-stimulated outward dopamine transport (Johnson et al., 2005; Chen et al., 2009). Moreover, it has been suggested that PKCα regulates the PMA-reduced uptake of glycine in C6 glioma cells (Morioka et al., 2008). To elucidate further the regulatory mechanism of glycine uptake and GlyT1 phosphorylation in FH-GlyT1b/PAE cells, we performed an extensive pharmacological study by using a variety of kinase inhibitors and assayed their effects on glycine uptake. The GlyT1b was selected as representative of all the isoforms given that similar results were obtained for isoforms GlyT1a and GlyTc. In agreement with previous studies on other SLC6 members, an incubation of FH-GlyT1b/PAE cells with PKA (KT5720) or CaMKII (KN93) inhibitors did not prevent the PMA-induced reduction in glycine uptake that could be abolished by the specific PKC inhibitor BIM, demonstrating that PKA and CaMKII activities do not participate in the downregulation of glycine uptake (data not shown). Given that PMA activates novel and conventional PKCs, we then examined the effect of different concentrations of rottlerin, an inhibitor of the novel PKCδ, on glycine uptake. As shown in Fig. 4A, preincubation of FH-GlyT1b/PAE cells with rottlerin at concentrations below and more than 100 fold above (10 μM) the IC50 (3 nM) did not affect the downregulation induced by PMA, demonstrating that novel PKCs do not play a role in the regulation of GlyT1. Moreover, to determine the role of conventional PKCs on GlyT1 activity, we incubated FH-GlyT1b/PAE cells with increasing concentrations of the selective PKCα, β, γ and δ inhibitor Gö6983. As expected, preincubation of the cells with increasing concentrations of Gö6983, above the IC50 (7 nM) gradually prevented the PMA-induced reduction in uptake to levels similar to those found in vehicle or BIM/PMA treated cells (Fig. 4B). These experiments clearly demonstrate the involvement of conventional PKCs in the regulation of GlyT1 activity.
Figure 4. Effect of protein kinase C inhibitors on the PMA-dependent reduction of glycine uptake.
FH-GlyT1b/PAE cells were incubated for 30 min at 37 °C with either of the following inhibitors, before treatment with 1 μM PMA, (A) Rottlerin (novel PKCδ inhibitor), (B) Gö6983 (inhibitor of conventional PKCs), (C) Gö6976 (PKCα/β inhibitor), (D) HBDDE (PKCα/γ inhibitor), (E) PKCβ inhibitor or (F) LY333532 (PKCβ inhibitor). After PMA treatment for 1 h, glycine uptake was measured as described in “Experimental Procedures”. Data bars represent the mean ± SE, n=3-8, *, +, p=<0.001, ++, p=0.048, *, p=0.008, **, p=0.006, ***, p=0.002 compared to cells treated with vehicle (DMSO).
Similar findings were obtained with Gö6976, a potent and more selective inhibitor of classical PKCα and PKCβ. As shown in Fig. 4C, incubation of FH-GlyT1b/PAE cells with 3 nM Gö6976, at a concentration below the IC50 value (∼6 nM) did not prevent the effect of PMA; by contrast, at concentration of 10 nM or above was sufficient to completely abolish the PMA-induced reduction in glycine uptake. These results together suggest that conventional PKCα and/or β are responsible for the downregulation of glycine transport. We further analyzed the effect of more specific inhibitors to PKCα and PKCβ on the GlyT1 activity. As shown in Fig. 4, panels C-F, incubation of the cells with varying concentrations of the PKCβ inhibitors (referred as PKCβ inhibitor and LY333531) or the PKCα/γ (HDBBE) inhibitors did not prevent the reduction of glycine uptake triggered by PMA, suggesting that PKCα and PKCβ together regulate GlyT1 activity. These data together demonstrate that only incubation with Gö6976 was able to protect the reduction of glycine uptake stimulated by PMA and suggest that PKC regulates the activity of those transporters.
3.5. Expression of PKCα and PKCβ in PAE and C6 glioma cells
Although the pharmacological analysis provided information about the PKC isozymes responsible for the regulation of GlyT1 activity, it does not show the presence and the amount of PKC isosymes contained within the cell. To determine whether PKCα and PKCβ were expressed in PAE cells, control tissue lysate from rat cerebellum and forebrain, or varying amounts of FH-GlyT1b/PAE cell lysates were subjected to SDS-PAGE and immunoblotting with specific antibodies to PKCα, PKCβI or PKCβII, obtained from different suppliers. Cerebellum and forebrain are two well known CNS regions to express both PKCα and PKCβ. As shown in Fig. 5A and 5B, blotting with the anti-PKCα and PKCβ antibodies respectively, readily identified an immunoreactive ∼75 kDa band corresponding to PKCα and PKCβ from cerebellum, forebrain and PAE cells, in agreement with the predicted size of the polypeptide. Given that PKCα and PKCβ migrated with similar molecular weight in SDS-PAGE, the identity of each isozyme was corroborated further by performing two-dimensional gel electrophoresis followed by Western blotting with the corresponding antibodies. Consistent with the previous results, PKCα and PKCβ1 were detected at the same molecular weight but were separated by their difference in iso-electric point values (Fig. 5C). These findings confirmed the expression of both isozymes in PAE cells. A recent study by Morioka et al. (Morioka et al., 2008) reported that GlyT1 activity is regulated by PKCα and their conclusion was based on the observation that the anti-PKCβ antibodies used in their study did not detect PKCβ in C6 glioma cells. By contrast, our results pointed to PKCα/β as responsible for regulating the downregulation on Gly uptake. To address this discrepancy, we tested the abilities of different antibodies to recognize the PKC isozymes. Different quantities of total lysate from PAE and C6 glioma cells were subjected to Western blotting with anti-PKC antibodies from different sources. As mentioned above, PKCα was detected in control and PAE cell lysates with the anti-PKCα antibody, consistent with earlier observations in C6 glioma cells by Morioka et al. (Morioka et al., 2008). By contrast, blotting with monoclonal (E3) or a polyclonal (C-16, not shown) anti PKCβ1 antibodies readily detected a ∼75 kDa band in controls and C6 glioma cells corresponding to PKCβ1, showing the expression of this isozyme and to similar levels in both C6 and PAE cells. In addition, using specific polyclonal anti-PKCβII antibodies (C-18), we detected expression of PKCβII in C6 glioma cells and to a lower level in PAE cells, indicating the presence of both PKCβ isozymes (Fig. 5B). However, to clarify some of the confusion related to the expression of PKCβ in C6 glioma cells reported by Morioka et al.(Morioka et al., 2008), we tested additional lysate samples with the same anti-PKCβ antibody (BD Bioscience, PKCβ 126-324). As illustrated at the bottom panel of Fig. 5B, PKCβ was detected by the anti-PKCβ antibody at the predicted molecular weight in both PAE and C6 glioma cells demonstrating the expression of PKCβ in C6 glioma cells. These results together and the pharmacological studies confirmed the expression of the conventional PKCα, as well as PKCβIand PKCβII in PAE and C6 glioma cells.
Figure 5. Immunodetection of PKCα and PKCβ isoenzymes in PAE and C6 glioma cells.
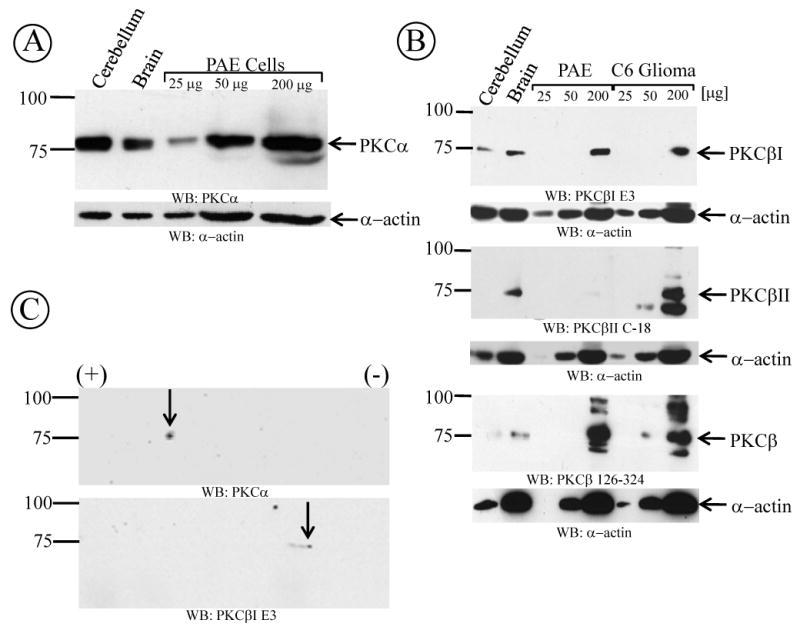
(A) PAE or C6 glioma cells were lysed and different quantities of lysates were subjected to Western blotting with different PKCα or (B) different PKCβ antibodies. Blotting with actin antibodies was used as loading control. As a positive control, 50 μg of freshly prepared rat brain or cerebellum homogenate was used. (C) PAE cell lysates were subejected to isoelectric focusing separation followed by SDS-polyacrylamide gel electrophoresis. Proteins were subjected to Western blotting with PKCα and PKCβ antibodies from different sources (described in “experimental procedures”). Total protein concentration was determined by the Bradford assay and depicted in each pannel is a represenative of 3-6 different experiments.
3.6. PKCβ triggers GlyT1 and DAT phosphorylation in PAE cells
We have determined that PKCα and β are responsible for the changes in glycine uptake; however, it is not known whether a sole PKC isozyme is responsible for the changes in transporter phosphorylation. To determine whether PKCα and/or PKCβ could be involved in the enhanced GlyT1 phosphorylation, we performed a metabolic labeling assay of FH-GlyT1/PAE cells with 32P-orthophosphate and explored the effects of different PKC inhibitors on GlyT1 phosphorylation. As shown in Fig 6A, when GlyT1 was purified from vehicle treated cells, low levels of phosphorylation were detected, suggesting that some GlyT1 molecules in the population are phosphorylated at a steady state. In agreement with the previous results, when GlyT1 was purified from cells exposed to PMA for 1 h, phosphorylation was dramatically increased (up to∼3-4 folds, p=<0.001). The PKC dependency was confirmed by pretreatment of the cells for 30 min with 1 μM BIM, which resulted in the abolishment of GlyT1 phosphorylation (Fig. 6A, lane 3). We previously showed that the conventional PKC inhibitor Gö6976 prevented the PMA-dependent decrease in glycine uptake; therefore, we preincubated the FH-GlyT1/PAE cells with 1 μM Gö6976 for 30 min followed by incubation in the presence of 1 μM PMA for 1 h. Not surprisingly, the inhibitor blocked the PKC-dependent GlyT1 phosphorylation (Fig. 6A, lane 4). Furthermore, 30 min pretreatment of the cells with either of the two PKCβ specific inhibitors (200 nM of PKCβ inhibitor or 200 nM LY333531) prevented the activation of PKCβ by PMA and abolished the increased on GlyT1 phosphorylation (Fig. 6A, lanes 5 and 6). By contrast, enhanced GlyT1 phosphorylation was observed after incubation with 50 μM of the specific PKCα/γ inhibitor HDBBE and further stimulation with PMA, suggesting that PKCβ is the only responsible enzyme to induce GlyT1 phosphorylation (Fig. 6A, lane 7). For the dopamine transporter it has been demonstrated the role of PKCβ in dopamine efflux; however the involvement of PKCβ on DAT phosphorylation has not been demonstrated. To investigate the role of PKCβ on DAT phosphorylation and compare this process to GlyT1, FH-DAT/PAE cells were subjected to the same treatment and metabolic labeling. Interestingly, similar findings were obtained in PAE cells expressing the human dopamine transporter (Fig.6B). The hDAT phosphorylation was inhibited by pre-treatment of FH-hDAT/PAE cells with 1 μM Gö6976 or 200 nM of PKCβ inhibitor, consistent with those results obtained for GlyT1. These results may provide a direct link between PKCβ dependent DAT phosphorylation and published results that demonstrate a role of PKCβ in amphetamine-dependent dopamine efflux (Johnson et al., 2005). Taken together, these findings demonstrate that PKCα/β are responsible for the PMA-induced inhibition of glycine uptake whereas PKCβ regulates the increase in GlyT1 phosphorylation.
Figure 6. GlyT1 and DAT phosphorylation are dependent on PKCβ activation.
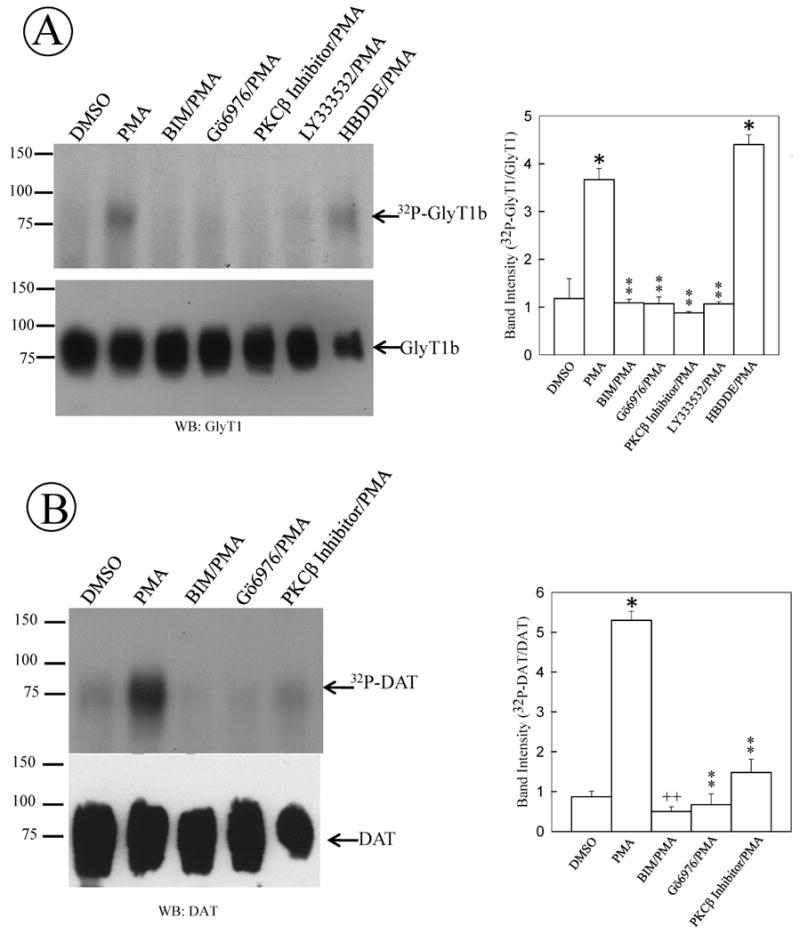
(A) FH-GlyT1b/PAE or (B) FH-hDAT/PAE cells were grown to 80-90% confluency and labeled with 50 μCi 32P-orthophosphate/ml for 2 h. After labeling, cells were incubated for 60 min in the presence of the following selective PKC inhibitors: Gö6976 (PKCα/β), PKCβ inhibitor or LY333531 (PKCβ inhibitors) or HDBBE (PKCα/γ inhibitor) before incubation with 1 μM PMA for 1 h. After incubation, GlyT1 and DAT were purified by affinity chromatography and analyzed by autoradiography and Western blotting with GlyT1 or DAT antibodies. A representative of 3-5 different experiments is shown. Data bars represent the mean ± SE, *, p=<0.001, ++, p=0.035, **, p≥0.082, compared to intensity obtained from cells treated with vehicle (DMSO).
4. Discussion
Post-translational modifications such as phosphorylation and ubiquitination are known to regulate the activity of many proteins including receptors, channels and transporters (Miranda and Sorkin, 2007). To date, numerous studies indicate that several members of the SLC6 family of neurotransmitter transporters such as the dopamine, serotonin and norepinephrine transporters, are phosphorylated at a steady state and enhanced phosphorylation is observed in response to PKC activation. So far, no direct experimental evidence has been provided to demonstrate that GlyT1 is phosphorylated, indeed it has been only speculated based on the presence of putative phosphorylation sites along the GlyT1 sequence (Sato et al., 1995). In this study, we present the first solid biochemical evidences that demonstrate GlyT1 phosphorylation. By expressing an amino-terminal Flag, His-tagged GlyT1a, b and c isoforms in PAE cells, we showed that these tagged-GlyT1 transporters trafficked through the biosynthetic pathway and were effectively delivered to the plasma membrane. Moreover, the FH-GlyT1 retained the functional properties and the ability to respond to phorbol ester as demonstrated by glycine uptake. This is in agreement with previous studies which have shown that tagging the amino-terminal end of GlyT1 does not affect processing and function of the transporter when expressed in different heterologous expression systems (Cubelos et al., 2005). Expression of the FH-tagged GlyT1 in PAE cells showed glycine uptake with kinetic parameters similar to those described for untagged GlyT1 (Olivares et al., 1994; Gomeza et al., 1995; Geerlings et al., 2000). In addition, the inhibition of GlyT1 activity observed after activation of PKC by phorbol ester was not affected by the presence of the tags, given that PKC activation by PMA led to a reduction of the Vmax and no significant changes in the Km values, as has been reported for other neurotransmitter transporters such as those for transport of dopamine (Huff et al., 1997; Vaughan et al., 1997; Chi and Reith, 2003; Sorkina et al., 2003), norepinephrine (Apparsundaram et al., 1998) and serotonin (Qian et al., 1997; Jayanthi et al., 2005).
This heterologous expression model system allowed us to study phosphorylation of the transporter by using a GlyT1 preparation purified by double affinity chromatography to near homogeneity. We demonstrated by metabolic labeling that the three amino-terminal GlyT1 isoforms (a, b and c) are phosphorylated in a PKC- and time-dependent mechanism. Similar to the findings obtained for all the monoamine transporters, GlyT1 is phosphorylated at basal levels in non stimulated cells and further activation of PKC with phorbol ester dramatically enhances phosphorylation. Not surprisingly, pre-incubation with the PKC inhibitor BIM blocked the enhanced GlyT1phosphorylation. Although several neurotransmitter transporters are phosphorylated at steady state and PKC appears to enhance their phosphorylation, the functional role of such phosphorylation and whether transporters are directly phosphorylated by PKC or another PKC-dependent kinase, are questions that still remain to be answered. Previous studies have demonstrated that amphetamines elicit release of dopamine into the media via DAT, but the molecular mechanism underlying this process is still unclear (Parker and Cubeddu, 1988; Sulzer et al., 1995; Cowell et al., 2000; Johnson et al., 2005). Moreover, previous studies in a DAT mutant lacking the first 22 amino acids of the N-terminus or a mutant in which five N-terminal serine residues were mutated to alanine greatly reduced the PKC-dependent phosphorylation and mostly eliminated most of the AMPH-induced dopamine efflux, suggesting a tight link between transporter phosphorylation and DA efflux (Granas et al., 2003; Khoshbouei et al., 2004). Interestingly these mutations did not affect the inward DA uptake, inhibitor binding or PKC-dependent endocytosis. Based on these findings, the authors proposed that phosphorylation of one or more serine residues in the N-terminus of DAT is/are needed for the AMPH-induced DAT mediated efflux. Given the remarkable sequence similarity between GlyT1 and the other members of the Na+/Cl--dependent neurotransmitter family such as the DAT and NET, we can speculate that they share similar structures and regulatory mechanisms, thus supporting a role for PKC-dependent phosphorylation in glycine efflux. The GlyT1 and GlyT2 have been described to have the capacity for reverse transport; however, whether GlyT1 phosphorylation contributes to the reversal of glycine transport and the location of the phosphorylation sites are interesting questions awaiting elucidation (Luccini and Raiteri, 2007). Interestingly, we have identified phosphorylated serine residues at the C-terminus of GlyT1a and GlyT1b by mass spectrometry, pointing to this tail as a possible regulatory domain (unpublished results).
The ability of phorbol ester to stimulate PKC and induce GlyT1 phosphorylation and down-regulation of activity in PAE cells is clear; however, the PKC isozyme responsible for these modifications needed to be further characterized for GlyT1. Our detailed pharmacological study shows the role of conventional PKCs in phosphorylation and uptake. Inhibition of phosphorylation with two highly selective PKCβ inhibitors (LY333532 and PKCβ inhibitor) demonstrates the involvement of this isoform in the PKC-dependent phosphorylation of GlyT1 and DAT. Interestingly, accumulating evidences support a direct interaction of PKCβI and PKCβII with the DAT in co-immunoprecipitation experiments from rat striatal synaptosomes or ectopically expressed DAT in PC12 cells. In addition, they showed inhibition of amphetamine-mediated dopamine efflux by treatment of rat striatal slices with another PKCβ inhibitor, LY379196, or a dramatic reduction of dopamine efflux in striatal synaptosomes prepared from a PKCβ knockout mice. Together, these findings clearly reveal a key role of PKCβ in regulation of transporter phosphorylation and dopamine efflux (Johnson et al., 2005; Chen et al., 2009). Although it has not been demonstrated whether PKCβ is directly responsible for the phosphorylation of the GlyT1 or DAT, additional evidence suggests a role for other kinases in the complex processes of efflux and phosphorylation. For example, a marked reduction in cell surface and DAT phosphorylation were obtained after inhibition of the MEK1/2 kinases or PI3-kinase and more recent findings implicate CaMKII in the regulation of DA efflux (Carvelli et al., 2002; Fog et al., 2006; Dipace et al., 2007). These data suggest that different kinases, linked to several signaling pathways, could potentially be responsible for transporter phosphorylation, although additional evidence is needed to support this hypothesis. It is noteworthy that the two specific PKCβ inhibitors did not prevent the reduction of glycine uptake induced by PMA, suggesting that PKCβ-dependent GlyT1 phosphorylation may not be involved in the regulation of Gly inward uptake but more likely in the efflux of substrate. By contrast, inhibition of PKCα and PKCβ with the inhibitor Gö6976 abolished completely the effect of PMA on glycine transport. It is possible that the reduction of glycine uptake is due to ubiquitination followed by GlyT1 endocytosis, given that PMA triggers a change in Vmax with little or no change in the affinity for substrate (Km). In previous studies on DAT, we reported that endocytosis is triggered by a PKC-dependent transporter ubiquitination process. Therefore, it is possible that enhanced ubiquitination and endocytosis are dependent on activation of PKCα and PKCβ whereas transporter phosphorylation is an independent process that is triggered by PKCβ activation. Several published data support this hypothesis (Johnson et al., 2005; Chen et al., 2009; Robertson et al., 2009). In recent years, many membrane proteins such as receptors, channels and transporters are described to be phosphorylated and ubiquitinated, these modifications may represent regulatory mechanisms for controlling one or multiple properties of the protein. For the receptor tyrosine kinase VEGFR-2, serine phosphorylation in the PEST domain is required for ubiquitination, endocyosis and further degradation of the receptor (Meyer et al., 2011), similarly EGFR phosphorylation of Ser-991 and Tyr-998 seem to be required for further ubiquitination and degradation of receptor (Tong et al., 2009). Whether GlyT1 phosphorylation is required for substrate efflux or ubiquitination, is a question that will require further investigation and extensive site-directed mutagenesis and analysis of phosphorylation sites.
Given the main role of GlyT1 in controlling extracellular glycine concentration and therefore modulating NMDA receptor activation, it will be critical to study whether GlyT1 phosphorylation is implicated in glycine efflux and the transition of GlyT1 from an inward to an outward conformation. Shifting GlyT1 to an outward conformation could allow an increase in extracellular glycine and further NMDA receptor activation. These studies could potentially uncover an alternative therapeutic approach for intervention in disorders involving the NMDA receptor, such as schizophrenia and bipolar disorder, without affecting inward transport.
Highlights.
The plasma membrane glycine transporter is phosphorylated in response to PKCβ activation.
PKCα/β are responsible for regulation of glycine transport by GlyT1
GlyT1 phosphorylation is transient and follows a bell shape-like behavior.
Transporter phosphorylation is independent of ubiquitination
Acknowledgments
We thank the staff of the Cell Culture and High Throughput Screening (HTS) and the Biomolecule Core Facility for facilities provided. This study was supported by grant NIMH 5SC1MH 086070-02 to MM and also in part by the grant 5G12RR008124 to the Border Biomedical Research Center (BBRC)/University of Texas at El Paso) from the National Center for Research Resources (NCRR, NIH). The authors are grateful to Drs. Detlev Bioson and Dietmar Benke (Federal Institute of Technology, Switzerland) for providing the GlyT1 antibodies, and Dr. Zhang and his laboratory members for technical assistance with 2D gels. We thank Drs. Arshad Khan and Manuel Llano for critical reading of the manuscript and helpful suggestions.
Abbreviations
- PMA
4-α-phorbol 12-myristate 13-acetate
- PKC
protein kinase C
- GlyT
glycine transporter
- PAE
Porcine Aortic Endothelial cells
- DAT
dopamine transporter
- NET
norepinephrine transporter
- SERT
serotonin transporter
- BIM
bisindolylmaleimide I
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apparsundaram S, Schroeter S, Giovanetti E, Blakely RD. Acute regulation of norepinephrine transport: II. PKC-modulated surface expression of human norepinephrine transporter proteins. J Pharmacol Exp Ther. 1998;287:744–751. [PubMed] [Google Scholar]
- Aragon MC, Gimenez C, Mayor F. Stoichiometry of sodium- and chloride-coupled glycine transport in synaptic plasma membrane vesicles derived from rat brain. FEBS Lett. 1987;212:87–90. doi: 10.1016/0014-5793(87)81562-4. [DOI] [PubMed] [Google Scholar]
- Aubrey KR, Vandenberg RJ, Clements JD. Dynamics of Forward and Reverse Transport by the Glial Glycine Transporter, Glyt1b. Biophys J. 2005;89:1657–1668. doi: 10.1529/biophysj.105.061572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Hoffman BJ. Analysis of a Gene Encoding Two Glycine Transporter Variants Reveals Alternative Promoter Usage and a Novel Gene Structure. J Biol Chem. 1998;273:29077–29085. doi: 10.1074/jbc.273.44.29077. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LM, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002;81:859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang M, Kim MN, Gereau RWt, Leitges M, Gnegy ME. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Ther. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther. 2003;307:729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Kantor L, Hewlett GH, Frey KA, Gnegy ME. Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. Eur J Pharmacol. 2000;389:59–65. doi: 10.1016/s0014-2999(99)00828-6. [DOI] [PubMed] [Google Scholar]
- Cubelos B, Gimenez C, Zafra F. Localization of the GLYT1 Glycine Transporter at Glutamatergic Synapses in the Rat Brain. Cereb Cortex. 2005;15:448–459. doi: 10.1093/cercor/bhh147. [DOI] [PubMed] [Google Scholar]
- Dipace C, Sung U, Binda F, Blakely RD, Galli A. Amphetamine induces a calcium/calmodulin-dependent protein kinase II-dependent reduction in norepinephrine transporter surface expression linked to changes in syntaxin 1A/transporter complexes. Mol Pharmacol. 2007;71:230–239. doi: 10.1124/mol.106.026690. [DOI] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Vaughan RA. Dopamine Transporters Are Phosphorylated on N-terminal Serines in Rat Striatum. J Biol Chem. 2002;277:25178–25186. doi: 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Pauly-Evers M, Schwerdel C, Lentz M, Bluethmann H, Vogt K, Alberati D, Mohler H, Boison D. Enhancement of the NMDA receptor function by reduction of glycine transporter-1 expression. Neurosci Lett. 2005;373:79–84. doi: 10.1016/j.neulet.2004.09.064. [DOI] [PubMed] [Google Scholar]
- Geerlings A, Lopez-Corcuera B, Aragon C. Characterization of the interactions between the glycine transporters GLYT1 and GLYT2 and the SNARE protein syntaxin 1A. FEBS Lett. 2000;470:51–54. doi: 10.1016/s0014-5793(00)01297-7. [DOI] [PubMed] [Google Scholar]
- Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: molecular function of important drug targets. Trends in Pharmacological Sciences. 2006;27:375–383. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Zafra F, Olivares L, Gimenez C, Aragon C. Regulation by phorbol esters of the glycine transporter (GLYT1) in glioblastoma cells. Biochim Biophys Acta. 1995;1233:41–46. doi: 10.1016/0005-2736(94)00249-o. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Hulsmann S, Ohno K, Eulenburg V, Szoke K, Richter D, Betz H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron. 2003a;40:785–796. doi: 10.1016/s0896-6273(03)00672-x. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Ohno K, Hulsmann S, Armsen W, Eulenburg V, Richter DW, Laube B, Betz H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron. 2003b;40:797–806. doi: 10.1016/s0896-6273(03)00673-1. [DOI] [PubMed] [Google Scholar]
- Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U. N-terminal Truncation of the Dopamine Transporter Abolishes Phorbol Ester- and Substance P Receptor-stimulated Phosphorylation without Impairing Transporter Internalization. J Biol Chem. 2003;278:4990–5000. doi: 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- Hanley JG, Jones EMC, Moss SJ. GABA Receptor rho 1 Subunit Interacts with a Novel Splice Variant of the Glycine Transporter, GLYT-1. J Biol Chem. 2000;275:840–846. doi: 10.1074/jbc.275.2.840. [DOI] [PubMed] [Google Scholar]
- Huff RA, Vaughan RA, Kuhar MJ, Uhl GR. Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J Neurochem. 1997;68:225–232. doi: 10.1046/j.1471-4159.1997.68010225.x. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Ramamoorthy S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J Biol Chem. 2004;279:19315–19326. doi: 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- Johnston GA, Iversen LL. Glycine uptake in rat central nervous system slices and homogenates: evidence for different uptake systems in spinal cord and cerebral cortex. J Neurochem. 1971;18:1951–1961. doi: 10.1111/j.1471-4159.1971.tb09601.x. [DOI] [PubMed] [Google Scholar]
- Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther. 1998;284:592–598. [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Kingsmore SF, Han H, Yang-Feng TL, Godinot N, Seldin MF, Caron MG, Giros B. Cloning of the human glycine transporter type 1: molecular and pharmacological characterization of novel isoform variants and chromosomal localization of the gene in the human and mouse genomes. Mol Pharmacol. 1994;45:608–617. [PubMed] [Google Scholar]
- Lin Z, Zhang PW, Zhu X, Melgari JM, Huff R, Spieldoch RL, Uhl GR. Phosphatidylinositol 3-Kinase, Protein Kinase C, and MEK1/2 Kinase Regulation of Dopamine Transporters (DAT) Require N-terminal DAT Phosphoacceptor Sites. J Biol Chem. 2003;278:20162–20170. doi: 10.1074/jbc.M209584200. [DOI] [PubMed] [Google Scholar]
- Luccini E, Raiteri L. Mechanisms of [(3)H]glycine release from mouse spinal cord synaptosomes selectively labeled through GLYT2 transporters. J Neurochem. 2007;103:2439–2448. doi: 10.1111/j.1471-4159.2007.04967.x. [DOI] [PubMed] [Google Scholar]
- Meyer RD, Srinivasan S, Singh AJ, Mahoney JE, Gharahassanlou KR, Rahimi N. PEST motif serine and tyrosine phosphorylation controls VEGFR-2 stability and downregulation. Mol Cell Biol. 2011 doi: 10.1128/MCB.01006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Wu CC, Sorkina T, Korstjens DR, Sorkin A. Enhanced Ubiquitylation and Accelerated Degradation of the Dopamine Transporter Mediated by Protein Kinase C. J Biol Chem. 2005;280:35617–35624. doi: 10.1074/jbc.M506618200. [DOI] [PubMed] [Google Scholar]
- Miranda M, Sorkin A. Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Mol Interv. 2007;7:157–167. doi: 10.1124/mi.7.3.7. [DOI] [PubMed] [Google Scholar]
- Morioka N, Abdin JM, Morita K, Kitayama T, Nakata Y, Dohi T. The regulation of glycine transporter GLYT1 is mainly mediated by protein kinase Calpha in C6 glioma cells. Neurochem Int. 2008;53:248–254. doi: 10.1016/j.neuint.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: poised to signal. American Journal of Physiology - Endocrinology And Metabolism. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Vol. 422. 2003. pp. 302–307. [DOI] [PubMed] [Google Scholar]
- Olivares L, Aragon C, Gimenez C, Zafra F. Carboxyl terminus of the glycine transporter GLYT1 is necessary for correct processing of the protein. J Biol Chem. 1994;269:28400–28404. [PubMed] [Google Scholar]
- Olivares L, Aragon C, Gimenez C, Zafra F. Analysis of the Transmembrane Topology of the Glycine Transporter GLYT1. J Biol Chem. 1997;272:1211–1217. doi: 10.1074/jbc.272.2.1211. [DOI] [PubMed] [Google Scholar]
- Parker EM, Cubeddu LX. Comparative effects of amphetamine, phenylethylamine and related drugs on dopamine efflux, dopamine uptake and mazindol binding. J Pharmacol Exp Ther. 1988;245:199–210. [PubMed] [Google Scholar]
- Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J Biol Chem. 1998;273:2458–2466. doi: 10.1074/jbc.273.4.2458. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacology & Therapeutics. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Sato K, Adams R, Betz H, Schloss P. Modulation of a recombinant glycine transporter (GLYT1b) by activation of protein kinase C. J Neurochem. 1995;65:1967–1973. doi: 10.1046/j.1471-4159.1995.65051967.x. [DOI] [PubMed] [Google Scholar]
- Sorkina T, Doolen S, Galperin E, Zahniser NR, Sorkin A. Oligomerization of Dopamine Transporters Visualized in Living Cells by Fluorescence Resonance Energy Transfer Microscopy. J Biol Chem. 2003;278:28274–28283. doi: 10.1074/jbc.M210652200. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Taylor P, Peterman SM, Prakash A, Moran MF. Epidermal Growth Factor Receptor Phosphorylation Sites Ser991 and Tyr998 Are Implicated in the Regulation of Receptor Endocytosis and Phosphorylations at Ser1039 and Thr1041. Molecular & Cellular Proteomics. 2009;8:2131–2144. doi: 10.1074/mcp.M900148-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem. 1997;272:15541–15546. 15542. doi: 10.1074/jbc.272.24.15541. Huff RA et al. Phorbol esters increase dopam…[PMID: 8978729]Related Articles, Links. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Zafra F, Aragon C, Gimenez C. Molecular biology of glycinergic neurotransmission. Mol Neurobiol. 1997;14:117–142. doi: 10.1007/BF02740653. [DOI] [PubMed] [Google Scholar]