Abstract
BACKGROUND
Surgery often causes prolonged post-operative pain for which the mechanisms are unknown. Here we investigate the role of p38, a pain-associated Mitogen Activated Protein Kinase, in induction and maintenance of such pain.
METHODS
Male rats were subjected to the Skin Muscle Incision Retraction procedure at the saphenous region that causes ~4 weeks of secondary tactile hyperalgesia in the ipsilateral plantar region, indicating central sensitization. Spinal cord was sectioned from L3 and L4+5 vertebral segments and stained for activated p38 (P-p38) at post-operative day 3 (POD3), just as secondary hyperalgesia develops, at POD10-12, the time of maximum hyperalgesia, and at POD35, after the resolution of hyperalgesia. Some sections were co-stained for microglia, astrocytes and neurons. Intrathecal injections of a P-p38 inhibitor, occurred at POD2 or POD9, and subsequent changes in pain monitored.
RESULTS
Skin Muscle Incision Retraction increased the numbers of dorsal horn P-p38 positive cells in L3 by ~3-fold and in L4+5 by ~7-fold, from POD3 to POD11-12. This increase was accompanied by a shift from microglia to neurons, resulting in a ~20-fold increase in P-p38 positive neurons in L4−5 over this time. No P-p38 was detected in astrocytes. A P-p38 inhibitor given at POD2 prevented development of secondary hypersensitivity, but when given at POD9 the same dose gave weak relief of pain for <3h.
CONCLUSIONS
Spinal P-p38 Mitogen Activated Protein Kinase, activated after incision-retraction, is important for the induction of prolonged pain, but despite increased levels near the time of maximum pain, its functional importance for the maintenance of pain is not great.
INTRODUCTION
Post-operative pain of various durations can follow different surgical procedures. Minor plastic surgery causes resting pain for 48–72h, whereas thoracotomies and herniorrhaphies often result in chronic pain, lasting 6 months or longer1,2. Prolonged pain slows recovery, causing personal discomfort and social withdrawal.
The reasons for these different pain durations are unknown. Both primary hyperalgesia, at the site of injury, and secondary hyperalgesia, at regions not directly affected by the surgical procedure, contribute to the course of postoperative pain. Secondary hyperalgesia results from “central sensitization” in the spinal cord and brain which account for the longer duration pain that follows peripheral injury. Surgical incision through the skin and muscles of the foot 3 or back 4 lead to 3–5 days of acute post-incisional pain. Animal models show the involvement in the spinal cord of elevated glutamate,5 ALPHA-AMINO-3-HYDROXY-5-METHYL-4-ISOXAZOLE PROPRIONATE/kaianate6,7 and metabotropic glutamate (mGluR5) receptors,8 and of activated p38 (P-p38) Mitogen Activated Protein Kinase (MAPK)9–11 and chemokine CCL-212 in this acute pain model. Peripheral treatments that cause more prolonged pain, such as nerve injury or inflammation, involve some but not all of these factors, i.e., N-methyl-D-aspartate type glutamate receptors are involved in inflammatory and some nerve-injury pain, as are chemokines other than CCL-2.13 However, surgical procedures resulting in prolonged post-operative pain have not been similarly examined to identify the factors responsible for the chronic as well as the acute phases.
In the present study we have used the skin muscle incision retraction (SMIR) model that causes 4–5 weeks of post-operative secondary hyperalgesia and allodynia, in order to examine the role of activated p38 in prolonged postoperative pain14. Incision and retraction of the skin and muscle, entrapping the saphenous nerve for I hr, results in mechanical hypersensitivity detected on the plantar surface of the ipsilateral paw, far from the site of incision/retraction. Importantly, there are no indications of any peripheral nerve injury in this model, 15,16. Systemic morphine and gabapentin can reverse the mechano-sensitivity from SMIR17.
The saphenous nerve enters the spinal cord primarily at L3, with a lesser component entering at L414. The L4 spinal segment is predominantly innervated by the sciatic nerve, whereas L5 exclusively receives input from the sciatic nerve18, which terminates in the plantar paw, the tested area for tactile hypersensitivity in after SMIR. Tests of altered plantar sensitivity after saphenous manipulations thus reveal coupling between sciatic and saphenous nerves resulting from neuroplasticity due to central sensitization.
We hypothesized that the course of mechano-hyperalgesia from the SMIR procedure would be paralleled by a change in P-p38 expression in microglia, where it was known to be elevated after paw incision. By examining spinal cord sections from L3 and L4+L5 segments taken from rats at different postoperative pain stages we can correlate P-p38 expression with secondary hypersensitivity. Co-staining for glia and neurons in the dorsal horn allows the identification of the cell types expressing P- p38. Intrathecal delivery of an inhibitor of P-p38 near these spinal segments at different pain stages indicates its functional importance in post-operative pain induction and maintenance.
MATERIALS and METHODS
Animals
All animal experimentation was approved by the Harvard Medical Area Standing Committee on Animals, Boston, MA. Male Sprague-Dawley rats were purchased from Charles River Laboratory (Wilmington, MA) and kept in the supervised animal housing facilities, with controlled humidity (20%–30% relative humidity), room temperature (24°C), a 12-hour (6:00 AM–6:00 PM) light-dark cycle, and unlimited access to food and water. Before all behavioral experiments, the animals were handled to familiarize them with the behavioral investigator, experimental environment, and specific experimental procedures for reduction of stress during experiments. At the time of surgery, rats weighed approximately 250–300g, and after the longest post-surgical period, 13 days, they weighed 315–345 g.
Skin/muscle incision and retraction (SMIR) surgery
SMIR surgery was performed as developed by Flatters14. Rats (250–300 g) were anesthetized with intraperitoneal Nembutal® (sodium pentobarbital, 50 mg/ml, Sigma-Aldrich Chemical Co., St. Louis MO), at doses of 65–75 mg/kg, laid supine and the medial side of left lower limb shaved. The shaved skin was sterilized with beta-iodine, then alcohol to enable visualization through the skin of the saphenous vein. A 1.2 –1.5 cm skin incision was made approximately 4 mm medial to the saphenous vein to reveal the muscle of the leg. An incision (7–10 mm long) was then made in the superficial muscle layer, approximately 4 mm medial to the saphenous nerve. The superficial muscle was then parted further, by spreading it with blunt scissors within the muscle incision site, to allow the insertion of a micro-dissecting retractor with four prongs, spaced over an 8 mm distance, each prong being 4 mm deep (Cat. No. 13-1090, Biomedical Research Instruments Inc., Silver Spring, MD). The retractor was inserted into the incision site on the thigh so as to position all prongs underneath the superficial gracilis muscle and above the adductor magnus muscle. The skin and this muscle were then retracted by 2 cm, revealing the fascia of the underlying muscles. Covered by a sterile Phosphate Buffered Saline-soaked dressing over the open wound, this retraction was maintained for 1 h. The animals were closely monitored during the retraction period and, if the rat began to stir or blink its eyes, additional anesthesia was provided by muzzle inhalation of the volatile general anesthetic sevoflurane (Ultane, Abbott Labs, Abbott Park, IL). During this period the animals were also completely covered (apart from the head) with a large absorbent bench pad (VWR International, Cat. No. 56616-031, West Chester, PA) to minimize heat loss. After removal of the retractor, muscle and skin wounds were closed with 4-0 Vicryl® (Myco Medical, Cary, NC) and 3-0 silk sutures (Angiotech; Surgical Specialties Corp., Reading PA), respectively. Sham-operated rats underwent the same incisional procedure, with the same general anesthetic, but without the retraction. Following recovery from anesthesia, which occurred in 1–2 hours, all animals could ambulate normally and rise up on their hind limbs to reach food and water.
Immunocytochemistry
At 3, 11–12, and 35 days postoperative, animals were terminallly anesthetized with pentobarbital (100 mg/kg, intraperitoneal) and trans-cardially perfused with phosphate buffered saline at room temperature, followed by cold 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4, 4°C). The L3, L4 and L5 spinal cord segments were dissected, post-fixed overnight in the same perfusion fixative, and then transferred to 20% sucrose-phosphate buffered saline for cryo-protection, and incubated overnight at 4°C.
For the different post-operative days and conditions, the numbers of rats used for P-p38 staining alone were as follows: POD3: SMIR=6, Sham=5; POD11-12: SMIR=8, Sham=6; POD35: SMIR=4, Sham=4; Naïve=4. For each rat, 6–10 sections were usually counted to give an average number for that rat, and these averages were then averaged to give a mean value for that condition.
For the each of the co-localization experiments, with for microglia, astrocytes or neurons, 3 sections each from 4 rats were taken at POD3 and 7 sections from a total of 4 rats were taken at POD 10–12.
Spinal cord segments were then frozen at −20°C in a cryostat and transverse free-floating sections were cut to 30μm thickness and collected in 0.1 M phosphate buffer. After blocking with 2% normal goat serum containing 0.3% Triton X-100 for 1 h at room temperature, all the sections were incubated with either a primary rabbit antibody to P-p38, to Iba (or, occasionally, to OX-42, for microglial staining) to Glial Fibrillary Acid Protein (GFAP; for astrocyte staining), to Neuron Nuclear specific protein (NeuN) or with no primary antibody. (Sections that were incubated in no primary antibody, but the usual titer of secondary antibody, showed no significant staining of individual cells, documenting the specificity of the secondary antibody.) After thorough washing to remove excess primary antibody, sections were incubated with secondary antibodies for 1hr at 20–24°C. Sections were washed free of secondary antibody, placed on glass slides, dried, one drop of Vectashield (Vector Laboratories, Burlingame CA) placed over them and then covered with glass cover slips. Antibodies, and their respective titers were as follows: P-p38 primary antibody: (Cell Signaling, Phospho-p38 MAPK(Thr180/Tyr182 Rabbit mAb, #9215; Thr180/Tyr182 Mouse mAb, #9216); 1:400. P-p38 secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA); 1:800. CD11b primary (Clone OX-42): (AbD monoclonal antibody, Serotec, Raleigh, NC); 1:250. CD11b secondary antibody (Jackson ImmunoResearch laboratories); 1:200. Iba-1 primary antibody (Wako Chemicals USA, Richmond, VA) 1:5000; Iba-1 secondary antibody (Jackson ImmunoResearch Laboratories, Bar Harbor, ME)1:400. GFAP primary monoclonal antibody (Millipore Bioscience Research Reagents, Millipore Corp., Woburn, MA);1:4,000. GFAP secondary antibody (Jackson ImmunoResearch); 1:400. Neuronal-specific nuclear protein (NeuN) primary antibody (Millipore Bioscience Research Reagents) 1:3000; Neuron Nuclear protein secondary antibody (Jackson ImmunoResearch Laboratories)1:400. For double immunofluorescence, sections were incubated with a mixture of primary antibodies: rabbit polyclonal antibody against phosphorylated p38 (P-p38), mouse monoclonal antibody against P-p38, GFAP, OX-42, or Iba-1 for 36–48 h at 4°C, followed by a mixture of goat anti-mouse fluorescein isothiocyanate–conjugated and goat anti-rabbit indocarbocyanine–conjugated secondary antibodies for 1 h at room temperature. The staining pattern with the rabbit polyclonal Ab against P-p38 was comparable to that obtained with the mouse monoclonal Ab, although a quantitative comparison was not conducted.
Fluorescence was viewed with an Olympus BX50 fluorescence microscope (Olympus America, Inc. Center Valley, PA), and the computer images captured and manipulated by Image-J software (NIH Image, National Institutes of Health, Bethesda MD). Positive cells were identified by bright spots at >5X background intensity and those in the dorsal horn (DH) were counted by hand. Cell bodies were selected by only counting “circular” bright areas and avoiding more diffuse of undefined bright spots. Nearly the same instrument and software settings, adjusted slightly to give virtually equal cell luminosity, were applied to determine the number of P-p38-positive cells in the DH (lamina I-III) of L3 and L4+5 segments. These latter segments were merged for analysis because we lacked a clear demarcation between them during sectioning.
Behavioral testing of mechanical sensitivity
All animals to be tested behaviorally were habituated three times to the testing environment on three sequential days just prior to testing. Throughout the behavioral testing time courses, the experimenter was blind to the injected agents (P-p38 inhibitor or vehicle solution).
Animals were placed on an elevated wire mesh floor and confined underneath individual overturned plastic boxes. Mechanical allodynia/hyperalgesia was assessed using four von Frey filaments (VON FREY HAIR) with bending forces of 4 g, 6 g, 10 g and 15 g (Touch-Test™ Sensory Evaluators, Stoelting Co., Wood Dale, IL). In ascending order of force, each VON FREY HAIR filament was applied 10 times, to the mid-plantar/central area of the hindpaw encircled by tori/footpads. Withdrawal responses to each of the VON FREY HAIR filaments from both hindpaws were counted and recorded. Although significant increases in withdrawal response frequency occurred for tests using the 6, 10 and 15g filaments, only the hyperalgesic response to the 15g VON FREY HAIR is reported here. The three baseline measurements of mechanical sensitivity were taken on separate days and then averaged to provide the pre-surgery baseline response, denoted by the value graphed on POD 0.
For each time of testing and treatment group, the following numbers of rats were used: Early injection of SB203580 (on POD2): SB treated rats, n=8; vehicle treated rats, n=6. Late injection of SB203580 (on POD9): SB-injected rats, n=7; vehicle injected rats, n=4.
Statistics
Data are presented as means ± S.D. For immunocytochemistry, numbers of P-p38 positive cells, averaged for each individual rat (see Methods, above) were further averaged to give grand mean ± S.D. The density of these cells, counted in lamina I-III of the ipsilateral dorsal horn, were compared between SMIR and Sham rats using a generalized linear model (PROC GLM), with simultaneous comparisons between treatment groups (SMIR vs Sham), among the three post-operative days (3 vs 11 vs 35), and between spinal segments (L3 vs L4–L5). This was followed by pair-wise comparisons when allowed, using Tukey’s multiple comparison procedure (SAS Software, Cary N.C.).
Response values from the behavioral experiments were compared between drug (SB203580) –injected and vehicle-injected rats using one-Way ANOVA.
For all analyses, the significance minimum is reported for all comparison, which were two-tailed with significance assigned at p<0.05 for pair-wise comparisons, and at adjusted values for comparison among larger groups.
RESULTS
IMMUNOCYTOCHEMISTRY
Numbers of P-p38-positive cells increase with hyperalgesia
The SMIR procedure results in a tactile hyperalgesia of the ipsilateral hindpaw that first develops at POD3-4, reaches a maximum at POD10-14 and has resolved to the pre-operative level by POD3514. A direct test of the primary incision area is not possible in awake rats, but a sham procedure similar to this one, that includes incision but, importantly, omits the retraction step, on the rat’s back showed an acute elevation of the primary response over only the first 5–7 days4. The experiments in this paper are directed towards understanding the secondary hypersensitivity that indicates central sensitization following surgical procedures.
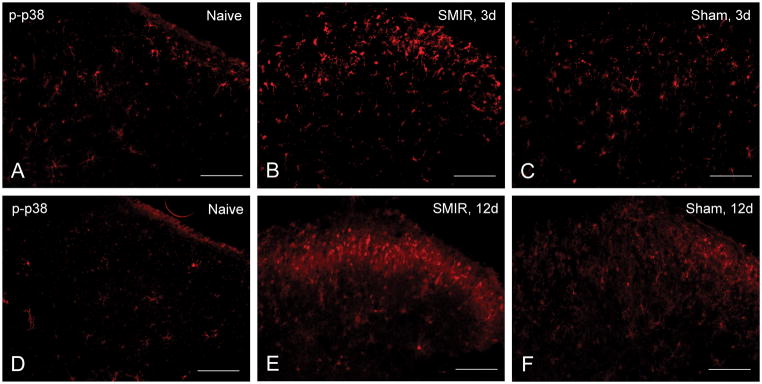
Spinal cord sections were taken from rats undergoing SMIR or sham procedures at these respective post-operative time periods and stained for P-p38 using phospho-specific antibodies (Figures 1A, B, C). Positively stained cells were apparent in the ipsilateral DH from L3 segments at POD3 (Figure 1B), primarily in lamina I–III, and were elevated over the numbers in the L3 DH taken at POD3 from naive (Figure 1A) and from sham rats (Figure 1C). The L3 spinal segment receives the major input (~75%) of the saphenous nerve, with the remaining 25% entering at L4, which also receives afferents from the sciatic nerve 14, 18. No staining above the naïve level was observed in the contralateral L3 DH (data not shown).
Figure 1.
Immunofluorescence of dorsal horn from an ipsilateral L3 spinal segment taken A. from a naïve rat (of the same age and weight as for B and C), B. at post-operative day 3 after Skin Muscle Incision Retraction treatment, and C. 3 days after Sham treatment. By comparison, segments from L3 were also taken from E SMIR-treated rats at post-operative day 12, and F.12 days after sham treatment. The naïve control for this second group, D, shows a similar low density of positive cells. Scale bar = 100μm.
Stained segments taken at the time of maximum pain are shown in Figures 1D, E, F). P-p38-positive cells were more abundant in the L3 DH from SMIR rats at the time of maximum pain, POD11-12 (Figure 1E, Table 1). Segments from L3 of sham-treated rats were less densely stained (Figure 1F), but still greater than those from naïve rats of the same weight and age (Figures 1A and 1D). In addition to the cell bodies, that are distinguished by bright spots, axons passing through the DH, shown by a more diffuse fluorescence, also were positive for P-p38 at POD12, for both SMIR and sham rats (Figure 1E, F). Contralateral segments of SMIR and sham rats taken at POD12 remained stained only to the level of the naïve DH (data not shown).
Table 1.
Numbers of P-p38-Positive Cells in Lamina I–III of Dorsal Horn of Lumbar Segments from SMIR and Sham Operated Rats (mean ± S.D.)
| Post-Operative Day | DAY 3 | DAY 11 | DAY 35 | ||||
|---|---|---|---|---|---|---|---|
| Spinal Cord Segment | L3 | L4+L5 | L3 | L4+L5 | L3 | L4+L5 | |
| Procedure | SMIR | 27 ± 4.8 (4)# | 7.4 ± 4.7 (7)# | 74.3 ± 9.8 (7)*,+,# | 52.5 ± 5 (4)*,+,# | 40.5 ± 7.1 (4)# | 32.2 ± 3.4 (8)# |
| Sham | 12 ± 2.2 (4)# | 9.3 ± 2.4 (7)# | 35 ± 5 (5)+,# | 27.5 ± 5.2 (6)+,# | 31.7 ± 3.5 (3) | 28 ± 2.4 (6) | |
SMIR: Skin-Muscle Incision Retraction
L3: lumbar spinal segment 3
L4+L5: lumbar spinal segments 4 and 5.
Significant differences, by the three-way procedure described in Statistics, Methods:
SMIR vs Sham in L3 (P = 0.017) and in L4+L5 (P = 0.034) on Day 11;
L3 vs L4+L5, for SMIR on Day 11, P = 0.031;
Day 3 vs Day 11 vs Day 35, P < 0.05 for all SMIR in L3 and also in L4 + L5, and for Sham between Day 3 and Day11 in L3 and also in L4+L5.
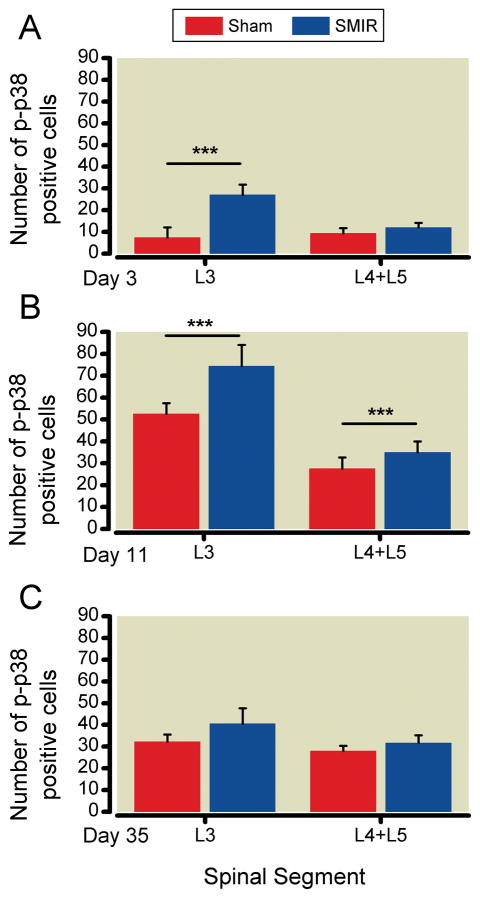
Cells positive for P-p38 in lamina I-III of spinal cord segments from L3 and L4+L5 were counted separately, for both SMIR and sham rats. (Segments from naïve rats were also examined, but so few positive cells were detected that those numbers are not reported here.) Average values for numbers of P-p38 positive cells for the separate segments and conditions are graphed in Figure 2, and collected, with significance parameters, in Table 1. At POD3 the number of P-p38 positive cells in the L3 segment from SMIR rats exceeds that in sham rats, but is equal between the two procedures in L4+5. At POD11, in both L3 and L4+L5 segments, the number of positive cells is greater in SMIR than in sham segments. The number of these cells is also greater in L3 than in L4+L5 at POD11 in SMIR animals. Finally, there is a significant difference in the number of P-p38-positive cells between POD3, POD11 and POD35 for both L3 and L4+L5 in SMIR rats and in L3 of sham rats, and between POD3 and POD11 in L4+L5 of sham rats (Table 1).
Figure 2.
Average values (± SD) of numbers of P-p38 positive cells in lamina I–III of the dorsal horn of ipsilateral L3 and merged L4+L5 segments taken from rats treated by the Skin Muscle Incision Retraction (textured bars) and Sham (open bars) procedures. A. Post-operative day 3 (before pain is elevated), B. Postoperative day 11 (at the hyperalgesic maximum), C. Post-operative day 35 (when all hyperalgesia has resolved). SMIR and Sham values compared for the same time and segment, by one-way ANOVA; *** p<0.001.
By POD11, at the time of maximum post-operative mechano-hyperalgesia, the number of positive cells in L3 of SMIR rats had approximately tripled over POD3. A similar fold increase in positive cells in sham rats had also occurred, although the numbers remain lower than in SMIR rats (Figure 2, Table 1). Segments from L4+L5 taken at these times showed a seven-fold increase in positive cells from SMIR rats, and a 3-fold increase in those from sham rats (Table 1). By POD 35, when allodynia had disappeared, the numbers of positive cells in L3 from SMIR rats was approximately halved over that at POD11-12, and similarly reduced in L4+L5 segments, so that these densities were now equal (Figure 2). In contrast, the number of positive cells in L3 of sham treated rats did not change between POD11-12 and POD35, and the same was true for those in L4+L5 (Table 1). Consequently, by POD 35 the numbers of P-p38-positive cells is the same in both segments, for both procedures (Figure 2C).
P-p38 distribution changes in microglia and neurons over time after surgery
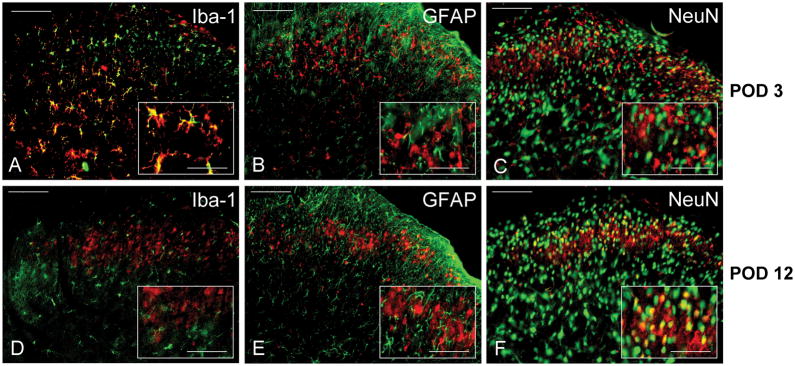
Counter-staining of P-p38 segments with antibodies that identify activated microglia, OX-42 (data not shown), or all microglia (Iba), or activated astrocytes, GFAP, allowed the identification of p38 activation among these different cell types. At POD3, the time of first detection of secondary hyperalgesia, there was strong co-localization of P-p38 and microglia in the L3 dorsal horn (Figure 3A). No P-p38 cells were identified as astrocytes, as shown by the absence of co-localization with GFAP (Figure 3B). Of the many neurons in the dorsal horn, identified by Neuron Nuclear Specific Protein staining, a small but significant number were positive for P-p38 (Figure 3C, Table 2). (Although we did not co-stain segments from naïve rats, the morphology of the few P-p38-positive cells that were detected was like that of microglia and not of neurons.)
Figure 3.
Immunofluorescence of P-p38 stained (red) ipsilateral dorsal horn L3 spinal segments taken from POD3 Skin Muscle Incision Retraction rats (top row, A–C) or POD 12 SMIR rats (bottom row, D–F), and counter-stained (green) for A, D. the microglial marker Iba-1, B, E. the astrocyte marker glial fibrillary acid protein (GFAP), and C,F. the neuronal marker NeuN (Neuron Specific Nuclear Protein). Scale bar = 100μm for the large panels, and 50μm for the magnified insets.
Table 2.
Percent of P-p38-Positive Neurons* After the SMIR Procedure
| Post-Operative Day | DAY 3 | DAY 10 – 12 |
|---|---|---|
| % NeuN Positive Cells | 14.7 + 0.8 % | 32.4 + 0.5 % |
Mean ± S.D. for numbers of co-localized cells from 3 sections from each of 4 animals for post-SMIR days 3 and 7 sections from 4 animals at post-SMIR day 10–12.
Stained by Neuron Nuclear Specific Protein (NeuN). SMIR: Skin Muscle Incision Retraction.
However, by the time that postoperative hyperalgesia was maximum, this pattern of cellular distribution had shifted. In segments taken at POD10-12 there was virtually no P-p38 detected in microglia (Figure 3D), and P-p38 continued to be absent from astrocytes (Figure 3E). Remarkably, neuronal expression of P-p38 had increased markedly by that time (Figure 3F), and a quantitative analysis showed that the fraction of P-p38 positive cells that were neurons had risen 2–3 fold from POD3 to POD 10–12 (Table 2).
BEHAVIORAL PHARMACOLOGY
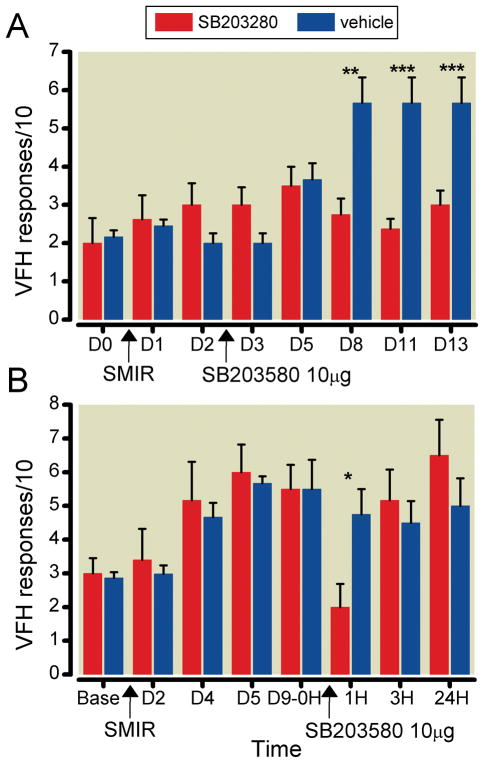
The early intrathecal delivery of an inhibitor of P-p38 was able to prevent the development of SMIR-induced mechano-sensitivity. Injection of 10 μg of SB203580 at the L4–L5 region on POD2 prevented any significant rise of response to VON FREY HAIR stimulation over the next 11 days (the duration of testing), to the time of maximum response (Figure 4A). (The significance of differences between treated and control responses at D8, D11 and D13, established in pair-wise comparisons, disappears when adjusted for comparisons among all the measured days in both groups.) In contrast, when the same dose of this inhibitor was given at POD9, after the mechano-sensitivity was well developed, the pain relieving effects were very brief (Figure 4B). (This significant difference between the treated SB203580-treated and the vehicle-treated groups, analyzed pair-wise, also disappeared when adjusted for comparison of all 8 sets of data.) The response to tactile stimulation fell to pre-operative levels, but only at 1 hr after the injection; by 3h the post-operative hyper-sensitivity had returned fully to its pre-drug level.
Figure 4.
Effect of p-p38 inhibitor on behavioral responses after Skin Muscle Incision Retraction (SMIR). Rats were stimulated by a 15 g von Frey filament (VFH) pressed against the mid-plantar paw surface of the leg ipsilateral to the surgical procedure; the vertical axis plots the number of withdrawal responses per 10 VFH stimulations (mean ± SD). A. Intrathecal injection of 10 μg of SB203580 at post-operative day 2 keeps the response level at the pre-operative baseline (denoted at day 0, D0), and, at post-operative days 8–13, significantly below that for the control, hyperalgesic rats that received only a vehicle injection. B. Intrathecal injection of 10 μg of SB203580 at post-operative day 9, after hyperalgesia has developed, results in only a brief reduction of the elevated response. Comparison of vehicle- and SB203580-injected rats on the same days: * p<0.05, ** p<0.01, *** p<0.001 (one-way ANOVA).
DISCUSSION
The results of this paper show that the activated form of p38 MAPKinase, P-p38, appears in an increased number of cells in the dorsal horn following skin-muscle incision and retraction. The patterns of distribution of P-p38 change after surgery, among the different cell types in the DH, and between segments receiving input exclusively from the saphenous nerve (L3) or from the saphenous and the sciatic nerve(L4) or the sciatic nerve alone (L5). At the earliest time of observation, POD3, when secondary hypersensitivity may be just detected but when primary post-incisional pain is almost certainly present (but not testable in this model), P-p38 appears mostly in microglia. During peak secondary hyperalgesia, at POD11-12, a time when primary post-incisional pain in other models has disappeared 3,4, 11, 12, P-p38 positive cells increase markedly in L3 and even more in L4+L5. In addition to this increase there is a shift in the distribution of P-p38 from microglia to neurons; this 2–3-fold increase in the proportion of positive cells that are neurons, coupled with the 7-fold increase in total P-p38-positive cells in L4+L5, results in an approximately 20-fold increase in the number of P-p38 positive neurons in L4+L5, from the very beginning of secondary hypersensitivity to its maximum. Since L4+L5 segments contain the inputs of the sciatic nerve from the behavioral test area of the plantar hindpaw, it is noteworthy that such a large increase in neuronal P-p38 occurs over the time when secondary hyperalgesia develops in an area innervated by the sciatic, far removed from the locus of saphenous nerve manipulation. The L3 spinal segments, exclusively innervated by the saphenous nerve, show the same numbers of P-p38 positive cells at POD3 after SMIR and POD11 after sham, both conditions when secondary hyperalgesia is absent. These same, lower numbers of P-p38-positive cells are present in L4+L5 in both SMIR and sham rats at POD 35, a time when secondary hyperalgesia (and probably primary hypersensitivity, too 3,4) has fully resolved. Although these numbers are above the baseline values seen in naïve rats, they may reflect changes in spinal circuitry that is inadequate to drive the secondary hypersensitivity from central sensitization. Thus the rising numbers of P-p38-positive cells in L3 and in L4+L5 over the first ~ two weeks appear to correlate with the induction of functional central sensitization after retraction, but not after incision alone, and the retention of P-p38 levels above the baseline at a time beyond the resolution of pain implies that p38 activation per se is not tightly coupled to the maintenance of hyperalgesia.
The importance of P-p38 for pain induction is supported by the functional effects of an inhibitor of this MAPKinase. Injected intrathecally a few days after the SMIR procedure, this agent is able to virtually prevent secondary hypersensitivity. Injected a week later, at a time near the peak of the induced secondary pain, it is marginally and only transiently effective.
How do these results compare with other studies about P-p38 involvement in injury- or inflammation-induced pain hypersensitivity, and with studies on the role of P-p38 in the acute responses to (paw) incision-induced pain, and how can we explain these findings in terms of the a model for the induction and resolution of prolonged post-operative pain?
Role of P-p38 in models of nerve injury and inflammation
P-p38 is elevated in response to peripheral nerve injury and inflammation19. Chronic constriction injury 20, and spinal nerve ligation 21,22 increase the activated P-p38 in spinal cord microglia, for at least 2 weeks. Activation of p38 occurs in spinal cord microglia after inflammation, with significant increases after the injection of formalin19, 23, carrageenan23 or FREUND’S COMPLETE ADJUVANT24 into the rat’s foot pad. Such activation is quite rapid, appearing within minutes, and quite transient25–26. Plantar injection of FREUND’S COMPLETE ADJUVANT activates p38 only in peripheral neurons, in the DorsaL Root Ganglia, and not in spinal neurons 27,28, cells where the Extracellular Signal-Regulated MAPKinase, is activated early after inflammation29 and incision30. These findings suggest a temporal sequence of activation of different MAPKinase, as well as other kinases, first in peripheral and then in central structures, and moving from sensory neurons to spinal glia31–33. Activated p38, in turn, is known to increase peripheral neuronal TRANSIENT RECEPTOR PROTEIN VANNILOID 1 expression, and to facilitate the release of cytokines and growth factors that in turn enhance release of excitatory amino acid transmitters essential for pain transmission in the spinal cord, as well as to sensitize their receptors on post-synaptic neurons34.
Inhibitors of P-p38 are generally effective in the prevention and the reversal of thermal and mechanical hypersensitivity after nerve injury,15,20,35,36 but are selectively effective on the thermal but not the mechano-hypersensitivity after inflammation23,25,36. An inhibitor of P-p38 (SB203580, as used here) given daily intrathecally starting just before SPINAL NERVE LIGATION, delays development of mechanical allodynia, with the strongest effect occurring 1 day after nerve ligation and a much weaker effect 10 days later21. Later bolus administration of the inhibitor gives only transient relief, which is longer lasting for injections at day 1 then at day 10 post-SPINAL NERVE LIGATION21. This time-dependent effectiveness parallels the behavior observed with the SMIR-induced pain reported in the present paper, despite the absence of nerve injury in the SMIR model14.
Post- Incision pain and P-p38
Incision of the plantar paw leads to activation of spinal microglia and astrocytes10,11 and to elevation of P-p38 that is coupled to the relatively short-lasting post-incisional mechanical allodynia 9,11. The density of P-p38 positive cells in the ipsilateral dorsal horn is maximum at 1and 2 day after paw incision9,11, declining by ~ 20 % at POD3, and to insignificant elevation over pre-operative levels at POD79. This time-course roughly parallels the time course of mechano-allodynia and thermal hyperalgesia3,8,9 although the resolution of this pain is quite variable11,36,37 and appears to depend, among other factors, on the anesthetic used during the surgical procedure38,39.
Post-incisional P-p38 has been localized during the acute period of hypersensitivity primarily in microglia, but this activated MAPkinase is also seen in some spinal neurons10 and also in astrocytes11. Blocking the activation of p38 by pre-operative intrathecal injection of an inhibitor of the upstream, activating kinase, MITOGEN ACTIVATED PROTEIN KINASE KINASES, significantly suppressed mechano-allodynia from paw incision through POD2, but had a much smaller effect, and only at 1h post-operative, on thermal hyperalgesia10. Intrathecal administration of the glial inhibitor fluorocitrate at POD1 resulted in a partial but long-lasting (up to POD5) reversal of established mechano-allodynia11. In contrast to this result, the intraperitoneal injection of antagonists/inverse agonists of cannabinoid receptors to rats after paw incision enhanced the expression of P-p38, in astrocytes, elevated GFAP, and concomitantly delayed the resolution of post-incisional pain9. Perhaps this treatment results in a shift of the cause of post-incisional pain from microglia to astrocytes, akin to the progression of pain-associated cell loci during nerve injury-induced pain31.
The present report is the first using a model of prolonged post-operative pain to study the role of MAPKinases. The current results extend the previous work on P-p38 and paw incision, showing that the continuously activated form of this MAPKinase is not essential for maintaining elevated pain. In the SMIR model, secondary tactile allodynia and hyperalgesia that typically involve central sensitization40,41 appear after the initial rise of P-p38 in the spinal cord, an early event which also occurs after Sham incision, without retraction. This suggests that P-p38 is elevated by both procedures during the initial insult, the incision, and is further increased at later times when retraction was also used during surgery.
The spread of P-p38 activation from L3 into L4+L5, and the approximately 20-fold increase in P-p38-expressing DH neurons in L4+L5 correlates with the development of secondary hypersensitivity. Early activation of p38, in microglia, may be necessary for hypersensitivity to appear, judging by the effectiveness of the inhibitor given at POD2 in preventing the elevation of the pain response. However, the increase in P-p38 in neurons, if it does contribute to later hypersensitivity, appears to be not alone sufficient to sustain it, as shown by the relatively weak effectiveness of P-p38 inhibitors to reverse this sensitivity once it is established.
Thus, in SMIR-induced tactile hypersensitivity, even in the absence of peripheral nerve injury, there appears to be adequate afferent input to cause long-term sensitization of central pathways. Accordingly, intrathecal inhibitors of P-p38 may have time-sensitive effectiveness for two reasons; first, P-p38 per se may be more important for the induction of hypersensitivity than for its maintenance, or second, the locus of activated P-p38 essential for hypersensitivity may shift over days from spinal cord to brain. In this second explanation, inhibition of spinal P-p38 may temporarily interrupt the pain facilitating afferent throughput to the brain, but does not reverse the essential, altered brain state. Once the inhibitor has been removed or degraded, the conditions necessary for hypersensitivity are restored and elevated postoperative pain re-emerges. If this second hypothesis is true, then studies of glial and neuronal changes in spinal cord alone are not adequate for a complete understanding of the mechanisms underlying prolonged postoperative pain. Persistent or chronic pain’s maturation may require a temporary stage of neuroplasticity where spinal processes are critical, but the stable phenotypic changes in the brain are likely to be the essential targets to target for treatment of chronic pain that occurs after surgery.
Summary Statement.
During prolonged, post-operative pain, expression of activated Mitogen Activated Protein Kinase p38 shifts from microglia to neurons. Inhibition of P-p38 at early times prevents later post-operative pain but later inhibition causes only transient pain relief.
Acknowledgments
Thanks to Zheng-Zhong Xu, PhD (Instructor, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham & Women’s Hospital, Boston, MA) for help teaching the methods for spinal cord sectioning and staining, and to Sarah Flatters, PhD, Academic Fellow, Wolfson Centre for Age-Related Diseases, Kings College, London, United Kingdom, for demonstrating the SMIR surgical procedure.
Mr. Jamie Bell, Information Technologist (Department of Anesthesiology, Perioperative and Pain Medicine, Brigham & Women’s Hospital, Boston, MA), aided with the final formatting of the micrographs.
Supported by funds from the BWH Foundation for Education and Research, c/o Department of Anesthesiology, Perioperative and Pain medicine, 75 Francis Street, Boston, MA 02115, USA., and a grant from the National Institutes of Health and the National Cancer Institute, Bethesda MD; 2 R01 CA080153 (to GS).
References
- 1.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery: A review of predictive factors. Anesthesiology. 2000;93:1123–33. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 3.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 4.Duarte AM, Pospisilova E, Reilly E, Mujenda F, Hamaya Y, Strichartz GR. Reduction of post-incisional allodynia by subcutaneous bupivacaine: Findings with a new model in the hairy skin of the rat. Anesthesiology. 2005;103:113–25. doi: 10.1097/00000542-200507000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Zahn PK, Sluka KA, Brennan TJ. Excitatory amino acid release in the spinal cord caused by plantar incision in the rat. Pain. 2002;100:65–76. doi: 10.1016/s0304-3959(02)00241-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee HJ, Pogatzki-Zahn EM, Brennan TJ. The effect of the AMPA/kainate receptor antagonist LY293558 in a rat model of postoperative pain. J Pain. 2006;7:768–77. doi: 10.1016/j.jpain.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Pogatzki EM, Niemeier JS, Sorkin LS, Brennan TJ. Spinal glutamate receptor antagonists differentiate primary and secondary mechanical hyperalgesia caused by incision. Pain. 2003;105:97–107. doi: 10.1016/s0304-3959(03)00169-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhu CZ, Hsieh G, EI-Kouhen O, Wilson SG, Mikusa JP, Hollingsworth PR, Chang R, Moreland RB, Brioni J, Decker MW, Honore P. Role of central and peripheral mGluR5 receptors in the post-operative pain in rats. Pain. 2005;114:195–202. doi: 10.1016/j.pain.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Alkaitis MS, Solorzano C, Landry RP, Piomelli D, DeLeo JA, Romero-Sandoval EA. Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain. PLoS ONE. 2010;5:1–15. e10891. doi: 10.1371/journal.pone.0010891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, Kohno T, Sun WZ, Wang CC. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009:110155–65. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- 11.Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M. Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J Pain. 2006;7:816–22. doi: 10.1016/j.jpain.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Peters CM, Eisenach JC. Contribution of the chemokine (C-C motif) ligand 2 (CCL2) to mechanical hypersensitivity after surgical incision in rats. Anesthesiology. 2010;112:1250–8. doi: 10.1097/ALN.0b013e3181d3d978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji R-R, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: Implications for the initiation and maintenance of pathological pain. Neurobiol Disease. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 14.Flatters SJL. Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR) Pain. 2008;135:119–30. doi: 10.1016/j.pain.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–22. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cloburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–75. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 17.Flatters SJL. Effect of analgesic standards on persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR) Neurosci Lett. 2010;477:43–7. doi: 10.1016/j.neulet.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neur. 1985;231:66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- 19.Svensson CI, Yaksh TL, Sorkin LS. The role of p38 in microglial regulation of spinal pain processing. In: DeLeo JA, Sorkin LS, Watkins LR, editors. Immune and Glial Regulation of Pain. IASP Press; Seattle: 2007. pp. 297–317. [Google Scholar]
- 20.Kim SY, Bae JC, Kim JY, Lee HL, Lee KM, Kim DS, Cho HJ. Activation of the p38 MAP kinase in the rat dorsal root ganglia and spinal cord following peripheral inflammation and nerve injury. Neuroreport. 2002;13:2483–6. doi: 10.1097/00001756-200212200-00021. [DOI] [PubMed] [Google Scholar]
- 21.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003:4017–22. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- 23.Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–40. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- 24.Boyle DL, Jones TL, Hammaker D, Svensson CI, Rosengren S, Albani S, Sorkin L, Firestein GS. Regulation of peripheral inflammation by spinal p38 MAP kinase in rats. PLoS Med. 2006;3:e338. doi: 10.1371/journal.pmed.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson CI, Masala M, Westlund A, Calcutt NA, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–44. doi: 10.1046/j.1471-4159.2003.01969.x. Calpha-amino-3-hydroxy-5-methyl-4-isoxazole proprionatena WM. [DOI] [PubMed] [Google Scholar]
- 26.Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL. Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J Neurochem. 2005;92:1508–20. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- 27.Beloeil H, Ji RR, Berde CB. Effects of bupivacaine and tetrodotoxin on carrageegan-induced hind paw inflammation in rats (Part 2): Cytokines and p38 mitogen-activated protein kinases in dorsal root ganglia and spinal cord. Anesthesiology. 2006;105:139–45. doi: 10.1097/00000542-200607000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. P38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRANSIENT RECEPTOR PROTEIN VANNILOID 1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 29.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of EXTRACELLULAR RECEPTOR ACTIVATED KINASE in spinal neurons contributes to pain hypersensitivy. Nat Neurosci. 1999;2:1114–9. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 30.Campillo A, González-Cuello A, Cabañero D, Garcia-Nogales P, Romero A, Milanés MV, Laorden ML, Puis MM. Increased spinal dynorphin levels and phosphor-extracellular signal-related kinases 1 and 2 and c-Fos immunoreactivity after surgery under remifentanil anesthesia in mice. Mol Pharmacol. 2010;77:185–94. doi: 10.1124/mol.109.059790. [DOI] [PubMed] [Google Scholar]
- 31.Ji RR, Gereau RW, 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–48. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Zhang YQ. Protein kinases as potential targets for the treatment of pathological pain. Handb Exp Pharmacol. 2007;177:359–89. doi: 10.1007/978-3-540-33823-9_13. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y-J, Ji R-R. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–40. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang FE, Cao JL, Zhang CL, Zeng YM. Activation of p38 mitogen-activated protein kinase in spinal cord contributes to chronic constriction injury-induced neuropathic pain. Sheng Li Xue Bao. 2005;57:545–51. [PubMed] [Google Scholar]
- 36.Mizushima T, Obata K, Yamanaka H, Dai Y, Fukuoka T, Tokunaga A, Mashimo T, Noguchi K. Activation of p38 MAPK in primary afferent neurons by noxious stimulation and its involvement in the development of thermal hyperalgesia. Pain. 2005;113:51–60. doi: 10.1016/j.pain.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 37.Romero-Sandoval A, Chai N, Nutile-McMenemy N, Deleo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–26. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horvath RJ, Landry RP, Romero-Sandoval EA, DeLeo JA. Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience. 2010;169:843–54. doi: 10.1016/j.neuroscience.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cabañero D, Célérier E, Garcia-Nogales P, Mata M, Roques BP, Maldonado R, Puig MM. The pro-nociceptive effects of remifentanil or surgical injury in mice are associated with a decrease in delta-opioid receptor mRNA levels: Prevention of the nociceptive response by on-site delivery of enkephalins. Pain. 2009;141:88–96. doi: 10.1016/j.pain.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Dirks J, Moiniche S, Hilsted KL, Dahl JB. Mechanisms of postoperative pain: Clinical indications for a contribution of central neuronal sensitization. Anesthesiology. 2002;97:1591–6. doi: 10.1097/00000542-200212000-00035. [DOI] [PubMed] [Google Scholar]
- 41.Kawamata M, Koshizaki M, Shimada SG, Narimatsu E, Kozuka Y, Takahashi T, Namiki A, Collins JG. Changes in response properties and receptive fields of spinal dorsal horn neurons in rats after surgical incision in hairy skin. Anesthesiology. 2005;102:141–51. doi: 10.1097/00000542-200501000-00023. [DOI] [PubMed] [Google Scholar]