Abstract
Binge eating and substance dependence are disorders characterized by a loss of control over consummatory behaviors. Given the common characteristics of these two types of disorders, it is not surprising that the comorbidity between eating disorders and substance abuse disorders is high (20–40%; Conason et al., 2006). It is unknown, however, whether loss of control in one disorder predisposes an individual to loss of control in the other. The present study, therefore, used a rodent model to test whether a history of binge eating would augment subsequent responding for cocaine. Using the limited access protocol described by Corwin et al. (1998), 45 adult male Sprague-Dawley rats were maintained on one of four dietary protocols for a period of six weeks: chow only (Chow; n=9), continuous access to an optional source of dietary fat (Ad Lib; n=12), 1-h access to an optional source of dietary fat daily (Daily; n=12), or 1-h access to an optional source of dietary fat on Monday, Wednesday, and Friday (MWF; n=12). All four groups also had unrestricted access to a nutritionally complete diet of chow and water. Fat-bingeing behaviors developed in the MWF rats, the group with the most restricted access to the optional fat. Thereafter, cocaine-seeking and –taking behaviors were assessed in all rats using a self-administration protocol modified from that described by Deroche-Gamonet et al. (2004), which focus on the motivation for and preoccupation with obtaining and consuming drug (assessed using a progressive ratio (PR) schedule of reinforcement) and persistence in responding for drug during periods of signaled drug non-availability (SNA). Rats with the MWF history tended to take more cocaine late in fixed ratio (FR) training, they persisted in their efforts to obtain cocaine in the face of signaled non-availability, worked harder for cocaine on a PR schedule of reinforcement, and exhibited more goal-directed behavior towards the cocaine-associated operandum. These results demonstrate a link between binge-type intake of fat and the development of drug-seeking and -taking behaviors, suggesting that a history of fat bingeing may predispose individuals to exhibit more robust “addiction-like” behaviors toward a substance of abuse. Thus, it appears that conditions promoting excessive behavior toward one substance (e.g., a palatable fatty food) beget excessive behavior toward another (e.g., cocaine).
Keywords: addiction, bingeing, cocaine, high-fat diet, self-administration
Introduction
Drug addiction persists as a major problem in the United States. In fact, 18% of Americans suffer from some form of substance dependence in their lifetime (Gillin and Drummond, 2000), which leads substance abuse to incur an estimated $484 billion in annual expenses to our nation (National Institute on Drug Abuse, 2005). Likewise, excessive food intake (especially foods high in sugar and fat) has become problematic. One form of intake of particular concern is intermittent, excessive, dysfunctional appetitive behavior such as binge eating (National Institutes of Health, 2004). The lifetime prevalence of binge eating (subthreshold bingeing, as well as the binge-related eating disorders) is estimated to be approximately 5% (Hudson et al., 2007; Swanson et al., 2011), and the 12-month prevalence (2.1%) is similar to that of illicit drug dependence (2.8%; Hudson et al., 2007; SAMHSA, 2009). Episodes of binge eating characterize several eating disorders, including binge eating disorder (BED), which is thought by many to be the most common eating disorder, affecting as many as 4% of Americans (American Psychiatric Association, 2000; National Women’s Health Information Center, 2000). While binge eating and obesity can be co-expressed clinically, nearly 67% of those who binge (including those suffering from BED, bulimia nervosa, and subthreshold BED) are not obese (BMI < 30; Hudson et al., 2007). Even so, binge eating is a devastating form of disordered eating and is highly associated with potentially life-threatening comorbid disturbances (Hudson et al., 2007; Swanson et al., 2011).
Substance abuse and binge eating are both characterized by loss of control over consummatory behaviors. Not surprisingly, these disorders share high comorbidity (Conason et al., 2006; Hudson et al., 2007; Swanson et al., 2011), particularly in relation to alcohol and cocaine dependence (Brewerton et al., 1995; Bulik et al., 2002; Bushnell et al., 1994; Johnson et al., 1997; Jonas et al., 1987; Wiederman and Pryor, 1996; Wilson, 1993). In addition, expression of bulimic symptoms has been shown to predict the onset of Alcohol Use Disorder (Franko et al., 2005). As such, the possibility that one maladaptive behavior (e.g., compulsive food intake) may serve as a gateway for the development of the other (e.g., drug addiction) has been proposed. A similar progression in use of increasingly serious substances of abuse has been characterized (Degenhardt et al., 2008).
Substantial behavioral evidence linking either sugar or fat intake with the intake of drugs of abuse lends support to this idea. For example, a correlation between preference for sweets and drugs of abuse has been identified in humans (Pelchat, 2002). In addition, several studies in rats suggest that repeated exposure to sucrose enhances behavioral sensitization to cocaine (Gosnell, 2005) and increases cocaine self-administration (Gosnell, 2000). In addition, individual differences in sucrose preference have been shown to predict individual differences in amphetamine self-administration (DeSousa et al., 2000). Likewise, preexisting preferences for fat have been shown to predict differences in ethanol consumption in rats (Krahn et al., 1991). Conversely, several studies demonstrate that it may not be intake, per se, but the manner in which sugar is consumed that leads to differences in responsiveness to drugs of abuse in rats. For example, intermittent excessive intake of sucrose has been shown to enhance behavioral sensitization to amphetamine (Avena and Hoebel, 2003) and to increase ethanol consumption (Avena et al., 2004). The onset of withdrawal may contribute to these behavioral changes as abrupt discontinuation of intermittent excessive intake of sucrose has been shown to produce opiate-like withdrawal symptoms (Colantuoni et al., 2002) and to exacerbate the expression of morphine withdrawal (Schoenbaum et al., 1990). Finally, there is evidence that the composition of the diet itself can affect the intake of drugs of abuse, as diets promoting fat consumption have been shown to increase ethanol intake (Carrillo et al., 2004).
Offering further support for the connection between the intake of sugar and fat and the intake of drugs of abuse are several studies investigating the neuroanatomical and neurochemical changes that accompany sucrose and fat consumption. Not surprisingly, these sugar and fat consumption-induced changes occur in the mesocorticolimbic dopamine system, a major component of the brain’s reward pathway (see Kelley and Berridge, 2002 and Berridge et al., 2010 for reviews), and many of the changes mimic those that occur following exposure to drugs of abuse (see Avena et al., 2008 and Koob and Volkow, 2010 for reviews), including turnover and release of DA (Hajnal and Norgren, 2002; Rada et al., 2005), D2 receptor binding and expression (Bello et al., 2002; Johnson and Kenny, 2010), and dopamine transporter (DAT) binding and expression (Bello et al., 2003). In addition, differential responsiveness in rats bingeing on fat has been reported when a D2 receptor antagonist is administered peripherally (Corwin and Wojnicki, 2009), or directly into the prefrontal cortex (PFC; Corwin and Babbs, In press). Finally, when shown food stimuli, individuals suffering from BED exhibit increases in extracellular dopamine in the caudate that are correlated with binge eating score, not BMI (Wang et al., 2011).
While chronic intake of sugar, fat, and drugs of abuse has been shown to exert similar effects on the mesocorticolimbic dopamine system, as alluded to above, it may be the manner in which the substance is consumed, rather than the substance itself, that results in those alterations (see Avena et al., 2008; Corwin and Babbs, In Press; Corwin et al., In Press for reviews). Additionally, although there is evidence in rats that binge-type consumption of sucrose leads to increased consumption of ethanol (Avena et al., 2004), it is not known whether binge-type consumption of fat also will lead to increased consumption (in this case, i.v. self-administration) of a substance of abuse. Therefore, the current study was designed to systematically and operationally investigate whether the manner in which a fatty substance is consumed will predispose subjects to self-administer a drug of abuse, in this case, cocaine. Finally, it is not known whether addicts have “addictive personalities” (i.e., traits) that predispose them to “addiction-like” behaviors in many venues (e.g., eating, drinking, drug taking, gambling), or if experience in one environmental context with one type of addiction can induce a state that then makes an individual more prone to other addictions in a different context. To shed light on this debate we employed two separate behavioral paradigms. The first was the limited access protocol developed by Corwin et al. (1998), which was used to promote fat bingeing. The second paradigm was a drug self-administration protocol modified from that described by Deroche-Gamonet et al. (2004) which assesses compulsive drug-seeking and -taking behaviors, such as persistent responding for drug during periods of signaled non-availability (SNA) and the motivation for, and preoccupation with, obtaining and consuming drug during progressive ratio (PR) testing. Successive use of these two paradigms allows for the direct assessment of whether binge-type consumption of a fatty food will influence the development of “addiction-like” behaviors for cocaine.
Methods
Subjects
This study was run in two replications. The subjects were 81 (n=36 for Replication 1 and n=45 for Replication 2) naïve, male Sprague-Dawley rats (Harlan, Indianapolis, IN), 60 days of age at the beginning of the experiment. Due to complications during surgery, four rats were eliminated from the study. An additional eight rats were eliminated due to loss of catheter patency, leaving 69 rats for self-administration training and experimental testing (described below). Due to the length of the study, an additional 24 rats (7 from the Ad Lib group, 8 from the Daily group, and 9 from the MWF group) were eliminated during the course of the experiment due to loss of catheter patency or unexpected death. In these cases, data contributed to the analyses across as many trials as possible. Forty-five subjects, then, completed the study in full. A similar profile (i.e., nearly identical figures and statistical results), however, was obtained whether the analyses were conducted on these 45 rats or on all 69 subjects using data from as many trials as possible. Only data from the 45 rats that completed the study are reported below. For both the binge eating induction phase and drug self-administration phase of the experiment, rats were housed individually in hanging wire mesh cages in a colony room with temperature, humidity, and ventilation controlled automatically. They were allowed ad lib access to a nutritionally complete commercial laboratory rodent chow (Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN; percent of calories as protein: 28.05%, fat: 12.14%, carbohydrate: 59.81%; 3.3 kcal/g) and water, except where otherwise noted. In addition, the rats were maintained on a 12-h light-dark cycle.
Limited Access Protocol
After a one-week adaptation period, the rats were matched for body weight, average three-day chow intake, and overnight shortening intake, and assigned to four dietary protocol groups in the Corwin laboratory, Department of Nutritional Sciences, The Pennsylvania State University, University Park, Pennsylvania. Briefly, the rats had continuous access to the rodent diet described above and water throughout the study, and, in this portion of the study, were provided additional access to a jar of hydrogenated vegetable shortening (optional fat; Crisco® All-Vegetable shortening, J. M. Smucker Co., Orrville, OH) clipped to the front of the home cage under one of the following four conditions for a period of six weeks: no optional fat access (i.e., chow only; Chow; n=9), continuous access to the optional fat (Ad Lib; n=19), daily 1-h access to the optional fat (Daily; n=20), or 1-h access to the optional fat on Monday, Wednesday, and Friday (MWF; n=21). One-h fat access was provided 2.5 h prior to dark onset. Fresh shortening was provided at least once per week. Body weight and intake of shortening (1-h and 24-h) were measured. Immediately following this six-week period, all rats were transported 90 miles away to the Grigson laboratory in the Department of Neural and Behavioral Sciences, The Pennsylvania State University College of Medicine, Hershey, Pennsylvania, where they remained for the duration of the study. From this point until the completion of the study, access to the optional fat was no longer provided.
Catheter Construction and Implantation
Self-administration catheter
Intra-jugular catheters were custom-made in the Grigson laboratory as described by Twining et al. (2009).
Catheter implantation
Rats were anesthetized using an intraperitoneal (i.p.) injection of ketamine/xylazine and catheters were implanted into the jugular vein as described by Twining et al. (2009). Following surgery, rats were allowed at least two days to recover. General maintenance of catheter patency involved daily examination and flushing of catheters with heparinized saline (0.2 ml of 30 IU/ml heparin). Catheter patency was verified, as needed, using 0.2 ml of propofol (Diprivan 1%) administered intravenously.
Apparatus
Each rat was trained in one of twelve identical operant chambers (MED Associates, St. Albans, VT) as described previously (Grigson and Twining, 2002; Twining et al., 2009). Each chamber measured 30.5 cm in length × 24.0 cm in width × 29.0 cm in height, and was individually housed in a light- and sound-attenuated cubicle. The chambers consisted of a clear Plexiglas top, front, and back wall. The side walls were made of aluminum. Grid floors consisted of nineteen 4.8-mm stainless steel rods, spaced 1.6 cm apart (center to center). Each chamber was equipped with three retractable sipper spouts that entered through 1.3-cm diameter holes, spaced 16.4 cm apart (center to center). A stimulus light was located 6.0 cm above each tube. Each chamber was also equipped with a houselight (25 W), a tone generator (Sonalert Time Generator, 2900 Hz, Mallory, Indianapolis, IN), and a speaker for white noise (75 dB). Cocaine reinforcement was controlled by a lickometer circuit that monitored empty spout licking to operate a syringe pump (Model A, Razel Scientific Instruments, Stamford, CT). A coupling assembly attached the syringe pump to the catheter assembly on the back of each rat and entered through a 5.0-cm diameter hole in the top of the chamber. This assembly consisted of a metal spring attached to a metal spacer with Tygon tubing inserted down the center, protecting passage of the tubing from rat interference. The tubing was attached to a counterbalanced swivel assembly (Instech, Plymouth Meeting, PA) that, in turn, was attached to the syringe pump. Events in the chamber and collection of data were controlled on-line with a Pentium computer that used programs written in the Medstate notation language (MED Associates).
Drug Preparation
Individual 20-ml syringes were prepared for each rat prior to each daily session by diluting cocaine HCl stock solution (1.24 g cocaine HCl + 150 ml saline) with heparinized saline (0.1 ml 1000 IU heparin/60.0 ml saline) for a dose of 0.8 mg/kg (Deroche-Gamonet et al., 2004).
Data Collection
Habituation, self-administration training, and progressive ratio testing were conducted during the light phase of the light/dark cycle.
Habituation Procedure
Rats were water-deprived for approximately 16 h and then were habituated to the operant chambers during a single 15-min session the day before the beginning of self-administration training. During this session, water was available in the right (“active”) spout within the operant chamber, while the left (“inactive”) spout was empty. Thereafter, rats were returned to ad libitum access to water for the duration of the study.
Self-Administration Training Procedure
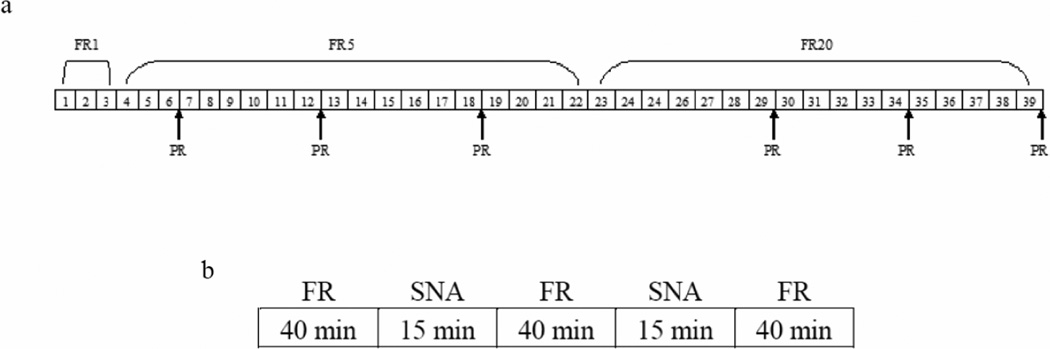
Self-administration training began immediately following the habituation phase. Each rat was trained during daily 150-min sessions, as described by Deroche-Gamonet et al. (2004), for 39 days (see Figure 1a). Each 150-min session consisted of three drug periods, separated by two signaled non-availability (SNA) periods (see Figure 1b). Specifically, rats were placed in the operant chambers in darkness. Immediately upon initiation of the 150-min session, the white noise was turned on, the right and left empty spouts advanced into the chamber, and the cue light above the right spout was illuminated. Rats were then allowed to self-administer cocaine (0.8 mg/kg) for 40 minutes. The right spout was termed the “active” spout, while the left spout was termed the “inactive” spout. A 15-min SNA period followed the 40-min drug period, during which time the cue light above the right spout was turned off, a light on the chamber wall opposite the spouts was illuminated, and the infusion pump was turned off. Responding on the “active” spout was without consequence during SNA periods. A fixed ratio (FR) 1 schedule of reinforcement was implemented initially (Trials 1–3). During this time, completion of a single lick on the “active” empty spout was followed by a single intravenous (i.v.) infusion of cocaine over six seconds. Drug delivery was signaled by offset of the stimulus light, retraction of the “active” spout, and onset of the tone and houselight. The tone and houselight remained on for a 20-sec timeout period. Responding on the “inactive” spout was without consequence throughout each 150-min session. The reinforcement schedule was increased to FR5 (Trials 4–22) and then to FR20 (Trials 23–39) to fully distinguish between active and inactive responding. Following each self-administration training session, the rats were returned to their home cages. The number of infusions self-administered during drug periods was evaluated throughout self-administration training. In addition, responding during the final phase of FR training (i.e., across the seventeen FR20 trials) was assessed. Thus, the number of infusions self-administered and goal-directed behavior (calculated by subtracting the total number of inactive responses made from the total number of active responses made) were assessed across the final FR20 trials. Responding was assessed similarly and independently across the intervening SNA periods.
Figure 1.
Overview of daily fixed ratio (FR) training sessions and timeline of behavioral training and experimental testing. a) Each daily 150-min FR training trial was divided into three 40-min active drug periods that were separated by two 15-min signaled drug non-availability (SNA) periods. b) During the 39 days of training, FR1 (Trials 1–3), FR5 (Trials 4–22), and FR20 (Trials 23–39) schedules of reinforcement were used (i.e., either 1, 5, or 20 responses were required, respectively, to receive a single infusion of cocaine). Progressive ratio (PR) tests were conducted intermittently (approximately every 7 days), during which the number of responses required to receive each infusion increased relative to the last infusion received.
Progressive Ratio Testing
In addition to FR training, a progressive ratio (PR) schedule of reinforcement, as described by Deroche-Gamonet et al. (2004), was implemented periodically to test the impact of a history of fat bingeing on the rats’ willingness to work for the drug. Thus, PR testing was conducted approximately every seven days (see Figure 1a). During PR testing, rats were placed in the operant chambers with conditions identical to those of self-administration training, except the number of active responses required to receive the first infusion started at 10 and then progressively increased by a multiple of 10 (except for the third infusion where there is only an increase of 5) every third infusion (10, 10, 10+5=15, 15, 15+10=25, 25, 25+10=35, etc.). During PR sessions, rats were allowed to self-administer cocaine (0.8 mg/kg) until a period of 30 min elapsed without receipt of an infusion. Terminal (i.e., during the final PR test) break point (the highest ratio completed) and terminal goal-directed behavior were measured, as PR responding tends to stabilize across several sessions. The PR testing conditions and the dose of cocaine used are consistent with those used by Deroche-Gamonet et al. (2004).
Data Analysis
All data, except the correlational analyses, were analyzed with Statistica (StatSoft, Tulsa, OK) using one-way and mixed factorial analysis of variance (ANOVA) tests. Newman-Keuls post hoc tests were conducted on significant ANOVAs, when appropriate, with α set at 0.05. Fisher’s Least Significant Difference (LSD) tests were used when indicated. Pearson correlation coefficients were determined using SAS v9.1 (SAS Institute, Cary, NC). Formulas described by Thalheimer & Cook (2002) were used to calculate the effect size of the fat access histories relative to chow.
Results
Fat bingeing
The following independent measures were analyzed: one-hour intake during Week 6 of the special dietary protocols, total fat intake across the entire 6-week period of special dietary protocols, and change in fat intake from Week 1 to Week 6 of the special dietary protocols (i.e., escalation). The results of a one-way ANOVA on 1-h intake during Week 6 of the special dietary protocols revealed a significant main effect of Group, F(3, 41)=65.90, p < 0.01 (see first column of Table 1). Post hoc Newman-Keuls tests indicated that the 1-h intake of the Daily group exceeded that of the Chow and Ad Lib groups (*; ps < 0.01) and that the 1-h intake of the MWF group was greater than that of all other groups (**; ps < 0.01). These results show that, consistent with previous reports, rats in the MWF group (the group with the most restricted access) developed a binge-type intake of fat by consuming more than any of the other groups during the 1-h fat access period.
Table 1.
Intake During Maintenance on the Limited Access Protocol
| Group | Intake Parameter (kcal) | |||
|---|---|---|---|---|
| 1-h Intake | 24-h Fat Intake | Total Fat Intake | Escalation of Fat Intake | |
| Chow | 4.2 (+/− 0.70) | NA | NA | NA |
| Ad Lib | 3.3 (+/− 0.63) | 50.2 (+/− 2.58)* | 2176.0 (+/− 78.63)* | −35.2 (+/− 4.62) |
| Daily | 23.3 (+/− 1.96)* | NA | 1149.5 (+/− 130.08) | 2.1 (+/−1.27)* |
| MWF | 63.2 (+/− 6.17)** | NA | 924.7 (+/− 85.70) | 35.2 (+/− 5.19)** |
Mean (+/− SEM) of 1-h intake during the 1-h shortening access period of Week 6 of access to optional fat, 24-h intake during Week 6 of access to optional fat, total cumulative shortening intake across the entire 6-week dietary protocol, and change in shortening intake from Week 1 to Week 6 of access to optional fat.
1-h Intake. 1-h chow intake of the Chow group and 1-h fat intake of the other groups. * denotes statistical significance (ps < 0.01) compared to the 1-h intakes of the Chow and Ad Lib groups and
denotes statistical significance (ps < 0.01 and 0.03) compared to the 1-h intakes of the Chow, Ad Lib, and Daily groups and the 24-h intake of the Ad Lib group, respectively.
24-h Intake. * denotes statistical significance (p < 0.01) compared to the 1-h intake of the Daily group.
Total Fat Intake. * denotes statistical significance (ps < 0.01) compared to the Daily and MWF groups.
Escalation of Fat Intake. * denotes statistical significance (p < 0.01) compared to the Ad Lib group and
denotes statistical significance (ps < 0.01) compared to the Ad Lib and Daily groups.
In addition, the 24-h intake of fat in the Ad Lib group during Week 6 of the special dietary protocols was compared to the 1-h fat intake of the Daily and MWF groups (see first and second columns of Table 1). The results of a one-way ANOVA also revealed a significant main effect of Group, F(2, 33)=25.63, p < 0.01. Post hoc Newman-Keuls tests indicated that the 24-h intake of fat by the Ad Lib group was greater than the 1-h intake of fat by the Daily group (*; p < 0.01) and that the 1-h intake of fat by the MWF group was greater than both the 1-h intake of fat by the Daily group and the 24-h intake of fat by the Ad Lib group (**; ps < 0.03). These results indicate that MWF rats, in fact, consumed more fat in a 1-h period than did Ad Lib rats in a 24-h period. This finding is remarkable, given the fact that the Ad Lib group had access to the optional fat for 24 h per day every day of the week, while the MWF group had access for only 1 h on each of three days per week.
The results of a one-way ANOVA on total fat intake across the entire 6-week period of special dietary protocols revealed a significant main effect of Group, F(2, 33)=43.85, p < 0.01 (see third column of Table 1). Post hoc Newman-Keuls tests indicated that the total intake of fat by the Ad Lib group was greater than all other groups (*; ps < 0.01), while there was no significant difference between the Daily and MWF groups (ps > 0.05). These results indicate that, despite the 1-h and 24-h fat intake data, the Ad Lib group consumed the most total fat across the entire six weeks of maintenance on the dietary protocol, while the MWF group consumed the least. These data may seem contradictory to the other intake data; however, they make sense given the differing lengths of access to optional fat provided to each group. In spite of the differences in overall fat consumption, significant differences in body weight did not develop (Mean Chow: 380 g (+/− 7.86 g); Mean Ad Lib: 384 g (+/− 5.23 g); Mean Daily: 381 g (+/− 6.90 g); Mean MWF: 391 g (+/− 8.07 g)). These group differences in overall fat consumption allow us to separate the impact of high fat intake (greatest in the Ad Lib group) from that of binge intake (greatest in the MWF group).
Finally, the results of a one-way ANOVA on the change in 1-h fat intake from Week 1 to Week 6 (i.e., escalation in fat intake) of the special dietary protocols also revealed a significant main effect of Group, F(2, 33)=75.24, p < 0.01 (see fourth column of Table 1). Post hoc Newman-Keuls tests revealed that shortening intake escalated more over the 6-week period in the Daily group than the Ad Lib group (*; p < 0.01) and in the MWF group than all other groups (**; ps < 0.01). These results show that 1-h intake of fat escalated in rats that were maintained on restricted access diets (Daily and MWF groups), with MWF rats showing greater escalation than Daily rats, overall.
Cocaine intake during FR active drug periods
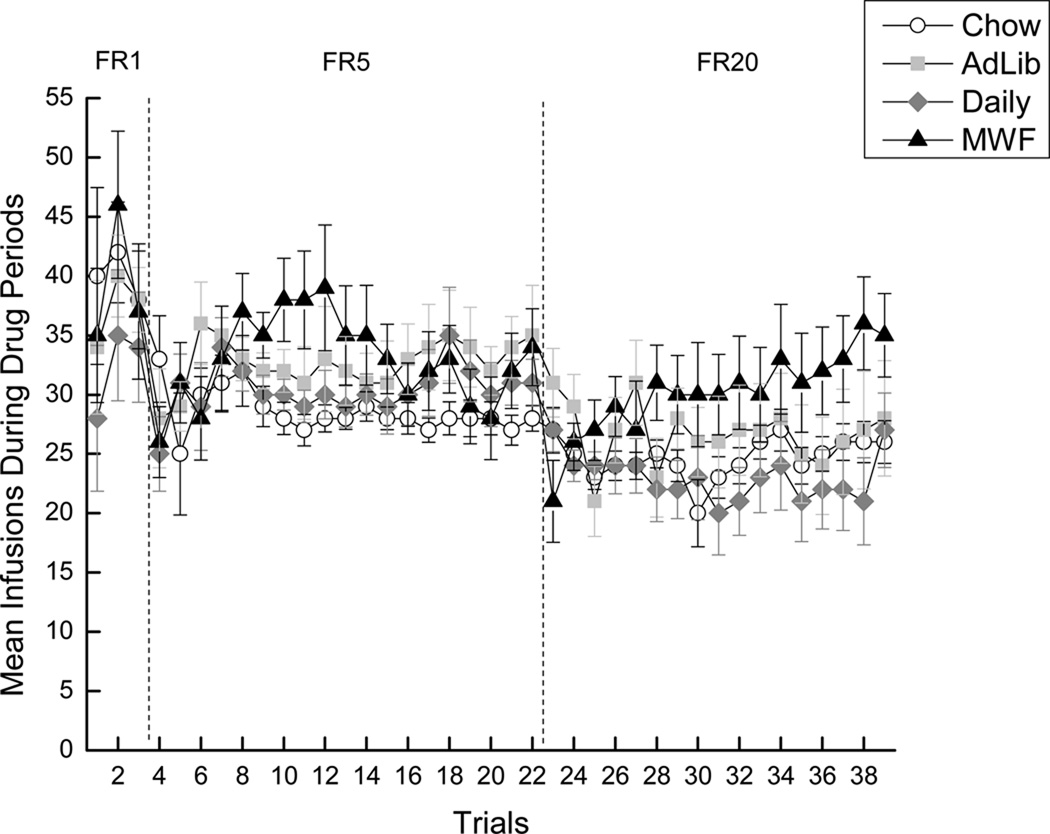
Responding during FR1 trials (trials 1–3) was highly variable and, as such, was not analyzed (see left panel of Figure 2). Upon analysis of the data from the FR5 trials (trials 4–22), the results of a 4 × 19 mixed factorial ANOVA varying Group (Chow, Ad Lib, Daily, or MWF) and Trials (4–22) revealed a significant main effect of Trials, F(18, 954)=2.50, p < 0.01, indicating that the number of cocaine infusions self-administered increased across trials (see the center panel of Figure 2). Neither a main effect of Group nor the Group × Trials interaction were significant, ps > 0.05. Analysis of the FR20 trials (trials 23–39) using a 4 × 17 mixed factorial ANOVA varying Group (Chow, Ad Lib, Daily, or MWF) and Trials (23–39) also revealed a significant main effect of Trials, F(16, 640)=1.63, p = 0.05, indicating that the number of cocaine infusions self-administered increased across trials, and a significant Group × Trials interaction, F(48, 640)=1.63, p < 0.01 (see the right panel of Figure 2). Post hoc Newman-Keuls tests of the two-way interaction, however, did not add to the interpretation of the data. These results indicate that, overall, rats with a history of fat exposure (i.e., Ad Lib, Daily, and MWF) tended to self-administer more cocaine than Chow rats during the FR5 trials, though these results did not reach statistical significance. Moreover, when the schedule of reinforcement was increased to FR20, responding initially converged among the groups. However, by the end of FR20 training, the MWF group (the fat-bingeing group) began to emerge as the group that self-administered the most cocaine. Again, this tendency did not attain statistical significance. While differences in the overall intake of cocaine may be intuitively expected, the absence of an effect during FR sessions in this paradigm is consistent with Deroche-Gamonet et al. (2004), where differences in drug intake did not manifest until challenged with SNA or PR testing.
Figure 2.
Mean (+/− SEM) cocaine infusions self-administered during the 40-min signaled active drug periods. White circles represent the Chow group, light gray squares represent the Ad Lib group, gray diamonds represent the Daily group, and black triangles represent the MWF group.
Compulsive drug seeking during terminal SNA periods
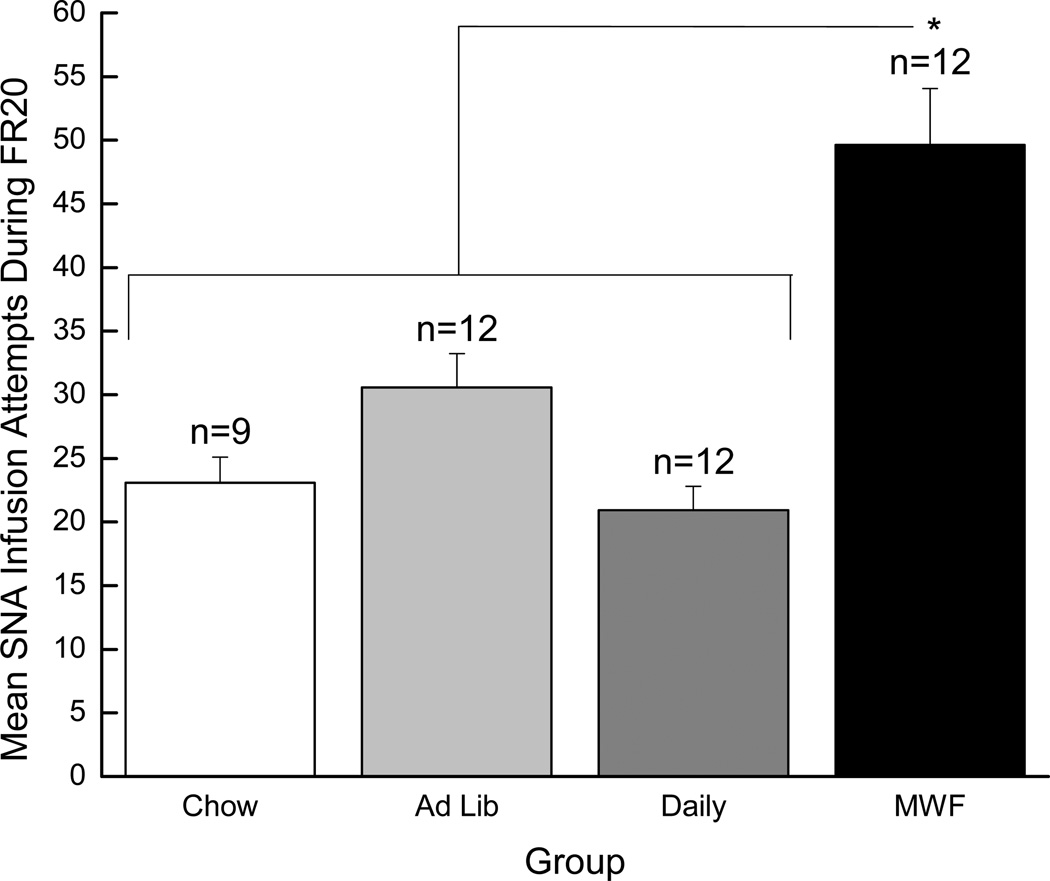
One of the revealing manipulations in the Deroche-Gamonet paradigm was responding during the daily SNA periods. In accordance, throughout FR trials SNA responding was tracked daily. Here we report on SNA responding across the seventeen FR20 trials. These trials were analyzed on the basis of the observation made by Deroche-Gamonet et al. (2004) that differences in SNA responding between “addiction-prone” and “addiction-resistant” groups emerge and stabilize over time. Also, it was clear that MWF rats were responding the most overall as FR20 trials progressed (see the right panel of Figure 2). Here and elsewhere, a 4 × 17 mixed factorial ANOVA varying Group (Chow, Ad Lib, Daily, and MWF) and trials (23–39) failed to obtain a significant main effect of Group (p > 0.05), likely because responding by the three “control” groups (Chow, Ad Lib, and Daily) was so uniform (see Figure 3). Effect size analysis was consistent with this interpretation (i.e., the Ad Lib and Daily histories did not have much of an effect on SNA responding when compared to a history of Chow only). Specifically, relative to the Chow group, the Ad Lib and Daily histories had small effects (Cohen’s d 0.23 and −0.09, respectively), whereas the effect of the MWF history was much larger (Cohen’s d 0.62; moderate effect). Given this pattern of data, and our a priori hypotheses, we then conducted Fisher’s LSD tests and determined that the rats in the MWF condition made more infusion attempts during the SNA period than did rats in the Daily condition, p < 0.05, and responding by rats in the Daily group did not differ from responding by rats in either the Ad Lib or the Chow condition, which did not differ from one another, ps < 0.05. On this basis, these three groups (Chow, Ad Lib, and Daily) were combined and compared to the MWF group using a 2 × 17 mixed factorial ANOVA. The results of the 2 × 17 ANOVA revealed a significant main effect of Group, F(1, 43)=6.17, p < 0.02, confirming that the rats in the MWF group made more infusion attempts during the FR20 SNA periods than did rats from all other groups combined. Also, there was a significant main effect of Trials, F(16, 688)=4.24, p < 0.01, as well as a significant Group × Trials interaction, F(16, 688)=2.35, p < 0.01. Post hoc Newman-Keuls tests on the two-way interaction, however, did not find significant differences in responding across trials, ps > 0.05.
Figure 3.
Mean (+/− SEM) infusion attempts made during the 15-min signaled non-availability (SNA) periods while training with an FR20 schedule of reinforcement. The white bar represents the Chow group, the light gray bar represents the Ad Lib group, the gray bar represents the Daily group, and the black bar represents the MWF group. * denotes statistical significance compared to the other three groups combined (p < 0.02).
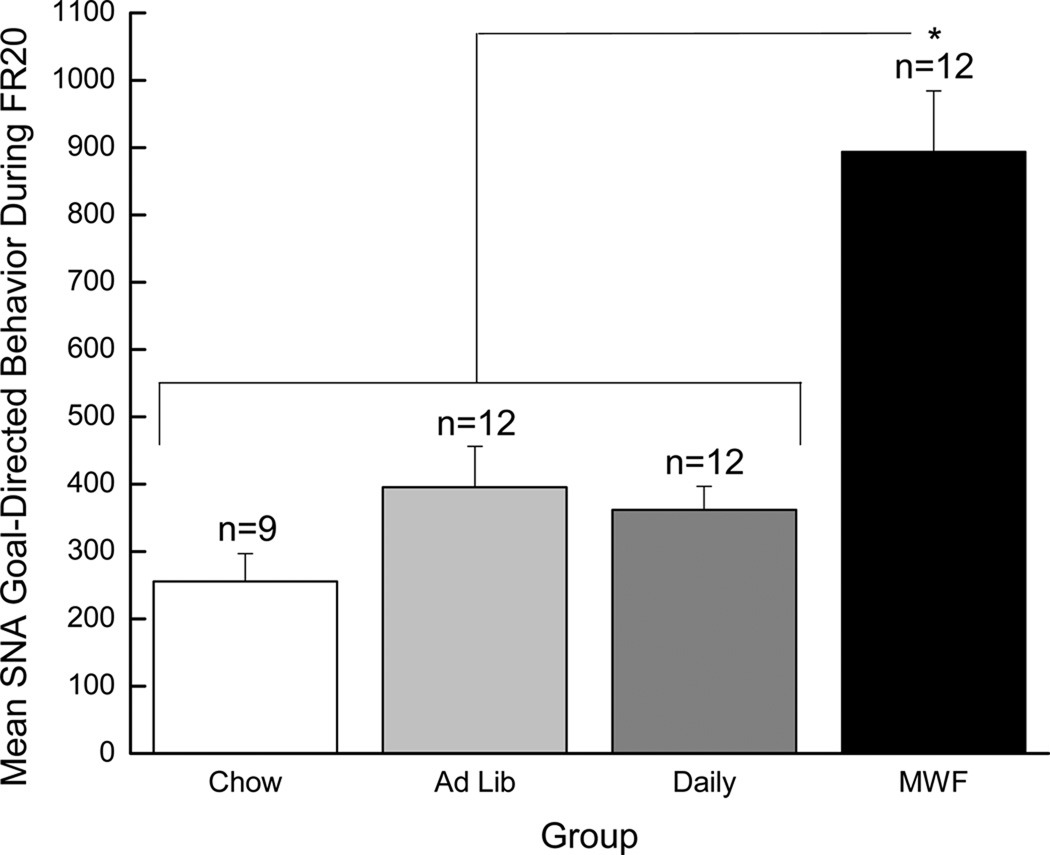
A similar analysis was conducted on the goal-directed behavior (calculated by subtracting the total number of inactive responses from the total number of active responses) exhibited during FR20 SNA periods. This measure of goal-directed behavior is of interest because it has been found to be greater in large drug-takers than in small drug-takers and is augmented in small drug-takers by acute sleep deprivation (Puhl et al., 2009). Once again, the 4 × 17 mixed factorial ANOVA was not significant, F (2,41) = 2.32, p > 0.05. However, the MWF effect again was again moderate (Cohen’s d 0.60), while the Ad Lib and Daily effects again were small (Cohen’s d 0.17 and 0.05, respectively), relative to the effect of Chow only. As with the previous measure, Fischer LSD tests confirmed that rats in the MWF group exhibited greater goal-directed behavior (i.e., greater seeking) during the SNA period than did rats in either the Daily or the Chow condition, ps < 0.05, and responding by rats in the Daily, Chow, and Ad Lib conditions did not differ from one another, ps > 0.05 (see Figure 4).
Figure 4.
Mean (+/− SEM) goal-directed responding (total active responses minus total inactive responses) made during the 15-min signaled non-availability (SNA) periods while training with an FR20 schedule of reinforcement. The white bar represents the Chow group, the light gray bar represents the Ad Lib group, the gray bar represents the Daily group, and the black bar represents the MWF group. * denotes statistical significance compared to the other three groups combined (p < 0.01).
The rats in these three groups were then collapsed and compared to the MWF group using a 2 × 17 mixed factorial ANOVA. This analysis revealed a significant main effect of Group, F(1, 43)=7.12, p = 0.01, indicating that rats in the MWF group focused more attention on the cocaine-associated spout during the FR20 SNA periods than did rats from the other three conditions combined. In addition, a significant main effect of Trials, F(16, 688)=4.87, p < 0.01, and a significant Group × Trials interaction, F(16, 688)=1.72, p < 0.05, were obtained. Post hoc tests on the two-way interaction, however, did not reveal significant group differences across trials, ps > 0.05. Taken together, these data indicate that, compared to all other subjects, rats in the MWF condition selectively persisted in responding on the cocaine-associated operandum, even when signaled that cocaine was not available.
Pearson correlation coefficients were calculated in order to determine if the individual propensity to binge during the 1-h fat access period (data from first column of Table 1) or if total fat intake (data from third column of Table 1) predicted SNA responding and goal-directed responding. The results showed that intake during the 1-h access period was significantly and positively associated with SNA responding (r = 0.32; p < 0.03) and goal-directed behavior (r = 0.34, p < 0.03) -- rats that ate the most (binged) during the 1-h fat access period were more likely to respond for cocaine during periods when cues clearly indicated that cocaine was not available. In contrast, the total shortening that previously had been consumed was neither associated with SNA attempts (r = −0.07, p > 0.2) nor goal-directed behavior (r = −0.18, p > 0.2). Thus, the previous history of bingeing on fat, rather than the total amount of fat consumed, predicted subsequent persistence in responding for cocaine during periods of signaled non-availability.
Motivation to work for cocaine during terminal PR testing
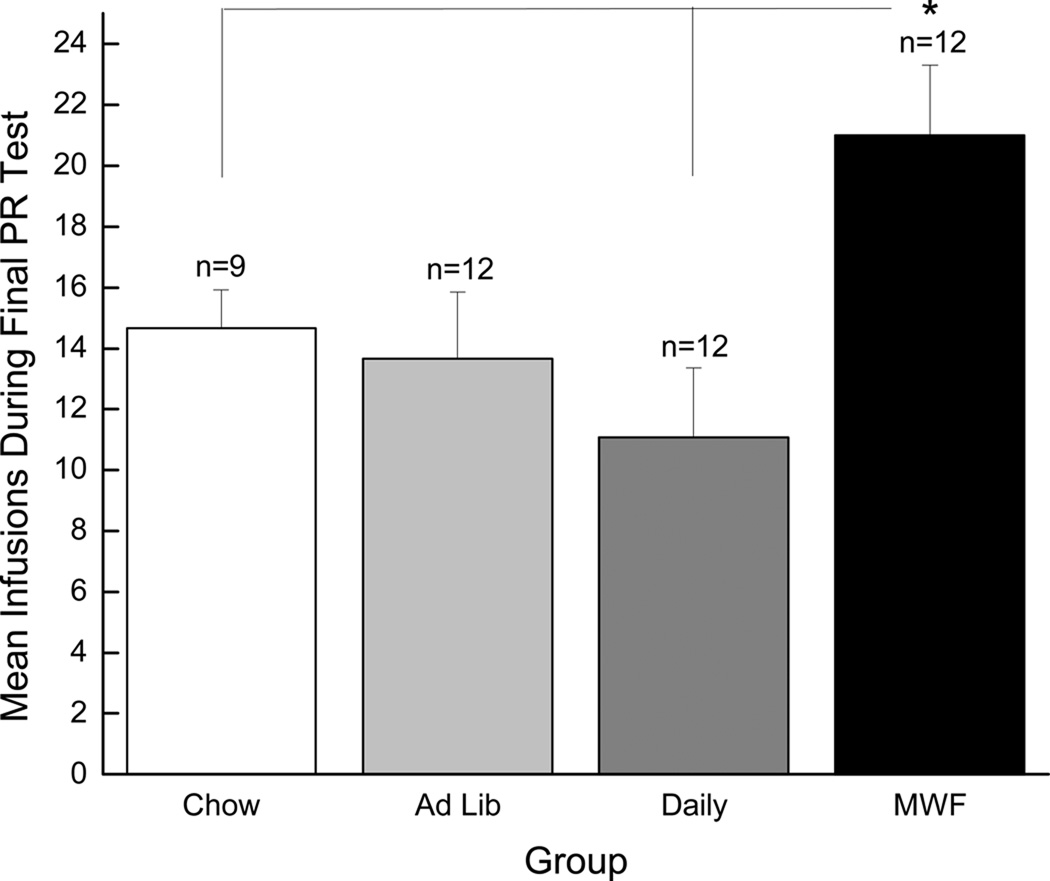
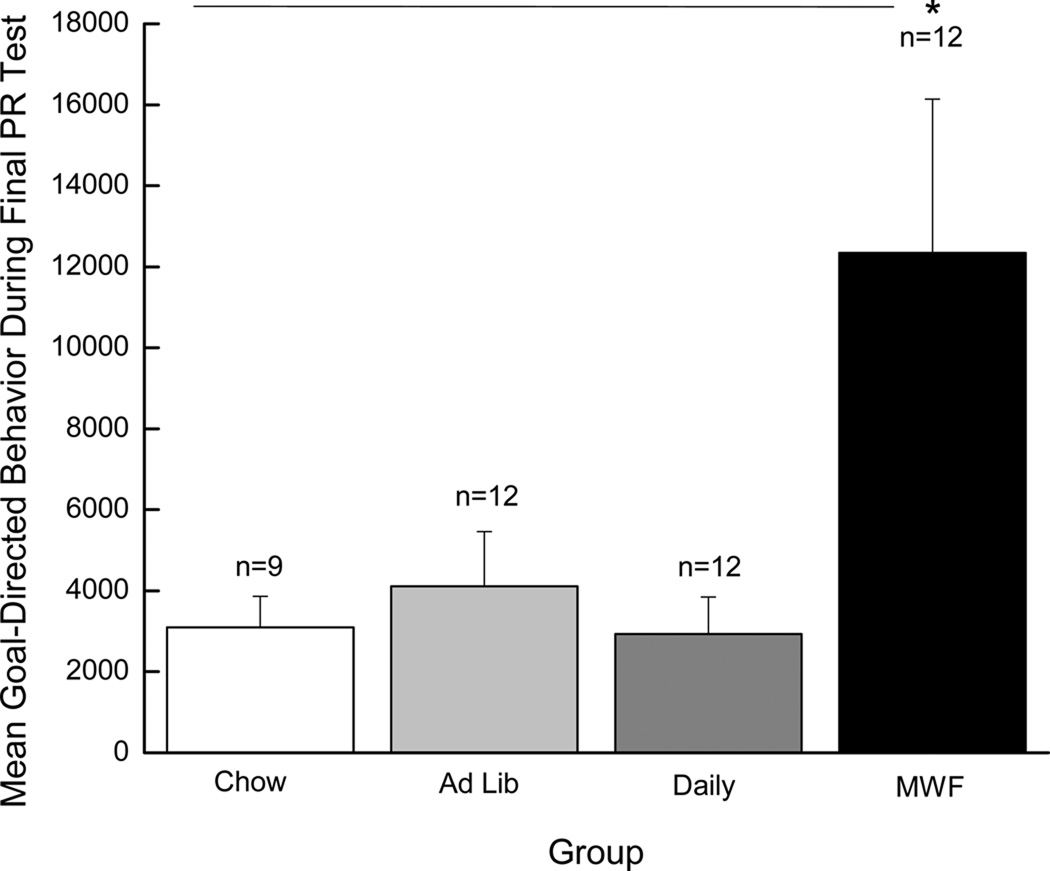
In addition, the motivation to work for drug was repeatedly probed using a progressive ratio (PR) schedule of reinforcement (again, see Figure 1a for an overview of behavioral testing). During PR testing, rats are required to make progressively more licks on the “active” spout to receive each subsequent infusion. Here we report on the number of cocaine infusions self-administered during the final PR test (PR test #6). Significant differences in responding were not observed during the previous five PR tests. Again, emergence of such differences late in training is consistent with data obtained by Deroche-Gamonet et al. (2004). The results of a one-way ANOVA revealed a significant main effect of Group, F(3, 41)=4.10, p < 0.02, and post-hoc Newman-Keuls tests showed that rats in the MWF group took a greater number of infusions (i.e., exhibited higher break points) for cocaine during the final PR test than the Chow and Daily groups, ps < 0.05 (see Figure 5). In addition, goal-directed behavior (calculated by subtracting the total number of inactive responses from the total number of active responses) during the final PR test was examined. The results of a one-way ANOVA revealed a significant main effect of Group, F(3, 41)=4.75, p < 0.01 (see Figure 6). Post hoc Newman Keuls tests confirmed that rats in the MWF group focused more attention on the cocaine-associated empty spout during the final PR test than did rats from any other group, ps < 0.02. Collectively, these data indicate that rats in the MWF condition had greater motivation to seek and take drug than did rats from all other groups.
Figure 5.
Mean (+/− SEM) cocaine infusions self-administered during the final PR test (PR test #6). The white bar represents the Chow group, the light gray bar represents the Ad Lib group, the gray bar represents the Daily group, and the black bar represents the MWF group. * denotes statistical significance compared to the Chow and Daily groups (p < 0.05).
Figure 6.
Mean (+/− SEM) goal-directed responding (total active responses minus total inactive responses) made during the final PR test. The white bar represents the Chow group, the light gray bar represents the Ad Lib group, the gray bar represents the Daily group, and the black bar represents the MWF group. * denotes statistical significance compared to all the other groups (p < 0.02).
To determine if the individual propensity to binge predicted individual PR infusions and goal-directed behavior, Pearson correlation coefficients were determined between the PR data and total fat as well as 1-h intake. Results were similar to those reported above for SNA. Previous intake during the one-hour fat access period was significantly and positively associated with PR infusions (r = 0.34, p < 0.03) as well as PR goal-directed behavior (r = 0.42, p < 0.01). Thus, the propensity to binge predicted the propensity to expend effort for cocaine. Notably, the total shortening that the rats had previously consumed was not associated with PR performance (r = −0.15 and −0.28 for PR infusions and goal-directed behavior, respectively; ps > 0.1). Taken together, these findings show that the manner in which the fat was consumed, but not the total amount of fat ingested, predicts how much an animal will seek, and how hard an animal will work for, cocaine.
“Addiction-like” Behaviors
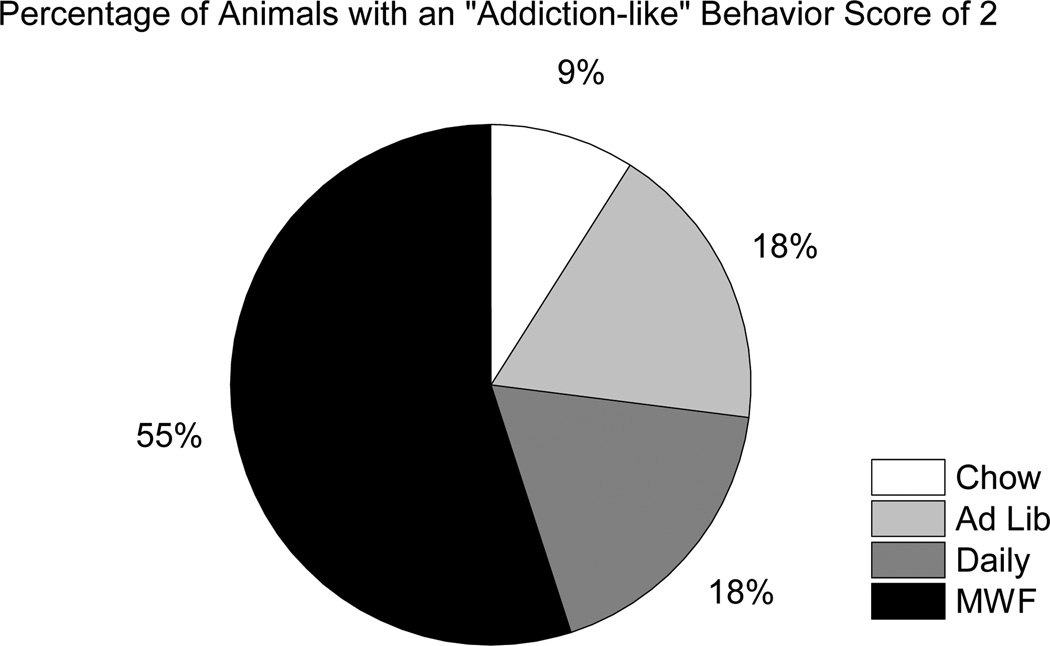
Similar to Deroche-Gamonet et al. (2004), behavioral measures of cocaine seeking and cocaine taking were used to determine “addiction-like” behavior scores for each animal. First, all rats were ranked on the basis of the number of responses made on the “active” empty spout during SNA periods of FR20 trials (trials 23–39) and also on the basis of the number of infusions self-administered during the final PR test. Those rats that ranked in the top 33rd percentile for each measure were considered positive for that particular criterion. All rats, then, received an “addiction-like” behavior score of 0, 1, or 2, depending on the number of criteria they met. Twenty-four percent of all the animals (11 out of 45) scored a 2. Interestingly, of those animals that scored a 2, 55% were from the MWF group, while only 9% were from the Chow group, 18% were from the Ad Lib group, and 18% were from the Daily group (see Figure 7). In addition, 50% (6 out of 12) of MWF rats scored a 2, compared to 17% (2 out of 12) of Daily rats, 17% (2 out of 12) of Ad Lib rats, and 11% (1 out of 9) of Chow rats. These data illustrate that a history of bingeing on a fatty food (in this case, vegetable shortening) can predispose rats to express “addiction-like” behaviors toward a substance of abuse (in this case, cocaine). Total intake of fat, on the other hand, had no impact on the expression of these “addiction-like” behaviors.
Figure 7.
Percentages of animals with an “addiction-like” behavior score of 2. All animals were ranked on the basis of the number of responses made on the “active” empty spout during the 15-min signaled non-availability (SNA) periods across FR20 trials (trials 23–39), as well as the number of cocaine infusions self-administered during the final progressive ratio (PR) test. Those animals in the top 33rd percentile of each ranking were considered positive for that particular criterion. The white portion represents the Chow group, the light gray portion represents the Ad Lib group, the gray portion represents the Daily group, and the black portion represents the MWF group. Rats in the MWF group represented the largest proportion of all rats with an “addiction-like” behavior score of 2.
General Discussion
Here we report that a history of bingeing on fat renders rats more vulnerable to subsequent “addiction-like” behavior for cocaine, even after several months of abstinence from the fat binge protocol. A history of bingeing on fat, then, can lead to long-term behavioral vulnerability, presumably due to long term alterations in neuronal function. A key finding in this study is that consumption of fat in-and-of itself did not increase the likelihood of subsequent “addiction-like” behavior for cocaine. Rather, the intermittent binge-type manner in which the fat was consumed proved critical.
While epidemiological studies in humans are useful for identifying associations between binge eating and substance abuse, the development and use of animal models is critical for systematically identifying the maladaptive behaviors and, ultimately, the underlying neural mechanisms involved in the expression of these disorders. The limited access protocol reliably produces binge-type eating of fat in rats (Corwin, 2004; Corwin et al., 1998; Davis et al., 2007; Dimitriou et al., 2000; Thomas et al., 2002; Wojnicki et al., 2008a, b). In addition, under this model, rats exhibit compulsive fat-seeking and -consuming behaviors that are reminiscent of the drug-seeking and -taking behaviors displayed in drug self-administration models. For example, as shown here, intake of fat escalates to a greater extent in fat-bingeing rats (Corwin et al., 1998; Dimitriou et al., 2000; Wojnicki et al., 2008b), much like the escalation of drug intake now recognized as a behavioral indicator of drug addiction (Ahmed et al., 2002). Also, when previously restricted rats are given prolonged access to fat, bingeing rats consume more than controls (Wojnicki et al., 2008b). This is similar to the increase in self-administration of cocaine under conditions of prolonged access in “addiction-prone” rats (Deroche-Gamonet et al., 2004). Finally, progressive ratio (PR) responding for fat increases after exposure to the MWF fat access protocol (Wojnicki et al., 2006) and fat-bingeing rats exhibit higher breakpoints for fat compared to controls (Wojnicki et al., 2010). This is akin to the escalation of, and ultimately higher, breakpoints seen when “addiction-prone” rats are tested under PR schedules of reinforcement for cocaine (Deroche-Gamonet et al., 2004; Roberts et al., 2007).
Consistent with the studies cited above, the current study demonstrates that restricted access to fat in non-food-deprived rats leads to the development of fat-bingeing behaviors. Not only did the MWF group (the fat-bingeing group) consume more fat than the Chow, Ad Lib, and Daily groups in a given 1-h period, they also consumed more fat in a 1-h period than the Ad Lib group did in an entire 24-h period. Interestingly, a simple history of exposure to a diet high in fat (whether Ad Lib, Daily, or MWF) resulted in the tendency to self-administer more cocaine when the reinforcement schedule was easy (i.e., FR5). However, when shifted to a more difficult reinforcement schedule (i.e., FR20), the MWF group tended to self-administer more cocaine relative to the other groups.
This finding is in keeping with other data obtained from rats in the MWF group in the present study. Specifically, the MWF group exhibited high levels of responding for drug and responded more exclusively on the drug-associated spout during FR20 SNA periods, compared to all other groups. This high level of responding, as well as the highly goal-directed nature of that responding, despite signaling that drug was no longer available, characterize the compulsive drug-seeking behavior described in rats by Deroche-Gamonet et al. (2004) and seen among human drug addicts (American Psychiatric Association, 2000). Furthermore, the MWF group also exhibited higher breakpoints and greater goal-directed responding during terminal PR testing. High motivation to seek and take drug also is characteristic of drug addiction (American Psychiatric Association, 2000). The fact that these compulsive drug-seeking and -taking behaviors appeared only after prolonged, chronic self-administration training is consistent with previous findings (Deroche-Gamonet et al., 2004).
In the present study, however, the expression of “addiction-like” behaviors for cocaine is not attributable merely to genetic differences, as reported by Deroche-Gamonet et al. (2004), but is, instead, driven by prior intermittent, excessive consumption of a fatty food. In particular, these “addiction-like” behaviors for cocaine occurred predominantly in the rats with a history of fat bingeing. In fact, when “addiction-like” behavior scores were calculated, nearly 50% of the rats from the MWF group were positive for both criteria. These data are consistent with studies showing that intermittent excessive intake of sucrose enhances behavioral sensitization to amphetamine (Avena and Hoebel, 2003) and increases ethanol consumption (Avena et al., 2004). Also, it is interesting to note that, while the rats in the Ad Lib group ultimately consumed more fat than all other groups overall (representing about 53% of dietary energy during Week 6 of the fat access protocols), they were three times less likely to exhibit “addiction-like” behavior for cocaine than the MWF group (total dietary fat ~31% energy), the group with the most restricted access to fat (i.e., only 2 out of 12 Ad Lib rats scored a 2, while 6 out of 12 MWF rats scored a 2).
That said, all rats with a history of having consumed fat were more likely to exhibit “addiction-like” behavior (i.e., to score a 1 or a 2) for cocaine compared to rats in the Chow group (see Figure 10). These results appear contrary to other reports in which maintenance on a high-fat diet attenuated the dopaminergic response to reward (Davis et al., 2008) and, of more direct relevance here, slowed acquisition of cocaine self-administration (Wellman et al., 2007). However, unlike the procedures used in the current report, fat was not optional in these previous studies (i.e., the rats had no choice about what to eat). Maintenance on a single diet versus a diet that allows for choice has been reported to differentially affect brain serotonin and dopamine (Thibault, 1992). Therefore, the dietary protocols used in previous research versus the current study may have contributed to the different effects obtained.
In addition, the high-fat diet was simultaneously available with cocaine in the Wellman et al. study (i.e., it was available in both the home cage and in the test chamber). As such, it may not have been fat intake, per se, but the simultaneous availability of the alternative reward that reduced acquisition of drug-taking via a contrast effect (Flaherty, 1996; Grigson, 2008). In support, the near simultaneous presentation of a sweet fully blunts the dopamine response to morphine (Grigson & Hajnal, 2007) and acquisition of cocaine self-administration is markedly disrupted by the simultaneous presentation of a sweet (Carroll and Lac, 1993; Lenoir et al., 2007) or by housing in an enriched environment (Puhl et al., submitted). In the present study, on the other hand, well more than a month elapsed between exposure to fat and the start of self-administration testing. These data offer further support for the notion that it is not the consumption of fat, per se, that is responsible for the effects seen here, but rather the manner in which the fat is presented and consumed. Collectively, these data indicate that exposure to conditions that promote excessive behavior toward one substance (in this case, fat) predisposes excessive behavior toward another substance (in this case, cocaine).
Differences in body composition are unlikely to have contributed to the present results. Body weights did not differ among the groups and previous research has shown that the body composition of MWF rats does not differ from chow controls (Corwin et al., 1998; Dimitriou et al., 2000). Since the total cumulative fat intake by the MWF group was less than that of the other groups with fat access, fat intake alone also cannot account for these results. Rather, as stated, it appears that the intermittent fat access history and/or the manner in which the fat was consumed (i.e., bingeing and/or the loss of control), endangered the MWF rats.
While one can reasonably speculate on underlying mechanisms, one point is clear from the behavioral data: a history of bingeing on fat changed the brain and/or physiology in a manner that predisposed these rats to seek and take drug when tested more than a month later. The neuronal alterations that occur as a function of intermittent opportunities to consume optional palatable foods are only beginning to be elucidated. Hoebel and colleagues have reported that chronic intermittent access to sugar solutions, but not chronic continuous access to the sugar, provokes neurological and behavioral changes similar to those reported for drugs of abuse, including opioid-like withdrawal symptoms, as well as alterations in brain dopamine and acetylcholine (Avena et al. 2008; Umberg and Pothos 2011). Similar to what is reported here, these studies emphasize the importance of how the palatable food is consumed rather than how much is consumed. However, in the Hoebel et al. reports, intermittency consisted of 12-h access to sugar every day, whereas in the present study, intermittency consisted of brief daily access to fat. Indeed, the greatest “addiction-like” behavior was revealed when access to fat was highly restricted, allowing for only 1 h access on MWF.
While differences in the protocols used in different labs make mechanistic comparisons challenging, circuitry including the PFC may be involved. Intermittent opportunities to consume palatable foods may engage midbrain dopamine neurons in a manner that is different from continuous and or repeated daily access, particularly when cues predicting those opportunities are ambiguous. Since the MWF rats in the present study were housed in the same room as the other groups, cues predicting shortening access were ambiguous or uncertain in the MWF group. This may be similar to the uncertainty surrounding binge episodes among human binge eaters: binge eating often is not planned and can be triggered by the uncertain occurrence of a variety of events (American Psychiatric Association, 2000). Midbrain dopamine neurons respond differentially to certain and uncertain cues associated with food (Fiorillo et al. 2003). These neurons project to several brain regions including the PFC and amygdala, which also have been shown to be responsive to uncertainty (Schultz et al., 2008). In addition, dopamine increases in the PFC when uncertainty is associated with food reward delivery (Richardson & Gratton, 1998; Stefani and Moghaddam, 2006). Recent data from the Corwin lab indicate alterations in PFC function, which appear to involve D2-like receptors, in rats maintained on the MWF binge protocol relative to rats that have daily fat access (Babbs and Corwin, 2011; Corwin and Babbs, 2011). Additional evidence is accumulating for the involvement of the PFC and related circuitry in addiction (e.g. Feil et al., 2010). Thus, the MWF rats in the present study may have been primed to respond more robustly for cocaine due to alterations in dopamine signaling within the PFC provoked, in part, by their previous history of uncertain fat access opportunities.
Stress-related pathways also may be involved. In a manner similar to that proposed for drug addiction, corticotropin releasing factor (CRF) has been proposed to create an aversive state during periods of palatable food abstinence, which then provokes further intake when the palatable food becomes available (Cottone et al. 2009; Koob 2010; Koob and Zorrilla 2010). Indeed, consumption of palatable fatty and sugary foods has been shown to attenuate stress responsivity (Christiansen et al. 2011; Kinzig et al. 2008; Pecoraro et al. 2004; Ulrich-Lai et al. 2010). When opportunities to consume the palatable food are no longer available, however, other “addiction-like” behaviors may develop, as is reported here. Interestingly, CRF can activate VTA dopamine neurons, either directly (Wanat et al 2008), or indirectly via activation of hypothalamic orexin neurons (Korotkova et al. 2003; Moorman and Aston-Jones 2010; Winsky-Sommerer et al. 2004). Furthermore, elevations of dopamine within the PFC, but not within the NAc, result when orexin is administered into the VTA (Vittoz & Berridge, 2006). Thus, midbrain dopamine neurons may be differentially activated by binge uncertainty as well as by orexin signaling associated with CRF during binge abstinence. This would disrupt signaling in the PFC, resulting in loss of control during a binge episode, and may ultimately increase vulnerability for other “addiction-like” behaviors. Future studies must test the merits of this hypothesis.
In summary, these findings provide behavioral evidence that bingeing on food can change the brain, leading to increased vulnerability for “addiction-like” behaviors for drug in rats. As such, the data offer an operational explanation for the co-morbid expression of binge-type disorders and substance abuse in humans. Due to the overlap in neural substrates involved in the processing of the rewarding nature of foods (especially highly palatable foods, such as those rich in fat and sugar) and drugs of abuse, the brain responds similarly to conditions promoting excessive, possibly dysfunctional, food intake and compulsive drug abuse. Unfortunately, it seems plausible that once the neural mechanisms mediating addiction have been “turned on” in the brain for one stimulus, the individual is much more likely to develop dysregulated, compulsive responding for another stimulus. Thus, when one such behavior spirals out of control, circuitry in the brain is altered, predisposing the development of similar dysfunctional consummatory behaviors for other rewarding stimuli. Accordingly, the data from the present study highlight the critical importance of behavior and experience in shaping the aberrant consummatory behaviors that are born of addiction. While approximately 17% of subjects exposed to cocaine are found to develop “addiction-like” behaviors for cocaine (Deroche-Gamonet et al., 2004; Anthony et al., 1994), the likelihood of such “addiction-like” behavior tripled for rats with a history of having binged on fat. This experience, then, shifted the odds, likely due to neuronal alterations that occurred, not from effects of the fat itself, but from the learning that took place during its intermittent, and possibly uncertain, exposure.
Together, these results also offer a means to better explore parameters that may increase vulnerability to, or conversely, prevention of the development of such “addiction-like” behaviors. Importantly, the present study provides compelling evidence that fat is not addictive, but the way in which fat is consumed can promote long-term behavioral vulnerability to addiction-like behavior. While future studies will need to examine the general nature of this phenomenon (e.g., whether loss of control for sugar or a mixture of sugar and fat also will promote “addiction-like” behavior for cocaine or heroin, or whether ‘bingeing’ on drug also can increase vulnerability for bingeing on fat) and its neuronal underpinnings, the present data indicate the need to be mindful of the turnstile nature of these behaviors, even in a clinical setting focused on treatment and relapse prevention.
Acknowledgements
The authors would like to thank the National Institute on Drug Abuse for generously supplying the cocaine HCl used in this study. This work was supported by grants DA09815 and DA023315 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nature Neuroscience. 2002;5(7):625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of the Mental Disorders 4th Edition-Text Revision. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental & Clinical Psychopharmacology. 1994;2(3):244–268. [Google Scholar]
- Avena NM, Carrillo CA, Needham L, Leibowitz SF, Hoebel BG. Sugar-dependent rats show enhanced intake of unsweetened ethanol. Alcohol. 2004;34(2–3):203–209. doi: 10.1016/j.alcohol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122(1):17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience & Biobehavioral Reviews. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs RK, Corwin RL. Exacerbation of binge eating by inactivation of prefrontal cortex. Society for Neuroscience Abstract. (accepted) [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF. Galanin and consummatory behavior: Special relationship with dietary fat, alcohol, and circulating lipids. EXS. 2010;102:87–111. doi: 10.1007/978-3-0346-0228-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002;13(12):1575–1578. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2003;284(5):R1260–R1268. doi: 10.1152/ajpregu.00716.2002. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Research. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewerton TD, Lydiard RB, Herzog DB, Brotman AW, O’Neill PM, Ballenger JC. Comorbidity of axis I psychiatric disorders in bulimia nervosa. Journal of Clinical Psychiatry. 1995;56(2):77–80. [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Medical and psychiatric morbidity in obese women with and without binge eating. International Journal of Eating Disorders. 2002;32(1):72–78. doi: 10.1002/eat.10072. [DOI] [PubMed] [Google Scholar]
- Bushnell JA, Wells JE, McKenzie JM, Hornblow AR, Oakley-Browne MA, Joyce PR. Bulimia comorbidity in the general population and in the clinic. Psychological Medicine. 1994;24(3):605–611. doi: 10.1017/s0033291700027756. [DOI] [PubMed] [Google Scholar]
- Carrillo CA, Leibowitz SF, Karatayev O, Hoebel BG. A high-fat meal or injection of lipids stimulates ethanol intake. Alcohol. 2004;34(2–3):197–202. doi: 10.1016/j.alcohol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: Effects of nondrug alternative reinforcers on acquisition. Psychopharmacology. 1993;110(1–2):5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Christiansen AM, Dekloet AD, Ulrich-Lai YM, Herman JP. "Snacking" causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiology & Behavior. 2011;103(1):111–116. doi: 10.1016/j.physbeh.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obesity Research. 2002;10(6):478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Conason AH, Brunstein-Klomek A, Sher L. Recognizing alcohol and drug abuse in patients with eating disorders. Quarterly Journal of Medicine. 2006;99(5):335–339. doi: 10.1093/qjmed/hcl030. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42(2):139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Babbs RK. Rodent models of binge eating: Are they models of addiction? Institute for Laboratory Animal Research Journal. doi: 10.1093/ilar.53.1.23. (In Press) [DOI] [PubMed] [Google Scholar]
- Corwin RL, Babbs RK. Exacerbation of binge eating by blockade of prefrontal cortical D2 receptors. Society for Neuroscience Abstract. (accepted) [Google Scholar]
- Corwin RL, Wojnicki FH. Baclofen, raclopride, and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behavioural Pharmacology. 2009;20(5–6):537–548. doi: 10.1097/FBP.0b013e3283313168. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Avena NM, Boggiano MM. Feeding and reward: Perspectives from three rat models of binge eating. Physiology and behavior. 2011;104(1):87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FHE, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiology & Behavior. 1998;65(3):545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Melhorn SJ, Shurdan JD, Heiman JU, Tschöp MH, Clegg DJ, Benoit SC. Comparison of hydrogenated vegetable shortening and nutritionally complete high-fat diet on limited access-binge behavior in rats. Physiology & Behavior. 2007;92(5):924–930. doi: 10.1016/j.physbeh.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Tschöp MH, Lipton JW, Clegg DJ, Benoit SC. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behavioral Neuroscience. 2008;122(6):1257–1263. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Conway K, Dierker L, Glantz M, Kalaydjian A, Merikangas K, Sampson N, Swendsen J, Kessler RC. Does the 'gateway' matter? Associations between the order of drug use initiation and the development of drug dependence in the National Comorbidity Study Replication. Psychological Medicine. 2008;39(1):157–167. doi: 10.1017/S0033291708003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza V. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- DeSousa NJ, Bush DE, Vaccarino FJ. Self-administration of intravenous amphetamine is predicted by individual differences in sucrose feeding in rats. Psychopharmacology. 2000;148(1):52–58. doi: 10.1007/s002130050024. [DOI] [PubMed] [Google Scholar]
- Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. International Journal of Eating Disorders. 2000;28(4):436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yücel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neuroscience Biobehavioral Reviews. 2010;35(2):248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299(5614):1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Incentive Relativity. In: Gray J, editor. Problems in the Behavioural Science. New York: Cambridge University Press; 1996. [Google Scholar]
- Franko DL, Dorer DJ, Keel PK, Jackson S, Manzo MP, Herzog DB. How do eating disorders and alcohol use disorder influence each other? International. Journal of Eating Disorders. 2005;38(3):200–207. doi: 10.1002/eat.20178. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Drummond SPA. Medication and substance abuse. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Saunders; 2000. pp. 1176–1195. [Google Scholar]
- Gosnell BA. Sucrose intake predicts rate of acquisition of cocaine self-administration. Psychopharmacology. 2000;149(3):286–292. doi: 10.1007/s002130000375. [DOI] [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Research. 2005;1031(2):194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Reward comparison: The Achilles’ heel and hope for addiction. Drug Discovery Today: Disease Models. 2008;5(4):227–233. doi: 10.1016/j.ddmod.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. Neuroreport. 2002;13(17):2213–2216. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JG, Spitzer RL, Williams JBW, Kroenke K, Linzer M, Brody D, deGruy F, Hahn S. Psychiatric comorbidity, health status, and functional impairment associated with alcohol abuse and dependence in primary care patients: Findings of the Prime MD-1000 study. In: Marlatt GA, VandenBos GR, editors. Addictive Behaviors. Washington, D.C: American Psychological Association; 1997. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010;13(5):529–531. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JM, Gold MS, Sweeney D, Pottash AL. Eating disorders and cocaine abuse: A survey of 259 cocaine abusers. Journal of Clinical Psychiatry. 1987;48(2):47–50. [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. Journal of Neuroscience. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiology & Behavior. 2008;95(1–2):108–113. doi: 10.1016/j.physbeh.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Research. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsycopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Current Opinion in Investigational Drugs. 2010;11(1):63–71. [PMC free article] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA. Fat-preferring rats consume more alcohol than carbohydrate-preferring rats. Alcohol. 1991;8(4):313–316. doi: 10.1016/0741-8329(91)90465-9. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. Overconsumption of dietary fat and alcohol: Mechanisms involving lipids and hypothalamic peptides. Physiology & Behavior. 2007;91(5):513–521. doi: 10.1016/j.physbeh.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2(1):e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. http://www.nih.gov.
- National Institute on Drug Abuse. http://www.nida.nih.gov.
- National Women’s Health Information Center. http://www.womenshealth.gov.
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pelchat ML. Of human bondage: food craving, obsession, compulsion, and addiction. Physiology & Behavior. 2002;76(3):347–352. doi: 10.1016/s0031-9384(02)00757-6. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Gratton A. Changes in medial prefrontal cortical dopamine levels associated with response-contingent food reward: An electrochemical study in rat. Journal of Neuroscience. 1998;18(21):9130–9138. doi: 10.1523/JNEUROSCI.18-21-09130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Progess in Neuro-psychopharmacology & Biological Psychiatry. 2007;31(8):1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum GM, Martin RJ, Roane DS. Discontinuation of sustained sucrose-feeding aggravates morphine withdrawal. Brain Research Bulletin. 1990;24(4):565–568. doi: 10.1016/0361-9230(90)90160-2. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105(3):535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Preuschoff K, Camerer C, Hsu M, Fiorillo CD, Tobler PN, Bossaerts P. Explicit neural signals reflecting reward uncertainty. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2008;363(1511):3801–3811. doi: 10.1098/rstb.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens, and dorsal striatum. Journal of Neuroscience. 2006;26(34):8810–8818. doi: 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Swanson CJ, Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behavioral Brain Research. 1998;93(1–2):43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10–4586. Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health, Vol I. Summary of National Findings. [Google Scholar]
- Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents: Results from the National Comorbidity Survey Replication Adolescent Supplement. Archives of General Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.22. DOI:10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalheimer W, Cook S. How to calculate effect sizes from published research articles: A simplified methodology. 2002 Retrieved May 12, 2011 from http://work-learning.com/effect_sizes.htm. [Google Scholar]
- Thibault L. Influence of feeding paradigm in rats on temporal pattern of: II. Brain serotoninergic and catecholaminergic systems. Chronobiology International. 1992;9(1):19–34. doi: 10.3109/07420529209064513. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Rice HB, Weinstock D, Corwin RL. Effects of aging on food intake and body composition in rats. Physiology & Behavior. 2002;76(4–5):487–500. doi: 10.1016/s0031-9384(02)00800-4. [DOI] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behavioral Neuroscience. 2009;123(4):913–925. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberg EN, Pothos EN. Neurobiology of aversive states. Physiology & Behavior. 2011;104(1):69–75. doi: 10.1016/j.physbeh.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux With the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31(2):384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, Galanti K, Selig PA, Han H, Zhu W, Wong CT, Fowler JS. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity. 2011 doi: 10.1038/oby.2011.27. DOI:10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman PJ, Nation JR, Davis KW. Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet. Pharmacology, Biochemistry & Behavior. 2007;88(1):89–93. doi: 10.1016/j.pbb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederman MW, Pryor T. Substance use among women with eating disorders. International Journal of Eating Disorders. 1996;20(2):163–168. doi: 10.1002/(SICI)1098-108X(199609)20:2<163::AID-EAT6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Wilson GT. Binge eating and addictive disorders. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment, and Treatment. New York: Guilford Press; 1993. pp. 97–120. [Google Scholar]
- Wojnicki FH, Roberts DC, Corwin RL. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food-deprived rats. Pharmacology, Biochemistry & Behavior. 2006;84(2):197–206. doi: 10.1016/j.pbb.2006.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FH, Charny G, Corwin RL. Binge-type behavior in rats consuming trans-fat-free shortening. Physiology & Behavior. 2008a;94(4):627–629. doi: 10.1016/j.physbeh.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FH, Johnson DS, Corwin RL. Access conditions affect binge-type shortening consumption in rats. Physiology & Behavior. 2008b;95(5):649–657. doi: 10.1016/j.physbeh.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FH, Babbs RK, Corwin RL. Reinforcing efficacy of fat, as assessed by progressive ratio responding, depends upon availability not amount consumed. Physiology & Behavior. 2010 doi: 10.1016/j.physbeh.2010.03.004. DOI: 10.1016/j.physbeh.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]