SYNOPSIS
Adiponectin is protective against hepatic fibrosis, while leptin promotes fibrosis. In hepatic stellate cells (HSCs), leptin signals via a Janus Kinase 2/Signal Transducers and Activators of Transcription 3 (Jak2/Stat3) pathway, producing effects that enhance extracellular matrix deposition. Suppressors of Cytokine Signaling-3 (SOCS-3) and Protein Tyrosine Phosphatase-1B (PTP1B) are both negative regulators of Jak/Stat signaling, and recent studies demonstrated a role for adiponectin in regulating SOCS-3 expression. In this study we investigated mechanisms whereby adiponectin dampens leptin signaling and prevents excess ECM production. We treated culture-activated rat HSCs with recombinant adiponectin, leptin, both or neither, and also treated adiponectin knockout (Ad−/−) and wild-type mice with leptin and/or carbon tetrachloride (CCl4), or saline. We analyzed Jak2 and Ob-Rb phosphorylation, and PTP1B expression and activity. We also explored potential mechanisms through which adiponectin regulates SOCS-3/Ob-Rb association. Adiponectin inhibited leptin-stimulated Jak2 activation and Ob-Rb phosphorylation in HSCs, while both were increased in Ad−/− mice. Adiponectin stimulated PTP1B expression and activity, in vitro, while PTP1B expression was lower in Ad−/−mice than in wild-type mice. Adiponectin also promoted SOCS-3/Ob-R association, and blocked leptin-stimulated formation of extracellular TIMP-1/MMP-1 complexes, in vitro. These data suggest two novel mechanisms whereby adiponectin inhibits hepatic fibrosis: by promoting binding of SOCS-3 to Ob-Rb, and stimulating PTP1B expression and activity, thus inhibiting Jak2-Stat3 signaling at multiple points.
Keywords: Fibrosis, Adiponectin, Leptin, SOCS-3, PTP1B, Jak2
INTRODUCTION
Hepatic fibrosis, results from excess deposition of extracellular matrix (ECM) proteins such as type I collagen, and is characteristic of chronic liver injury, regardless of etiology [1]. The accumulation of excess ECM leads to the formation of fibrous scar tissue that causes portal hypertension, and ultimately chronic liver failure. Hepatic fibrosis is considered part of the wound-healing program in response to liver injury and may be reversed if the initiating insult is resolved sufficiently early, but protracted fibrosis, specifically that which accompanies nonalcoholic fatty liver disease (NAFLD), may lead to cirrhosis and potentially hepatocellular carcinoma [2, 3].
Hepatic stellate cells (HSC) of healthy liver are normally quiescent, residing in the Space of Disse. They serve as storage repositories for retinoic acid in the form of retinyl esters. During the wound-healing response to chronic liver injury, however HSCs are ‘activated’, becoming proliferative and increasing their production of ECM proteins [4]. Although other hepatic cell types may also display fibrogenic properties, the activated HSC appears to be the primary actor responsible for e excessive ECM present in the fibrotic liver [3].
Leptin, the 16kDa adipocytokine product of the ob gene, known largely for its role in influencing hypothalamic control of appetite, insulin secretion, and glucose metabolism, displays multiple profibrogenic properties [5, 6]. Synthesized and secreted by white adipose tissue, leptin promotes expression of α2 (I) collagen [7, 8], the excessive deposition of which is a key feature of hepatic fibrosis. Leptin also suppresses the expression and activity of the collagen-degrading matrix metalloproteinase-1 (MMP-1) [9-11], and promotes expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) [9], an important negative regulator of this MMP. Leptin also promotes maintenance of HSCs in the ‘activated’ phenotype by stimulating their proliferation and suppressing apoptosis [12]. Evidence supporting a role for leptin in promoting hepatic fibrogenesis is provided by Saxena et al’s observation that lean mice, when compared with their leptin non-producing ob/ob counterparts, display elevated deposition of collagen in the carbon tetrachloride (CCl4) model of hepatic fibrosis [8] while the CCl4-treated ob/ob mice failed to develop liver fibrosis.
Leptin signal transduction is conducted via a Janus-Activated Kinase 2 (Jak2)/Signal Transducer and Activator of Transcription 3 (Stat3) tyrosine kinase pathway, initiated by leptin binding at the cell surface to the long form leptin receptor, Ob-Rb [13, 14]. Leptin binding to Ob-Rb results in activation of the receptor-associated Jak2 by autophosphorylation [15, 16]; Jak2 subsequently activates Ob-Rb by phosphorylation at Tyr985 (Y985) and Tyr1138 (Y1138) [17]. Phosphorylation of Ob-Rb at Tyr1138 is required to induce binding by Stat3 [17], which is then also phosphorylated and activated by Jak2 [18]. Once activated, Stat3 can form homodimers and translocate to the nucleus, where it acts in concert with other cellular and microenvironment-specific factors, ultimately regulating transcription of respective target genes. Leptin-stimulated transcriptional activation of both type I collagen and TIMP-1 involve Jak/Stat signal transduction [9].
Adiponectin is a 30kDa protein that, like leptin, is synthesized and secreted primarily by white adipose tissue. Adiponectin circulates in the blood as low, medium, or high molecular weight oligomers, and its serum levels correlate inversely with body fat [5, 19]. Two receptors that propagate signal transduction in response to adiponectin have been identified: AdipoR1, expressed primarily in skeletal muscle and AdipoR2, expressed abundantly in liver [19]. Adiponectin signal transduction in the liver is conducted primarily through activation of adenosine monophosphate-activated kinase (AMPK) by phosphorylation at Thr172, although there is also evidence of a role for peroxisome proliferator-activated receptor alpha (PPAR-α) mediated signaling in response to adiponectin [20, 21]. LKB1 has been identified as an upstream activator of AMPK, but the molecular events involved in adiponectin signal transduction have not been completely defined [22], particularly as related to biological actions not related to the regulation of cellular energy stores and insulin sensitivity.
Whereas leptin generates profibrogenic effects, multiple studies indicate that adiponectin is antifibrogenic, although the mechanisms of this protective effect have not yet been described [5]. Overexpression of adiponectin in rat HSCs suppresses proliferation and reduces expression of proliferating cell nuclear antigen (PCNA) and α smooth muscle actin (αSMA), a marker for HSC activation [23]. Treatment with adiponectin also stimulates apoptosis in activated, but not quiescient HSCs, suggesting that adiponectin may act to maintain HSCs in the quiescient state, thereby reducing their fibrogenic actions [23]. In humans, reduced serum adiponectin levels are associated with several negative physiological consequences, including poor liver histology, inflammation, and fibrosis [5, 24-26]. Moreover, mice overexpressing adiponectin via adenoviral delivery are less vulnerable than lac z-expressing mice to CCl4-induced hepatic fibrosis [27]. Extending this paradigm, recent experiments from our laboratory demonstrate that adiponectin knockout mice (Ad−/−) are more susceptible to CCl4-induced hepatic fibrosis than wild-type mice, and that Ad−/− mice are also more vulnerable to leptin-induced fibrosis [6, 10]. A potential clue from these experiments suggests that a plausible mechanism explaining the increased vulnerability of the Ad−/− mice to leptin-mediated hepatic fibrosis is their comparatively decreased expression of the Suppressors of Cytokine Signaling 3 (SOCS-3) protein, an important negative regulator of leptin signaling. These data imply a functional loss of the ability of Ad−/− mice to inhibit leptin signaling, leading to the enhanced hepatic fibrosis observed therein. Although leptin and adiponectin clearly represent a mutually antagonistic paradigm regulating hepatic extracellular matrix deposition, very little is known about the molecular mechanisms of crosstalk between these two important pathways. In the current study, we investigated mechanisms by which adiponectin can antagonize leptin signaling to provide protection against leptin-stimulated hepatic fibrosis.
EXPERIMENTAL
Antibodies and Chemical Reagents
Recombinant human adiponectin was purchased from Biovendor (Candler, NC). Dulbecco’s modified Eagle’s medium (DMEM), trypsin-EDTA, and penicillin-streptomycin were all purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from (HyClone, Logan, UT). Puromycin, polybrene, recombinant human leptin, and antibodies against β-actin were purchased from Sigma Chemical Co. (St. Louis, MO). SOCS-3, Ob-Rb (K-20), phospho Ob-Rb (Y985) and phospho Ob-R (Y1138) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for Jak2 and phospho Jak2 (Y1007/1008) were purchased from Cell Signaling Technology (Danvers, MA); the antiserum against phospho Jak2 (S523) was a generous gift from Martin Myers at the University of Michigan. PTP1B antibody was purchased from Abcam (Cambridge, MA). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from GE Healthcare (Piscataway, NJ).
Isolation of hepatic stellate cells
Quiescent HSCs were isolated as described previously [8, 28]. Sprague–Dawley rats were purchased from Charles River (Boston, MA). All rats received humane care, and the Emory University Institutional Animal Care and Use Committee approved the HSC isolation protocol. In brief, in situ perfusion of the liver with 20 mg/dl of Pronase (Boehringer Mannheim, Indianapolis, IN) was followed by collagenase (Crescent Chemical, Hauppauge, NY) perfusion. Dispersed cell suspensions were layered on a discontinuous density gradient of 8.2% and 15.6% Accudenz (Accurate Chemical and Scientific, Westbury, NY). The resulting upper layer consisted of more than 95% HSCs. Cells were cultured in Medium 199 containing 20% (v/v) FBS (Flow Laboratories, Naperville, IL). Purity of activated HSCs was assessed by immunolocalization of α-SMA in the monolayer, and by intrinsic auto fluorescence in freshly isolated HSCs. HSC viability was verified by propidium iodide exclusion and for in vitro experiments was greater than 95%. Sub-confluent activated HSCs in culture, 10 d after isolation and initial plating, were washed twice with PBS and were cultured in DMEM. Growth media was replaced every other day, and activated HSCs were split 1:3 every 7 d, by trypsinization. Only cells between passage 2 and 5 were used in experiments.
In vitro leptin and adiponectin treatments
Culture-activated HSCs were treated with adiponectin (10 μg/ml), leptin (100 ng/ml), both or neither after 16 h serum deprivation in DMEM supplemented with 2.5% FBS (v/v) and 1% penicillin-streptomycin (v/v). Treatment durations are indicated in the appropriate figure legends.
Animal care/in vivo studies with carbon tetrachloride and recombinant leptin
Adiponectin knock-out (Ad−/−) mice, generated as previously described [29], were a gift from the laboratory of Dr. Kenneth Walsh (Boston University School of Medicine, Boston, MA). The animals were cared for in accordance with protocols approved by the Animal Care and Use Committee of Emory University. Animals were housed in a temperature-controlled environment (20 to 22°C) with a 12:12 h light: dark cycle, and fed ad libitum with Purina Laboratory Chow (Ralston Purina, St. Louis, MO) and water.
The studies included three cohorts of mice: control mice being administered sterile saline, another group being administered only CCl4 for the duration of the study, and a third group that was administered CCl4 for the duration of the study and recombinant human leptin for the final six weeks. Six-week old male littermates of Ad-/- mice and wild-type mice of the same background were administered CCl4 (2 ml/kg) with olive oil (1:1 ratio) twice weekly by gavage for eight weeks. Leptin was administered concomitantly by intraperitoneal (IP) injection every 36 hours for 6 weeks at a dosage of 5 mg/kg. All mice were euthanized and liver tissue collected for molecular analysis as described subsequently.
Protein Lysate Production
At the end of the in vitro experiments, HSCs were washed in PBS and suspended in ice-cold RIPA buffer (10 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 1% Nonidet P-40 (v/v), 0.5% sodium deoxycholate, 0.1% SDS containing 20 μl/ml protease inhibitor cocktail (Research Products, International, Prospect, IL) for 30 min on ice. Lysates were centrifuged at 12,000 × g for 30 min at 4°C. Supernatant was collected and protein concentrations were determined using the Bradford reagent (Sigma) [30].
At the conclusion of the in vivo studies, one hundred mg of harvested liver previously frozen at the time of necropsy was cut into 0.5 cm × 0.5 cm pieces, allowed to thaw at 4°C in 3 ml lysis buffer [RIPA buffer containing 20 μl/ml protease inhibitor cocktail (Research Products, International, Prospect, IL)] per gram of tissue, and tissue dispersion was enhanced by sonication, incubated on ice for 30 min, and centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was collected and centrifuged a second time for 10 min at 10,000 × g; the resulting supernatant constituted the lysates used in subsequent analyses.
Immunoblotting
Equal amounts of protein were resolved on 4-20% SDS-PAGE [31] and immobilized on PVDF membranes by wet transfer. After blocking for 30 min in 5% (w/v) nonfat dry milk in TBS-Tween 20 (20 mM/l Tris-Cl (pH 7.5), 137 mM/l NaCl, 0.05% (w/v) Tween-20), the membranes were exposed overnight, at 4°C, to primary antibody, then for 2 h at room temperature to the corresponding HRP-conjugated secondary antibody. Equal protein loading was controlled by immunoblot of ß-actin. Immunoreactive proteins were visualized using the HyGlo Chemiluminescent HRP Antibody Detection Reagent (Denville Scientific, Metuchen, NJ), and exposure to X-Ray film (Kodak, Rochester, NY). Band density was analyzed using AlphaEase FC Software, version 4.0.1 (Alpha Innotech Corp., San Leandro, CA).
Immunoprecipitation
Equal amounts of protein were incubated with 20 μl of primary antibody for 2 h at 4°C. Twenty ml of protein A/G Plus-Agarose (Santa Cruz Biotechnology) was added to the mixture, and the suspension was incubated at 4°C on a rocking platform overnight. The immunoprecipitates were collected by centrifugation at 1000 × g for 30 s at 4°C, and washed twice in PBS, and centrifugation was repeated following each wash. The supernatant was discarded, and the pellet was resuspended in electrophoresis sample buffer. Immunodetection was conducted as described for immunoblotting.
Quantitative RT-PCR
RNA was extracted using RNeasy® (Qiagen). Primers were designed for PTP1B, AdipoR1, and AdipoR2 using NIH Primer-BLAST, such that the resulting PCR products would be 100–200 base pairs in length and bridge two separate exons. cDNA synthesis was conducted using Bio-Rad’s iScript™ cDNA Synthesis kit, according to the manufacturer’s recommended parameters. First-strand cDNA synthesis was carried out in 20 μl reaction volumes containing 1 μg of total RNA, 4ml 5× iScript reaction mix, 1 ml iScript reverse transcriptase, and nuclease-free water. Real-time quantitative PCR for PTP1B was conducted using IQ™ SYBR® Green Supermix (Bio-Rad), according to the manufacturer’s protocol. PCR was performed in 25 μl reaction volumes containing nuclease-free water, 1 μl aliquots of cDNA and gene-specific primer pairs, and 12.5 ml SYBR Green Supermix in a MyIQ™ One Color Real Time PCR Detection System. The PCR cycle parameters were set at 95 °C for 20 s, 55 °C for 45 s and 72 °C for 30 s, for 40 cycles. Relative amounts of the target cDNA were estimated by the Ct (threshold cycle) number, and compared with GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Three independent samples were analyzed for each condition in triplicate.
Lentivirus-mediated knockdown of adiponectin receptors in rat HSCs
Adiponectin receptor (AdipoR1 and AdipoR2) and non-targeting shRNA lentivirus particles were purchased from Santa Cruz Biotechnology. Rat HSCs were seeded at 1×105 cells per ml in 100 mm3 tissue culture plates. HSCs at 50-70% confluence were incubated for 16 h with lentiviral particles (MOI = 2) in the presence of 5μg/ ml polybrene. The infected cells were cultured in complete media for 48h, and stable clones were selected by culture for several days in complete media containing 2μg/ml puromycin. Receptor knockdown was confirmed by RT-PCR and western blot.
ELISA for MMP-1/TIMP-1 Complexes
The human MMP-1/TIMP-1 Complex ELISA DuoSet® (Cat. No. DY1550) and all assay reagents were purchased from R&D Systems (Minneapolis, MN). Primary rat HSCs were treated as described in the appropriate figure legend for each experiment. Conditioned media was collected from treated HSC cultures, and the detection of TIMP-1/MMP-1 complexes from rat HSCs was conducted according to the manufacturer’s instructions. Briefly, to immobilize capture antibody, 160ng/well goat anti-MMP-1 was added to a 96-well plate, the plate was sealed and incubated overnight at room temperature. To pull down MMP-1, 100μg protein from conditioned media was added per well to the microplate, and the plate was incubated 1h. To detect MMP-1/TIMP-1 complexes, 1.8μg biotinlylated anti-TIMP-1 antibody was added per well to the plate, and the plate incubated 2 h at room temperature. Captured anti-TIMP-1 was detected by adding Streptavidin-conjugated horseradish peroxidase (HRP) and an HRP substrate solution (R&D, # DY999). Optical density of each well was determined with a Bio-Tek Synergy 2 plate reader (Winooski, VT) set to 450 nm. The concentration of MMP-1/TIMP-1 complex in each sample was determined from a standard curve constructed using the measured optical densities of known concentrations of MMP-1/TIMP-1 complex.
Statistical Analysis
Animal experiments were performed with 8 animals in each treatment and control group. All in vitro data is reported as the result of three independent experiments including three replicates per experiment. The data are presented as means ± SE. Statistical analysis was performed using Graphpad® Prism 4 software (www.graphpad.com), statistical differences between groups were tested using parametric tests (paired Student’s T test or one-way ANOVA). In all analyses, only p values of less than 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Adiponectin inhibits leptin-stimulated activation of Jak2
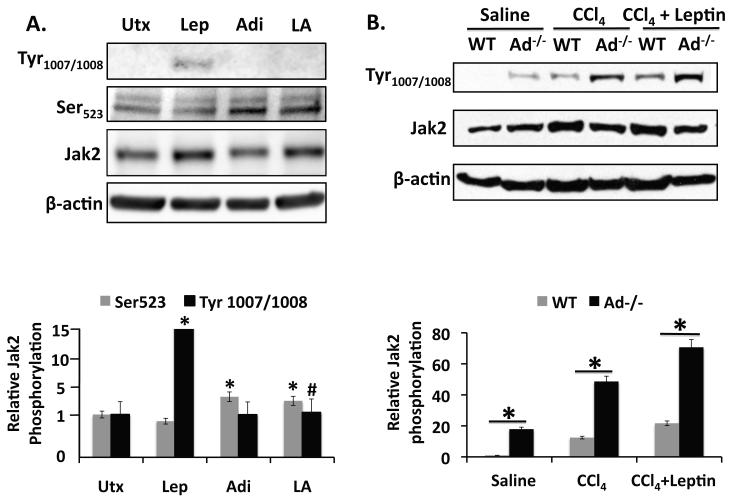
Leptin initiates signaling by binding to its receptor, Ob-Rb, at the cell surface. Ligand binding to the receptor causes activation of the receptor-associated Jak2 by autophosphorylation at Tyr1007/1008 [13, 32]. Jak2 can also be inhibited by phosphorylation at Tyr570 or Ser523, but only phosphorylation at Ser523 inhibits Jak2-dependent signaling by Ob-Rb [33]. As Jak2 activation represents the most upstream event in leptin signal transduction, we examined first whether adiponectin had any effect on phosphorylation of Jak2 at Tyr1007/1008, and Ser523. As anticipated, leptin stimulated phosphorylation of Jak2 at Tyr1007/1008, while adiponectin did not (p < 0.05). Importantly, however, adiponectin blocked leptin-stimulated Tyr1007/1008 phosphorylation when both adipocytokines were co-administered (p < 0.05, compared with leptin treated). In addition, leptin tended to suppress phosphorylation of Jak2 at Ser523 compared with untreated cells but the decrease was not statistically significant. By contrast, adiponectin stimulated Ser523 phosphorylation, whether administered alone or in the presence of leptin (p < 0.05, compared to untreated). These data indicate that adiponectin can block leptin signal transduction by two upstream mechanisms, inhibiting activation of Jak2 at Tyr1007/1008, and suppressing propagation of leptin-mediated downstream signal transduction by promoting the inhibitory phosphorylation of Jak2 at Ser523 (Figure 1A).
Figure 1. Adiponectin blocks leptin-mediated activation of Jak2.
A) Top Panel: Western blot of phospho-Jak2[Tyr1007/1008 and Ser523] in lysates prepared from rat HSCs. Thirty minute leptin treatment (Lep, 100ng/ml) increased Jak2 (Tyr1007/1008) phosphorylation significantly (*p < 0.05 vs. untreated) compared to untreated samples (Utx). Co-administration of leptin and adiponectin (LA) inhibited leptin-stimulated Jak2 (Tyr1007/1008) phosphorylation (#p < 0.05 vs. leptin). Adiponectin (10μg/ml) stimulated Jak2 (Ser523) phosphorylation whether administered alone or in the presence of leptin (*p < 0.05 vs. untreated). Bottom Panel: Quantitation of data presented in top panel (A). B) Top Panel: Western Blot of phospho-Jak2 (Tyr1007/1008) from liver tissue collected from Adiponectin knockout (Ad−/−) and wild-type (WT) mice. CCl4 intoxication (2 ml/kg) and CCl4+leptin co-administration both increased Jak2 phosphorylation significantly over saline-treated control mice. Jak2 phosphorylation was always greater in Ad-/- mice compared with wild-type mice, regardless of treatment (*p < 0.05 vs. WT). Bottom Panel: Quantitation of data presented in top panel (B).
We also examined Jak2 Tyr1007/1008 phosphorylation in livers from Ad−/− and wild-type mice to investigate regulation of leptin signal transduction by adiponectin in vivo. Ad−/− mice displayed greater Jak2 Tyr1007/1008 phosphorylation than wild type mice (p < 0.05) in every experimental condition tested, consistent with the implications of our earlier work suggesting that Ad−/− mice are incapable of attenuating leptin signaling rendering them more vulnerable to hepatic fibrosis. Indeed, the negative consequences of this impaired signaling became more pronounced in mice treated with CCl4, and the co-administration of CCl4 and leptin. When compared with saline treatment, CCl4, and CCL4/leptin co-administration increased Jak2 Tyr1007/1008 phosphorylation in both wild-type and Ad−/− mice; and, as anticipated, CCl4/leptin co-administration produced an increase in Jak2 Tyr1007/1008 phosphorylation over CCl4 alone (Figure 1B).
Adiponectin inhibits leptin activation of Ob-Rb
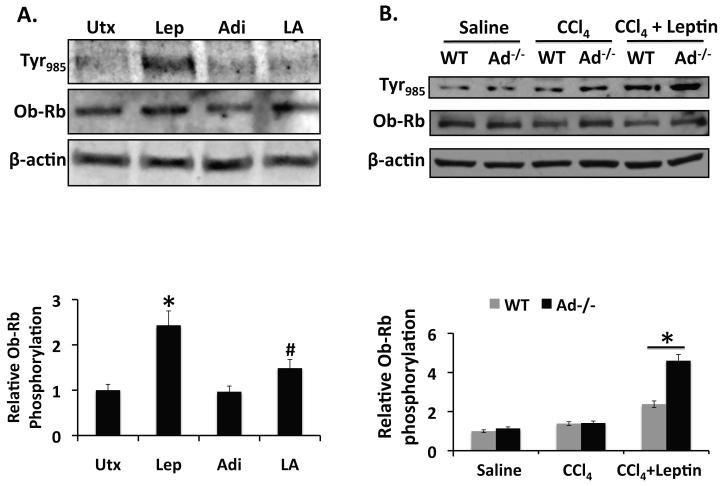
The inhibition of leptin-stimulated Jak2 activation by adiponectin would suggest that downstream propagation of the leptin signal might be mitigated. After Jak2 activation, the leptin signal is conducted via Jak2 phosphorylation of Ob-Rb at Tyr985 and Tyr1138. Phosphorylation of Ob-Rb at Tyr1138 recruits binding by Stat3, which is subsequently also phosphorylated by Jak2. We, and others have already reported that adiponectin reduces leptin-stimulated phosphorylation of Stat3 in vitro [10]. It thus follows from the data presented here and elsewhere that adiponectin may also suppress leptin-stimulated phosphorylation of Ob-Rb.
To test this hypothesis, we examined the effect of adiponectin on Ob-Rb phosphorylation in rat HSCs and Ad−/− mouse livers using experimental designs identical to those used to investigate Jak2 phosphorylation. In both series of studies we determined whether or not phosphorylation of Ob-RbY985, one of the tyrosine residues implicated in receptor activation, would be prevented by adiponectin.
As anticipated, leptin stimulated phosphorylation of Ob-Rb at Tyr985 in rat HSCs, while adiponectin failed to do so. In contrast Ob-Rb phosphorylation after leptin and adiponectin co-administration was less than in the presence of leptin alone (p < 0.05), and more comparable to the levels associated with adiponectin treatment (Figure 2A). In mice, after CCl4 treatment there was a trend towards increased Ob-RbY985 phosophorylation in both wild-type and Ad−/− liver. Leptin co-administration with CCl4 also produced a trend towards increase in Ob-RbY985 phosphorylation above that detected with CCl4 alone, but these differences were not statistically significant. Importantly however, leptin/CCl4 co-administration increased Ob-RbY985 phosphorylation significantly more in livers from Ad−/− mice than in liver from wild-type mice (p < 0.05, Figure 2B). These data provide evidence that adiponectin negatively regulates leptin-stimulated Ob-Rb activation. We are aware of no other reports that describe adiponectin regulation of Ob-Rb, therefore these data further corroborate the emerging paradigm describing adiponectin as a negative regulator of leptin signal transduction. As implied earlier, these results are not surprising in light of the observed effects of adiponectin on Jak2 activation and our earlier work showing that adiponectin inhibits leptin-mediated Stat3 phosphorylation. Negative regulation by adiponectin of Jak2, the upstream kinase responsible for activating Ob-Rb, predicts resulting inhibitory effects on the downstream elements of the leptin signal transduction, including the previously reported inhibition of leptin-mediated Stat3 phosphorylation. As such, the reduction in Ob-Rb activation described here is presumably indirect, resulting from adiponectin inhibition of Jak2 or other factors acting upstream of Ob-Rb and Stat3 phosphorylation, although these data do not preclude the possibility that adiponectin directly regulates Ob-Rb or Stat3. It is also interesting that the changes observed in Jak2 phosphorylation are not recapitulated at the level of Ob-Rb phosphorylation. While Ob-Rb phosphorylation is leptin-dependent, Jak2 phosphorylation is not. We suspect that the differences observed in Jak2 and Ob-Rb phosphorylation reflect the ligand-dependent nature of Ob-Rb, becoming activated only when leptin is introduced into the experimental system, while Jak2 phosphorylation may be more broadly activated by fibrogenic stimuli.
Figure 2. Adiponectin blocks leptin-stimulated Ob-Rb phosphorylation.
A) Top Panel: Western blots of phospho-Ob-Rb[Tyr985] in lysates prepared from rat HSCs. Thirty-minute leptin treatment (Lep, 100ng/ml) increased Jak2 (Tyr985) phosphorylation significantly (*p < 0.05) compared to untreated samples (Utx). Co-administration of leptin (100ng/ml) and adiponectin (10μg/ml) (LA) inhibited leptin-stimulated Ob-Rb(Tyr985) phosphorylation (#p < 0.05 vs. leptin). Bottom Panel: Quantitation of data presented above. B) Top Panel: Western Blots of phospho-Ob-Rb (Tyr985) from liver tissue collected from Ad−/− mice and wild-type (WT) mice. Ob-Rb phosphorylation was significantly increased in CCl4 + leptin-treated Ad−/− mice, compared to similarly treated wild-type mice (*p < 0.05 vs. WT). Bottom Panel: Quantitation of data presented (2A) and (2B).
These data again underscore the increased vulnerability of Ad−/− mice to hepatic fibrosis in general and leptin-mediated hepatic fibrosis in particular. They also further substantiate the role for adiponectin as a protective factor in fibrogenesis, demonstrating a novel antagonistic feature of adiponectin with respect to leptin signal transduction. Importantly, these data not only demonstrate that adiponectin inhibits leptin signal transduction at the earliest known points, but to our knowledge, are the first data revealing the critical intersection of adiponectin regulation of Jak2 and Ob-Rb signaling. And in conjunction with data showing that, when compared with lean patients, obese individuals have higher circulating leptin levels [34, 35] and lower circulating adiponectin levels [26, 36, 37], these data also provide a plausible molecular explanation for why obese humans may be at higher risk for advanced liver disease than lean individuals—regardless of etiology.
Adiponectin signaling via AdipoR1 promotes PTP1B expression and activity
Protein tyrosine phosphatase 1B (PTP1B) dephosphorylates and thus deactivates Jak2, acting upstream of Ob-Rb and Stat3 to negatively regulate leptin signal transduction [38, 39]. PTP1B is an important inhibitor of leptin signaling in the hypothalamus, where PTP1B participates in the regulation of glucose and fat metabolism [33]. Although the mechanisms and effects of hypothalamic PTP1B activity on metabolism have been well studied, much less is known about its role in non-metabolic functions, including hepatic fibrogenesis. Because PTP1B plays a critical role in the negative regulation of Jak-Stat signaling in other tissues, we examined whether adiponectin’s anti-fibrogenic effects involve PTP1B.
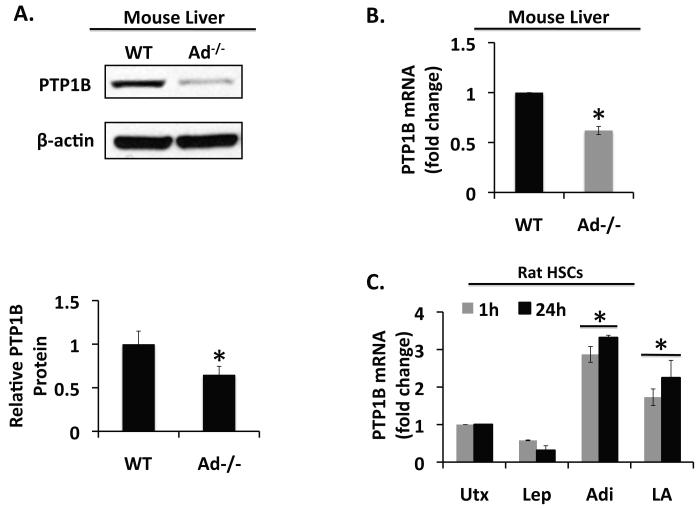
We detected significantly less PTP1B protein (Figure 3A, top panel) and PTP1B mRNA expressed (Figure 3B) in livers from Ad−/− mice compared with liver lysates from wild-type mice (p < 0.05). PTP1B mRNA expression was also significantly increased in rat HSCs exposed to adiponectin (p < 0.05). The stimulatory effect of adiponectin, evident at 1h and 24h, occurred whether adiponectin was administered alone, or in the presence of leptin. By contrast, leptin tended to suppress PTP1B expression in HSCs, although the difference was not statistically significant (Figure 3C). These data provide strong evidence that adiponectin promotes PTP1B expression in liver, and particularly HSCs.
Figure 3. Adiponectin promotes PTP1B expression.
A) Western blot and (B) quantitative RT-PCR analysis of PTP1B expression in Ad−/− mice and wild-type (WT) mice demonstrates reduced PTP1B expressed in Ad−/− mice compared with wild-type mice. (*p < 0.05 vs. WT). C) Data from quantitative RT-PCR shows PTP1B expression in rat HSCs after treatment with leptin (Lep, 100 ng/ml), adiponectin (Adi, 10 μg/ml), both (LA) or neither (Utx). Leptin suppressed PTP1B mRNA expression, while adiponectin stimulated PTP1B mRNA expression, whether administered alone or in the presence of leptin (*p < 0.05 vs. untreated).
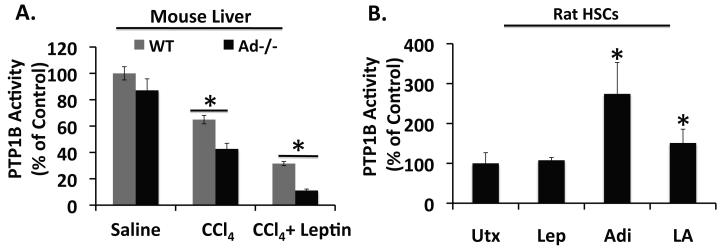
In addition to promoting PTP1B expression, adiponectin treatment also stimulated PTP1B activity in HSCs. Leptin treatment alone in vitro did not change basal PTP1B activity, but co-administration of adiponectin and leptin increased PTP1B activity significantly above that measured in control samples (p < 0.05). Although the PTP1B activity detected in the presence of leptin and adiponectin was appreciably less than in the presence of adiponectin alone, the difference was not statistically different (Figure 4B). We also examined the regulation of hepatic PTP1B activity by adiponectin and leptin within the framework of the CCl4 in vivo experimental model. CCl4 gavage suppressed PTP1B activity in the livers of both wild-type and Ad−/− mice, but the suppression was significantly greater in the knockout mice (p < 0.05, Figure 4A). There was also significantly less PTP1B activity in liver lysates from wild-type and Ad−/− mice co-administered CCl4 and leptin, when compared with saline-treated (control) mice or mice gavaged with CCl4 alone (p < 0.05). Importantly, PTP1B activity in leptin-treated Ad−/− mice was significantly less than the PTP1B activity measured in liver from leptin-treated wild-type mice (p < 0.05). These data provide new insights to explain why Ad−/− mice are exquisitely susceptible to fibrogenic stimuli when compared to wild-type mice. The results indicate that while leptin may suppress PTP1B expression, adiponectin stimulates hepatic PTP1B expression and activity. Taken together, these data strongly suggest that adiponectin inhibits leptin signaling at least partially by enhancing PTP1B activity, thus promoting Jak2 desphosphorylation and preventing Ob-Rb activation. Such a mechanism would represent a novel molecular link accounting for the hepato-protective inhibition of leptin signal transduction by adiponectin in the context of hepatic fibrosis.
Figure 4. Adiponectin promotes PTP1B activity.
A) Analysis of PTP1B activity in liver tissue collected from livers of wild-type (WT) and Adiponectin knockout mice (Ad−/−). Administration of CCl4 (2 ml/kg) reduced PTP1B activity in both wild-type and Ad−/− mice. Leptin administration (5 mg/kg) further suppressed PTP1B activity. In both treatments, PTP1B activity was significantly less in Ad−/− mice than in wild-type mice. *p < 0.05 vs. WT B) In vitro analysis of PTP1B activity in rat HSCs treated for 30 min with leptin (Lep), adiponectin (Adi), both (LA), or neither. Adiponectin stimulated PTP1B activity whether administered alone or in the presence of leptin. *p < 0.05 vs. Untreated
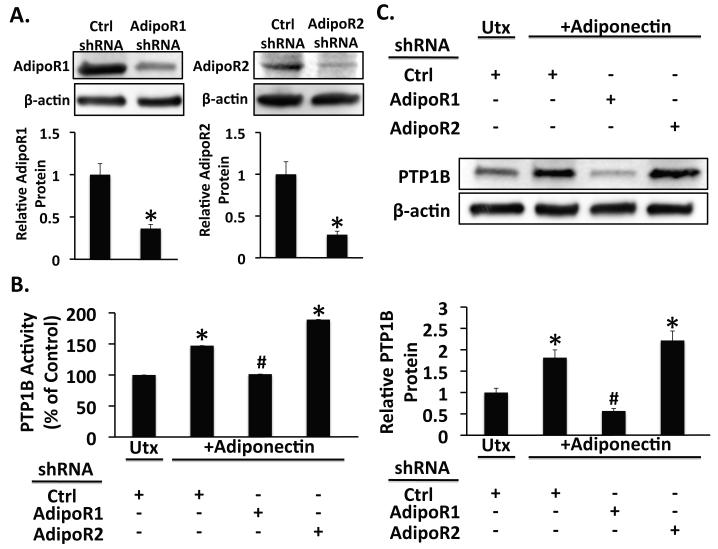
To further elucidate the molecular events involved in attenuation of hepatic fibrosis by adiponectin, we also investigated the role of the adiponectin receptors in regulating PTP1B expression and activity. In these studies we silenced expression of the adiponectin receptors by transfection of culture-activated rat HSCs with non-targeting short-hairpin RNA (shRNA), or shRNA targeting AdipoR1 or AdipoR2. After confirming knockdown (Figure 5A), we treated the cells for 1h with leptin (100 ng/ml), or adiponectin (10 μg/ml), both, or neither. Consistent with the data from experiments using untransfected HSCs, adiponectin increased PTP1B expression (Figure 5C) and activity (Figure 5B) in HSCs transfected with non-targeting shRNA (p < 0.05 vs. control shRNA without adiponectin). Adiponectin also stimulated PTP1B activity and expression when AdipoR2 was silenced (p < 0.05 vs. control shRNA without adiponectin). However, silencing of AdipoR1 blocked the adiponectin-induced increase in PTP1B protein and activity (p < 0.05 vs. control shRNA + adiponectin). Together, these data suggest that in HCSs, signaling via AdipoR1, but not AdipoR2 mediates adiponectin-induced stimulation of PTP1B expression and activity.
Figure 5. Silencing of AdipoR1, but not of AdipoR2 blocks stimulation of PTP1B expression and activity by adiponectin.
A) Western blots from lysates of rat HSCs infected with lentivirus expressing a non-targeting short hairpin RNA, or short hairpin RNA targeting either AdipoR1 (shAdipoR1) or AdipoR2 (shAdipoR2) show decreased receptor protein levels in cells only when infected with the appropriate targeting shRNA. B) Analysis of PTP1B in cell extracts from untreated (Utx) or adiponectin-treated (+Adiponectin) rat HSCs infected with either control shRNA (Ctrl), shAdipoR1, or shAdipoR2. Adiponectin stimulated PTP1B activity in control-infected HSCs (*p < 0.05 vs. untreated), but silencing of AdipoR1 prevented stimulation of PTP1B activity by adiponectin (#p < 0.05 vs. ctrl shRNA + Adiponectin). Silencing AdipoR2 did not affect adiponectin-stimulated PTP1B activity. C) Western blots of lysates from untreated (Utx) or adiponectin-treated (+Adiponectin) rat HSCs infected with lentivirus expressing shRNA targeting either AdipoR1 (shAdipoR1) or AdipoR2 (shAdipoR2), or non-targeting (Ctrl) shRNA show increased PTP1B protein in control and AdipoR2 shRNA-expressing cells (*p < 0.05 vs. untreated). PTP1B protein was not increased in AdipoR1 shRNA-expressing rat HSCs (#p < 0.05 vs. ctrl shRNA + Adiponectin)
While the question of which specific effectors are acting downstream of AdipoR1 to regulate PTP1B will require further investigation, recent reports by others suggest that AMPK is not involved. AMPKα−/− mice are as sensitive to CCl4 –induced hepatic fibrosis as AMPKα+/+ are, and while HSCs derived from AMPKα−/− mice initially show impaired proliferation, they nevertheless become activated in culture and upon passage proliferate normally [40]. Moreover, recent work by Miller and colleagues [41] showing that adiponectin can suppress hepatocyte gluconeogenic gene expression independent of LKB1-AMPK signaling underscores our limited understanding of the molecular details involved in the regulation of hepatic function. PPAR-α, and the identification of several AdipoR-interacting proteins [42, 43], particularly APPL1 (adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif) [44, 45], provide attractive targets for future investigations.
Delibegovic et al have generated liver-specific PTP1B knockout mice and report improved metabolism [46]. While the authors do not describe the state of hepatic fibrosis in these animals, the improvement in glucose homeostasis and increase in insulin sensitivity of livers from Alb-Cre-PTP1B−/− mice suggest potential negative metabolic consequences associated with increased hepatic PTP1B activity caused by adiponectin. Furthermore, in light of adiponectin’s reported insulin sensitizing function [36, 47-49], our findings might seem to present a paradox. We propose that the effects described here are cell-specific, occurring only in activated hepatic stellate cells, which are the primary mediator of extracellular matrix metabolism and are present normally only during hepatic wound-healing. In contrast, the metabolic effects of adiponectin in the liver are presumed to occur in hepatocytes, mainly responsible for conducting the liver’s metabolic functions and the predominant (>80%) resident liver cell type. Such divergence of function would explain the apparent paradoxical nature of the actions of adiponectin in non-parenchymal liver cells.
Although experiments directly testing this hypothesis are clearly necessary, some of the data we present here is consistent with a cell-specific response to adiponectin in the context of hepatic fibrogenesis. Although hepatic PTP1B expression was reduced in Ad−/− mice when compared with wild-type mice (Figure 3, A and B), there was no significant difference in hepatic PTP1B activity between Ad−/− and wild-type mice when no fibrogenic stimulus was introduced (Figure 4A). As mentioned previously and reviewed thoroughly elsewhere [3, 4], HSCs are normally quiescient in healthy liver, comprising a relatively small percentage of total liver cells. However, during the wound healing response to liver injury, HSCs are activated to produce ECM and proliferate, temporarily increasing their percentage of the total hepatic cellular population, and presumably their contribution to any hepatic phenotype. Consistent with this paradigm, induction of hepatic fibrosis by CCl4 intoxication suppresses PTP1B activity significantly more (p < 0.05) in Ad−/− mice than in wild-type mice. Moreover, this difference was exacerbated by leptin (Figure 4A). Our data thus demonstrate an important distinction: in the absence of liver injury, and therefore when HSCs are quiescent, there is no difference in hepatic PTP1B activity between Ad−/− and wild-type mice. However, after a fibrogenic stimulus, with subsequent activation of HSCs, the ability of Ad−/− mice to promote hepatic PTP1B activity is reduced when compared with wild-type mice.
The in vitro data further underscore the role of PTP1B in culture-activated rat HSCs. In such experiments our data show that in response to adiponectin, PTP1B expression increased 3.5 fold (Figure 3C) and PTP1B activity 200% (Figure 4B). Therefore, while conventional thought suggests that adiponectin serves only as a cellular energy sensor acting via AMPK activation, this mechanism may not be as critical for the role of adiponectin as an antagonist to hepatic fibrosis in a myofibroblastic or portal fibroblastic phenotype—cells associated with fibrosis but not glucose homeostasis or other metabolic functions typically carried out in liver by hepatocytes.
Adiponectin promotes SOCS-3 association with Ob-Rb
Leptin induces expression of Supressors of Cytokine Signaling 3 (SOCS-3), which in turn negatively regulates leptin signaling. Neural cell-specific SOCS-3 knockout mice, as well as mice with SOCS-3 haploinsufficiency exhibit greater sensitivity to leptin while being resistant to diet-induced obesity, when compared to wild-type mice [50-52]. SOCS-3 inhibits leptin signaling by binding Ob-Rb and preventing Jak2 phosphorylation of Stat3, and targeting the activated receptor complex for degradation. Bjorbaek et al. showed that SOCS-3 binds specifically to phosphorylated Tyr985 of Ob-Rb and that SOCS-3 fails to inhibit transcriptional activation from an erythropoietin receptor/Ob-Rb chimera when Tyr985 of the chimera is mutated [53].
Recent evidence from our laboratory suggests that adiponectin also regulates SOCS-3 expression, positively, but whether the mechanism is transcriptional or post-translational is unclear [10]. Ad−/− mice express less hepatic SOCS-3 mRNA and protein than wild-type mice, suggesting a transcriptional mechanism. Conversely, the kinetics of the change in SOCS-3 protein level from adiponectin treated or leptin/adiponectin treated rat HSCs suggest that adiponectin may also function to stabilize SOCS-3 protein, at least in vitro. These data, along with the previously mentioned SOCS-3 knockout mouse experiments suggest a mechanism whereby an increase in adiponectin increases the available pool of SOCS-3, thereby promoting the binding of SOCS-3 to Ob-Rb, resulting in leptin signaling inhibition. To investigate this hypothesis, we examined the influence of adiponectin on SOCS-3 binding to Ob-Rb.
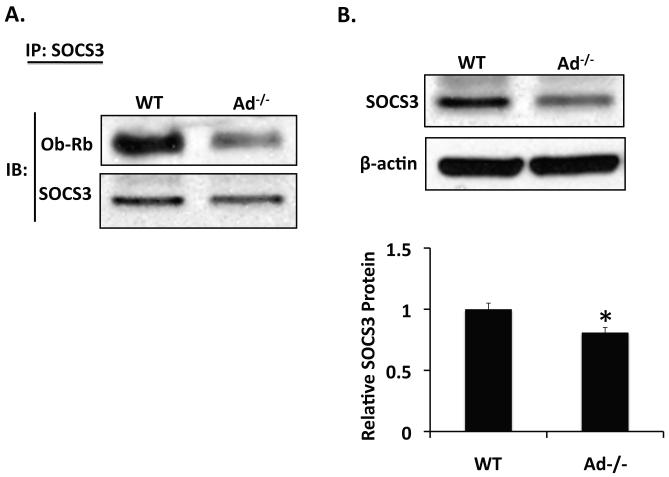
Consistent with the role of adiponectin as a negative regulator of HSC leptin signaling, less SOCS-3 was bound to Ob-Rb in liver from Ad−/− mice than in liver from wild-type mice (Figure 6A). As demonstrated previously [10], hepatic expression of SOCS-3 protein was also decreased in livers from Ad−/− mice, when compared with liver from wild-type mice (p < 0.05, Figure 6B). The difference in SOCS-3 protein expression presumably accounts for the difference in Ob-Rb-bound SOCS-3 that we observed, and is consistent with our hypothesis that, by increasing the available pool of SOCS-3, adiponectin promotes binding of SOCS-3 to Ob-Rb, thus inhibiting leptin signal transduction.
Figure 6. Adiponectin promotes SOCS-3/OB-Rb association, in vivo.
A) Western Analysis for Ob-Rb after immunoprecipitation of SOCS-3 from Adiponectin knockout (Ad−/−) and wild-type (WT) mouse liver. Ad−/− mice display decreased SOCS-3/Ob-Rb association compared with wild-type mice. B) Western blot of SOCS-3 from liver tissue collected from Adiponectin knockout (Ad−/−) and wild-type (WT) mice. SOCS-3 protein is decreased in liver from Ad−/− mice, compared to wild-type mice. (*p < 0.05 vs. wild-type)
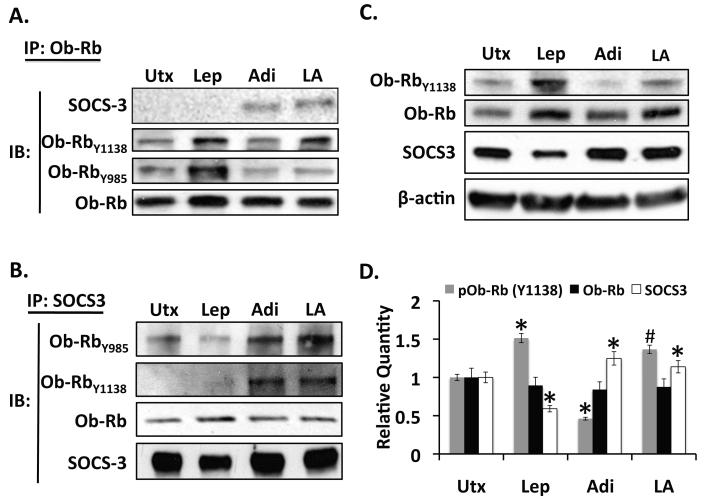
In vitro, 1h adiponectin treatment stimulated SOCS-3 binding to Ob-Rb, even in the presence of leptin. We detected no SOCS3/Ob-Rb association in lysates from untreated HSCs or HSCs treated with only leptin (Figure 7A) for 1h. In studies using immunoprecipation of SOCS-3, however, we did indeed detect SOCS-3/Ob-Rb association in lysates from untreated and leptin-only treated HSCs (Figure 7B). We suspect that the discrepancy between the two sets of experiments results from differences in the relative sensitivities of the two assays based on the relative lack of abundance of our bait proteins in these experiments, Ob-Rb and SOCS-3. Because Ob-Rb is a low-abundance protein, using it as bait in co-ip studies results in a fairly insensitive assay, hence our inability to detect any binding between Ob-Rb and SOCS-3 in the experiment presented in Figure 7A. But because SOCS-3 is a relatively abundant protein, the results represented in figure 7B are not subject to this limitation.
Figure 7. Adiponectin promotes SOCS-3/OB-Rb association, in vitro.
A) Western Analysis for SOCS-3 after immunopreciptitation of Ob-Rb from rat HSCs treated for 1h with leptin (Lep, 100ng/ml), adiponectin (Adi, 10μg/ml), both (LA), or neither. No SOCS-3/Ob-Rb associated was detected in untreated or leptin-treated samples. Adiponectin stimulated SOCS-3/Ob-Rb association whether administered alone or in combination with leptin. B) Western Analysis for Ob-Rb[Tyr1138 and Tyr985] after immunoprecipitation of SOCS-3 from rat HSCs treated for 1h with leptin, adiponetin, both or neither. In the presence of leptin, SOCS was associated with Ob-Rb phosphorylated at Tyr985, but not at Tyr1138. In the presence of adiponectin, or adiponectin and leptin however, SOCS-3 was associated with Ob-Rb phosphorylated both Tyr985 and Tyr1138. C) Western blot of pOb-Rb(Tyr1138), total Ob-Rb, and SOCS-3 from culture-activated rat HSCS treated for 1h with leptin (Lep, 100ng/ml), adiponectin (Adi, 10μg/ml), both (LA), or neither. Compared with untreated samples, adiponectin treatment suppressed Ob-Rb (Tyr1138) phosphorylation. Leptin stimulated Ob-Rb(Tyr1138) phosphorylation, but the stimulation was attenuated when leptin and adiponectin were administered together. Compared to untreated, SOCS-3 protein levels decreased in the presence of leptin, but increased in the presence of adiponectin. D) Quantitation of data presented in (7C) (*p < 0.05 vs. untreated; #p < 0.05 vs. leptin)
In HSCs, Ob-Rb protein levels were similar in all conditions tested, although there was a trend towards decrease in the presence of adiponectin, and a trend towards increase after leptin, or leptin/adiponectin co-administration. As anticipated, leptin treatment stimulated Ob-Rb phosphorylation at Tyr1138, while adiponectin reduced Ob-RbY1138 phosphorylation (p < 0.05, vs. untreated). Compared with untreated samples, adiponectin and leptin co-administration also increased Ob-RbY1138 phosphorylation, but the stimulation was attenuated compared with leptin-only treated samples. (p < 0.05, Figure 7, C and D). These data are consistent with both the findings reported here on Ob-RbY985 phosphorylation and our earlier work showing that adiponectin inhibits leptin-induced Stat3 phosphorylation [10], and suggest that steady-state Ob-Rb protein levels alone do not account for the difference in SOCS-3/Ob-Rb binding that we observed.
In contrast, SOCS-3 protein levels were again consistent with increased available pools of SOCS-3 producing increased SOCS-3/Ob-Rb binding. Adiponectin increased SOCS-3 protein levels (p < 0.05), whether it was administered alone or in the presence of leptin (p < 0.05), while SOCS-3 protein levels were decreased in HSCs by 1h exposure to leptin (p < 0.05; Figure 7, C and D). The binding characteristics here are consistent with the kinetics of SOCS-3 protein expression described in our earlier work, wherein we observed stabilization of SOCS-3 as early as 5 min after the administration of adiponectin, followed by sustained high expression for at least 24h. By contrast, in those studies leptin-only treatment initially decreased the expression of SOCS-3 protein, which did not begin to recover until 1h after leptin treatment was begun. These kinetics are consistent with a negative feedback loop as described in the literature, wherein prolonged Ob-Rb activation leads to SOCS-3 transcriptional activation [17, 51-53]. In chinese hamster ovary cells stably expressing the long form of the leptin receptor, SOCS-3 protein levels were maximal after 2-3h of leptin treatment and remained elevated at 20h [54]. Indeed, in our studies, the negative feedback loop was also intact in rat HSCs, as SOCS-3 protein levels were also elevated after 24h of leptin treatment (data not shown).
We also examined the phosphorylation status of SOCS-3-bound Ob-Rb in rat HSCs. In the presence of leptin alone, SOCS-3 was bound to Tyr985-phosphorylated Ob-Rb (Figure 7B), consistent with the earlier reports describing phosphorylation of Ob-Rb at Tyr985 being required for SOCS-3 binding and feedback inhibition of leptin signal transduction. We detected no SOCS-3 association with Tyr1138-phosphorylated Ob-Rb in the presence of leptin alone by IP analysis. Interestingly, regardless of whether adiponectin was administered to HSCs by itself, or in the presence of leptin, IP analysis revealed that SOCS-3 was associated with Ob-Rb phosphorylated at both Tyr985 and Tyr1138 (Figure 7B). These studies may explain, with respect to hepatic fibrosis, why the leptin-SOCS-3 feedback loop is insufficient to block the fibrogenic potential of leptin. Mutation studies by Bjorbaek demonstrated that Tyr985 of Ob-Rb is essential for negative feedback inhibition of leptin signaling, but not essential for Stat3-mediated transcription in response to leptin, as HEK293 cells expressing a mutant Ob-Rb containing leucine at position 985 (instead of tyrosine) promotes Stat3-mediated transcription in response to leptin as well as cells expressing wild-type Ob-Rb, but they are incapable of producing negative feedback inhibition of leptin signal transduction [53]. Conversely, several laboratories have reported data showing that mutation of Ob-RbY1138 abrogates Stat3-mediated transcription initiated by Ob-Rb [55-57]. The requirement for phosphorylation of Ob-Rb at Tyr1138 for Stat3-mediated transcriptional activation during leptin signaling results from its role as the site for Stat3 binding to Ob-Rb: when Stat3 is not bound to Ob-Rb, it is not phosphorylated by ligand-activated Jak2. Our data suggest that, by stimulating binding of SOCS-3 to Ob-Rb at both Tyr985 and Tyr1138, adiponectin exploits the distinct importance of each of these phosphorylation sites to inhibit leptin signal transduction. While inhibiting leptin signal transduction by binding of SOCS-3 to Ob-Rb at Tyr985 is similar to the negative feedback loop originally described to occur in the hypothalamus, inhibition of leptin signaling by causing SOCS-3 to bind Ob-Rb at Tyr1138 represents a novel mechanism of action not only for adiponectin, but also for SOCS-3. Taken together, these data suggest that adiponectin promotes SOCS-3/Ob-Rb association independent of the negative feedback mechanism originally associated with leptin signaling.
Adiponectin inhibits leptin-stimulated formation of TIMP-1/MMP-1 complexes
Matrix Metalloproteinases (MMPs) regulate ECM homeostasis by catalyzing the degradation of various ECM components [2]. MMP-1, or collagenase, is produced by activated HSCs and catalyzes proteolysis of fibrillar collagens. Tissue Inhibitors of Metalloproteinases (TIMPs), on the other hand, regulate ECM homeostasis by binding a particular MMP to prevent its activity. Leptin, in addition to its other profibrogenic properties, stimulates TIMP-1 secretion by rat HSCs, and decreases extracellular MMP-1 activity [10]. These effects presumably correlate with increased formation of TIMP-1/MMP-1 complexes, in which MMP-1 would be deactivated because it is bound by the TIMP-1 molecule. Conversely, the findings suggest that the antifibrogenic inhibition of leptin signaling by adiponectin may reduce leptin-stimulated formation of extracellular TIMP-1/MMP-1 complexes.
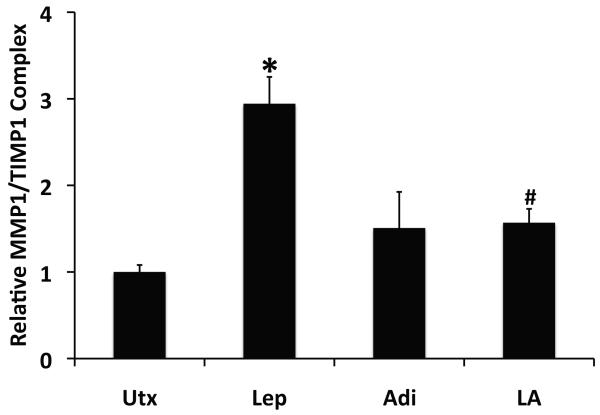
To test these hypotheses, we used ELISA to measure the amount of TIMP-1/MMP-1 complexes present in conditioned media from rat HSCs treated with leptin, adiponectin, both, or neither. As anticipated, leptin increased the amount of such complexes significantly, nearly threefold over untreated samples (p <0.05). The presence of adiponectin, however, significantly reduced leptin-stimulated formation of TIMP-1/MMP-1 complexes (p < 0.05), to levels statistically equal to those measured in the presence of adiponectin alone (Figure 8).
Figure 8. Adiponectin blocks leptin-stimulated formation of MMP-1/TIMP-1 complexes.
Data from ELISA of conditioned media from rat HSCs treated for 24h with leptin (Lep, 100ng/ml), adiponectin (Adi, 10μg/ml), both (LA), or neither demonstrated that leptin stimulated formation of MMP-2/TIMP-1 complexes (*p < 0.05 vs. untreated), while co-administration of adiponectin in the presence of leptin significantly reduced the formation of MMP-1/TIMP-1 complexes compared with leptin alone (#p < 0.05 vs. leptin).
Taken together, these data provide evidence of multiple mechanisms by which adiponectin can inhibit leptin signal transduction to reduce its profibrogenic effects. Our data suggest that, signaling via AdipoR1, adiponectin targets Jak2 and Ob-Rb directly to inhibit the early events of leptin signal transduction and produce a cellular environment wherein leptin signaling would be attenuated. By preserving the inhibitory phosphorylation of Jak2 at Ser523 and blocking the activating phosphorylations at Tyr1007/1008, adiponectin can inhibit leptin signal transduction at the earliest events in the pathway, as supported by the reduced activation of the downstream signaling elements, Ob-Rb and Stat3. Understanding the mechanisms of these effects require further investigation, but the stimulation of PTP1B activity by adiponectin in HSCs provides a plausible explanation for how adiponectin negatively regulates Tyr1007/1008 phosphorylation. To target Ob-Rb, adiponectin increases SOCS-3 expression and subsequent binding to Ob-Rb, in a mechanism that involves Tyr1138 phosphorylation of Ob-Rb. We propose that this mechanism is distinct from the negative feedback loop of leptin signaling in which SOCS-3 also participates, which requires only Tyr985 phosphorylation of Ob-Rb, however the specific roles of Ob-Rb phosphorylation at Tyr985 and Tyr1138 in adiponectin regulation of leptin signaling require further investigation. Ultimately, these varied effects converge to reduce the strength of the leptin signal, inhibiting molecular events like the formation of TIMP-1/MMP-1 complexes, that leptin produces to promote excessive deposition of ECM.
Our understanding of the role of adiponectin plays in stellate cell biology and liver fibrosis is evolving. On the one hand adiponectin appears to suppress HSC mitosis and increase susceptibility to apoptosis [23], effects that may be mediated by the conventional AMPK signaling axis. The data here, however, coupled with the recent report that AMPK knockout mice are still exquisitely sensitive to hepatic fibrosis [40], support pleiotropic effects of adiponectin, based on differential receptor and downstream signaling pathway activation. While one may be involved with regulating the life cycle of activated HSCs, another is involved downregulation of fibrogenic stimuli, including leptin. In either case, the emerging data provide additional molecular details that must be addressed, while raising the opportunity for targeting molecular therapy in human liver disease.
ACKNOWLEDGEMENT
The authors wish to thank Dr. Martin Myers at the University of Michigan for his generous sharing of the anti-phospho Jak2 (Ser523) anti sera.
Supported by (R24)DK064399, (R01)DK062092, (R01)DK075397 and (K08) DK076742
Abbreviations Used
- Jak2
Janus-Activated Kinase 2
- SOCS-3
Supressors of Cytokine Signaling 3
- Stat3
Signal Transducer and Activator of Transcription 3
- PTP1B
Protein Tyrosine Phosphatase 1B
- HSC
hepatic stellate cell
- TIMP-1
Tissue Inhibitor of Metalloproteinase 1
- MMP-1
Matrix Metalloproteinase 1
- ECM
Extracellular Matrix
- AMPK
adenosine monophosphate-activated kinase
- PPARα
Peroxisome Proliferator-Activated Receptor Alpha
Footnotes
AUTHOR CONTRIBUTION Jeffrey Handy participated in all aspects of this work. Ping Fu and Pradeep Kumar contributed to the execution of the experiments and acquisition of data. Jamie Mells participated in the design of the experiments and interpretation of data. Shvetank Sharma participated in interpretation of data and manuscript preparation. Neeraj Saxena and Frank Anania participated in experimental design, data interpretation and manuscript preparation.
REFERENCES
- 1.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo J, Friedman SL. Hepatic fibrogenesis. Sem. Liver Dis. 2007;27:413–426. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- 3.Bataller R, Brenner D. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc. Natl. Acad. Sci. U.S.A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolani C, Marra F. The role of adipokines in liver fibrosis. Pathophysiology. 2008;15:91–101. doi: 10.1016/j.pathophys.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, Zhang YJ, Lang T, Fukuda T, Yamashina S, Kitamura T, Sato N. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122:1399–1410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- 7.Saxena NK, Saliba G, Floyd JJ, Anania FA. Leptin induces increased alpha2(I) collagen gene expression in cultured rat hepatic stellate cells. J. Cell. Biochem. 2003;89:311–320. doi: 10.1002/jcb.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin S, Saxena NK, Ding X, Stein LL, Anania FA. Leptin increases tissue inhibitor of metalloproteinase I (TIMP-1) gene expression by a specificity protein 1/signal transducer and activator of transcription 3 mechanism. Mol. Endo. (Baltimore, Md. 2006;20:3376–3388. doi: 10.1210/me.2006-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handy JA, Saxena NK, Fu P, Lin S, Mells JE, Gupta NA, Anania FA. Adiponectin activation of AMPK disrupts leptin-mediated hepatic fibrosis via suppressors of cytokine signaling (SOCS-3) J. Cell. Biochem. 2010;110:1195–1207. doi: 10.1002/jcb.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Q, Mak KM, Lieber CS. Leptin represses matrix metalloproteinase-1 gene expression in LX2 human hepatic stellate cells. J. Hepatol. 2007;46:124–133. doi: 10.1016/j.jhep.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. Faseb J. 2004;18:1612–1614. doi: 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahima RS, Osei SY. Leptin signaling. Physiol. Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Ihle JN. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 15.Muraoka O, Xu B, Tsurumaki T, Akira S, Yamaguchi T, Higuchi H. Leptin-induced transactivation of NPY gene promoter mediated by JAK1, JAK2 and STAT3 in the neural cell lines. Neurochem. Int. 2003;42:591–601. doi: 10.1016/s0197-0186(02)00160-2. [DOI] [PubMed] [Google Scholar]
- 16.Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol. Endo. 1997;11:393–399. doi: 10.1210/mend.11.4.9907. [DOI] [PubMed] [Google Scholar]
- 17.Banks AS, Davis SM, Bates SH, Myers MG., Jr. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 18.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr., Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat. Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 22.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 23.Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, Anania FA. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Amer. J. Path. 2005;166:1655–1669. doi: 10.1016/S0002-9440(10)62476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balmer ML, Joneli J, Schoepfer A, Stickel F, Thormann W, Dufour JF. Significance of serum adiponectin levels in patients with chronic liver disease. Clin. Sci. 2010;119:431–436. doi: 10.1042/CS20100008. (1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savvidou S, Hytiroglou P, Orfanou-Koumerkeridou H, Panderis A, Frantzoulis P, Goulis J. Low serum adiponectin levels are predictive of advanced hepatic fibrosis in patients with NAFLD. J. Clin. Gastroenterol. 2009;43:765–772. doi: 10.1097/MCG.0b013e31819e9048. [DOI] [PubMed] [Google Scholar]
- 26.Pilz S, Horejsi R, Moller R, Almer G, Scharnagl H, Stojakovic T, Dimitrova R, Weihrauch G, Borkenstein M, Maerz W, Schauenstein K, Mangge H. Early atherosclerosis in obese juveniles is associated with low serum levels of adiponectin. J. Clin. Endocrinol. Metab. 2005;90:4792–4796. doi: 10.1210/jc.2005-0167. [DOI] [PubMed] [Google Scholar]
- 27.Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H, Okamoto Y, Kihara S, Miyagawa J, Shinomura Y, Funahashi T, Matsuzawa Y. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–1807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal. Biochem. 1987;161:207–218. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- 29.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nature. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Friedman S, Jones HW, 3rd, Golbetz HV, Lee JA, Little HL, Myers BD. Mechanisms of proteinuria in diabetic nephropathy. II. A study of the size-selective glomerular filtration barrier. Diabetes. 1983;32(Suppl 2):40–46. doi: 10.2337/diab.32.2.s40. [DOI] [PubMed] [Google Scholar]
- 33.Ishida-Takahashi R, Rosario F, Gong Y, Kopp K, Stancheva Z, Chen X, Feener EP, Myers MG., Jr. Phosphorylation of Jak2 on Ser(523) inhibits Jak2-dependent leptin receptor signaling. Mol. Cell. Biol. 2006;26:4063–4073. doi: 10.1128/MCB.01589-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura R, Sano H, Matsudaira T, Miyashita Y, Morimoto A, Shirasawa T, Takahashi E, Kawaguchi T, Tajima N. Childhood obesity and its relation to serum adiponectin and leptin: a report from a population-based study. Diabetes Res. Clin. Pract. 2007;76:245–250. doi: 10.1016/j.diabres.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 36.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J .Clin. Endocrinol. Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 37.Asayama K, Hayashibe H, Dobashi K, Uchida N, Nakane T, Kodera K, Shirahata A, Taniyama M. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obes. Res. 2003;11:1072–1079. doi: 10.1038/oby.2003.147. [DOI] [PubMed] [Google Scholar]
- 38.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 39.Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 40.da Silva Morais A, Abarca-Quinones J, Guigas B, Viollet B, Starkel P, Horsmans Y, Leclercq IA. Development of hepatic fibrosis occurs normally in AMPK-deficient mice. Clin. Sci. 2010;118:411–420. doi: 10.1042/CS20090293. [DOI] [PubMed] [Google Scholar]
- 41.Miller RA, Chu Q, Le Lay J, Scherer PE, Ahima RS, Kaestner KH, Foretz M, Viollet B, Birnbaum MJ. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J. Clin. Invest. 2011;121:2518–2528. doi: 10.1172/JCI45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heiker JT, Kosel D, Beck-Sickinger AG. Molecular mechanisms of signal transduction via adiponectin and adiponectin receptors. Biol. Chem. 2010;391:1005–1018. doi: 10.1515/BC.2010.104. [DOI] [PubMed] [Google Scholar]
- 43.Buechler C, Wanninger J, Neumeier M. Adiponectin receptor binding proteins--recent advances in elucidating adiponectin signalling pathways. FEBS Lett. 2010;584:4280–4286. doi: 10.1016/j.febslet.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 44.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 45.Tu Q, Zhang J, Dong LQ, Saunders E, Luo E, Tang J, Chen J. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. J. Biol. Chem. 2011;286:12542–12553. doi: 10.1074/jbc.M110.152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong EG, Cho YR, Kim JK, Kahn BB, Neel BG, Bence KK. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes. 2009;58:590–599. doi: 10.2337/db08-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 48.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 49.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 50.Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell. Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat. Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 52.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nature. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 53.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 54.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J. Biol. Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 55.Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tartaglia LA. The leptin receptor. J. Biol. Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 57.White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J. Biol. Chem. 1997;272:4065–4071. doi: 10.1074/jbc.272.7.4065. [DOI] [PubMed] [Google Scholar]