Abstract
Background
While corticosteroid use in Acute Hemorrhagic Stroke (AHS) is not widely adopted, management with intravenous dexamethasone (IVDxM) has been standard of care at the University Hospital of Heraklion, Crete (UH-Crete) with observed outcomes superior to those reported in literature. To explore this further, we conducted a retrospective, multivariable-adjusted two-center study.
Methods
We studied 391 AHS cases admitted to UH-Crete between 1/1997 and 7/2010 and compared them with 510 AHS cases admitted to Massachusetts General Hospital, Boston from 1/2003 to 9/2009. Of the Cretan cases, 340 received a tapering scheme of IVDxM, starting with 16–32 mg/day, while the Boston patients were managed without steroids.
Results
The two cohorts had comparable demographics and stroke severity on admission, although anticoagulation was more frequent in Boston. The in-hospital mortality was significantly lower on Crete (23.8%, n=340) than in Boston (38.0 %, n=510; p<0.001) as was the 30-day mortality (Crete: 25.4%, n=307; Boston: 39.4%, n=510; p<0.001). Exclusion of patients on anticoagulants showed even greater differences (30-day mortality: Crete 20.8%; n=259; Boston 37.0%; n=359; p<0.001). The improved survival on Crete was observed three days after initiation of IVDxM and was pronounced for deep-seated hemorrhages. After adjusting for AHS volume/location, GCS, hypertension, diabetes mellitus, smoking, coronary artery disease and statin, antiplatelet and anticoagulant use, IVDxM treatment was associated with better functional outcomes and significantly lower risk of death at 30-days (odds ratio 0.357; 95% C.I. 0.174–0.732).
Conclusions
This study suggests that IVDxM improves outcome in AHS and supports a randomized clinical trial using this approach.
Keywords: ICH, Dexamethasone, stroke management
Introduction
Acute hemorrhagic stroke (AHS) often carries a poor prognosis, as effective treatments are lacking.1 While the use of corticosteroids in intra-cerebral hemorrhage (ICH) is widely discouraged,2–7 management with intravenous dexamethasone (IVDxM) has been standard of care at the University Hospital of Heraklion, Crete (UH-Crete). As review of the data indicated better outcomes than historical non-treated controls, we conducted a retrospective, multivariable-adjusted two-center study comparing outcomes in a Cretan cohort treated with corticosteroids to a U.S. cohort in Boston managed without corticosteroids.
Methods
Of the 391 AHS cases admitted to UH-Crete between 1/1997 and 7/2010, 340 are known to have received IVDxM (Figure S1). These were compared with all AHS patients admitted to the Stroke Service of Massachusetts General Hospital, Boston from 1/2003 to 9/2009. This study was approved by the Institutional Review Boards of both centers.
Brain imaging studies from Boston were sent to Crete in digital form and were compared with those from Crete regarding ICH location and volume (for details see supplementary data at http://stroke.ahajournals.org). On Crete, AHS patients were placed on tapering IVDxM scheme within two hours from admission, with the starting dose being 16–32 mg/day, given in four divided doses. Every two days afterwards, IVDxM was decreased to half and was discontinued within 10–12 days from start. The decision to use 16mg, 24mg or 32 mg IVDxM initially was made by the attending neurologist using the hemorrhage size and the patient clinical status as a guide (Table S1). To prevent hyperglycemia, patients were kept under an insulin sliding scheme. Prophylaxis from ulcer was provided with the use of an intravenous proton pump inhibitor and from deep venous thrombosis with low molecular weight heparin and/or elastic stockings.
Neurological evaluations were performed on admission and at regular intervals afterwards or as needed. Neurological status was assessed by the Glasgow Coma Scale (GCS) and the modified Rankin Scale (mRS). For the Boston cohort, one- and three-month mortality was established by follow-up phone call to patient or next-of-kin or review of the US Social Security Death Index. For the Cretan patients, data were obtained from the records of the outpatient Stroke Clinic, by follow-up phone call between May 2009 and July 2010 and by search of computerized hospital records.
Statistical analyses were performed with the PASW software, using Pearson’s chi-square statistics and independent samples t-test or the corresponding Mann-Whitney test. Adjusted ORs were estimated using multiple logistic regression models including age, sex, anticoagulant, antiplatelet and statin use, hematoma volume and location, admission GCS, hypertension, diabetes mellitus, coronary artery disease, smoking in the last 5 years and dexamethasone administration.
Results
The two cohorts were similar regarding patients’ demographics, smoking habits, history of diabetes mellitus, admission blood glucose levels and previous ICH (Table S2). Also, the size and location of the ICH, and the severity of stroke (as judged by the GCS) on admission were similar. Moreover, the time from symptom onset to ER or to CT was comparable. However, hypertension, coronary artery disease, atrial fibrillation and use of statins, antiplatelets and anticoagulants were more frequent in the Boston cohort (Table S2).
The in-hospital mortality was significantly lower on Crete (23.8%; n=340) than in Boston (38.0 %; n=510; p<0.001) as was the 30-day mortality (Crete: 25.4%; n=307; Boston: 39.4%; n=510; p<0.001; Table 1). Similarly, the three-month mortality was significantly lower on Crete than in Boston (Table 1). After excluding patients on anticoagulants from both centers, the in-hospital and 30-day mortality were again lower on Crete (19.2%; n=291 and 20.8%; n=259, respectively) than in Boston (35.4% and 37.0%; respectively; n=359; p<0.001) (Table 2). Limiting the analysis to patients admitted in Crete over the same time period as in Boston (1/2003–9/2009) gave similar results (Tables 1 and 2). Differences in AHS mortality remained significant even when all 391 AHS patients admitted to UH-Crete were included (intention to treat analysis, Table S3). The functional status of AHS survivors, as judged by the discharge mRS, was better on Crete (Tables 1 and 2, and Fig. S2). However, the average in-hospital stay was longer on Crete than in Boston (Tables 1 and 2), as the Cretan patients needed to stay in the hospital for their IVDxM course to be completed.
Table 1.
Clinical outcomes
| Boston (n=510) 2003–2009 |
Crete (n=340) 1997–2010 |
Crete (n=190) 2003–2009 |
|||
|---|---|---|---|---|---|
| Death in hospital-no. (%) | 194 (38.0%) | 81 (23.8%) | <0.001 | 48 (25.3%) | 0.002 |
| Death (30 days)– no. (%) | 201 (39.4%) | 78 (25.4%) | <0.001 | 47 (26.0%) | 0.001 |
| Death (90 days)– no. (%) | 223 (43.7%) | 92 (32.7%) | 0.003 | 55 (33.3%) | 0.018 |
| Average in hospital stay-days (mean ± SD) | 8.8 ± 11.8 | 14.0 ± 11.4 | <0.001 | 15.0 ± 12.9 | <0.001 |
| Mod. Rankin on discharge (mean ± SD) | 4.5 ± 1.5 | 3.8 ± 1.8 | <0.001 | 3.9 ± 1.8 | <0.001 |
Table 2.
Clinical outcomes for AHS patients not receiving anticoagulants
| Boston (n=359) 2003–2009 |
Crete (n=291) 1997–2010 |
Crete (n=159) 2003–2009 |
|||
|---|---|---|---|---|---|
| Death in hospital-no. (%) | 127 (35.4%) | 56 (19.2%) | <0.001 | 33 (20.8%) | 0.001 |
| Death (30 days)– no. (%) | 133 (37.0%) | 54 (20.8%) | <0.001 | 33 (22.0%) | 0.001 |
| Death (90 days)– no. (%) | 143 (39.8%) | 65 (27.7%) | 0.002 | 39 (28.9%) | 0.025 |
| Average in hospital stay-days (mean ± SD) | 8.9 ± 12.8 | 14.5 ± 11.3 | <0.001 | 15.2 ± 12.8 | <0.001 |
| Mod. Rankin on discharge (mean ± SD) | 4.4 ± 1.6 | 3.7 ± 1.8 | <0.001 | 3.7 ± 1.8 | <0.001 |
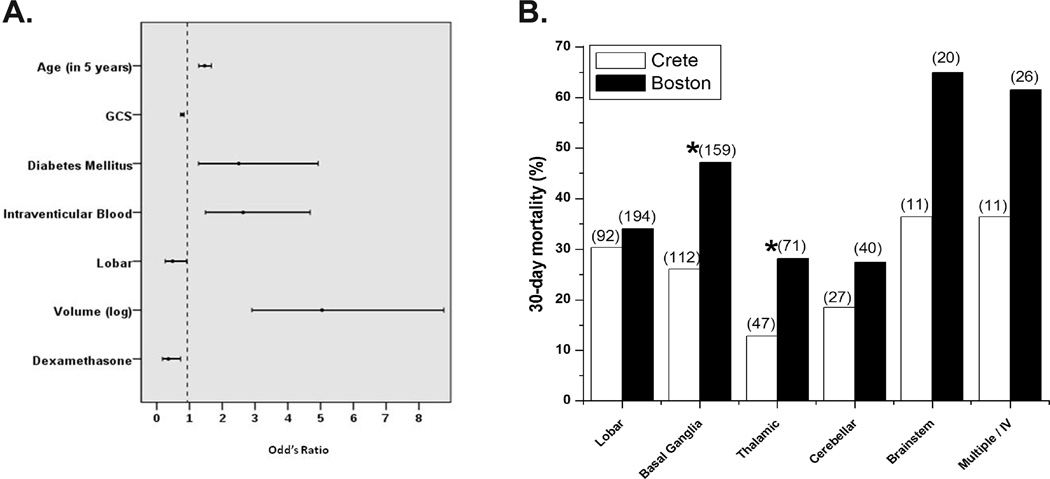
Multiple logistic regression analysis adjusting for various factors revealed that IVDMx decreased the risk of death at 30 days (odds ratio 0.357; 95% C.I. 0.174–0.732) (Figure 1A). The beneficial effect of IVDMx occurred three or more days after treatment initiation (Figure S3). Analyses based on ICH location revealed that the decreased mortality on Crete was mainly due to improved outcomes for thalamic and basal ganglia, but not for lobar, hemorrhages (Figure 1B; Table S4). IVDxM was relatively well tolerated, with serious non-infectious adverse events occurring rather rarely (Table S5).
Fig. 1. A. Odds ratio for death at 30 days. B. 30-day mortality according to the ICH location.
Numbers in parentheses above each column represent the total number of patients per category. * p<0.05
Discussion
This retrospective, multivariable-adjusted two-center study revealed that AHS patients treated acutely with IVDxM had lower mortality rates and better functional outcomes than those managed without corticosteroids. We acknowledge, however, that the two cohorts differed in their genetic and cultural background and variations in patient care of the two hospitals may have affected the observed outcomes. Notwithstanding these limitations, the mortality of IVDMX-treated Cretan patients is still better than that of other Greek Centers in which ICH is managed without steroids.8 While our results are at variance with those of studies from USA4, Thailand5, India6 and Nigeria7, these previous reports included small AHS patient samples. Since we observed decreased mortality rates three or more days after starting the IVDxM treatment and since this effect was more pronounced for deep seated hemorrhages, it is possible that this beneficial outcome relates to the well-known action of steroids on vasogenic edema or on inflammation.3 Such edema and/or inflammation are shown to complicate ICH.1,9 While we cannot exclude that factors other than IVDxM could be operational, the encouraging results of this retrospective analysis support controlled clinical trials using this approach.
Supplementary Material
Acknowledgments
The help of Irini Tzanaki and Drs. Minas Tzagournissakis, Mihalis Mavridis, Vassilios Mastorodemos and Martha Spilioti is cordially acknowledged.
Funding Source
Association for Neurologic Disorders of Crete “EY ZHN”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Qureshi A, Mendelow D, Hanley D. Intracerebral hemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin V, Anderson N, Rinkel G, Algra A, van Gijn J, Bennett D. Corticosteroids for aneurysmal subarachnoid hemorrhage and primary intracerebral hemorrhage. Cochrane Database Syst Rev. 2005;3:CD004583. doi: 10.1002/14651858.CD004583.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Davis SM, Donnan GA. Steroids for Stroke: Another Potential Therapy Discarded Prematurely? Stroke. 2004;35:230–231. doi: 10.1161/01.STR.0000105932.01846.7B. [DOI] [PubMed] [Google Scholar]
- 4.Tellez H, Bauer R. Dexamethasone as treatment in cerebrovascular disease. A controlled study in intracerebral hemorrhage. Stroke. 1973;4:541–546. doi: 10.1161/01.str.4.4.541. [DOI] [PubMed] [Google Scholar]
- 5.Poungvarin N, Bhoopat W, Viriyavejakul A, Rodprasert P, Buranasiri P, Sukondhabhant S, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. N Engl J Med. 1987;316:1229–1233. doi: 10.1056/NEJM198705143162001. [DOI] [PubMed] [Google Scholar]
- 6.Desai P, Prasad K. Dexamethasone is not necessarily unsafe in primary supratentorial intracerebral haemorrhage. J Neurol Neurosurg Psych. 1998;65:799–800. doi: 10.1136/jnnp.65.5.799a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogun S, Odusote K. Effectiveness of high dose dexamethasone in the treatment of acute stroke. West Afr J Med. 2001;20:1–6. [PubMed] [Google Scholar]
- 8.Vemmos K, Takis C, Georgilis K, Zakopoulos N, Lekakis J, Papamichael CM, et al. The Athens stroke registry: results of a five-year hospital-based study. Cerebrovasc Dis. 2000;10:133–141. doi: 10.1159/000016042. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2006;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.