Abstract
We expressed rat Nav1.6 sodium channels in combination with the rat β1 and β2 auxiliary subunits in human embryonic kidney (HEK293) cells and evaluated the effects of the pyrethroid insecticides tefluthrin and deltamethrin on expressed sodium currents using the whole-cell patch clamp technique. Both pyrethroids produced concentration-dependent, resting modification of Nav1.6 channels, prolonging the kinetics of channel inactivation and deactivation to produce persistent “late” currents during depolarization and tail currents following repolarization. Both pyrethroids also produced concentration dependent hyperpolarizing shifts in the voltage dependence of channel activation and steady-state inactivation. Maximal shifts in activation, determined from the voltage dependence of the pyrethroid-induced late and tail currents, were ~25 mV for tefluthrin and ~20 mV for deltamethrin. The highest attainable concentrations of these compounds also caused shifts of ~5–10 mV in the voltage dependence of steady-state inactivation. In addition to their effects on the voltage dependence of inactivation, both compounds caused concentration-dependent increases in the fraction of sodium current that was resistant to inactivation following strong depolarizing prepulses. We assessed the use-dependent effects of tefluthrin and deltamethrin on Nav1.6 channels by determining the effect of trains of 1 to 100 5-ms depolarizing prepulses at frequencies of 20 or 66.7 Hz on the extent of channel modification. Repetitive depolarization at either frequency increased modification by deltamethrin by ~2.3-fold but had no effect on modification by tefluthrin. Tefluthrin and deltamethrin were equally potent as modifiers of Nav1.6 channels in HEK293 cells using the conditions producing maximal modification as the basis for comparison. These findings show that the actions of tefluthrin and deltamethrin of Nav1.6 channels in HEK293 cells differ from the effects of these compounds on Nav1.6 channels in Xenopus oocytes and more closely reflect the actions of pyrethroids on channels in their native neuronal environment.
Keywords: voltage-gated sodium channel, Nav1.6 isoform, pyrethroid, deltamethrin, tefluthrin, HEK293 cells
Introduction
Pyrethroid insecticides are synthetic analogs of the pyrethrins, the insecticidal constituents of the botanical insecticide pyrethrum (Elliott, 1989). Pyrethroids have been widely used in agriculture for more than three decades, growing to represent approximately 18% of the dollar value of the world insecticide market by 2002 (Pickett, 2004). Certain pyrethroids are also essential components of worldwide efforts to combat malaria and other mosquito-borne diseases despite the existence of resistance in some vector populations (Ranson et al., 2011). Pyrethroids are also commonly employed in household insecticides and insect control products for companion animals, whose unregulated use increases the risk of exposure and adverse effects in the general population (Power and Sudakin, 2007).
Pyrethroids owe their insecticidal activity to their ability to disrupt electrical signaling in the nervous system by prolonging the opening of voltage-gated sodium channels (Soderlund, 1995). Voltage-gated sodium channels are also considered to be the primary targets for the central neurotoxic effects of pyrethroids in mammals (Soderlund et al., 2002; Soderlund, 2011). Acute intoxication in rats comprises two distinct syndromes of intoxication, designated T (tremor) and CS (choreoathetosis with salivation) (Verschoyle and Aldridge, 1980). The production of the T and CS syndromes is broadly correlated with chemical structure, so that Type I compounds (a diverse group lacking the α-cyano-3-phenoxybenzyl alcohol moiety) typically cause the T syndrome whereas Type II compounds (α-cyano-3-phenoxybenzyl esters) typically produce the CS syndrome (Soderlund et al., 2002; Breckenridge et al., 2009). The correlation of structure with intoxication syndrome, though strong, is not absolute because some Type II structures either cause the T syndrome or produce signs of intoxication that include elements of both syndromes (Verschoyle and Aldridge, 1980; Lawrence and Casida, 1982; Breckenridge et al., 2009).
Native sodium channels in the mammalian brain are heterotrimeric complexes of a large, pore-forming α subunit and two auxiliary β subunits that modulate channel gating and regulate channel trafficking and expression in the cell membrane (Goldin, 2001; Meadows and Isom, 2005). The α subunits of voltage-gated sodium channels in mammals comprise nine isoforms (designated Nav1.1 – Nav1.9) that are differentially distributed in excitable cells and tissues and exhibit distinctive functional and pharmacological properties (Catterall et al., 2005). Four of these isoforms (Nav1.1, Nav1.2, Nav1.3, and Nav1.6) are strongly expressed in the brain (Goldin, 2001) and represent putative targets for pyrethroid insecticides. There are four β subunit isoforms (β1 – β4) in mammals, all of which are expressed in the brain (Meadows and Isom, 2005). However, the widespread expression of the β1 and β2 subunits in the adult brain (Whitaker et al., 2000; Shah et al., 2001; Whitaker et al., 2001; Schaller and Caldwell, 2003) implies that the majority of brain sodium channels in adults are heterotrimers composed of an α subunit and the β1 and β2 subunits.
Several studies have employed transient expression in Xenopus laevis oocytes to assess the action of pyrethroids on individual sodium channel isoforms and defined subunit complexes. Among the five rat isoforms examined to date, the Nav1.3, Nav1.6 and Nav1.8 isoforms are relatively sensitive to pyrethroid modification whereas the Nav1.2 and Nav1.7 isoforms are resistant (Smith and Soderlund, 1998; Vais et al., 2000a; Smith and Soderlund, 2001; Soderlund and Lee, 2001; Choi and Soderlund, 2006; Meacham et al., 2008; Tan and Soderlund, 2009; Tan and Soderlund, 2010; Tan and Soderlund, 2011). The identification of Nav1.6 as a pyrethroid-sensitive isoform is of particular interest because Nav1.6 is the most abundantly-expressed sodium channel α subunit in the adult brain (Auld et al., 1988), where it is preferentially expressed in regions of brain axons associated with action potential initiation (Hu et al., 2009). Nav1.6 is also the predominant isoform at nodes of Ranvier and is expressed in presynaptic and postsynaptic membranes of the neocortex and cerebellum (Caldwell et al., 2000). This pattern of expression implies that Nav1.6 sodium channels play important roles in both electrical and chemical signaling in the brain.
Studies with insect and mammalian sodium channel isoforms in the Xenopus oocyte system also provide evidence for compound-specific, state-dependent modification of sodium channels by pyrethroids. With insect channels (reviewed in Soderlund, 2010), Type II compounds (e.g., deltamethrin and cypermethrin) produce exclusively use-dependent modification with little or no detectable modification of channels in the resting state, whereas Type I compounds (e.g., cismethrin and permethrin) produce significant modification of resting channels that in some cases is enhanced by repeated channel activation. With rat Nav1.2 and Nav1.6 channels, modification by deltamethrin is almost exclusively use-dependent whereas modification by tefluthrin (Type I) involves both resting and use-dependent effects (Tan and Soderlund, 2010). Tefluthrin also causes both resting and use-dependent modification of rat Nav1.3 and Nav1.7 channels in oocytes (Tan and Soderlund, 2009; Tan and Soderlund, 2011), whereas S-bioallethrin (Type I) modifies Nav1.6 channels exclusively via the resting state (Tan and Soderlund, 2010). The use-dependent effects of pyrethroids imply that these compounds bind preferentially to channels in the open state. The significance of use-dependent modification, particularly for insect channels, has led to the development of a high-resolution molecular model of the inner pore region of an insect sodium channel in the open configuration that identifies specific structural elements of the putative pyrethroid receptor (O'Reilly et al., 2006; Usherwood et al., 2007; Du et al., 2009).
Although the Xenopus oocyte system readily permits the manipulation of channel structure as an experimental variable, the biophysical and pharmacological properties of channels expressed in the oocyte membrane environment may not fully reproduce those in native neurons or mammalian cell expression systems due to species differences in membrane composition and post-translational modification (Goldin, 2006). The HEK293 cell line, derived from human embryonic kidney cells by transformation with sheared adenovirus type 5 DNA (Graham et al., 1977), is now widely employed as an alternative to Xenopus oocytes for the heterologous expression of a variety of proteins, including mammalian voltage-gated sodium channels (Thomas and Smart, 2005). Despite their origin HEK293 cells exhibit some characteristics of neurons, expressing more than 60 neuronal genes including neurofilament proteins and neuroreceptor and ion channel subunits (Shaw et al., 2002; Thomas and Smart, 2005). Moreover, some clonal populations of HEK293 cells exhibit small endogenous voltage-gated sodium currents that are associated primarily with the expression of the human Nav1.7 sodium channel isoform (He and Soderlund, 2010). To date there has been no comprehensive study of the action of pyrethroid insecticides on sodium channels of defined subunit structure expressed in HEK293 cells or any other mammalian cell expression system.
Here we describe the actions of tefluthrin and deltamethrin on rat Nav1.6 sodium channels stably expressed in HEK293 cells in combination with the rat β1 and β2 auxiliary subunits. Our results show that both compounds produced significant modification of Nav1.6 channels in the resting state that altered both the kinetics and voltage-dependent gating of the channel. Resting modification by deltamethrin, but not tefluthrin, was enhanced by repeated channel activation. These findings provide evidence that the actions of tefluthrin and deltamethrin on Nav1.6 channels in HEK293 cells differ from the effects of these compounds on Nav1.6 channels in Xenopus oocytes and more closely reflect the actions of pyrethroids on channels in their native neuronal environment.
Materials and methods
Sodium channel subunit cDNAs
Cloned rat voltage-gated sodium channel subunit cDNAs were obtained from the following sources: Nav1.6 from L. Sangameswaran (Roche Bioscience, Palo Alto, CA); and, β1 and β2 from W.A. Catterall (University of Washington, Seattle, WA). Each cDNA insert was subcloned into the vector pcDNA3.1 (Invitrogen, Carlsbad, CA) and the integrity of each clone was confirmed by DNA sequencing.
HEK-Nav1.6 cell lines
HEK293 cells (CRL-1573, lot number 7681666) were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (all from ATCC) in a humidified atmosphere of 5% CO2/95% air. Upon receipt cells were passaged twice and then frozen in DMEM+FBS with 5% dimethyl sulfoxide (DMSO) for future use; these stocks were considered to be at "laboratory passage one." One day before transfection, cells (passage five, 0.5×105 cells/100 µl growth medium without antibiotics) were transferred to a well of a 96-well plate and grown until ~80% confluent. Cells were transfected using 0.2 µg of plasmid DNA mixture (Nav1.6, β1 and β2 plasmids, 1:1:1 molar ratio) and Lipofectamine™2000 (Invitrogen) according to the manufacturer’s protocol. Cells were diluted 1:10 into 6-well plates 24 h after transfection, incubated in culture medium for an additional 24 h, and then selected for 15 days with culture medium containing G418 (Invitrogen; 800 µg/ml). Clonal colonies (derived from a single cell; ~50 cells/colony) of G418-selected cells were isolated using cloning rings (Sigma-Aldrich, St. Louis, MO) and maintained in continuous culture under G418 selection (400 µg/ml) for electrophysiological characterization. Clonal cell lines giving whole-cell peak transient sodium currents with amplitudes ≥2000 pA were saved as frozen stocks for further use.
Analysis of sodium channel subunit expression
First-strand cDNA from transfected sodium current-positive cell lines, synthesized using the SuperScript™ CellsDirect cDNA synthesis system (Invitrogen), was employed as the template in polymerase chain reaction (PCR) amplifications using pairs of oligonucleotide primers specific for the rat Nav1.6 α subunit and the rat β1 and β2 subunits (Table 1). Reactions were initiated by denaturing for 2 min at 94 °C followed by 40 cycles of amplification (30 sec at 94 °C, 30 sec at 62 °C, 1 min at 72 °C) and final extension for 7 min at 72 °C. Amplification products (151 – 294 bp) were analyzed by electrophoresis in 2% agarose gels and visualized by ethidium bromide staining.
Table 1.
Oligonucleotide primers employed in the PCR detection of rat sodium channel subunit transcripts expressed in HEK-Nav1.6 cells.
| Subunit | Primer sequences | Product |
|---|---|---|
| Nav1.6 | F: 5’-ACGATTTGAAGGGATGAGGGT-3’ | 294 bp |
| R: 5’-TGAAGGTTGCCACTTGAAGAA-3’ | ||
| β1 | F: 5’-TACGAGCACAACACCAGCGT-3’ | 151 bp |
| R: 5’-AGCAGTACACCATCTCCGCC-3’ | ||
| β2 | F: 5’-ACTCCACGGTGGCAGTCATC-3’ | 151 bp |
| R: 5’-TCCGTCTTGCCTTCCTCTTC-3’ |
Electrophysiology
On the day prior to assay, cells were plated at low density in 35-mm Petri dishes. For electrophysiological assays, cells (24 – 48 h after plating) were rinsed three times with extracellular perfusion medium that contained (mM): NaCl (140), KCl (5), CaCl2 (2), MgCl2 (1), and HEPES (10) at pH 7.40 (adjusted with 2M NaOH). Whole-cell patch clamp recordings were conducted at room temperature (23–27 °C) using an Axopatch 200B amplifier (Molecular Devices, Foster City, CA). Cells were perfused at ~350 µl/min with extracellular medium using a custom-fabricated passive perfusion manifold and a disposable plastic recording chamber insert (~240 µl volume; Warner Instruments, Hamden, CT). The intracellular solution contained (in mM): NaCl (35), CsF (105), MgCl2 (2), EGTA (10), and HEPES (10) at pH 7.20 (adjusted with 2M CsOH). The final osmolarity of both solutions was 295 – 305 mOsm. Fire-polished patch electrodes were fabricated from borosilicate glass capillaries (1.5 mm O.D.; 1.0 mm I.D.; World Precision Instruments Inc., Sarasota, FL) using a P-87 puller (Sutter Instruments, Novato, CA) to give a resistance of 1–2 MΩ when filled with intracellular solution. The ground electrode was a bridge of 1% agar in extracellular medium in a glass pipet. Output signals were filtered at 2 kHz and sampled at 50 kHz (DigiData 1322A; Molecular Devices). Voltage errors were minimized using 70–80% series resistance compensation. Leak currents were corrected using the P/4 method (Bezanilla and Armstrong, 1977). Membrane potentials were not corrected for junction potential (~2.3mV at 23.5°C). Data were acquired using pClamp 10.2 (Molecular Devices) software. Following the establishment of a stable holding potential (−120 mV) under voltage clamp, sodium currents were sampled using 40-ms step depolarizations to −15 mV at a frequency of 0.05 Hz for ~20 min to achieve stable sodium current amplitudes prior to initiating other protocols. To determine the voltage dependence of activation, cells were clamped at a membrane potential of −120 mV and currents were measured during a 40-ms depolarizing test pulse to potentials from −80 mV to 0 mV in 5-mV increments. To determine the voltage dependence of steady-state inactivation, cells were clamped at a membrane potential of −120 mV followed by a 100-ms conditioning prepulse to potentials from −120 mV to 0 mV in 5-mV increments and then a 40-ms test pulse to −15 mV. For determinations of use dependence, cells were given trains of 1 to 100 5-ms conditioning prepulses to 10 mV at frequencies of 20 or 66.7 Hz followed by a 40-ms test pulse to −15 mV. In some experiments, tetrodotoxin (TTX, Sigma; 500 nM final concentration) was used to visualize currents and voltage-clamp artifacts unrelated to sodium channel expression.
Assays with pyrethroids
Stock solutions of deltamethrin (99.5%; Bayer CropScience, Research Triangle Park, NC) and tefluthrin (98.8%; Syngenta, Bracknell, Berks., UK) in DMSO were diluted in extracellular medium to achieve final concentrations of 0.01 – 10 µM (deltamethrin) or 0.01 – 100 µM (tefluthrin) and applied through the perfusion system. The final concentration of DMSO in extracellular medium did not exceed 0.1%, a concentration that had no effect on sodium currents. Recording chamber inserts employed in experiments were used only once to prevent cross-contamination of cells. Following the characterization of control currents, each cell was clamped at −120 mV and sodium currents elicited by 40-ms pulses to −15 mV were sampled for 3 – 5 min at a frequency of 0.05 Hz to confirm the stability of sodium current amplitudes prior to experiments with insecticides. The last sampled control current from this series was used to normalize the amplitudes or conductances of pyrethroid-modified currents in each cell. Pyrethroids were applied by perfusion in extracellular medium and the development of pyrethroid modification was monitored until stable (~20 min) by assessing the increase in the sodium tail current observed following 40-ms test pulses from −120 mV to −15 mV at a frequency of 0.05 Hz. The voltage dependence of activation and steady-state inactivation and the effects of repeated stimulation on channel modification were measured as described above. All experiments with pyrethroids employed 10-s intervals between pulses or pulse trains to permit the complete decay of pyrethroid-modified currents.
Data analysis
Data were acquired and analyzed using pClamp 10.2 (Molecular Devices) and Origin 8.1 (OriginLab Corp., Northampton, MA). For each cell, currents from activation experiments were converted to sodium conductances and plotted as a function of test potential using the Boltzmann equation [y = (A1 – A2) / (1+e(x–x0)/dx) + A2] to give values for V0.5 (potential causing half-maximal activation) and K (slope factor). Similarly, currents from steady-state inactivation experiments with each cell were plotted as a function of prepulse potential and fitted to the Boltzmann equation. The initial conductance of the pyrethroid-induced sodium tail current, normalized to the conductance of the peak current measured in the same cell prior to pyrethroid exposure, was employed to calculate the fraction of pyrethroid-modified sodium channels (Tatebayashi and Narahashi, 1994). Statistical analyses were performed in Prism 5.0 (GraphPad Software, La Jolla, CA). Comparisons among three or more mean values employed one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for statistical significance. Comparisons with values of P < 0.05 were considered statistically significant.
Results
Expression, gating, and kinetics of sodium channels in HEK-Nav1.6 cells
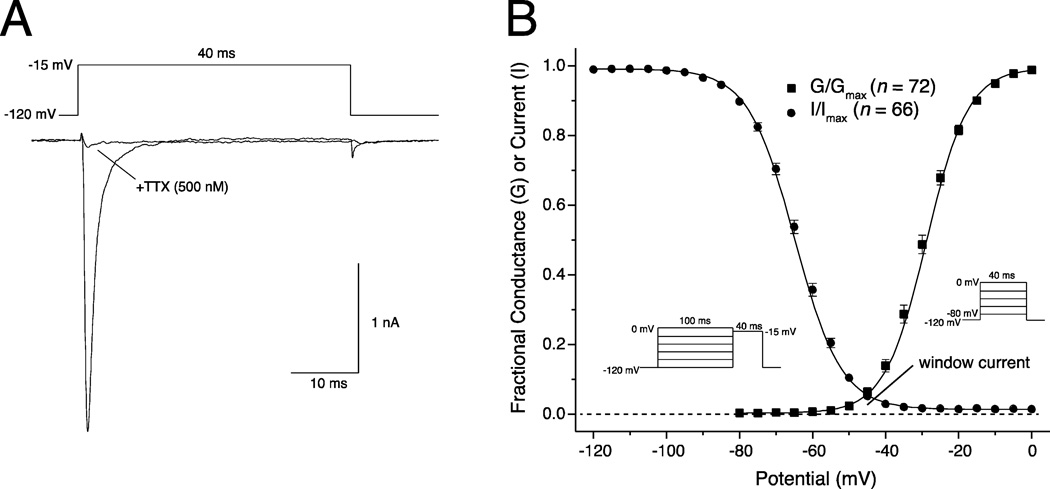
The experiments described here employed a single clonal population of HEK-Nav1.6 cells. These cells expressed peak transient sodium currents (Fig. 1A) with a mean amplitude of 4.1 ± 0. 3 nA (n = 71) and a mean sodium current density of 184.4 ± 11.7 pA/pF. These currents were completely inhibited by exposure of cells to 500 nM TTX (Fig. 1A). We confirmed the translational expression of the rat Nav1.6 α subunit and the β1 and β2 auxiliary subunits in HEK-Nav1.6 cells by RT-PCR (data not shown).
Fig. 1.
Properties of sodium currents expressed in HEK-Nav1.6 cells. (A) Representative current trace recorded during a 40-ms step depolarization from −120 mV to −15 mV before and after application of TTX (500 nM) in the perfusion medium. (B) Voltage dependence of activation and steady-state inactivation. For activation, normalized conductance (G/Gmax) was derived from the current-voltage relationship obtained using the indicated pulse protocol by dividing peak transient current (INa) by the driving force (V – Vrev) and normalizing to the maximum conductance observed in each cell. For inactivation, peak transient currents were measured using the indicated pulse protocol and normalized to the maximal peak transient current in each experiment (I/Imax). Values for G/Gmax and I/Imax were plotted as a function of test (activation) or prepulse (inactivation) potential and curves were drawn by fitting mean values to the Boltzmann equation. Values are the means of the indicated number of determinations with different cells; bars show SE values larger than the data point symbols. The dashed line indicates zero conductance or current.
Fig. 1A illustrates a representative sodium current recorded from a HEK-Nav1.6 cell. These currents activated and inactivated completely within ~10 ms of depolarization and did not exhibit any detectable residual or steady-state current at the end of a 40-ms depolarizing pulse. The decay of peak transient currents was best fit by two first order processes yielding two time constants, τfast and τslow (Table 2), for currents measured as shown in Fig. 1A.
Table 2.
Effects of tefluthrin and deltamethrin on the kinetics of fast inactivation and tail current decay of sodium channels expressed in HEK-Nav1.6 cells.
| Peak Current Inactivationb | Tail Current Decayc | ||||
|---|---|---|---|---|---|
| Conditiona | τfast | τslow | n | τ1 | n |
| Control | 0.98 ± 0.02 | 5.26 ± 0.19 | 66 | -- | |
| + Tefluthrin | 1.32 ± 0.12 d | 31.87 ± 0.81 d | 12 | 40.3 ± 3.6 | 17 |
| + Deltamethrin | 1.35 ± 0.06 d | 8.65 ± 0.93 d | 9 | NDe | |
Pyrethroids assayed at 10 µM.
Time constants (ms) for the fast (τfast) and slow (τslow) components of sodium current inactivation; values are means ± SE for the indicated number of replicate experiments with different cells.
Time constant (ms) for the fast (τ1) component of tail current decay; values are means ± SE for the indicated number of replicate experiments with different cells.
Values were significantly different from control values.
Not determined.
The voltage-dependent gating of sodium channels in HEK-Nav1.6 cells is illustrated in Fig. 1B and the statistical summary of these data is presented in Table 3. The V0.5 values for activation and steady-state inactivation of channels in HEK-Nav1.6 cells were shifted by ~5 mV in the direction of depolarization compared to values measured in another HEK293 cell line expressing the Nav1.6 α subunit without auxiliary β subunits (B. He and D. M. Soderlund, unpublished observations), thereby providing functional confirmation of the expression of heteromultimeric channel complexes in the HEK-Nav1.6 cell line employed in these studies. Steady-state inactivation of channels in HEK-Nav1.6 cells was incomplete, yielding a small component (~1.5% of the total current) that was resistant to inactivation following depolarization to potentials between −30 mV and 0 mV.
Table 3.
Effects of tefluthrin and deltamethrin on the voltage dependence of activation and steady-state inactivation of sodium channels expressed in HEK-Nav1.6 cells.a
| Activation | Inactivationb | |||||
|---|---|---|---|---|---|---|
| Condition | V0.5 | K | n | V0.5 | K | n |
| Control | −29.3 ± 0.6*c | 5.08 ± 0.13* | 72 | −64.2 ± 0.5* | 6.09 ± 0.09* | 66 |
| + Tefluthrin | ||||||
| 0.1 µM | −35.7 ± 1.1† | 5.11 ± 0.21* | 24 | −70.3 ± 1.0† | 6.77 ± 0.11† | 22 |
| 1 µM | −39.3 ± 1.3† | 6.30 ± 0.34† | 26 | −73.7 ± 1.0† | 6.44 ± 0.14* | 20 |
| 10 µM | −44.7 ± 1.9‡ | 6.61 ± 0.32† | 19 | −76.3 ± 1.0‡ | 6.25 ± 0.20* | 16 |
| 100 µM | −48.5 ± 1.7‡ | 7.21 ± 0.34† | 14 | −79.3 ± 1.0‡ | 6.28 ± 0.25* | 13 |
| + Deltamethrin | ||||||
| 0.1 µM | −31.9 ± 1.2*† | 5.31 ± 0.21* | 17 | −66.2 ± 1.3*† | 6.25 ± 0.24* | 14 |
| 1 µM | −34.2 ± 1.0† | 5.25 ± 0.28* | 15 | −68.4 ± 1.1†§ | 6.42 ± 0.26* | 15 |
| 10 µM | −36.0 ± 1.2† | 5.88 ± 0.26* | 16 | −71.6 ± 1.5§ | 6.32 ± 0.37* | 12 |
Values calculated from fits of the data from the indicated number of individual experiments to the Boltzmann equation; V0.5, midpoint potential (mV) for voltage-dependent activation or inactivation; K, slope factor.
Data for prepulse potentials from −55 mV to −25 mV were omitted from fits of inactivation data to the Boltzmann equation; see text for explanation.
Values in each column for control and concentrations of either tefluthrin or deltamethrin that are marked with the different symbols were significantly different.
Kinetic properties of pyrethroid-modified currents
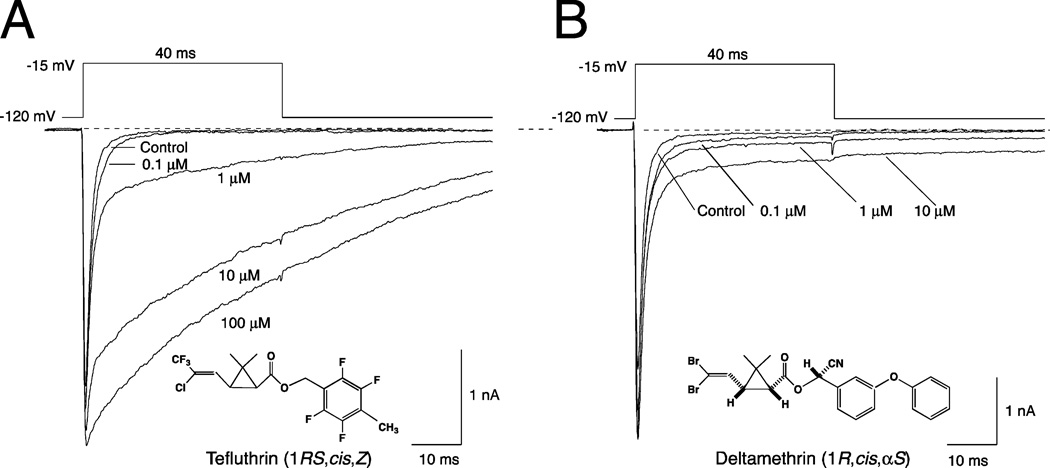
We assessed the effects of tefluthrin and deltamethrin on sodium channels in the resting state by equilibrating HEK-Nav1.6 cells with pyrethroid for 20–22 min at −120 mV and sampling peak transient currents and tail currents using low-frequency depolarizations. Figure 2 illustrates the concentration-dependent modification of sodium currents by tefluthrin and deltamethrin under these conditions obtained by the sequential equilibration of a single cell with increasing concentrations of insecticide. Tefluthrin (Fig. 2A) slowed the time course of transient current decay during a depolarizing pulse, significantly increasing τfast and τslow values for peak current decay relative to control values (Table 2) and producing a late current that persisted throughout a 40-ms depolarization. Tefluthrin also slowed the rate of channel deactivation following repolarization, producing a slowly-decaying sodium tail current. The threshold for the detection of tefluthrin-modified currents was 0.1 µM, and the relative amplitudes of the persistent currents and tail currents induced by tefluthrin increased with increasing concentration up to 100 µM, the highest concentration examined.
Fig. 2.
Concentration-dependent modification of sodium currents in HEK-Nav1.6 cells by tefluthrin (A) and deltamethrin (B). Traces for each compound were recorded from a single cell prior to pyrethroid exposure (control) and following equilibration with increasing concentrations of pyrethroid. Dashed lines indicate zero current.
Deltamethrin (Fig. 2B) also slowed the rates of channel inactivation and deactivation, but the deltamethrin-modified current was more persistent than that induced by tefluthrin. Therefore, we were unable to calculate time constants for the persistent component of the deltamethrin-modified current during depolarization and the deltamethrin-induced tail current following repolarization. Deltamethrin also significantly increased τfast and τslow values for peak current decay relative to control values (Table 2), but the rate constants obtained from these fits describe only the transient component of the current over the first 10 ms of depolarization and do not describe the sustained persistent current. The threshold concentration for the detection of deltamethrin-modified currents was also 0.1 µM, but overall the extent of channel modification by deltamethrin from the resting state at all concentrations examined (reflected in the relative amplitudes of the late current and peak transient current) was lower for deltamethrin than tefluthrin.
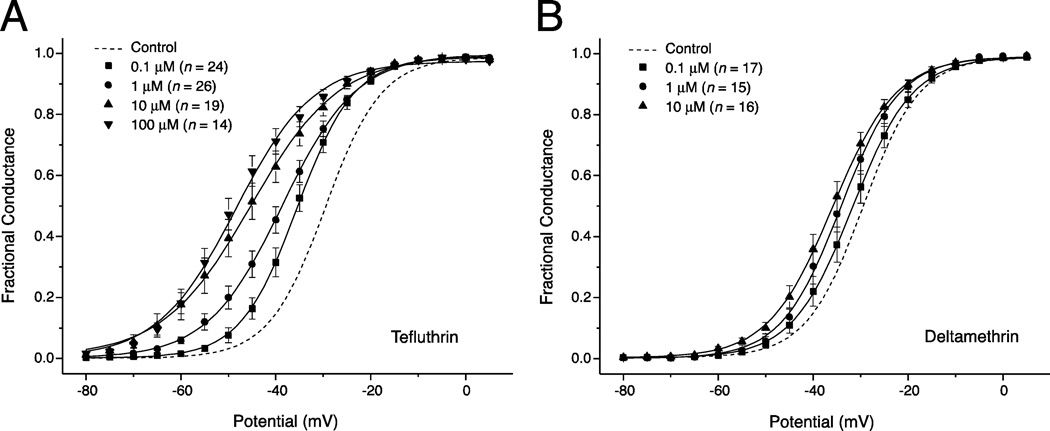
Effects of pyrethroids on voltage-dependent activation
Figure 3 illustrates the concentration-dependent effects of tefluthrin and deltamethrin on the voltage dependence of sodium channel activation in HEK-Nav1.6 cells and Table 3 summarizes the statistical analyses of these data. Both tefluthrin (Fig. 3A) and deltamethrin (Fig. 3B) caused a concentration-dependent hyperpolarizing shift in the V0.5 for channel activation that was accompanied by a reduction in the slope (increase in numerical slope factor) of the voltage response curve. Although the trend of both of these effects was consistent across concentrations, differences in V0.5 and K values were statistically significant only for some of the binary comparisons (Table 3).
Fig. 3.
Concentration-dependent modification of the voltage dependence of activation of sodium channels in HEK-Nav1.6 cells by tefluthrin (A) and deltamethrin (B). Values for the conductance of peak sodium current were plotted as a function of test potential and curves were drawn by fitting mean values to the Boltzmann equation. Values are the means of the indicated number of determinations with different cells; bars show SE values larger than the data point symbols. Dashed lines show the curve obtained by fitting mean control values to the Boltzmann equation (from Fig. 1B).
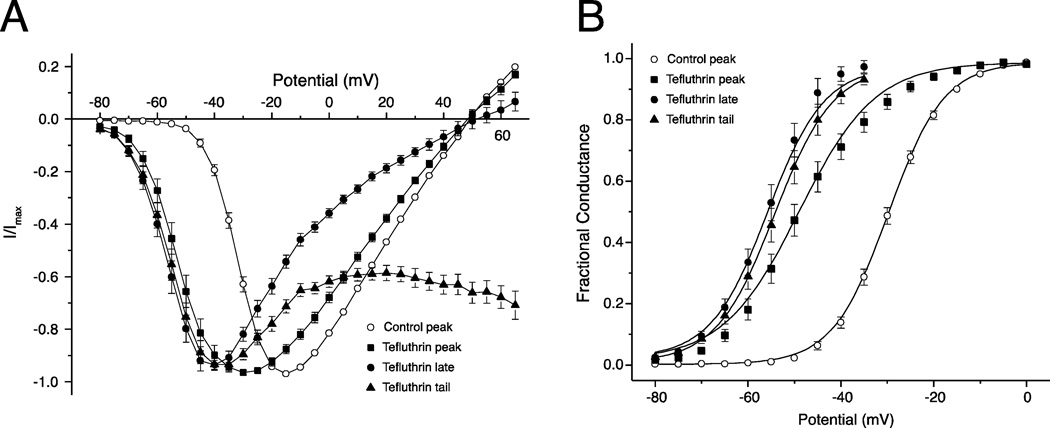
The concentration dependence of the activation parameters illustrated in Fig. 3 and Table 3 reflects the heterogeneous responses of mixed populations of modified and unmodified channels because the solubilities of tefluthrin and deltamethrin attainable in aqueous medium were insufficient to give concentrations that saturated the response (Fig. 2). To assess gating properties of only the pyrethroid-modified population of sodium channels, we measured the voltage dependence of the pyrethroid-induced late and tail currents and compared them to the control (unmodified) current and the pyrethroid-modified peak transient current. Figure 4 illustrates this approach with 100 µM tefluthrin. Figure 4A shows normalized current – voltage plots for control peak transient current, peak transient current measured in the presence of 100 µM tefluthrin, and the late and tail currents induced by 100 µM tefluthrin. In the absence of insecticide, depolarization to −15 mV gave the largest-amplitude peak transient currents. Exposure to 100 µM tefluthrin shifted the peak transient current maximum to −30 mV but shifted the maxima for late and tail currents to −40 mV. Plots of the conductance transformations of these data (Fig. 4B) show that the peak transient current measured in the presence of 100 µM tefluthrin underestimated the effect of tefluthrin on the voltage dependence of tefluthrin modified channels, which is reflected in the voltage responses of the late and tail currents. Moreover, the slopes of the transformed voltage response curves for the late and tail currents paralleled the slope for control channels, implying that the decreased slopes of the curve for tefluthrin-modified peak transient currents (Fig. 3A and Table 3) reflected the divergent voltage responses of modified and unmodified channels.
Fig. 4.
Comparison of the voltage dependence of the peak, late and tail currents induced by 100 µM tefluthrin. (A) Normalized current – voltage plots for control peak sodium currents and for the peak currents (as in Fig. 3A), late currents (measured at the end of a 40-ms depolarizing pulse) and tail currents (measured immediately following repolarization) following exposure to tefluthrin. Values are the means of 72 (control) or 14 (+tefluthrin) determinations with different cells; bars show SE values larger than the data point symbols. (B) Plots of the conductance transformations of data in Fig. 4A; curves were drawn by fitting mean values to the Boltzmann equation.
Table 4 gives V0.5 and K values for late and tail currents induced by 100 µM tefluthrin from the data in Fig. 4 as well as the corresponding values from similar analyses with 10 µM tefluthrin and 10 µM deltamethrin. Comparison of the results obtained with 10 µM and 100 µM tefluthrin shows that the voltage dependence of these currents, unlike the peak transient currents measured at the same time (Fig. 3A and Table 3), did not vary with insecticide concentration. This result confirms the hypothesis that the late and tail current parameters describe a single population of tefluthrin-modified channels. We infer from these data that the V0.5 for activation of channels modified by tefluthrin in the resting state was shifted in the direction of hyperpolarization by ~25 mV. Extension of this analysis to currents measured in the presence of 10 µM deltamethrin indicated that the corresponding hyperpolarizing shift for deltamethrin-modified resting channels was ~20 mV.
Table 4.
Voltage-dependent gating parameters for late currents and tail currents in HEK-Nav1.6 cells induced by tefluthrin and deltamethrin.a
| Late Current | Tail Current | ||||
|---|---|---|---|---|---|
| Condition | n | V0.5 | K | V0.5 | K |
| + Tefluthrin (10 µM) | 19 | −55.0 ± 1.5 | 4.33 ± 0.26 | −52.0 ± 1.9 | 5.18 ± 0.32 |
| + Tefluthrin (100 µM) | 14 | −55.2 ± 1.3 | 4.78 ± 0.22 | −53.6 ± 1.3 | 5.40 ± 0.21 |
| + Deltamethrin (10 µM) | 16 | −48.2 ± 1.5 | 4.97 ± 0.37 | −46.6 ± 1.4 | 5.93 ± 0.42 |
Values are means ± SE calculated from fits of individual data sets obtained in the presence of tefluthrin or deltamethrin to the Boltzmann equation (see Fig. 4) ; V0.5, midpoint potential (mV) for voltage-dependent activation or inactivation; K, slope factor; n, number of replicate experiments.
Effects of pyrethroids on steady-state inactivation
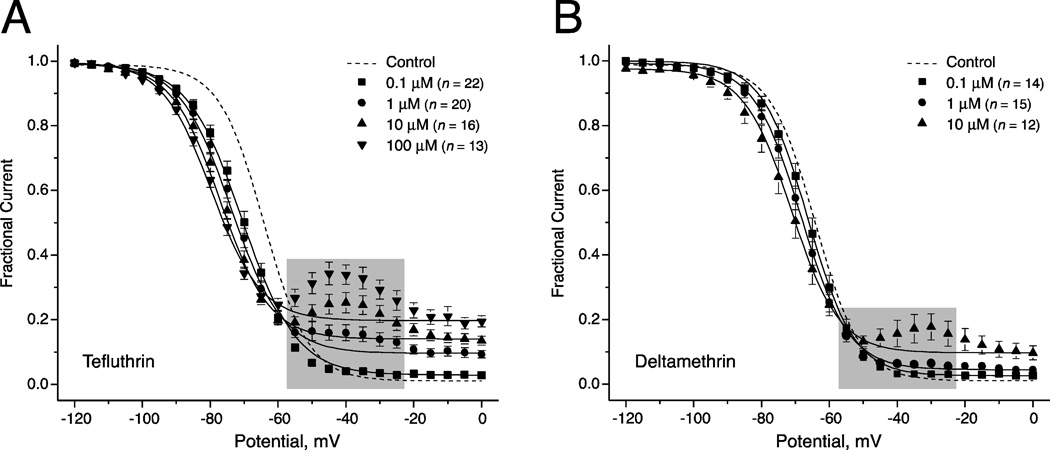
Figure 5 illustrates the effects of tefluthrin and deltamethrin on the voltage dependence of steady-state inactivation of sodium channels in HEK-Nav1.6 cells and Table 3 summarizes the statistical analyses of these data. Both tefluthrin (Fig. 5A) and deltamethrin (Fig. 5B) caused a concentration-dependent hyperpolarizing shift in the V0.5 for steady-state inactivation. However, the magnitude of these shifts was smaller than those found for activation and only some of the binary comparisons were statistically significant. Effects of insecticides on the slope of the voltage response curve for steady-state inactivation were significant only for 0.1 µM tefluthrin (Table 3).
Fig. 5.
Concentration-dependent modification of the voltage dependence of steady-state inactivation of sodium channels in HEK-Nav1.6 cells by tefluthrin (A) and deltamethrin (B). Normalized amplitudes of peak sodium currents were plotted as a function of test potential and curves were drawn by fitting mean values to the Boltzmann equation. Data points in the shaded regions were omitted from the fits of data obtained in the presence of pyrethroids (see text for details). Values are the means of the indicated number of determinations with different cells; bars show SE values larger than the data point symbols. Dashed lines show the curve obtained by fitting mean control values to the Boltzmann equation (from Fig. 1B).
The results shown in Fig. 5 also illustrate two additional effects of pyrethroids on steady-state inactivation. First, the voltage dependence of inactivation was reversed at conditioning potentials between −55 mV and −25 mV. The magnitude of this effect varied with pyrethroid concentration, thus implying an effect directly related to channel modification. With tefluthrin, the maximum reversal of inactivation occurred at potentials causing maximal activation of pyrethroid-modified channels (Fig. 4A). We postulate that the sodium currents measured during these steady-state inactivation experiments included components of sodium current resulting from the activation of pyrethroid-modified but non-inactivated channels during conditioning prepulses to potentials from −55 mV to −25 mV. To correct for this effect, we omitted the data from −55 mV to −25 mV from the fits of these results to the Boltzmann equation that resulted in the V0.5 and K values reported in Table 3. Second, both tefluthrin or deltamethrin increased the fraction of sodium current that was resistant to inactivation during strong depolarizing prepulses (e.g., to 0 mV). The magnitude of the inactivation-resistant current was concentration dependent, increasing from ~1.5% in the absence of insecticide to ~20% with 100 µM tefluthrin and ~10% with 10 µM deltamethrin (Fig. 4).
Effects of pyrethroids on sodium window currents
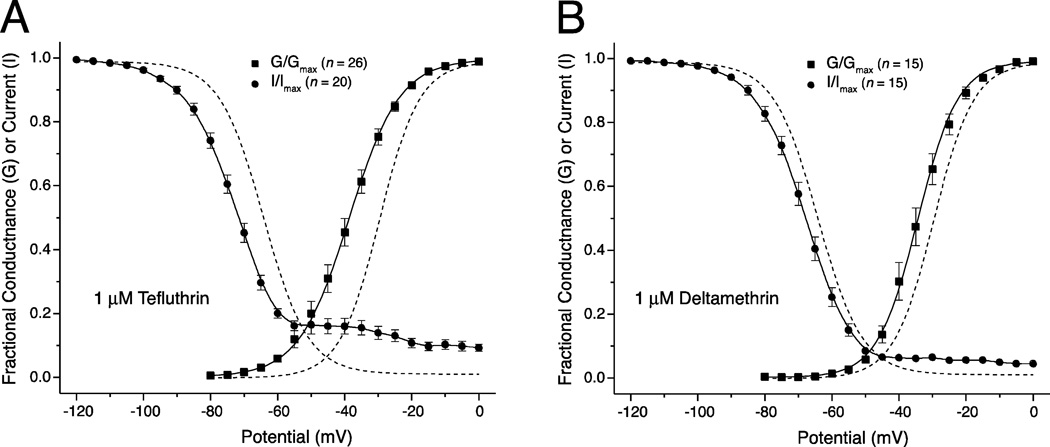
Figure 6 shows the combined impact of pyrethroid-induced hyperpolarizing shifts in activation and steady-state inactivation on sodium window currents using results obtained with 1 µM tefluthrin (Fig. 6A) and 1 µM deltamethrin (Fig. 6B). Tefluthrin at 1 µM produced a large increase in the availability of channels for activation at all potentials above −65 mV. The effects of deltamethrin at 1 µM were qualitatively similar but less dramatic, showing a more modest increase in the availability of channels for activation at potentials above −55 mV. These results show that both pyrethroids, but especially tefluthrin, significantly increased the probability of channel opening across a wide range of membrane potentials at which unmodified channels are either unresponsive or inactivated.
Fig. 6.
Effects of 1 µM tefluthrin (A) and 1 µM deltamethrin (B) on sodium window currents in HEK-Nav1.6 cells. Each panel shows voltage dependence plots for activation and steady-state inactivation in the presence of 1 µM pyrethroid taken from data in Figs. 3 and 5. Dashed lines show the curves obtained by fitting mean control values for activation and steady-state inactivation to the Boltzmann equation (from Fig. 1B).
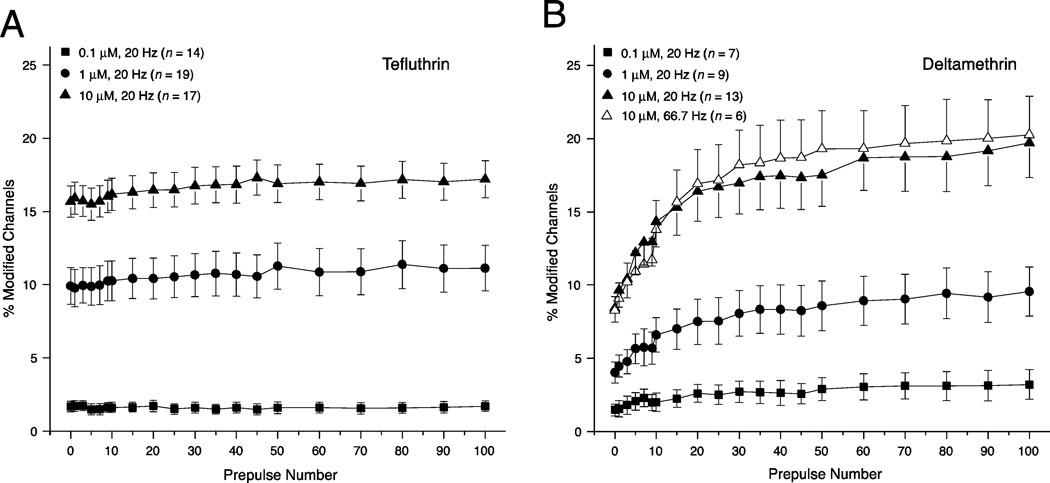
Relative importance of resting and use-dependent modification
Evidence for use-dependent effects of both tefluthrin and deltamethrin in assays of Nav1.6 sodium channels expressed in Xenopus oocytes (Tan and Soderlund, 2010) led us to explore the use-dependent modification of sodium channels in HEK-Nav1.6 cells. We found no evidence for an effect of repeated 5-ms depolarizations on the extent of channel modification caused by tefluthrin at a frequency of either 20 Hz (Fig. 7A) or 66.7 Hz (data not shown). By contrast, channel modification by deltamethrin increased with repeated depolarization at either 20 Hz or 66.7 Hz, reaching a steady-state level after approximately 50 prepulses (Fig. 7B). Repetitive depolarizations at a frequency of 20 Hz increased the extent of channel modification ~2.3-fold at all deltamethrin concentrations.
Fig. 7.
(A) Effects of repeated 5-ms depolarizing prepulses delivered at 20 Hz on the extent of modification of sodium channels in HEK-Nav1.6 cells by tefluthrin. (B) Effects of repeated 5-ms depolarizing prepulses delivered at 20 Hz or 66.7 Hz on the extent of modification of sodium channels in HEK-Nav1.6 cells by deltamethrin. Values are the means of the indicated number of determinations with different cells; bars show SE values larger than the data point symbols.
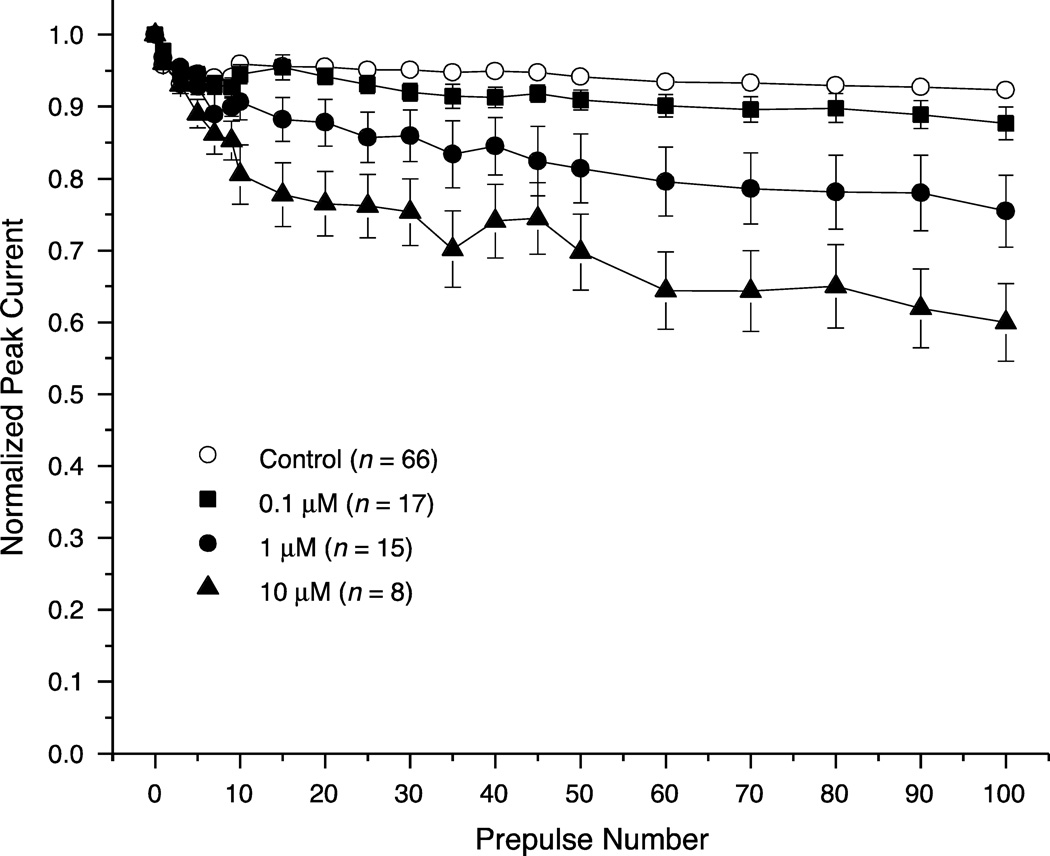
The use-dependent enhancement of channel modification by deltamethrin was accompanied by a corresponding use-dependent reduction in the amplitude of the peak transient current measured in the test pulse following a train of depolarizing prepulses (Fig. 8). In the absence of insecticide, peak currents declined rapidly to ~95% of the control current after 10 prepulses and then stabilized. In the presence of deltamethrin, peak currents declined at a rate and to an extent that correlated well with the use-dependent increase in channel modification (Fig. 7B). By contrast, repeated depolarization did not reduce peak current stability in the presence of tefluthrin (data not shown).
Fig. 8.
Effects of repeated 5-ms depolarizing prepulses delivered at 20 Hz on the normalized amplitude of peak sodium currents in HEK-Nav1.6 cells before (control) or after exposure to deltamethrin. Values are the means of the indicated number of determinations with different cells; bars show SE values larger than the data point symbols.
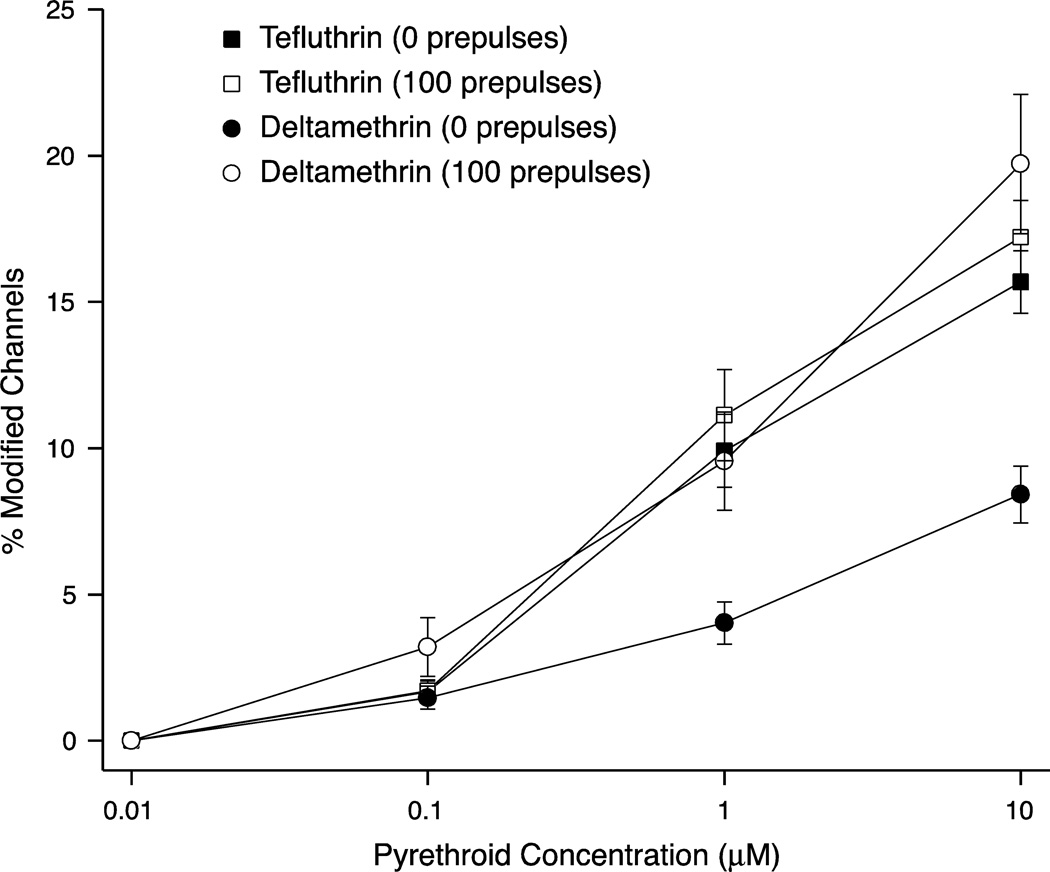
Figure 9 shows the concentration dependence of resting (0 prepulses) and maximal use-dependent (100 prepulses) modification of sodium channels in HEK-Nav1.6 cells by tefluthrin and deltamethrin. Tefluthrin was a more potent modifier of resting channels than deltamethrin, but the two compounds were equally active when use-dependent modification by deltamethrin is taken into consideration.
Fig. 9.
Concentration dependence of resting (0 prepulses) and maximal use-dependent (100 prepulses) modification of sodium channels in HEK-Nav1.6 cells by tefluthrin and deltamethrin. Data points for 0.1, 1 and 10 µM are taken from the plots in Fig. 7.
Discussion
Properties of rat Nav1.6 sodium channels expressed in HEK293 cells
The HEK-Nav1.6 cell line constructed and employed for these studies expressed the rat Nav1.6 sodium channel α subunit in combination with the rat β1 and β2 subunit. This combination is likely to be the most abundant Nav1.6-containing complex in the adult rat brain (Shah et al., 2001; Schaller and Caldwell, 2003) and also permits direct comparison of the results of the present study with our previous study of the action of pyrethroids on the rat Nav1.6+ β1+ β2 sodium channel complex in the Xenopus expression system (Tan and Soderlund, 2010). The mean sodium current density in HEK-Nav1.6 cells was more than tenfold higher than the density of endogenous sodium currents in the parental HEK293 cell line (He and Soderlund, 2010), thereby ensuring that the currents characterized in this study are carried predominantly by heterologously-expressed rat Nav1.6 channels rather than by endogenous human channels.
This study represents the first description of the properties of rat Nav1.6 sodium channels, and the only description of any mammalian Nav1.6 isoform coexpressed with the β1 and β2 auxiliary subunits, in a mammalian cell expression system. The activation and steady-state inactivation curves for channels in HEK-Nav1.6 cells were shifted by 12–14 mV in the direction of hyperpolarization compared to curves obtained by expressing the same channel complex in Xenopus oocytes (Tan and Soderlund, 2010). This difference is likely to reflect an effect of cell environment on gating because both the subunit composition of the Nav1.6 sodium channel complex and the electrophysiological protocols were otherwise identical in these two studies. The voltage dependence of activation of rat Nav1.6 channels in the HEK-Nav1.6 cell line was in good agreement with that measured for HEK293 cells expressing the human Nav1.6 ortholog in the absence of exogenous β subunits (Burbidge et al., 2002) but was shifted by 10–15 mV in the direction of hyperpolarization compared to the mouse Nav1.6 ortholog expressed in ND7/23 neuroblastoma cells in the absence of β subunits (Wittmack et al., 2004) or in tsA-201 cells either alone or with the rat β4 subunit (Chen et al., 2008). No meaningful comparison of inactivation gating is possible among the rat, human and mouse Nav1.6 orthologs due to the different inactivating prepulse protocols employed. Finally, sodium currents carried by rat Nav1.6 channels in HEK-Nav1.6 cells also lacked the pronounced persistent component of sodium current during depolarization observed for human (Burbidge et al., 2002) or mouse (Chen et al., 2008) Nav1.6 channels. It is not clear whether these differences in gating properties among mammalian Nav1.6 orthologs reflect intrinsic differences conferred by the structures of the subunits, differences due to the presence or absence of β subunits, or differences in the cellular environment.
Resting modification of sodium channels in HEK-Nav1.6 cells by tefluthrin and deltamethrin
Exposure of HEK-Nav1.6 cells to either tefluthrin or deltamethrin at membrane potentials that favor resting channel conformations profoundly altered both the kinetics and voltage-dependent gating observed upon subsequent depolarizations. Both compounds produced persistently open channels, evident in a slowing of the decay of the peak current during depolarization and the induction of a characteristic sodium tail current following repolarization. The decay of the peak and tail currents was slower in assays of Nav1.6 in HEK293 cells than in assays of these channels in oocytes under otherwise comparable conditions (Tan and Soderlund, 2010). With tefluthrin the time constants for the decay of peak and tail currents In HEK-Nav1.6 cells were 1.7-fold and 4.1-fold greater than those measured in assays of Nav1.6 channels in oocytes. With deltamethrin channel modification in HEK-Nav1.6 cells was essentially irreversible during the time frame of the experimental protocol, thereby preventing the determination of decay constants. The extreme persistence of deltamethrin-modified open channels in our assays with HEK-Nav1.6 cells mirrors the persistent modification of sodium channels by deltamethrin in both neuroblastoma cells and cultured neurons (Chinn and Narahashi, 1986; Tabarean and Narahashi, 1998; Motomura and Narahashi, 2001).
In addition to their effects on channel kinetics, tefluthrin and deltamethrin also modified the voltage dependence of activation and inactivation of sodium channels in HEK-Nav1.6 cells. Both insecticides caused a concentration-dependent hyperpolarizing shift in the voltage-dependence of channel activation. Using the voltage dependence of the pyrethroid-induced late and tail currents as indicators of the properties of only the fraction of channels modified by insecticide, we estimated the magnitude of the shift in activation curves to be ~25 mV with tefluthrin and ~20 mV with deltamethrin. These findings are consistent with previous reports of hyperpolarizing shifts on the order of ~10–20 mV in sodium channel activation curves for tefluthrin on the endogenous sodium channels of HEK293 cells (He and Soderlund, 2010) and sodium channels in rat GH3 pituitary tumor cells (Wu et al., 2009) and for deltamethrin and tetramethrin on the TTX-sensitive and TTX-resistant sodium currents of rat dorsal root ganglion neurons (Tatebayashi and Narahashi, 1994; Tabarean and Narahashi, 1998). By contrast, assays of cismethrin and cypermethrin action on rat Nav1.8 sodium channels expressed in Xenopus oocytes revealed only small (~5-mV) hyperpolarizing shifts in the voltage dependence of pyrethroid-modified late and tail currents compared to control peak transient currents (Smith and Soderlund, 1998). Moreover, there were no effects of tetramethrin or deltamethrin on the voltage dependence of peak transient current activation of rat Nav1.2, Nav1.3, Nav1.6, Nav1.7 and Nav1.8 sodium channels and human Nav1.3 sodium channels when assayed in the Xenopus oocyte system (Choi and Soderlund, 2004; Tan and Soderlund, 2009; Tan and Soderlund, 2010; Tan and Soderlund, 2011).
Tefluthrin and deltamethrin also caused hyperpolarizing shifts in the voltage dependence of steady-state inactivation of sodium channels in HEK- Nav1.6 cells, but the magnitude of these effects was much less than the effects on activation. These findings agree with the reported effects of tetramethrin on the TTX-sensitive and TTX-resistant sodium currents of rat dorsal root ganglion neurons (Tatebayashi and Narahashi, 1994). Deltamethrin also shifts the voltage dependence of steady-state inactivation of rat Nav1.8 sodium channels expressed in Xenopus oocytes in the direction of hyperpolarization (Choi and Soderlund, 2004), but neither tefluthrin nor deltamethrin affect the inactivation gating of other mammalian sodium channels expressed in this system (Tan and Soderlund, 2009; Tan and Soderlund, 2010; Tan and Soderlund, 2011). In addition to their effects on the voltage-dependent gating of sodium channels in HEK-Nav1.6 cells, both tefluthrin and deltamethrin caused concentration-dependent increases in the fraction of sodium current that was resistant to steady-state inactivation. This result is consistent with the effects of tefluthrin, deltamethrin and S-bioallethrin on Nav1.6 sodium channels expressed in Xenopus oocytes (Tan and Soderlund, 2010), but similar effects have not been reported for other channel isoforms expressed in oocytes.
Effects of pyrethroids on sodium window currents in HEK-Nav1.6 cells
The area of overlap between the voltage dependence curves for activation and steady-state inactivation is the window current (Fig. 2B), which describes the range of membrane potentials at which channels are predicted to be persistently open and conducting a current (Attwell et al., 1979). The magnitude of window currents differs among sodium channel isoforms; large window currents observed with the TTX-resistant Nav1.8 and Nav1.9 sodium channel isoforms are correlated with the significant persistent sodium currents produced by these isoforms in voltage clamp assays (Dib-Hajj et al., 2009). Similarly, mutations in Nav1.7 sodium channels that are associated with heritable pain disorders and increased sensory neuron excitability in humans cause hyperpolarizing shifts in the voltage dependence of channel activation and increases in the sodium window current (Dib-Hajj et al., 2009; Ahn et al., 2010).
The effects of tefluthrin and deltamethrin on the voltage dependence of activation and steady-state inactivation combined to cause a significant increase in the magnitude of the window current carried by sodium channels in HEK-Nav1.6 cells. The hyperpolarizing shifts in the activation curves for pyrethroid-modified channels increased the availability of channels for activation at membrane potentials below the activation threshold for unmodified channels. Moreover, these compounds prevented the complete inactivation of channels at more positive potentials. As a result, pyrethroids function in this system as sodium channel activators by creating a population of channels that open and remain open at membrane potentials that render unmodified channels refractory to activation. The effects of these two pyrethroids on channel availability at subthreshold potentials are therefore fully consistent with a recent study showing that tefluthrin, deltamethrin and other pyrethroids directly stimulate sodium uptake into cultured mouse cerebrocortical neurons (Cao et al., 2011).
Use-dependent modification of Nav1.6 channels in oocytes and HEK293 cells
Most studies of pyrethroid action on sodium channels in mammalian neurons under voltage clamp conditions (reviewed in Soderlund et al., 2002) were performed by equilibrating channels with pyrethroids at hyperpolarized membrane potentials and assessing the effects of pyrethroids upon depolarization. This approach is biased toward the detection of channel modification in the closed state. The discovery that modification by cypermethrin and deltamethrin of cloned insect sodium channels expressed in Xenopus oocytes is absolutely dependent on repeated depolarization (Smith et al., 1998; Vais et al., 2000b) has led to the conclusion that these compounds bind preferentially to open sodium channels. These findings have prompted renewed interest in the broader significance of pyrethroid binding to the open state of sodium channels and have led to the development of a new structural model for the molecular interactions between pyrethroids and the open state of the house fly sodium channel pore region (O'Reilly et al., 2006).
Studies with cloned mammalian sodium channels expressed in the Xenopus oocyte system also identify the importance of use-dependent channel modification for some pyrethroids. High concentrations of deltamethrin and cypermethrin produce low levels of resting modification of rat Nav1.2, Nav1.3 and Nav1.6 channels in oocytes, but repetitive depolarizations greatly increase the fraction of modified channels (Smith and Soderlund, 1998; Vais et al., 2000a; Meacham et al., 2008; Tan and Soderlund, 2010). Tefluthrin produces greater resting modification of these channel isoforms than deltamethrin but the extent of modification is also enhanced by repeated depolarization (Tan and Soderlund, 2009; Tan and Soderlund, 2010). In assays with Nav1.2 and Nav1.6 channels in oocytes, repetitive depolarization increases the apparent sensitivity of channels to tefluthrin modification by more than 10-fold (Tan and Soderlund, 2010). By contrast, repetitive depolarization has no effect on the extent of modification of Nav1.6 channels by S-bioallethrin.
The results of the present study with HEK- Nav1.6 cells, which are directly comparable to our previous study of the action of tefluthrin and deltamethrin on Nav1.6 channels in oocytes (Tan and Soderlund, 2010), suggest that use-dependent modification is less significant for channels expressed in HEK293 cells than for those expressed in oocytes. In contrast to their effects in oocytes, both tefluthrin and deltamethrin produced extensive resting modification of channels in HEK-Nav1.6 cells. Moreover, repetitive depolarization had no effect on tefluthrin modification and enhanced deltamethrin modification to a lesser extent than observed in oocytes. The differences between oocytes and HEK293 cells in the relative significance of resting and use-dependent modification imply that the relative affinities of the pyrethroid receptor on closed and open channels for a given pyrethroid is dependent in part on the cellular expression context. The greater relative significance of resting modification that we observed in assays with HEK-Nav1.6 cells is consistent with the large body of evidence documenting the resting modification of sodium channels in neurons by pyrethroids representing the both the Type I and Type II structural classes (Soderlund et al., 2002).
Relative pyrethroid sensitivity of Nav1.6 channels expressed in oocytes and HEK293 cells
One disadvantage of the oocyte expression system is that many pharmacological agents have been found to be less potent on ion channels expressed in oocytes than on channels expressed in mammalian cells or in their native cellular environment (Goldin, 2006). Studies with radiolabeled deltamethrin document extensive accumulation in the oocyte during exposure (Harrill et al., 2005), which may result in overestimates of the amount of insecticide available for interaction at the target site. However, we found the threshold for channel modification of Nav1.6 channels expressed in HEK293 cells by either tefluthrin or deltamethrin to be approximately 0.1 µM, a value close to that found previously in assays of these compounds on Nav1.6 channels expressed in oocytes (Tan and Soderlund, 2010). This value is also similar to the thresholds for tefluthrin- and deltamethrin-stimulated sodium uptake into mouse cerebrocortical neurons (Cao et al., 2011) and for deltamethrin modification of TTX-sensitive and TTX-resistant sodium currents in rat dorsal root ganglion neurons (Tabarean and Narahashi, 1998).
Taken together these results suggest that concentrations producing half-maximal effects of pyrethroids on mammalian sodium channels either in heterologous expression systems or in their native neuronal environment lie in the high nanomolar to micromolar range, depending on the channel and compound examined and the system and assay employed. Pyrethroids are therefore not exceptionally potent agents by these criteria. However, pyrethroid concentrations below the threshold of detectability in voltage clamp or sodium uptake assays are sufficient to disrupt normal neuronal function. For example, micromolar concentrations of tetramethrin were required to produce sufficient channel modification (5–25%) in rat Purkinje neurons to be detected readily under voltage clamp conditions, whereas 100 nM tetramethrin, a concentration calculated to modify fewer than 1% of sodium channels based on voltage clamp data, was sufficient to disrupt action potential generation in these cells (Song and Narahashi, 1996). Whereas conventional indices of the pharmacological potency of pyrethroids are useful to assess relative sensitivity among channel isoforms or relative potency among compounds, they underestimate the functional potency of pyrethroids on intact neurons.
Conclusions
The results of this study document substantial differences HEK293 cells and Xenopus oocytes as vehicles for studying the action of pyrethroid insecticides on individual sodium channel isoforms. The extensive resting modification of Nav1.6 sodium channels by tefluthrin and deltamethrin observed in the HEK293 cell system, involving effects not only on channel kinetics but also on voltage-dependent gating, corresponds closely to effects of pyrethroids described previously in native neurons. In particular, the resting modification caused by deltamethrin in our study parallels the action of this compound in mammalian neuronal preparations but contrasts sharply with the predominance of use-dependent modification found in numerous studies of the action of deltamethrin on insect and mammalian sodium channels in the oocyte expression system. At present, the close correspondence of the effects of pyrethroids on sodium channels expressed in HEK293 cells to effects on channels in native neurons is limited to data for two compounds and one channel isoform. Further studies with additional compounds and isoforms in this system are needed to fully validate the HEK293 cell system as a reliable model with predictive value for the action of pyrethroids on sodium channels in neurons.
The extensive use of Xenopus oocytes for studies of pyrethroid action on insect and mammalian sodium channels has accentuated the importance of use-dependent channel modification. Use-dependent modification has been interpreted as evidence that pyrethroids bind with higher affinity to open channels than to resting channels (Soderlund, 2010) and has led to the development of an elegant, high-resolution structural model of the pyrethroid receptor on an insect sodium channel in the open configuration (O'Reilly et al., 2006; Usherwood et al., 2007; Du et al., 2009). Whereas our results confirm the importance of use-dependent modification in the action of deltamethrin, our findings document effects of both tefluthrin and deltamethrin on the kinetics and gating of Nav1.6 sodium channels in HEK293 cells that can only result from channel modification in the resting state. We conclude that for some pyrethroids the highest affinity conformation of the receptor may occur in the resting state of the channel rather than the open state. Thus, there is a need to expand efforts to model the pyrethroid receptor to encompass both the resting and open conformations of the sodium channel.
Research Highlights.
We expressed rat Nav1.6 voltage-gated sodium channels in HEK293 cells.
Tefluthrin and deltamethrin caused resting modification of Nav1.6 channels.
Only deltamethrin exhibited use-dependent enhancement of modification.
State-dependent effects of pyrethroids are influenced by the cellular context.
Channels in HEK293 cells exhibit properties similar to native neuronal channels.
Acknowledgments
This work was supported in part by grant number R01-ES013686 from the National Institute of Environmental Health Sciences, National Institutes of Health. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences. We thank P. Adams and S. Kopatz for technical assistance and R. Araujo, S. McCavera, and R. von Stein for critical reviews of the manuscript.
Role of Funding Sources
The National Institute of Environmental Health Sciences provided financial support but no other input into this project or the manuscript derived from it.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statements for Authors
Neither B. He nor D. M. Soderlund have conflicts of interest regarding the research described in this manuscript.
References
- Ahn H-S, Dib-Hajj SD, Cox JJ, Tyrrell L, Elmslie FV, Clarke AA, Drenth JPH, Woods CG, Waxman SG. A new sodium channel gene mutation I234T in a child with severe pain. European Journal of Pain. 2010;14:944–950. doi: 10.1016/j.ejpain.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Attwell D, Cohen I, Eisner D, Ohba M, Ojeda C. The steady state TTX-sensitive ("window") sodium current in cardiac Purkinje fibres. Pflugers Arch. 1979;379:137–142. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ. A rat brain Na+ channel α subunit with novel gating properties. Neuron. 1988;1:449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. Inactivation of the sodium channel. J. Gen. Physiol. 1977;70:549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge CB, Holden L, Sturgess N, Weiner M, Sheets L, Sargent D, Soderlund DM, Choi J-S, Symington S, Clark JM, Burr S, Ray D. Evidence for a separate mechanism of toxicity for the Type I and Type II pyrethroid insecticides. Neurotoxicology. 2009;30:S17–S31. doi: 10.1016/j.neuro.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Burbidge SA, Dale TJ, Powell AJ, Whitaker WRJ, Xie XM, Romanos MA, Clare JJ. Molecular cloning, distribution and functional analysis of the Nav1.6 voltage-gated sodium channel from human brain. Mol. Brain Res. 2002;103:80–90. doi: 10.1016/s0169-328x(02)00188-2. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Nav1.6 is localized nodes of Ranvier, dendrites, and synapses. Proc. Natl. Acad. Sci. USA. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Shafer TJ, Crofton KM, Gennings C, Murray TF. Additivity of pyrethroid actions on sodium influx in cerebrocortical neurons in primary culture. Environ. Health Perspec. 2011 doi: 10.1289/ehp.1003394. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu FH, Sharp EM, Beacham D, Scheuer T, Catterall WA. Functional properties and differential modulation of Nav1.6 sodium channels. Mol. Cell. Neurosci. 2008;38:607–615. doi: 10.1016/j.mcn.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn K, Narahashi T. Stabilization of sodium channel states by deltamethrin in mouse neuroblastoma cells. J. Physiol. 1986;380:191–207. doi: 10.1113/jphysiol.1986.sp016280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J-S, Soderlund DM. Cylcosporin A and deltamethrin block the downregulation of Nav1.8 sodium channels expressed in Xenopus oocytes. Neurosci. Lett. 2004;367:389–393. doi: 10.1016/j.neulet.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Choi J-S, Soderlund DM. Structure-activity relationships for the action of 11 pyrethroid insecticides on rat Nav1.8 sodium channels expressed in Xenopus oocytes. Toxicol. Appl. Pharmacol. 2006;211:233–244. doi: 10.1016/j.taap.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Black JA, Waxman SG. Voltage-gated sodium channels: therapeutic targets for pain. Pain Med. 2009;10:1260–1269. doi: 10.1111/j.1526-4637.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Lee J-E, Nomura Y, Zhang T, Zhorov B, Dong K. Identification of a cluster of residues in transmembrane 6 of domain III of the cockroach sodium channel essential for the action of pyrethroid insecticides. Biochem. J. 2009;419:377–385. doi: 10.1042/BJ20082082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. The pyrethroids: early discovery, recent advances and the future. Pestic. Sci. 1989;27:337–351. [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu. Rev. Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Expression of ion channels in Xenopus oocytes. In: Clare JJ, Trezise DJ, editors. Expression and analysis of recombinant ion channels. Weinheim: Wiley VCH Verlag GmbH & Co.; 2006. [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–77. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Harrill JA, Meacham CA, Shafer TJ, Hughes MF, Crofton KM. Time and concentration dependent accumulation of [3H]-deltamethrin in Xenopus laevis oocytes. Toxicol. Lett. 2005;157:79–88. doi: 10.1016/j.toxlet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- He B, Soderlund DM. Human embryonic kidney (HEK293) cells express endogenous voltage-gated sodium currents and Nav1.7 sodium channels. Neurosci. Lett. 2010;469:268–272. doi: 10.1016/j.neulet.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Yang M, Hou H, Shu Y. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nature Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Lawrence LJ, Casida JE. Pyrethroid toxicology: mouse intracerebral structure-toxicity relationships. Pestic. Biochem. Physiol. 1982;18:9–14. [Google Scholar]
- Meacham CA, Brodfuehrer PD, Watkins JA, Shafer TJ. Developmentally-regulated sodium channel subunits are differentially sensitive to α-cyano containing pyrethroids. Toxicol. Appl. Pharmacol. 2008;231:273–281. doi: 10.1016/j.taap.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Meadows LS, Isom LL. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc. Res. 2005;67:448–458. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Motomura H, Narahashi T. Interaction of tetramethrin and deltamethrin at the single sodium channel in rat hippocampal neurons. Neurotoxicology. 2001;22:329–339. doi: 10.1016/s0161-813x(01)00023-7. [DOI] [PubMed] [Google Scholar]
- O'Reilly AO, Khambay BPS, Williamson MS, Field LM, Wallace BA, Davies TGE. Modelling insecticide binding sites at the voltage-gated sodium channel. Biochem. J. 2006;396:255–263. doi: 10.1042/BJ20051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett JA. New opportunities in neuroscience, but a great danger that some may be lost. In: Beadle DJ, Mellor IR, Usherwood PNR, editors. Neurotox '03: Neurotoxicological targets from functional genomics and proteomics. London: Society of Chemical Industry; 2004. pp. 1–10. [Google Scholar]
- Power LE, Sudakin DL. Pyrethrin and pyrethroid exposures in the United States: a longitudinal analysis of incidents reported to poison centers. J. Med. Toxicol. 2007;3:94–99. doi: 10.1007/BF03160917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheles mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Schaller KL, Caldwell JH. Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum. 2003;2:2–9. doi: 10.1080/14734220309424. [DOI] [PubMed] [Google Scholar]
- Shah BS, Stevens EB, Pinnock RD, Dixon AK, Lee K. Developmental expression of the novel voltage-gated sodium channel auxiliary subunit β3, in rat CNS. J. Physiol. 2001;534:763–776. doi: 10.1111/j.1469-7793.2001.t01-1-00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adeonviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Ingles PJ, Soderlund DM. Actions of the pyrethroid insecticides cismethrin and cypermethrin on house fly Vssc1 sodium channels expressed in Xenopus oocytes. Arch. Insect Biochem. Physiol. 1998;38:126–136. doi: 10.1002/(SICI)1520-6327(1998)38:3<126::AID-ARCH3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Action of the pyrethroid insecticide cypermethrin on rat brain IIa sodium channels expressed in Xenopus oocytes. NeuroToxicology. 1998;19:823–832. [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Potent actions of the pyrethroid insecticides cismethrin and cypermethrin on rat tetrodotoxin-resistant peripheral nerve (SNS/PN3) sodium channels expressed in Xenopus oocytes. Pestic. Biochem. Physiol. 2001;70:52–61. [Google Scholar]
- Soderlund DM. Mode of action of pyrethrins and pyrethroids. In: Casida JE, Quistad GB, editors. Pyrethrum Flowers: Production, Chemistry, Toxicology, and Uses. New York: Oxford University Press; 1995. pp. 217–233. [Google Scholar]
- Soderlund DM. State-dependent modification of voltage-gated sodium channels by pyrethroids. Pestic. Biochem. Physiol. 2010;97:78–86. doi: 10.1016/j.pestbp.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch. Toxicol. 2011 doi: 10.1007/s00204-011-0726-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. Mechanisms of pyrethroid toxicity: implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Lee SH. Point mutations in homology domain II modify the sensitivity of rat Nav1.8 sodium channels to the pyrethroid cismethrin. Neurotoxicology. 2001;22:755–765. doi: 10.1016/s0161-813x(01)00065-1. [DOI] [PubMed] [Google Scholar]
- Song J-H, Narahashi T. Modulation of sodium channels of rat cerebellar Purkinje neurons by the pyrethroid tetramethrin. J. Pharmacol. Exp. Ther. 1996;277:445–453. [PubMed] [Google Scholar]
- Tabarean IV, Narahashi T. Potent modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by the Type II pyrethroid deltamethrin. J. Pharmacol. Exp. Ther. 1998;284:958–965. [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Human and rat Nav1.3 voltage-gated sodium channels differ in inactivation properties and sensitivity to the pyrethroid insecticide tefluthrin. Neurotoxicology. 2009;30:81–89. doi: 10.1016/j.neuro.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Nav1.6 sodium channels. Toxicol. Appl. Pharmacol. 2010;247:229–237. doi: 10.1016/j.taap.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Action of tefluthrin on rat Nav1.7 voltage-gated sodium channels expressed in Xenopus oocytes. Pestic. Biochem. Physiol. 2011;101:21–26. doi: 10.1016/j.pestbp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J. Pharmacol. Exp. Ther. 1994;270:595–603. [PubMed] [Google Scholar]
- Thomas P, Smart TG. HEK293 cell line: a vehicle for the expression of recombinant proteins. J. Pharm. Toxicol. Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Usherwood PNR, Davies TGE, Mellor IR, O'Reilly AO, Peng F, Vais H, Khambay BPS, Field LM, Williamson MS. Mutations in DIIS5 and the DIIS4-5 linker of Drosophila melanogaster sodium channel define binding domains for pyrethroids and DDT. FEBS Lett. 2007;581:5485–5492. doi: 10.1016/j.febslet.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Vais H, Atkinson S, Eldursi N, Devonshire AL, Williamson MS, Usherwood PNR. A single amino acid change makes a rat neuronal sodium channel highly sensitive to pyrethroid insecticides. FEBS Lett. 2000a;470:135–138. doi: 10.1016/s0014-5793(00)01305-3. [DOI] [PubMed] [Google Scholar]
- Vais H, Williamson MS, Goodson SJ, Devonshire AL, Warmke JW, Usherwood PNR, Cohen CJ. Activation of Drosophila sodium channels promotes modification by deltamethrin: reductions in affinity caused by knock-down resistance mutations. J. Gen. Physiol. 2000b;115:305–318. doi: 10.1085/jgp.115.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschoyle RD, Aldridge WN. Structure-activity relationships of some pyrethroids in rats. Arch. Toxicol. 1980;45:325–329. doi: 10.1007/BF00293813. [DOI] [PubMed] [Google Scholar]
- Whitaker WRJ, Clare JJ, Powell AJ, Chen YH, Faull RLM, Emson PC. Distribution of voltage-gated sodium channel α-subunit and β-subunit mRNAs in human hippocampal formation, cortex, and cerebellum. J. Comp. Neurol. 2000;422:123–139. doi: 10.1002/(sici)1096-9861(20000619)422:1<123::aid-cne8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Whitaker WRJ, Faull RLM, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. Comparative distribution of voltage-gated sodium channel proteins in human brain. Mol. Brain Res. 2001;88:37–53. doi: 10.1016/s0169-328x(00)00289-8. [DOI] [PubMed] [Google Scholar]
- Wittmack EK, Rush AM, Craner MJ, Goldfarb M, Waxman SG, Dib-Hajj SD. Fibroblast growth factor homologous factor 2B: association with Nav1.6 and selective colocalization at nodes of Ranvier of dorsal root axons. J. Neurosci. 2004;24:6765–6775. doi: 10.1523/JNEUROSCI.1628-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-N, Wu Y-H, Chen B-S, Liu Y-C. Underlying mechanism of action of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology. 2009;2009:70–77. doi: 10.1016/j.tox.2009.01.009. [DOI] [PubMed] [Google Scholar]